Abstract
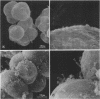
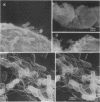
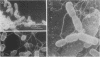
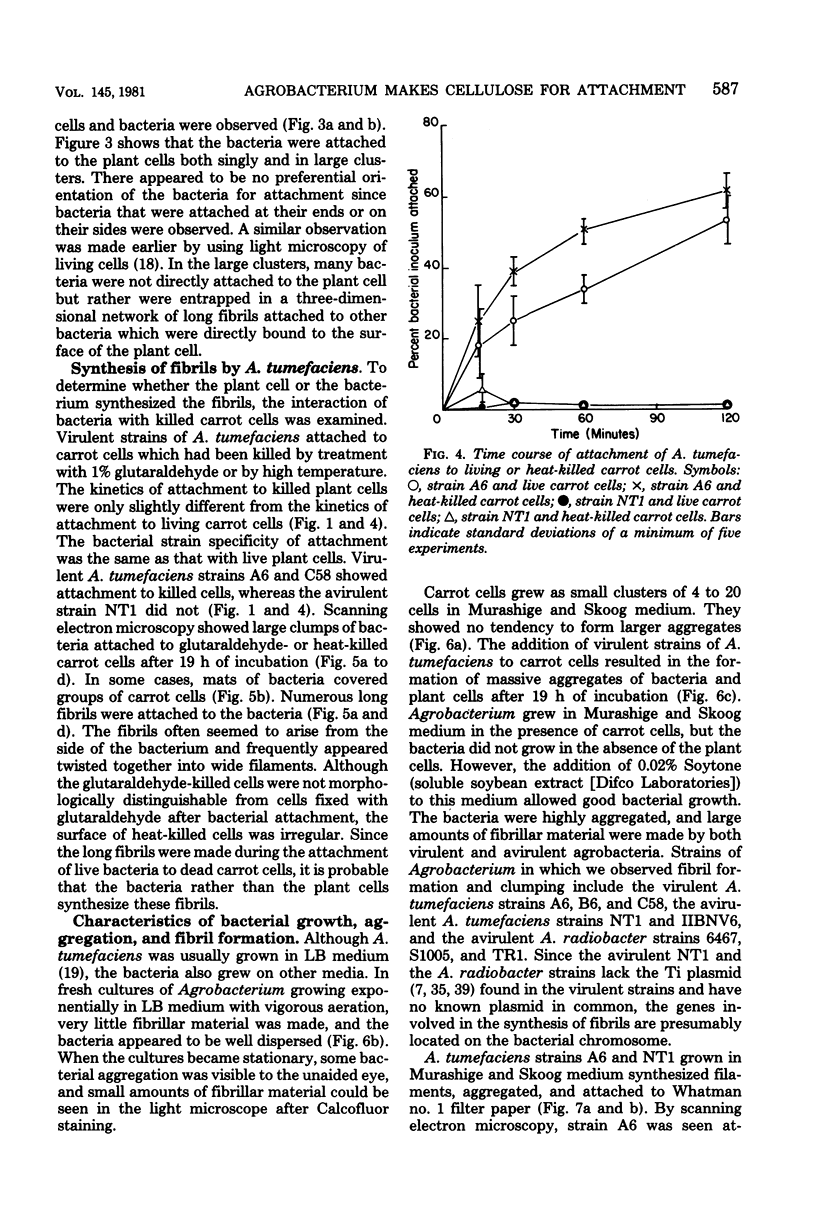
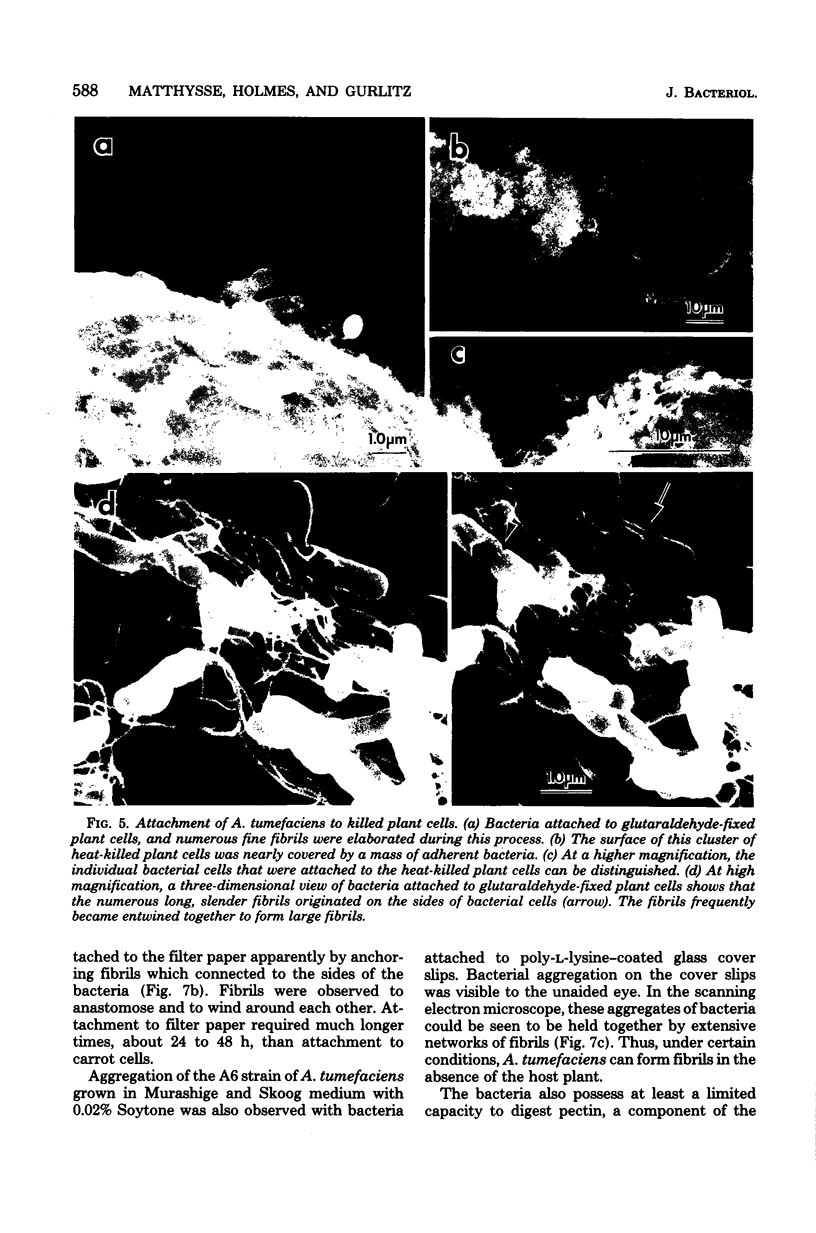
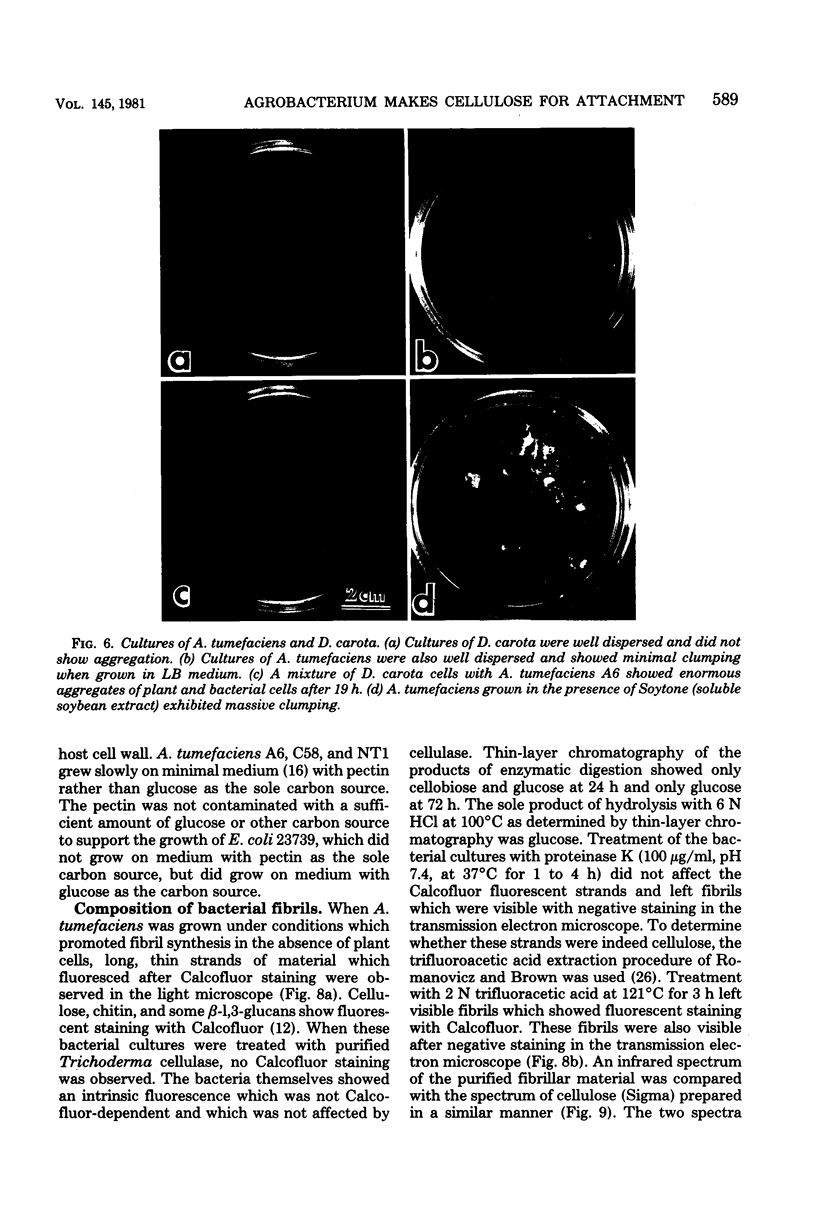
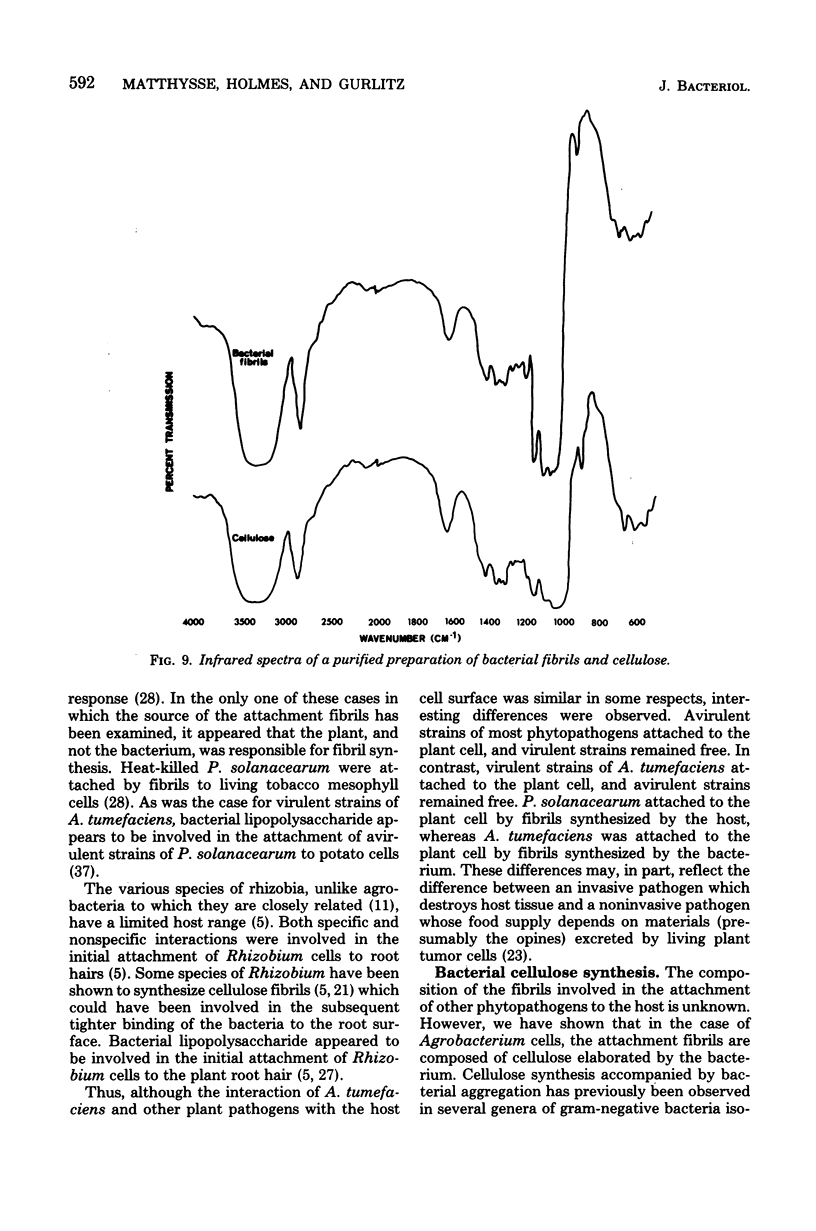
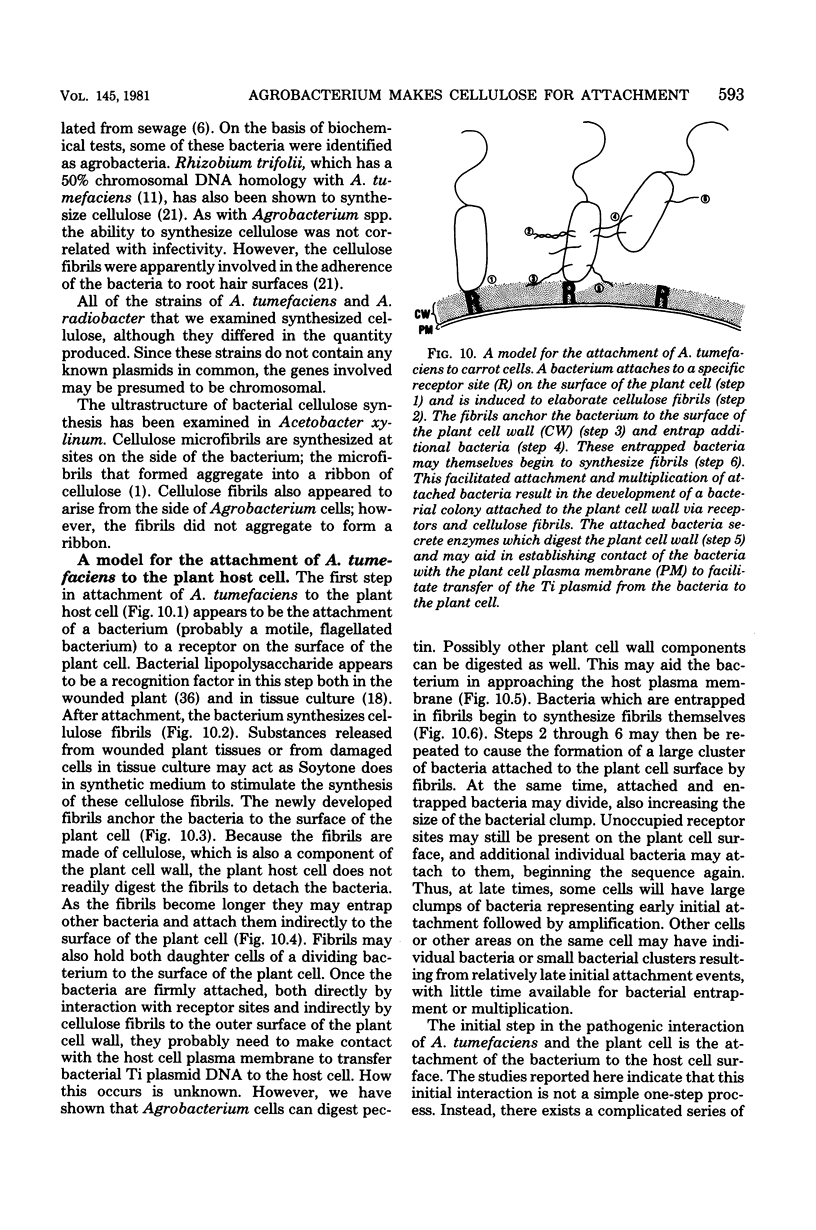
The attachment of virulent strains of Agrobacterium tumefaciens to plant cells is the first step in the bacterial induction of tumors. Binding of A. tumefaciens to carrot tissue culture cells occurred as a two-step process. The initial step was the attachment of the bacteria to the plant cell wall. Living plant cells were not required. Bacterial attachment to heat-killed or glutaraldehyde-fixed carrot cells proceeded with only slightly altered kinetics and unaltered bacterial strain specificity. After the bacteria bound to the carrot cell surface, scanning electron microscopy showed that fibrils developed, surrounded the bacteria, and anchored them to the plant cell surface. These fibrils were synthesized by the bacteria and not by the plant cell since they were also made after the attachment of A. tumefaciens to dead carrot cells and since under some conditions the bacteria synthesized fibrils in the absence of plant cells. Calcofluor staining, acid