Abstract
The apical transmembrane protein Crumbs is necessary for both cell polarization and the assembly of the zonula adherens (ZA) in Drosophila epithelia. The apical spectrin-based membrane skeleton (SBMS) is a protein network that is essential for epithelial morphogenesis and ZA integrity, and exhibits close colocalization with Crumbs and the ZA in fly epithelia. These observations suggest that Crumbs may stabilize the ZA by recruiting the SBMS to the junctional region. Consistent with this hypothesis, we report that Crumbs is necessary for the organization of the apical SBMS in embryos and Schneider 2 cells, whereas the localization of Crumbs is not affected in karst mutants that eliminate the apical SBMS. Our data indicate that it is specifically the 4.1 protein/ezrin/radixin/moesin (FERM) domain binding consensus, and in particular, an arginine at position 7 in the cytoplasmic tail of Crumbs that is essential to efficiently recruit both the apical SBMS and the FERM domain protein, DMoesin. Crumbs, Discs lost, βHeavy-spectrin, and DMoesin are all coimmunoprecipitated from embryos, confirming the existence of a multimolecular complex. We propose that Crumbs stabilizes the apical SBMS via DMoesin and actin, leading to reinforcement of the ZA and effectively coupling epithelial morphogenesis and cell polarity.
Keywords: epithelial polarity; zonula adherens; Drosophila; spectrin; DMoesin
Introduction
The functions of an epithelium depend on the polarized organization of its individual epithelial cells. The acquisition of a fully polarized phenotype involves a cascade of complex events including cell–cell adhesion, assembly of a lateral cortical complex, reorganization of the cytoskeleton, and polarized targeting of transport vesicles to the apical and basolateral membranes (Yeaman et al., 1999). Genetic studies in Drosophila have further revealed evidence for apical, lateral, and basal cues for epithelial polarization (Knust, 2000; Tanentzapf et al., 2000). Crumbs is an apical transmembrane protein that is responsible for organizing the apical pole in the fly and is expressed in all primary epithelia of Drosophila where it is concentrated just above the zonula adherens (ZA)* at the apical–lateral domain boundary (Tepass et al., 1990). Crumbs overexpression results in expansion of the apical domain (Wodarz et al., 1995), whereas loss of Crumbs disrupts the polarity of epithelial cells causing the breakdown of epithelial tissues (Tepass et al., 1990). In crumbs mutants, the ZA fail to coalesce at the apicolateral border, suggesting that Crumbs is involved in organizing this junctional structure, and thus in determining the location of the border between the apical and the lateral domains (Grawe et al., 1996; Tepass, 1996).
Surprisingly, most of the polarity functions in crumbs mutants are rescued by expression of its transmembrane and short cytoplasmic domains, suggesting that the major interactions regulating cell polarity and shape in the embryo are mediated by the 37 intracellular amino acids of this large (2,139 amino acids) protein (Wodarz et al., 1995). This hypothesis is reinforced by the observation that a nonsense mutation in the crumbs8F105 allele, preventing the translation of the last 23 amino acids of the cytoplasmic tail, produces a severe loss of function phenotype (Wodarz et al., 1993). Furthermore, it has been shown that the MAGUK family member Stardust (Sdt) binds to the last four amino acid residues (ERLI) of the cytoplasmic tail of Crumbs, along with the PSD-95/DLG/ZO-1 (PDZ) domain protein Discs-lost (Dlt) (Bhat et al., 1999; Klebes and Knust, 2000; Bachmann et al., 2001; Hong et al., 2001). These two proteins are both required for epithelial polarity, and thus Crumbs, together with Dlt and Sdt, defines a membrane–associated complex in the apical cytocortex of epithelial cells that is necessary for the proper generation of the polarized phenotype. Additional contributions from lateral proteins below the ZA, such as Scribble, are also involved in maintaining polarity and the integrity of the ZA. Because loss of scribble function results in a phenotype reminiscent of Crumbs overexpression, it has been suggested that the position and integrity of the ZA arises from a balance between the Crumbs-Dlt/Sdt complex at the apical border and the Scribble network on its basal side (Bilder and Perrimon, 2000).
Spectrins are long, tetrameric, F-actin–crosslinking proteins comprised of two α and two β subunits (for review see Bennett and Baines, 2001). The spectrin-based membrane skeleton (SBMS) is a branching cytoskeletal network of spectrin-crosslinked F-actin associated with the various membrane compartments in the cell. Each SBMS is bound to the membrane via interaction with integral membrane proteins and phospholipids (De Matteis and Morrow, 2000). At the plasma membrane, spectrin, in conjunction with cortical F-actin, provides a structural basis for modulating cell shape and membrane stability in both epithelial and nonepithelial cells. In Drosophila, a single α-spectrin isoform combines with either of two, structurally distinct β-isoforms (β-spectrin and βHeavy-spectrin [βH]) to produce (αβ)2 and (αβH)2 tetramers, respectively. In epithelial cells of Drosophila, (αβ)2 tetramers are restricted to the basolateral membrane, while the (αβH)2 tetramers localize to the apical membrane and the ZA (Dubreuil et al., 1997; Lee et al., 1997; Thomas et al., 1998; Thomas and Williams, 1999).
All three spectrin subunits are essential for normal development. βH, encoded by the karst locus, is an essential protein that is required for epithelial morphogenesis (Thomas et al., 1998). karst mutant cells exhibit altered shapes and disruption of the ZA indicating that (αβH)2 contributes to the integrity of the latter, but is not necessary for apicobasal polarity per se (Zarnescu and Thomas, 1999). Similarly, complex phenotypes are caused by mutations in the fly α- and β-spectrin genes as well as in the orthologous genes in Caenorhabditis elegans (Lee et al., 1993; de Cuevas et al., 1996; Dubreuil et al., 1998; McKeown et al., 1998; Dubreuil et al., 2000; Moorthy et al., 2000). Together, these studies indicate that the SBMS has an essential role in cell structure and morphogenesis (for review see Thomas, 2001), making the identification of proteins that recruit and/or organize this structure of considerable interest.
Spectrins are generally recruited to the membrane via adapter proteins that link the SBMS to integral membrane proteins (Bennett and Baines, 2001). Two families of such adapter proteins have been well characterized: ankyrins and protein 4.1 family members. The former binds to the midregion of the β-spectrin spectrin repeat array (Lombardo et al., 1994), whereas the latter forms a ternary complex between the actin-binding domain of β-spectrin and F-actin itself (Marfatia et al., 1997). Protein 4.1 is part of a larger superfamily of proteins containing protein 4.1/ezrin/radixin/moesin (FERM) domains (Chishti et al., 1998) that function to attach cortical F-actin to a variety of integral membrane proteins (Tsukita and Yonemura, 1999). The existence of multiple adapter protein genes, as well as alternatively spliced isoforms, generates great diversity in the number of proteins to which an SBMS can be attached (see De Matteis and Morrow, 2000 for a list of almost 50 spectrin associated proteins). The recruitment of conventional β-spectrins by adapter proteins is well characterized (e.g., Jenkins and Bennett, 2001); however, the cues recruiting spectrin to the apical domain are currently uncharacterized, as are the adapter proteins that associate with the βH isoform.
