Abstract
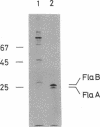
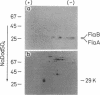
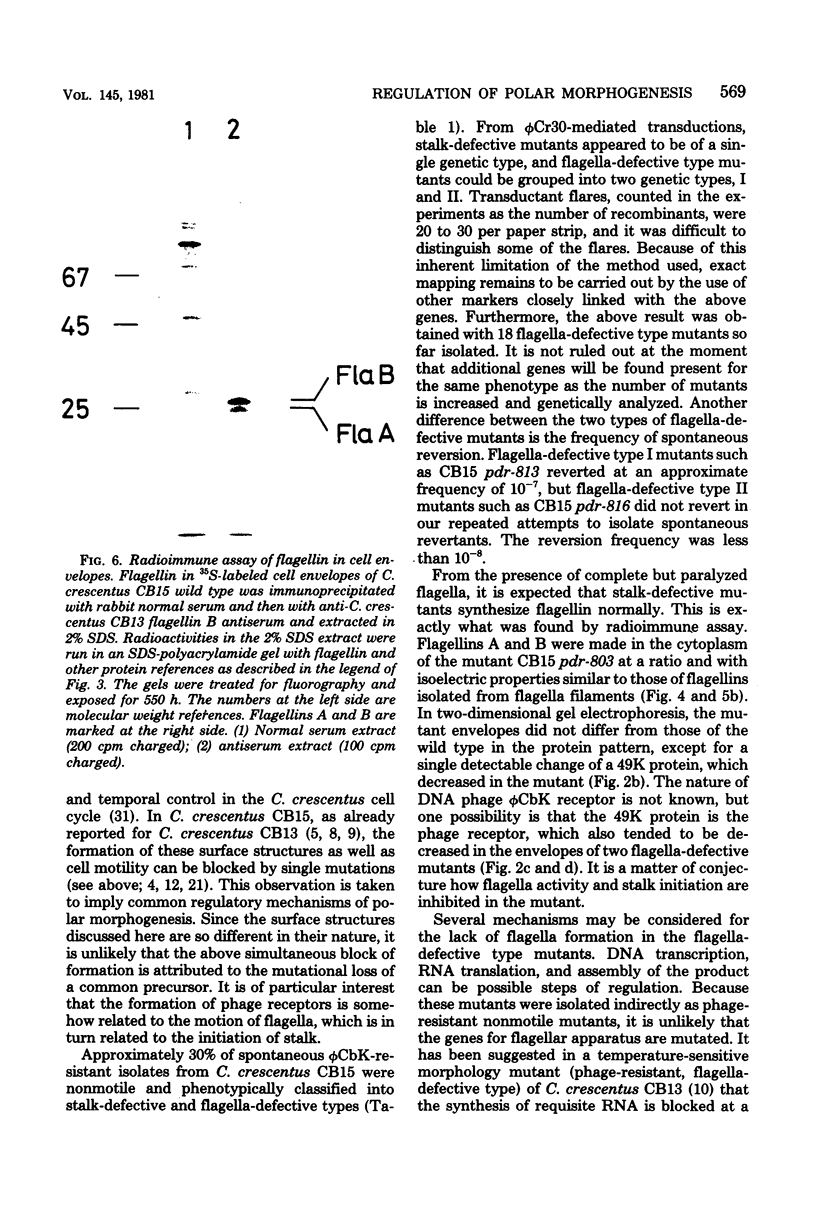
Deoxyribonucleic acid (DNA) phage phi CbK-resistant nonmotile mutants of Caulobacter crescentus CB15 were examined for their formation of polar surface structures (a stalk, a single flagellum, pili, and DNA phage receptors). These mutants were devoid of pili and DNA phage receptors and simultaneously defective either in both stalk formation and flagellar activity (stalk-defective type) or in the formation of normal flagella (flagella-defective type). DNA phage phi Cr30-mediated transductions revealed that stalk-defective mutants were of a single genetic type, whereas flagella-defective mutants were grouped into two different genetic types, I and II. To investigate how membrane proteins change in the above morphology mutants, cell envelopes pulse-labeled with L-[35S]methionine were analyzed by two-dimensional gel electrophoresis. No gross change of membrane proteins was observed in the stalk-defective mutant CB15 pdr-803, except a 49,000-molecular-weight (49K) protein which was found reduced. However, a 27K, two 28.5K, and a 70.5K protein were missing from the membrane of the flagella-defective type I mutant CB15 pdr-813. These proteins are most likely to be flagella-related protein, flagellins A and B, and hook protein, respectively. In another flagella-defective type II mutant, CB15 pdr-816, the 27K and two 28.5K proteins were similarly absent but the 70.5K protein was consistently present in the membrane. The synthesis of flagellin was next assayed radioimmunologically in the above 35S-labeled mutants. Stalk-defective CB15 pdr-803 synthesized flagellin normally, compared to the wild-type strain. Flagellins A (26K) and B (28K) formed multiple spots in isoelectric focusing. A 29K protein was also detected in the flagellin-specific radioactivity from the cytoplasm. Flagella-defective type I CB15 pdr-813 synthesized flagellin only at a basal level. Thus transcription or translation of flagellin appeared to be repressed in this mutant. Another flagella-defective type II strain, CB15 pdr-816, however, synthesized flagellin at an apparently enhanced rate compared with the wild type. Flagellin synthesized in CB15 pdr-816 was flagellin A and a smaller 22K flagellin. Flagellin B was not synthesized in the mutant. It then follows that flagellin B is not a precursor of flagellin A and the 22K flagellin. Flagella-defective type II CB15 pdr-816, without flagellin B, formed a stub structure with a hook attached to one end instead of normal flagella. In the wild-type membrane, flagellin B was the major flagellin, whereas flagellin A was major in the cytoplasm and the flagellar filament. It is suggested from these results that flagellin B is important in the assembly of normal flagella.