hydrolysis, enzymatic digestion studies, and infrared spectroscopy showed that the fibrils were composed of cellulose. The formation of these cellulose fibrils occurred during the attachment of virulent strains of A. tumefaciens to plant cells in vitro. The fibrils anchored the bacteria to the plant cell surface and entrapped additional bacteria. The multiplication of entrapped and attached bacteria resulted in the formation of large clusters of bacteria held close to the plant cell wall and plasma membrane by cellulose fibrils. This high concentration of bacteria may facilitate transfer of Ti plasmid deoxyribonucleic acid to the plant cell resulting in the formation of tumors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. M., Jr, Willison J. H., Richardson C. L. Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4565–4569. doi: 10.1073/pnas.73.12.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Deinema M. H., Zevenhuizen L. P. Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation. Arch Mikrobiol. 1971;78(1):42–51. doi: 10.1007/BF00409087. [DOI] [PubMed] [Google Scholar]
- Genetello C., Van Larebeke N., Holsters M., De Picker A., Van Montagu M., Schell J. Ti plasmids of Agrobacterium as conjugative plasmids. Nature. 1977 Feb 10;265(5594):561–563. doi: 10.1038/265561a0. [DOI] [PubMed] [Google Scholar]
- Glogowski W., Galsky A. G. Agrobacterium tumefaciens Site Attachment as a Necessary Prerequisite for Crown Gall Tumor Formation on Potato Discs. Plant Physiol. 1978 Jun;61(6):1031–1033. doi: 10.1104/pp.61.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley W. B., Kemp J. D., Albert M. J., Sutton D. W., Callis J. Transcription of Ti plasmid-derived sequences in three octopine-type crown gall tumor lines. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2828–2832. doi: 10.1073/pnas.76.6.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein G. T., De Ley J., Tijtgat R. Deoxyribonucleic acid homology and taxonomy of Agrobacterium, Rhizobium, and Chromobacterium. J Bacteriol. 1967 Jul;94(1):116–124. doi: 10.1128/jb.94.1.116-124.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., McCully M. E. The use of an optical brightener in the study of plant structure. Stain Technol. 1975 Sep;50(5):319–329. doi: 10.3109/10520297509117082. [DOI] [PubMed] [Google Scholar]
- Lippincott B. B., Lippincott J. A. Bacterial attachment to a specific wound site as an essential stage in tumor initiation by Agrobacterium tumefaciens. J Bacteriol. 1969 Feb;97(2):620–628. doi: 10.1128/jb.97.2.620-628.