Overexpression of Crumbs in the embryonic ectoderm causes an enlargement of the apical membrane and a concomitant expansion in the distribution of βH staining (Wodarz et al., 1995). This result suggested that this apical polarity cue might also be responsible for recruiting and/or organizing the apical SBMS. To investigate this possibility, we looked for genetic and physical interactions between βH and Crumbs. In this paper, we report that at least one allele of crumbs is a dominant enhancer of the karst phenotype, and that whereas the Crumbs distribution is unaffected in karst mutants, βH is mislocalized in the epithelial cells of crumbs8F105 mutants. Furthermore, overexpression of Crumbs led to redistribution of βH, DMoesin, and actin, indicating that Crumbs acts upstream of βH in organizing the apical SBMS. We also demonstrate that clustering of a chimeric-tagged form of Crumbs in Schneider 2 (S2) cells induces cocapping of βH and DMoesin. This provides evidence for a relationship between Crumbs and these two proteins under physiological conditions. This interaction is dependent on a consensus motif for the binding of proteins of the FERM family in the cytoplasmic tail of Crumbs. Finally, we show that Dlt, Crumbs, βH, and DMoesin coimmunoprecipitate, indicating that a multiprotein complex is recruited by Crumbs. These results indicate that Crumbs mediates a novel coordination between cell polarity, junctional stabilization, and morphogenesis.
Results
The membrane organization of βH depends on Crumbs
Previously, we have shown that βH exhibits a very close colocalization with the adhesion protein Drosophila epithelial (DE)-cadherin at the light microscope level throughout ZA formation and at the mature junction (Thomas et al., 1998; Thomas and Williams, 1999; Fig. 1 A for a schematic diagram of the organization of junctions in Drosophila epithelia). Not surprisingly, βH and Crumbs exhibit a similarly close apposition at this level of resolution (Fig. 1 B, left), as Crumbs is located at the apical margin of the ZA (marginal zone; Tepass, 1996). βH clearly colocalizes with DE-cadherin at times when Crumbs is not present (e.g., during early cellularization; see Thomas and Williams, 1999) and exhibits regulatory changes reflecting the area of the ZA itself in early eye development (Thomas et al., 1998). However, strenuous efforts to localize βH at the ultrastructural level have been unsuccessful for some time, leaving unresolved the issue of whether the βH domain only lies at the ZA or in the marginal zone, or encompasses both in mature epithelia.
Figure 1.

βH is mislocalized in crumbs8F105 embryos. (A) Schematic figure showing the organization of cell–cell junctions in the ectoderm of Drosophila. Arm, armadillo; SJ, septate junction; ZA, zonula adherens. Some key markers for the different structures are indicated. (B) Confocal micrographs showing part of the ectoderm from stage 11/12 embryos stained for βH (bottom) and Crumbs (top). In WT embryos, βH and Crumbs are highly concentrated in the apicolateral region of each cell (arrows). In crumbs8F105 embryos (crb8F105), βH is mislocalized to the whole apical membrane (asterisks), and to a lesser extent to the cytoplasm. Bar, 5 μm.
Overexpression of the Crumbs cytoplasmic tail causes a concomitant expansion in the distribution of βH (Wodarz et al., 1995), suggesting that Crumbs might be responsible for recruiting and/or organizing βH in normal cells. Therefore, we looked to see if the distribution of βH was perturbed in crumbs8F105 mutant embryos. Crumbs itself is mislocalized to the cytoplasm and to the whole surface of the ectodermal cells in these embryos as previously reported (Fig. 1 B, right; Tepass et al., 1990). In such embryos, we also find that βH is no longer concentrated in the apicolateral region, but is distributed over the whole of the apical domain at stages 11 and 12 (Fig. 1 B, right). At later stages, when epidermal cells are losing their polarized organization, βH is also found on the basolateral membrane (unpublished data). In contrast, Crumbs exhibits a normal localization in karst mutant embryos (unpublished data). Together, these data are consistent with the notion that a proper localization of Crumbs is required to recruit and/or restrict βH to the apicolateral zone, and that Crumbs lies of βH in organizing the apical domain of the cell.
crumbs is a dominant enhancer of karst
Given the close functional and spatial relationship between βH and the ZA (Thomas and Williams, 1999; Zarnescu and Thomas, 1999), we looked for a genetic interaction between karst and crumbs. Such an interaction is likely to be modest due to the existence of multiple pathways for recruiting βH (see Discussion). Furthermore, all karst alleles isolated to date exhibit variable expressivity necessitating a statistical approach. The interaction test was thus limited to the most readily quantified feature of the pleiotropic karst phenotype, the degree of lethality. Comparison of viability rates between karst crumbs/karst + and karst/karst genotypes reveals a statistically significant enhancement of lethality in the presence of one mutant crumbs allele (Fig. 2). Thus, halving the level of Crumbs further reduces the remaining functionality of the mutant βH protein. This defines crumbs as a dominant enhancer of karst and is the expected result if Crumbs lies upstream in the organization of the apical SBMS.
Figure 2.
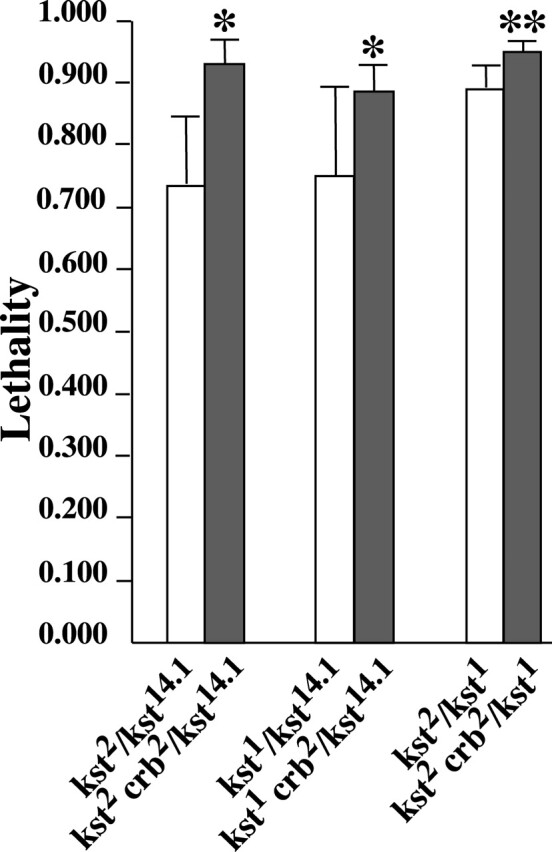
crumbs dominantly enhances karst lethality. Lethality is expressed as the fraction of Mendelian expectation. The values plotted were all estimated from multiple crosses with large sample sizes. From left to right the number of crosses/total number of flies scored were: 4/2,059; 19/10,013; 4/2,204; 5/3,655; 9/4,102; and 7/3,329. The presence of one mutant crumbs11A22 allele (crb2) significantly increased lethality in all genotypic combinations (* = P < 0.05; ** = P < 0.01). See Materials and methods for details on the statistical analysis of these data. Error bars represent 95% confidence intervals.
A Crumbs/DMoesin/βH complex in embryos
In order to test for a physical interaction between Crumbs and βH, we performed coimmunoprecipitations using an affinity-purified antibody to βH and embryo extracts (Fig. 3). The βH immunoprecipitates were probed for α-spectrin, a known partner of βH, or for Crumbs. Both proteins were present in βH immune complexes. As expected, Dlt also coimmunoprecipitated Crumbs under the same conditions. Control immunoprecipitations with a polyclonal rabbit anti–mouse (Fig. 3) or an irrelevant antiserum against Bub3 (unpublished data) did not bring down Crumbs, indicating that this interaction between Crumbs and βH was specific.
Figure 3.