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian-Keshishian N., Shapiro L. Stalked bacteria: properties of deoxriybonucleic acid bacteriophage phiCbK. J Virol. 1970 Jun;5(6):795–800. doi: 10.1128/jvi.5.6.795-800.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnen S. T., Newton A. Chromosome replication during development in Caulobacter crescentus. J Mol Biol. 1972 Mar 14;64(3):671–680. doi: 10.1016/0022-2836(72)90090-3. [DOI] [PubMed] [Google Scholar]
- Ely B., Johnson R. C. Generalized Transduction in CAULOBACTER CRESCENTUS. Genetics. 1977 Nov;87(3):391–399. doi: 10.1093/genetics/87.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Iba H., Okada Y. Stalkless mutants of Caulobacter crescentus. J Bacteriol. 1977 Jul;131(1):280–287. doi: 10.1128/jb.131.1.280-287.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Koyasu S., Okada Y. Characterization of two flagella-related proteins from Caulobacter crescentus. FEBS Lett. 1978 Nov 1;95(1):70–75. doi: 10.1016/0014-5793(78)80054-4. [DOI] [PubMed] [Google Scholar]
- Fukuda A., Miyakawa K., Iba H., Okada Y. A flagellotropic bacteriophage and flagella formation in Caulobacter. Virology. 1976 Jun;71(2):583–592. doi: 10.1016/0042-6822(76)90383-4. [DOI] [PubMed] [Google Scholar]
- Fukuda A., Miyakawa K., Iida H., Okada Y. Regulation of polar surface structures in Caulobacter crescentus: pleiotropic mutations affect the coordinate morphogenesis of flagella, pili and phage receptors. Mol Gen Genet. 1976 Dec 8;149(2):167–173. doi: 10.1007/BF00332885. [DOI] [PubMed] [Google Scholar]
- Fukuda A., Okada Y. Effect of macromolecular synthesis on the coordinate morphogenesis of polar surface structures in Caulobacter crescentus. J Bacteriol. 1977 Jun;130(3):1199–1205. doi: 10.1128/jb.130.3.1199-1205.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Fukuda A., Okada Y. Chromosome replication in Caulobacter crescentus growing in a nutrient broth. J Bacteriol. 1977 Mar;129(3):1192–1197. doi: 10.1128/jb.129.3.1192-1197.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979 Jan;137(1):627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Walsh M. P., Ely B., Shapiro L. Flagellar hook and basal complex of Caulobacter crescentus. J Bacteriol. 1979 Jun;138(3):984–989. doi: 10.1128/jb.138.3.984-989.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Kurn N., Ammer S., Shapiro L. A pleiotropic mutation affecting expression of polar development events in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3157–3161. doi: 10.1073/pnas.71.8.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Caulobacter crescentus pili: structure and stage-specific expression. J Bacteriol. 1977 Jul;131(1):340–346. doi: 10.1128/jb.131.1.340-346.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Caulobacter flagellar organelle: synthesis, compartmentation, and assembly. J Bacteriol. 