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott B. B., Whatley M. H., Lippincott J. A. Tumor induction by agrobacterium involves attachment of the bacterium to a site on the host plant cell wall. Plant Physiol. 1977 Mar;59(3):388–390. doi: 10.1104/pp.59.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J. A., Lippincott B. B. The genus Agrobacterium and plant tumorigenesis. Annu Rev Microbiol. 1975;29:377–405. doi: 10.1146/annurev.mi.29.100175.002113. [DOI] [PubMed] [Google Scholar]
- Matthysse A. G., Stump A. J. The presence of Agrobacterium tumefaciens plasmid DNA in crown gall tumour cells. J Gen Microbiol. 1976 Jul;95(1):9–16. doi: 10.1099/00221287-95-1-9. [DOI] [PubMed] [Google Scholar]
- Matthysse A. G., Wyman P. M., Holmes K. V. Plasmid-dependent attachment of Agrobacterium tumefaciens to plant tissue culture cells. Infect Immun. 1978 Nov;22(2):516–522. doi: 10.1128/iai.22.2.516-522.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Dazzo F., Hubbell D. Production of cellulose microfibrils by Rhizobium. Appl Microbiol. 1975 Jul;30(1):123–131. doi: 10.1128/am.30.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K., Pelcher L. E., Schaefer A. In Vitro Binding of Agrobacterium tumefaciens to Plant Cells from Suspension Culture. Plant Physiol. 1979 Feb;63(2):382–387. doi: 10.1104/pp.63.