Coimmunoprecipitation experiments reveal a CRB–βH complex. WT embryo lysates were immunoprecipitated (IP) with antibodies against βH or Dlt or with rabbit anti–mouse antibodies (RαM) and probed on immunoblots (Blot) with antibodies against α-spectrin (α-Sp) or Crumbs (Crb). Crumbs and α-spectrin coprecipitate with βH. Migration of markers is indicated in kD. Hom, a whole embryo extract loaded on the same gel as a control.
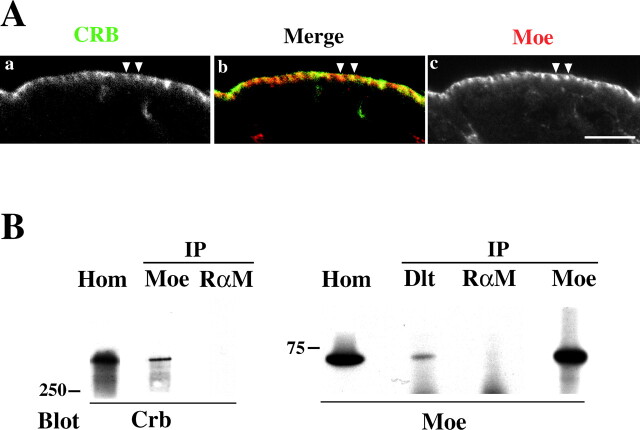
Spectrin is known to bind indirectly to transmembrane proteins via adapter proteins such as ankyrin and protein 4.1 family members (Introduction). Moreover, it has been speculated that the juxtamembrane region of the Crumbs cytoplasmic domain contains a consensus binding motif for a FERM domain protein (Klebes and Knust, 2000). The Drosophila FERM domain protein, DMoesin, localizes in the apical region of epithelial cells (McCartney and Fehon, 1996), and has been associated with the regulation of cell shape changes (Edwards et al., 1997). Double staining with DMoesin and Crumbs on WT embryos indicates that these two proteins are in close proximity (Fig. 4 A), suggesting that these two proteins could interact. Therefore, we immunoprecipitated DMoesin from WT embryos and probed the immunoprecipitates for the presence of Crumbs by immunoblotting (Fig. 4 B, left). The presence of Crumbs in the immune complex indicates that the two proteins are in a common protein complex. This was confirmed by the fact that immunoprecipitation of Dlt from embryonic extracts also brought down DMoesin (Fig. 4 B, right). It was not possible to test whether both DMoesin and βH coimmunoprecipitated with Crumbs, because the only available antibody against the extracellular domain of Crumbs fails to immunoprecipitate it (unpublished data).
Figure 4.
DMoesin interacts with Crumbs and βH. (A) Confocal micrographs showing part of the ectoderm from stage 11/12 embryos stained for Crumbs (a) and DMoesin (c). DMoesin and Crumbs are concentrated in the apicolateral domain (arrowheads). (B) Crumbs coimmunoprecipitates with DMoesin. Protein extracts from wild type embryos were immunoprecipitated (IP) with antibodies against DMoesin (Moe), Dlt, or rabbit anti–mouse antibodies (RαM). The resulting immunoprecipitates were immunoblotted with antibodies against DMoesin (Moe) or Crumbs (Crb). Migration of markers is indicated in kD. Hom, whole embryo extract as control.
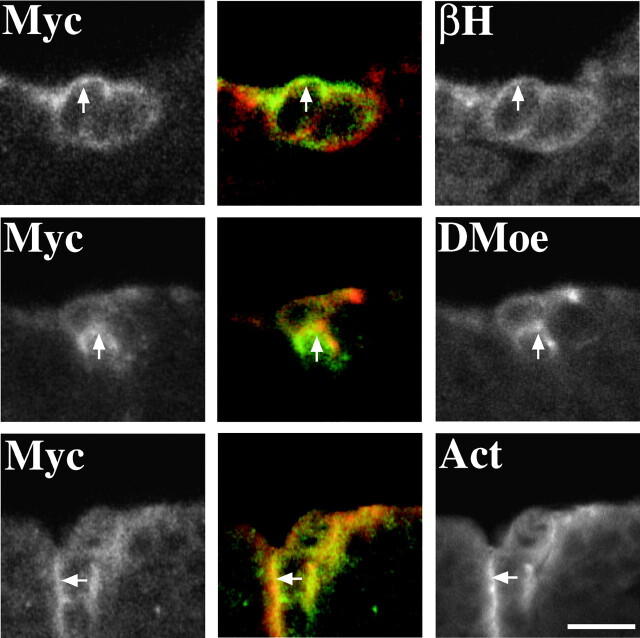
To determine if this complex exists in vivo, we examined embryos expressing the chimeric Crumbs protein, Crumbsmyc-intra (Wodarz et al., 1995) under the regulation of the Gal4 binary expression system (Brand and Perrimon, 1993). Crumbsmyc-intra is widely distributed on the cell membrane outside of the normal Crumbs domain when overexpressed in ectodermal cells (Wodarz et al., 1995). To provide a side-by-side comparison with normal cells we used the engrailed-Gal4 driver to limit expression to cells at the posterior border of each segment in the ectoderm of the embryo. We find that expression of this protein causes an identical mis-distribution of DMoesin, βH and actin (Fig. 5) indicating that this complex forms in vivo and that Crumbs is linked to the actin cytoskeleton.
Figure 5.
Overexpression of Myc-intraWTleads to the redistribution of βH, DMoesin, and actin. Confocal micrographs of part of the epidermis of a stage 13/14 embryo. Cells at the posterior margin of each segment are expressing Myc-intraWT driven by an engrailed-Gal4 driver. Embryos were stained for Myc and βH, Dmoesin, (DMoe), or actin (Act) as indicated. βH, DMoesin, and actin are all redistributed along with Myc-intraWT (arrows). Bar, 5 μm.
The Crumbs cytoplasmic domain induces accumulation of Discs lost, DMoesin, and βH in transfected S2 cells
Next, we turned to S2 cells in culture in order to try and understand how Crumbs, βH, and DMoesin interact in vivo. S2 cells do not express Crumbs (Wodarz et al., 1993), but have been shown to express βH (Dubreuil et al., 1997) and DMoesin (McCartney and Fehon, 1996). Because Dlt is a key component of the Crumbs pathway (Bhat et al., 1999), we used anti-Dlt antibodies to probe S2 homogenates by immunoblotting and found that Dlt is expressed at significant levels in S2 cells (unpublished data). Thus, S2 cells offer a suitable model to understand some aspects of Crumbs' molecular networks using transfected Crumbs constructs.
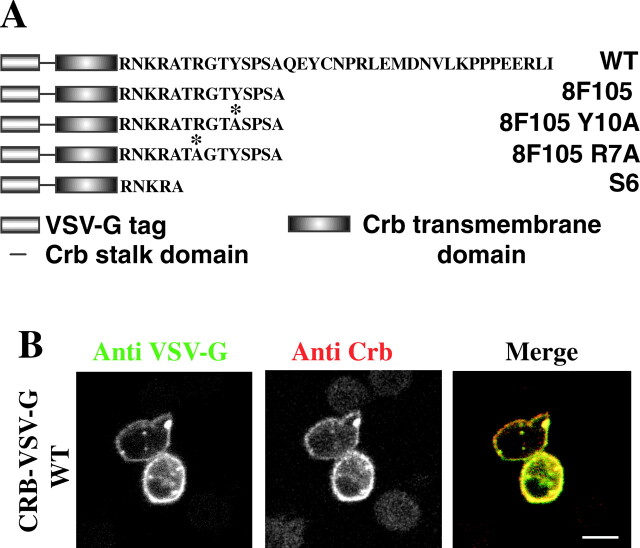
A Crumbs construct in which most of the extracellular domain was replaced by an epitope of vesicular stomatitis virus–protein G (VSV-G) (Fig. 6 A; recognized by the monoclonal antibody [mAb] P5D4), was stably expressed in S2 cells. The encoded crumbs (CRB)–VSV-G WT protein is transported to the cell surface where it is recognized by both the P5D4 mAb and a polyclonal antibody raised against the cytoplasmic domain of Crumbs (Fig. 6 B; see Materials and methods). This protein is equivalent to the Crumbsmyc-intra protein that we used in the overexpression experiments in embryos (Fig. 5). βH accumulates at the plasma membrane in adherent S2 cells (Dubreuil et al., 1997), but not when these cells are grown in suspension (Fig. 7, untransfected cells), whereas DMoesin is always associated with the plasma membrane. CRB–VSV-G WT expression caused no conspicuous change in the distribution of DMoesin or βH in adherent cells with both proteins colocalizing at the plasma membrane (unpublished data).