1978 Sep;135(3):1062–1069. doi: 10.1128/jb.135.3.1062-1069.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Physical characterization of Caulobacter crescentus flagella. J Bacteriol. 1976 Oct;128(1):435–444. doi: 10.1128/jb.128.1.435-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., DeMartini M., Agabian N. Isolation and characterization of Caulobacter crescentus flagellar hooks. J Bacteriol. 1978 Nov;136(2):795–798. doi: 10.1128/jb.136.2.795-798.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., Farmer S., Agabian N. Adsorption properties of stage-specific Caulobacter phage phiCbK. Virology. 1977 Mar;77(1):401–407. doi: 10.1016/0042-6822(77)90436-6. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Marino W., Ammer S., Shapiro L. Conditional surface structure mutants of Caulobacter crescentus temperature-sensitive flagella formation due to an altered flagellin monomer. J Mol Biol. 1976 Oct 25;107(2):115–130. doi: 10.1016/s0022-2836(76)80021-6. [DOI] [PubMed] [Google Scholar]
- Miyakawa K., Fukuda A., Okada Y. Isolation and characterization of RNA phages for Caulobacter crescentus. Virology. 1976 Sep;73(2):461–467. doi: 10.1016/0042-6822(76)90407-4. [DOI] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Cell-free synthesis and processing of a putative precursor for mitochondrial carbamyl phosphate synthetase I of rat liver. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5071–5075. doi: 10.1073/pnas.76.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osley M. A., Sheffery M., Newton A. Regulation of flagellin synthesis in the cell cycle of caulobacter: dependence on DNA replication. Cell. 1977 Oct;12(2):393–400. doi: 10.1016/0092-8674(77)90115-5. [DOI] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S. Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol. 1978 Sep;135(3):1141–1145. doi: 10.1128/jb.135.3.1141-1145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. M. Observations on the adsorption of Caulobacter bacteriophages containing ribonucleic acid. J Gen Microbiol. 1966 Nov;45(2):347–353. doi: 10.1099/00221287-45-2-347. [DOI] [PubMed] [Google Scholar]
- Shapiro L. Differentiation in the Caulobacter cell cycle. Annu Rev Microbiol. 1976;30:377–407. doi: 10.1146/annurev.mi.30.100176.002113. [DOI] [PubMed] [Google Scholar]
- Sheffery M., Newton A. Purification and characterization of a polyhook protein from Caulobacter crescentus. J Bacteriol. 1979 May;138(2):575–583. doi: 10.1128/jb.138.2.575-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffery M., Newton A. Reconstitution and purification of flagellar filaments from Caulobacter crescentus. J Bacteriol. 1977 Dec;132(3):1027–1030. doi: 10.1128/jb.132.3.1027-1030.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]