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker S. P., Brown R. D., Jr Characterization of endo-1,4-beta-D-glucanases purified from Trichoderma viride. Biochim Biophys Acta. 1978 Mar 14;523(1):147–161. doi: 10.1016/0005-2744(78)90017-7. [DOI] [PubMed] [Google Scholar]
- Sing V. O., Schroth M. N. Bacteria--plant cell surface interactions: active immobilization of saprophytic bacteria in plant leaves. Science. 1977 Aug 19;197(4305):759–761. doi: 10.1126/science.197.4305.759. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley M. H., Bodwin J. S., Lippincott B. B., Lippincott J. A. Role of Agrobacterium cell envelope lipopolysaccharide in infection site attachment. Infect Immun. 1976 Apr;13(4):1080–1083. doi: 10.1128/iai.13.4.1080-1083.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley M. H., Hunter N., Cantrell M. A., Hendrick C., Keegstra K., Sequeira L. Lipopolysaccharide Composition of the Wilt Pathogen, Pseudomonas solanacearum: CORRELATION WITH THE HYPERSENSITIVE RESPONSE IN TOBACCO. Plant Physiol. 1980 Mar;65(3):557–559. doi: 10.1104/pp.65.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Hegeman G. D. Tumor induction by Agrobacterium tumefaciens: specific transfer of bacterial deoxyribonucleic acid to plant tissue. J Bacteriol. 1971 Dec;108(3):973–979. doi: 10.1128/jb.108.3.973-979.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]