Figure 6.
Expression of CRB–VSV-G WT in S2 cells. (A) Sequences of the CRB–VSV-G fusion proteins expressed in this study. CRB–VSV-G WT is a fusion of the VSV-G epitope with the stalk region, transmembrane domain and the intracellular domain of Crumbs. Stop mutations in position 6 or 15 truncate the intracellular domain of Crumbs resulting in a cytoplasmic domain of 5 and 14 amino acids in variants CRB–VSV-G S6 and CRB–VSV-G 8F105, respectively. CRB–VSV-G 8F105 Y10A and R7A are CRB–VSV-G 8F105 constructs with point mutations (asterisk) replacing Tyr10 and Arg7 with an alanine, respectively. (B) CRB–VSV-G WT expression was induced in stably transfected S2 cells and cells were fixed and double labeled with a mouse anti–VSV-G and a rabbit anticytoplasmic domain of Crumbs antibodies followed by FITC-conju- gated anti–mouse (left) and TRITC- conjugated anti–rabbit (middle) antibodies. The two antibodies stained the same subcellular structures and in particular the plasma membrane (right). Bar, 10 μm.
Figure 7.

Dlt, βH, and DMoesin colocalize with capped CRB–VSV-G WT in transfected S2 cells. CRB–VSV-G WT (A–C, top) or S6 (A–C, bottom) were transiently expressed in S2 cells, followed by capping and staining with mouse anti–VSV-G antibody and fluorescein-conjugated anti–mouse antibody before fixation. After fixation and permeabilization, cells were additionally stained for either Dlt (A, Dlt), βH (B, βH), or DMoesin (C, Moe). Dlt, βH, and DMoesin were all redistributed to capped sites with CRB–VSV-G WT indicating a connection between these proteins and the cytoplasmic domain of Crumbs in S2 cells (arrows highlight specific examples). Bar, 10 μm.
We further investigated the possibility of a molecular association between Crumbs, DMoesin, and βH using the technique of capping. CRB–VSV-G WT was concentrated in patches on the surface of transfected S2 cells growing in suspension by treatment with the P5D4 mAb and a polyclonal anti-mouse antibody coupled to FITC. Capping with the secondary antibody was allowed to proceed for 5 minutes at room temperature (Fig. 7). Not only was Dlt recruited to the patches containing CRB–VSV-G WT as expected (Fig. 7 A, top), but both βH and DMoesin were also concentrated at such regions, indicating that there is indeed a link between Crumbs and these proteins in vivo (Fig. 7, B and C, top). Typically, a background level of ∼25% nonspecific capping was observed with this assay, and therefore was subtracted from all the percentages reported below. Using this procedure, a robust average of ∼50% capping was seen with CRB–VSV-G WT after removal of the background, despite the fact that S2 cells exhibit some heterogeneity in their level of expression of DMoesin and βH.
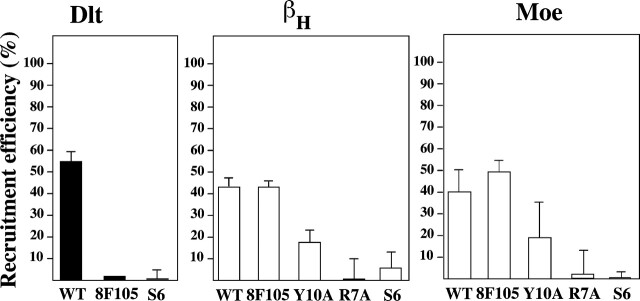
To investigate the role of the cytoplasmic domain of Crumbs for the interaction with DMoesin or βH, we expressed the truncated construct CRB–VSV-G S6 in which only the first five amino acids of the cytoplasmic domain remained (Fig. 6 A). This mutation prevented binding to Dlt in a GST pulldown assay (unpublished data) and essentially eliminated the ability to cluster Dlt as expected (Fig. 7 A, bottom). CRB–VSV-G S6 was also unable to efficiently recruit DMoesin and βH, indicating that the cytoplasmic domain of Crumbs was also necessary for the interaction with βH and DMoesin (Fig. 7 B and C, bottom).
The Crumbs FERM domain binding site is required to efficiently recruit both DMoesin and βH
The capping technique provides a readily quantifiable assay for interactions between the Crumbs cytoplasmic domain and other proteins. Thus, we next used it to determine which part of the cytoplasmic domain of Crumbs is necessary for the interaction with DMoesin and βH. A second truncation mutant, CRB–VSV-G 8F105 (Fig. 6 A), that mimics the crumbs8F105 allele with a stop codon at position 15 of the cytoplasmic domain (Wodarz et al., 1993), was able to recruit βH and DMoesin just as efficiently as CRB–VSV-G WT, but could no longer bind to Dlt as predicted (Fig. 8). These results indicate that the distal part of the Crumbs cytoplasmic domain is not crucial for the DMoesin/βH–Crumbs interaction, in contrast to the interaction between Crumbs and Dlt (Bhat et al., 1999; Klebes and Knust, 2000) and Crumbs and Sdt (Bachmann et al., 2001; Hong et al., 2001).
Figure 8.
Quantitative analysis of βH recruitment to CRB–VSV-G cap sites. S2 cells expressing the different CRB–VSV-G constructs were scored for Dlt, βH, or DMoesin (Moe) colocalization after capping with anti–VSV-G and secondary antibodies. Results are expressed as the mean percentage of cocapping seen for each protein from three independent experiments (except for Moe 8F105, Y10A, and S6, which were performed twice, and Dlt 8F105 which was done once).
Two motifs in the NH2-terminal and the COOH-terminal regions of the cytoplasmic domain of Crumbs are necessary for the rescue of a normal polarized phenotype in crumbs mutant epidermal cells (Klebes and Knust, 2000). The COOH-terminal motif, ERLI, is well defined and known to bind to Sdt and to recruit Dlt; however, little is known about the function of the first 15 amino acids near the membrane, beyond the essential nature of the tyrosine at position 10 (Fig. 6, Y10; Klebes and Knust, 2000). Because CRB–VSV-G 8F105 retains this residue, whereas CRB–VSV-G S6 does not, we mutated Y10 and the nearby arginine (R7) to alanine in the CRB–VSV-G 8F105 construct to see if either plays a role in recruiting DMoesin and βH. Both of these residues lie within the putative FERM domain binding site (Klebes and Knust, 2000). CRB–VSV-G R7A and Y10A were expressed in S2 cells and capping experiments were performed as above. CRB–VSV-G Y10A substantially reduced capping of DMoesin and βH, whereas CRB–VSV-G R7A behaved like the S6 truncation, showing severely reduced recruitment of both DMoesin and βH (Fig. 8). To ensure that the R7A mutation had not induced proteolytic degradation of the chimera, we confirmed that its size was the same as that of CRB–VSV-G 8F105 by SDS-PAGE (unpublished data). Thus, although the Y10 is important for recruiting DMoesin and βH, R7 appears to be an essential residue for this process. These experiments define the consensus sequence for the FERM domain binding site to be the critical domain via which Crumbs recruits DMoesin and βH.
Discussion
The crumbs–Dlt–Sdt pathway is essential for polarity and has been shown to be a major apical signal for establishing the ZA at the apical–lateral boundary (for reviews see Knust, 2000; Muller, 2000; Bilder, 2001). The observation that mutations affecting βH and Crumbs both cause a junctional phenotype, along with the close colocalization of both proteins in the marginal zone of epithelial cells, suggested a possible connection between the activities of these two proteins. Here we report evidence that Crumbs can recruit apical βH together with the FERM domain protein DMoesin and actin. Our data are in good agreement with the hypothesis that polarity cues are used to organize the SBMS (Yeaman et al., 1999), but this is the first time that this has been shown for an apical determinant.
crumbs lies upstream of karst, stabilizing the apical spectrin membrane skeleton
Several lines of evidence indicate that Crumbs can recruit βH into its complex: (a) βH is mislocalized in embryos mutant for the truncation allele crumbs8F105, in which the mutant Crumbs protein itself is mislocalized; (b) βH mislocalization can be induced by overexpression of the Crumbs transmembrane and cytoplasmic domains in vivo; (c) βH is recruited to Crumbs protein clusters in an S2 cell cocapping assay; (d) we can coimmunoprecipitate Crumbs with βH; and (e) the protein-null allele crumbs11A22 acts as a dominant enhancer of hypomorphic karst alleles, strongly indicating that a reduction in the normal amount of Crumbs reduces the level of partially functional βH at the membrane. Moreover, because the karst mutant alleles all produce COOH-terminally truncated proteins (see Materials and methods), these results further suggest that the Crumbs-βH interaction site lies in the NH2-terminal portion of the latter. Finally, in a paper that came out while this manuscript was under review, it was shown that loss of Crumbs eliminates βH from the stalk membrane of photoreceptors in the adult eye (Pellikka et al., 2002).
Current evidence indicates that βH can be recruited to the membrane in several additional ways. First, it can associate with the specialized basal adherens junctions during cellularization in a Crumbs-independent manner (Thomas and Williams, 1999). Second, it is found in the terminal web subtending brush borders in the midgut epithelium (Thomas et al., 1998) that does not express Crumbs (Tepass, 1997). Finally, it has also been shown that βH is only partially reduced in crumbs11A22 mutant follicle cell clones (Tanentzapf et al., 2000), indicating that in this Crumbs-expressing epithelium there are multiple mechanisms to recruit βH. These data provide a compelling explanation for the modest nature of the karst-crumbs genetic interaction. By reducing Crumbs, we are discretely affecting only one of these pathways. The observation that the karst1 allele produces readily detectable quantities of truncated product (Thomas et al., 1998), most of which is not recruited to the membrane in any of these epithelia, suggests that there is a general and essential role of the COOH-terminal half of βH in its stable membrane localization (Zarnescu and Thomas, 1999). Together, the above data are consistent with the multifunctional nature of spectrin membrane skeletons and with the idea that specific pathways recruit the SBMS to establish spatially distinct polarized membrane domains, whereas general COOH-terminal membrane association domains permit tight membrane association and network integration (Lombardo et al., 1994; Bennett and Baines, 2001).
The cytoplasmic domain of Crumbs recruits DMoesin and βH
The previously reported partial rescue of crumbs mutants by the crumbsmyc-intra construct (Wodarz et al., 1995) suggested that the transmembrane and cytoplasmic domains of Crumbs might be sufficient to concentrate βH to some areas of the apical membrane. We have confirmed and extended this result, showing that the critical region for recruiting βH is just 9 amino acids from position 6 through 14 of the cytoplasmic domain in the putative FERM domain binding site (Klebes and Knust, 2000). Within this region, a conserved tyrosine residue at cytoplasmic domain position 10 (crucial for Crumbs function in vivo; Klebes and Knust, 2000) and an arginine at position 7 are both required for this activity. It is worth noting that all Crumbs genes cloned so far contain a charged amino acid residue at position 7 in the cytoplasmic domain (see Klebes and Knust, 2000), suggesting that this is an evolutionarily conserved interaction site.
FERM domains are found in the protein 4.1 family of proteins which link the SBMS to cell-surface receptors (Hoover and Bryant, 2000) as well as several other proteins which organize the cortical actin (ezrin/radixin/moesin; Bretscher, 1999; Tsukita and Yonemura, 1999). The founding member of this group, protein 4.1, was originally identified as a major component of the erythrocyte SBMS where it facilitates the interaction of spectrin with actin and the transmembrane protein Glycophorin C (Marfatia et al., 1997). Therefore, the presence of a conserved FERM binding domain in the Crumbs cytoplasmic domain suggests that Crumbs may bind to βH via a FERM domain protein.
In Drosophila, the FERM domain family includes the proteins Coracle, DMerlin, DMoesin, and Expanded (McCartney and Fehon, 1996). Of these four proteins, Coracle is an unlikely candidate to bind to the Crumbs juxtamembrane domain since it is localized to the septate junctions basal to the ZA (Fehon et al., 1994). However, the DMerlin, DMoesin, and expanded proteins are localized in part or in whole at the ZA region in epithelia (McCartney and Fehon, 1996; Boedigheimer et al., 1997), and could thus be involved in the interaction between Crumbs and βH. The fact that none of protein 4.1 family members known in Drosophila contains a spectrin-binding domain as defined by the archetypal protein 4.1 does not necessarily abrogate this hypothesis. βH-spectrin is clearly recruited to the membrane by different mechanisms than its basolateral counterpart (Dubreuil and Grushko, 1999), and this specificity would likely be reflected in divergent interaction domains. In this work, we have found that βH and DMoesin can both coimmunoprecipitate Crumbs. Furthermore, our capping assay and embryo expression evidence provide in vivo support for this result. Not only will DMoesin cocap with the Crumbs cytoplasmic domain, it is dependent on exactly the same sequences that recruit βH. These results, together with the existence of the consensus binding site for a FERM domain protein in Crumbs, strongly support the hypothesis that DMoesin forms a bridge between Crumbs and the SBMS (see model in Fig. 9). A functional test of this relationship must wait until mutations in the DMoesin locus become available. Thus, the current data, although highly suggestive, do not formally distinguish between the possibility of a DMoesin bridge between Crumbs and the SBMS, and the existence of two separate complexes with direct interaction between Crumbs and βH or Dmoesin in each. Significantly, actin did not cap consistently with Crumbs in S2 cells and was not present in our immunoprecipitates (unpublished data). This suggests that other components present in epithelial cells are necessary for stabilization of the actin skeleton around the Crumbs complex. It also indicates that βH is specifically recruited to the proposed complex and is not merely a passive arrival along with bulk actin.
Figure 9.

Model for the protein interactions in the Crumbs complex. A model summarizing all the published data and those presented in this study is drawn to show the interactions occurring inside the Crumbs complex. The amino acids playing a crucial role for the interactions are indicated. See Discussion for details.
Our results indicate that Crumbs interacts with at least two different protein networks, a DMoesin/Spectrin/actin-based network and a PDZ protein scaffold (Dlt/Sdt). However, it is unclear at present whether Dlt/Sdt and DMoesin/βH/actin coexist in the same complex with Crumbs. In the erythrocyte model, glycophorin C is linked to spectrin via a ternary complex containing protein 4.1 and the PDZ domain protein p55 bound to a topologically similar pair of binding sites to the two functional regions identified in the Crumbs cytoplasmic domain (Marfatia et al., 1997). If such a ternary complex forms in association with Crumbs, then the observation that the Sdt-binding domain of Crumbs is not required for the interaction between Crumbs and βH, would indicate that the latter cannot be dependent on Dlt/Sdt for association with Crumbs in such a complex or that both interactions can coexist.
A model for Crumbs action in apical network formation
Because both the crumbs and karst phenotypes disrupt the ZA (Grawe et al., 1996; Zarnescu and Thomas, 1999), we hypothesize that Crumbs promotes the accumulation of βH to the apicolateral region during gastrulation to orchestrate the fusion of spot adherens junctions and/or to stabilize the ZA. Moreover, the observation that karst mutants exhibit morphogenetic defects without any loss of epithelial polarity (Zarnescu and Thomas, 1999), whereas dlt mutants exhibit a strong polarity phenotype (Bhat et al., 1999), suggests that the polarization and junction building functions of Crumbs are separate and parallel pathways. In support of this hypothesis, a paper appeared while this manuscript was under review indicating that the FERM domain binding region of Crumbs is indeed required for correct organization of the ZA (Izaddoost et al., 2002).
The loss of βH function causes defects in cell shape change that are associated with apical contraction driven by an apically located actomyosin contractile ring (McKeown et al., 1998; Zarnescu and Thomas, 1999; for review see Thomas, 2001). In this context the discovery that this spectrin isoform is complexed with DMoesin is particularly provocative, as the activity of the latter is strongly correlated with modulation of cell shape and the actin cytoskeleton (Edwards et al., 1997; Tsukita and Yonemura, 1999). Furthermore, the activity of moesin is modulated by phosphorylation in response to activation of Rho-associated kinase (ROK) in parallel with myosin II. Both Moesin and myosin light chain are activated by ROK phosphorylation and by ROK mediated inhibition of the myosin/moesin phosphatase (e.g., Fukata et al., 1998; Eto et al., 2000). Therefore, we speculate that βH is part of the cytoskeletal network that facilitates such cell shape changes, and that in organizing spectrin at the membrane, Crumbs would appear to be acting as a molecular coordinator of polarity and morphogenesis. Furthermore, the finding that in human, mutations in CRB1 lead to pathologies such as retinitis pigmentosa (RP12) (den Hollander et al., 1999) emphasizes the importance of deciphering the molecular networks associated with Crumbs in Drosophila. The human orthologue of βH, βV-spectrin, is strongly expressed in photoreceptor cells (Stabach and Morrow, 2000). This raises the exciting possibility that a similar interaction between CRB1 and βV-spectrin exists in these cells. This will be examined in future work.
Materials and methods
Fly stocks
The crumbs8F105, crumbs11A22, and P(UAS-Myc-IntraWT)38.14a (Wodarz et al., 1995) strains were provided by Dr. E. Knust (Heinrich Heine University, Düsseldorf, Germany), the karst alleles 1, 2, and 14.1 were originally described in Thomas et al. (1998). These alleles have now been sequenced and the specific lesions are as follows: karst1 is a nonsense mutation in codon 1919 producing a protein truncated near the end of segment 16 (see Thomas et al. [1997] for an explanation of the segment nomenclature); karst2 is a nonsense mutation in codon 1656 causing truncation of the protein in the middle of segment 14; karst14.1 is a small deletion that removes 22 bp from the third position of codon 1659–1666, inclusive. The resulting frameshift results in termination 5 amino acids downstream. This produces a protein truncated in the middle of segment 14 that is very similar in length to that of the karst2 allele. Therefore, all three alleles produce proteins of about half the size of native βH and lack both the tetramerization site and COOH-terminal PH domain region. Recombinant karst crumbs chromosomes were generated and verified by standard techniques. Oregon-R was used as WT stock. In the overexpression experiments the engrailed GAL4 driver line was used to activate expression of UAS-Myc-IntraWT.
Statistical analysis
The karst phenotype exhibits variable expressivity (Thomas et al., 1998; Zarnescu and Thomas, 1999), and thus enhancer/suppressor interactions must be characterized in replicate experiments with appropriate statistical comparisons. In this paper, viability to adulthood is expressed as a lethal fraction of the Mendelian expectation estimated using a maximum likelihood model to determine the cost of each allelic combination of karst. Because karst cannot be maintained for many generations over the TM3 chromosome (because the 63CD region is not effectively balanced and karst is rapidly lost through recombination), the more effective TM6 balancer is routinely used. However, TM6 itself exhibits a low level of dominant lethality. Thus, to accurately estimate karst viabilities in our crosses, we first estimated the cost of the TM6 chromosome (0.321 ± 0.138 [95% confidence interval]; 17 crosses; 6, 153 flies scored) and this figure was used in our estimates of karst viabilities. Testing for any increased lethality of karst crumbs/karst + versus karst alone utilized Microsoft Excel 98 (Microsoft Corporation) to perform a one tailed t-test on the mean lethality appropriate for equal or unequal variances (assessed using an F-test).
Antibodies
A serum raised against the cytoplasmic domain of Crumbs was affinity purified and used at a dilution of 1:50 for immunofluorescence. A mouse monoclonal anti-Crumbs antibody MabCq4 (provided by Dr. E. Knust) was used at a dilution of 1:2 for immunostaining and immunoblotting. A rabbit polyclonal anti Dlt antibody provided by Dr. M. Bhat (Mount Sinai School of Medicine, New York, NY) (Bhat et al., 1999) was used at a dilution of 1:3,000 and 1:300 for immunoblotting and immunofluorescence, respectively. A mouse monoclonal anti-VSV-G antibody P5D4 (Sigma-Aldrich) was used at a dilution of 1:500 and 1:300 for immunoprecipitation and immunofluorescence, respectively, and at 1:400 for capping experiments. Affinity-purified anti βH serum (#243) was prepared as previously described (Thomas and Kiehart, 1994) and used at 1:1,000 or 1:500 for immunoblots and immunofluorescence, respectively. Antibodies against DMoesin were provided by D. Kiehart (Duke University, Durham, NC), prepared as described (Edwards et al., 1997), and used at 1:500 for immunoprecipitations, 1:2,000 for immunofluorescence, and 1:20,000 for immunoblotting. The anti-myc mouse monoclonal antibody 9E10 (Santa Cruz Biotechnology, Inc.) was used at a dilution of 1:50 and TRITC-phalloidin (Sigma-Aldrich) was used at a dilution of 1:100.
Immunofluorescent staining of embryos and cells
Immunostaining of embryos (from 2 to 14 h) was performed as described (Muller and Wieschaus, 1996) using fluorescein isothiocyanate-conjugated goat anti–mouse IgG or rhodamine-conjugated goat anti–rabbit IgG as appropriate (Jackson ImmunoResearch Laboratories, Inc.) at a dilution of 1:100. Procedures for indirect immunofluorescence of S2 cells were as described for mammalian cells (Le Bivic et al., 1989). For intracellular staining, fixed cells were permeabilized with 0.05% saponin. Fluorescent secondary antibodies were used at a dilution of 1:200. For phalloidin staining, embryos were devitellinized in 80% ethanol.
Immunoblots and immunoprecipitations
For immunoprecipitations, 2 to 14 h wild-type Drosophila embryos (1 g) were homogenized in 6 ml of purification buffer (10 mM Tris, pH 7.5, 0.32 M sucrose, 3 mM MgCl2) supplemented with anti-proteases (1/1,000) and orthovanadate (0.2 mM) and centrifuged for 10 min at 1,500 g. Supernatant was collected and the pellet was resuspended in 4 ml of purification buffer, centrifuged and the supernatants were pooled. Supernatant was ultracentrifuged for 1 h at 40,000 rpm (Ti 70 rotor; Beckman Coulter) and pellet was resuspended in 3 ml of lysis buffer (1% Igepal, 50 mM Tris, pH 7.5, 10 mM EDTA, 3 mM MgCl2) supplemented with anti-proteases and orthovanadate as described above. After incubation for 30 min at 4°C, the lysate was centrifuged for 10 min at 14,500 g, incubated for 1 h with Pansorbin, and centrifuged at 14,500 g for 15 min. Lysates were immunoprecipitated for 2 h at 4°C using the anti-Moesin or anti-βH or rabbit anti–mouse antibodies (1:500; Compiègne) preabsorbed on protein A–Sepharose beads (Amersham Biosciences). Precipitates were fractionated by SDS-PAGE, electrophoretically transferred to nitrocellulose (Schleicher and Schuell GmbH), and incubated with appropriate primary and peroxidase-conjugated secondary antibodies (1:10,000; Immunotech SA). βH was analyzed as described previously (Thomas and Kiehart, 1994).
DNA constructs, transfections and cell culture
The chimeric construct CRB–VSV-G WT was obtained by amplifying a COOH-terminal Crumbs fragment containing the stalk region, transmembrane domain and cytoplasmic domain of Crumbs (amino acid 2074–2146) using the full-length crumbs cDNA as template, a gift of Dr. E. Knust, and cloning it into the pUC19 vector containing the VSV-G tag, a gift of Dr. P. Boquet (University of Nice, Nice, France). This fusion construct was subsequently subcloned into the EcoRV-BamHI sites of the pMK33/pMtHy plasmid with a metallothionein promoter, a gift of Dr. M. Koelle (Yale University, New Haven, CT). Mutant CRB–VSV-G constructs (Fig. 5) were derived by PCR and subcloned in the same vector. All constructs were verified by sequencing (Genome Express SA).
Drosophila S2 cells were transiently or stably transfected with constructs in pMK33/pMtHy plasmid using FuGENE 6 Transfection Reagent according to the manufacturer instructions (Roche Diagnostics GmbH). Stably transfected cells were selected and maintained with Hygromycin B (Roche Diagnostics GmbH) used at a concentration of 250 and 100 μg/ml, respectively. Expression of CRB–VSV-G constructs was induced by the addition of 1 mM CuCl2 to the growth medium for 17–24 h.
Capping experiments
Stably transfected S2 cells were processed as described (Jefford and Dubreuil, 2000), except that fluorescein isothiocyanate-conjugated goat anti-mouse IgG (1:200 in Drosophila Ringer's) was added for 5 min before being transferred to polylysine-coated slides. Once on slides, the cells were fixed and stained as above for Dlt, DMoesin, or βH and cells were examined with a Zeiss LSM 410 confocal microscope. Capped S2 cells expressing the CRB–VSV-G constructs were scored for the presence of fluorescent antibody-stain caps using the fluorescein channel and for Dlt, actin, DMoesin, or βH colocalization at caps using the rhodamine channel. About 50 VSV-G–positive cells were scored in each experiment, and results are expressed as a percentage of the cocapped cells found for each protein with the CRB–VSV-G S6 construct normalized at 0% (actual capping percentage, 25% for CRB–VSV-G S6 and 75% for CRB–VSV-G WT).
Acknowledgments
The authors would like to thank colleagues who shared reagents used in this study, Dr. Andrew Clark for statistical advice, Larry Ho and Cathy Liloia for their sterling efforts in hunting down the karst mutant sequences, and members of the Thomas and Le Bivic laboratories for critical comments on this manuscript. We would like to thank C. Faivre-Sarrailh for helpful discussions and comments during this study and on the manuscript. We are particularly grateful to E. Knust who provided antibodies, cDNAs, flies and precious advice during this work.
This work was supported by Centre National de la Recherche Scientifique CNRS 6156, Université de la Méditerranée, Institut de Biologie du Développement de Marseille, Fondation de France and Association pour la Recherche sur le Cancer 9297, and an EC grant (Crumbs therapeutics) to A. Le Bivic, and by National Institutes of Health grant #GM52506 to C.M. Thomas and a National Institutes of Health predoctoral fellowship GM20906 to J. Williams. This paper is dedicated to C. Goridis.
Footnotes
Abbreviations used in this paper: βH, βHeavy-spectrin; Crb: crumbs; DE, Drosophila epithelial; Dlt, discs lost; FERM, 4.1 protein/ezrin/radixin/moesin; mAb, monoclonal antibody; PDZ, PSD-95/DLG/ZO-1; ROK, Rho-associated kinase; S2, Schneider 2; SBMS, spectrin-based membrane skeleton; Sdt, stardust; VSV-G, vesicular stomatitis virus–protein G; ZA, zonula adherens.
References
- Bachmann, A., M. Schneider, E. Theilenberg, F. Grawe, and E. Knust. 2001. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 414:638–643. [DOI] [PubMed] [Google Scholar]
- Bennett, V., and A.J. Baines. 2001. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81:1353–1392. [DOI] [PubMed] [Google Scholar]
- Bhat, M.A., S. Izaddoost, Y. Lu, K.O. Cho, K.W. Choi, and H.J. Bellen. 1999. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 96:833–845. [DOI] [PubMed] [Google Scholar]
- Bilder, D. 2001. Cell polarity: squaring the circle. Curr. Biol. 11:R132–R135. [DOI] [PubMed] [Google Scholar]
- Bilder, D., and N. Perrimon. 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 403:676–680. [DOI] [PubMed] [Google Scholar]
- Boedigheimer, M.J., K.P. Nguyen, and P.J. Bryant. 1997. Expanded functions in the apical cell domain to regulate the growth rate of imaginal discs. Dev. Genet. 20:103–110. [DOI] [PubMed] [Google Scholar]
- Brand, A.H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118:401–415. [DOI] [PubMed] [Google Scholar]
- Bretscher, A. 1999. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr. Opin. Cell Biol. 11:109–116. [DOI] [PubMed] [Google Scholar]
- Chishti, A.H., A.C. Kim, S.M. Marfatia, M. Lutchman, M. Hanspal, H. Jindal, S.C. Liu, P.S. Low, G.A. Rouleau, N. Mohandas, et al. 1998. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem. Sci. 23:281–282. [DOI] [PubMed] [Google Scholar]
- de Cuevas, M., J.K. Lee, and A.C. Spradling. 1996. α-Spectrin is required for germline cell division and differentiation in the Drosophila ovary. Development. 122:3959–3968. [DOI] [PubMed] [Google Scholar]
- De Matteis, M.A., and J.S. Morrow. 2000. Spectrin tethers and mesh in the biosynthetic pathway. J. Cell Sci. 113:2331–2343. [DOI] [PubMed] [Google Scholar]
- den Hollander, A.I., J.B. ten Brink, Y.J. de Kok, S. van Soest, L.I. van den Born, M.A. van Driel, D.J. van de Pol, A.M. Payne, S.S. Bhattacharya, U. Kellner, et al. 1999. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat. Genet. 23:217–221. [DOI] [PubMed] [Google Scholar]
- Dubreuil, R.R., and T. Grushko. 1999. Neuroglian and DE-cadherin activate independent cytoskeleton assembly pathways in Drosophila S2 cells. Biochem. Biophys. Res. Commun. 265:372–375. [DOI] [PubMed] [Google Scholar]
- Dubreuil, R.R., P.B. Maddux, T.A. Grushko, and G.R. MacVicar. 1997. Segregation of two spectrin isoforms: polarized membrane-binding sites direct polarized membrane skeleton assembly. Mol. Biol. Cell. 8:1933–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil, R.R., J. Frankel, P. Wang, J. Howrylak, M. Kappil, and T.A. Grushko. 1998. Mutations of α spectrin and labial block cuprophilic cell differentiation and acid secretion in the middle midgut of Drosophila larvae. Dev. Biol. 194:1–11. [DOI] [PubMed] [Google Scholar]
- Dubreuil, R.R., P. Wang, S. Dahl, J. Lee, and L.S. Goldstein. 2000. Drosophila β spectrin functions independently of α spectrin to polarize the Na,K ATPase in epithelial cells. J. Cell Biol. 149:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, K.A., M. Demsky, R.A. Montague, N. Weymouth, and D.P. Kiehart. 1997. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev. Biol. 191:103–117. [DOI] [PubMed] [Google Scholar]
- Eto, M., L. Wong, M. Yazawa, and D.L. Brautigan. 2000. Inhibition of myosin/moesin phosphatase by expression of the phosphoinhibitor protein CPI-17 alters microfilament organization and retards cell spreading. Cell Motil. Cytoskeleton. 46:222–234. [DOI] [PubMed] [Google Scholar]
- Fehon, R.G., I.A. Dawson, and S. Artavanis-Tsakonas. 1994. A Drosophila homologue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development. 120:545–557. [DOI] [PubMed] [Google Scholar]
- Fukata, Y., K. Kimura, N. Oshiro, H. Saya, Y. Matsuura, and K. Kaibuchi. 1998. Association of the myosin-binding subunit of myosin phosphatase and moesin: dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J. Cell Biol. 141:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grawe, F., A. Wodarz, B. Lee, E. Knust, and H. Skaer. 1996. The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development. 122:951–959. [DOI] [PubMed] [Google Scholar]
- Hong, Y., B. Stronach, N. Perrimon, L.Y. Jan, and Y.N. Jan. 2001. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 414:634–638. [DOI] [PubMed] [Google Scholar]
- Hoover, K.B., and P.J. Bryant. 2000. The genetics of the protein 4.1 family: organizers of the membrane and cytoskeleton. Curr. Opin. Cell Biol. 12:229–234. [DOI] [PubMed] [Google Scholar]
- Izaddoost, S., S-C. Nam, M.A. Bhat, H.J. Bellen and K.-W. Choi. 2002. Drosophila Crumbs is a positional cue in photoreceptor adhererns junctions and rabdomeres. Nature. 416:178–183. [DOI] [PubMed] [Google Scholar]
- Jefford, G., and R.R. Dubreuil. 2000. Receptor clustering drives polarized assembly of ankyrin. J. Biol. Chem. 275:27726–27732. [DOI] [PubMed] [Google Scholar]
- Jenkins, S.M., and V. Bennett. 2001. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J. Cell Biol. 155:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebes, A., and E. Knust. 2000. A conserved motif in Crumbs is required for E-cadherin localisation and zonula adherens formation in Drosophila. Curr. Biol. 10:76–85. [DOI] [PubMed] [Google Scholar]
- Knust, E. 2000. Control of epithelial cell shape and polarity. Curr. Opin. Genet. Dev. 10:471–475. [DOI] [PubMed] [Google Scholar]
- Le Bivic, A., F.X. Real, and E. Rodriguez-Boulan. 1989. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc. Natl. Acad. Sci. USA. 86:9313–9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.K., R.S. Coyne, R.R. Dubreuil, L.S. Goldstein, and D. Branton. 1993. Cell shape and interaction defects in α-spectrin mutants of Drosophila melanogaster. J. Cell Biol. 123:1797–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.K., E. Brandin, D. Branton, and L.S. Goldstein. 1997. α-Spectrin is required for ovarian follicle monolayer integrity in Drosophila melanogaster. Development. 124:353–362. [DOI] [PubMed] [Google Scholar]
- Lombardo, C.R., S.A. Weed, S.P. Kennedy, B.G. Forget, and J.S. Morrow. 1994. βII-spectrin (fodrin) and β I epsilon 2-spectrin (muscle) contain NH2- and COOH-terminal membrane association domains (MAD1 and MAD2). J. Biol. Chem. 269:29212–29219. [PubMed] [Google Scholar]
- Marfatia, S.M., J.H. Morais-Cabral, A.C. Kim, O. Byron, and A.H. Chishti. 1997. The PDZ domain of human erythrocyte p55 mediates its binding to the cytoplasmic carboxyl terminus of glycophorin C. Analysis of the binding interface by in vitro mutagenesis. J. Biol. Chem. 272:24191–24197. [DOI] [PubMed] [Google Scholar]
- McCartney, B.M., and R.G. Fehon. 1996. Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J. Cell Biol. 133:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown, C., V. Praitis, and J. Austin. 1998. sma-1 encodes a βH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development. 125:2087–2098. [DOI] [PubMed] [Google Scholar]
- Moorthy, S., L. Chen, and V. Bennett. 2000. Caenorhabditis elegans beta-G spectrin is dispensable for establishment of epithelial polarity, but essential for muscular and neuronal function. J. Cell Biol. 149:915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, H.A. 2000. Genetic control of epithelial cell polarity: lessons from Drosophila. Dev. Dyn. 218:52–67. [DOI] [PubMed] [Google Scholar]
- Muller, H.A., and E. Wieschaus. 1996. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellikka, M., G. Tanentzapf, M. Pinto, C. Smith, C.J. McGlade, D.R. Ready, and U. Tepass. 2002. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 416:143–149. [DOI] [PubMed] [Google Scholar]
- Stabach, P.R., and J.S. Morrow. 2000. Identification and characterisation of βV spectrin, a mammalian ortholog of Drosophila βH spectrin. J. Biol. Chem. 275:21385–21395. [DOI] [PubMed] [Google Scholar]
- Tanentzapf, G., C. Smith, J. McGlade, and U. Tepass. 2000. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J. Cell Biol. 151:891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass, U. 1996. Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev. Biol. 177:217–225. [DOI] [PubMed] [Google Scholar]
- Tepass, U. 1997. Epithelial differentiation in Drosophila. Bioessays. 19:673–682. [DOI] [PubMed] [Google Scholar]
- Tepass, U., C. Theres, and E. Knust. 1990. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 61:787–799. [DOI] [PubMed] [Google Scholar]
- Thomas, G. 2001. Spectrin: The ghost in the machine. Bioessays. 23:152–160. [DOI] [PubMed] [Google Scholar]
- Thomas, G.H., and D.P. Kiehart. 1994. βHeavy-spectrin has a restricted tissue and subcellular distribution during Drosophila embryogenesis. Development. 120:2039–2050. [DOI] [PubMed] [Google Scholar]
- Thomas, G.H., and J.A. Williams. 1999. Dynamic rearrangement of the spectrin membrane skeleton during the generation of epithelial polarity in Drosophila. J. Cell Sci. 112:2843–2852. [DOI] [PubMed] [Google Scholar]
- Thomas, G.H., E.C. Newbern, C.C. Korte, M.A. Bales, S.V. Muse, A.G. Clark, and D.P. Kiehart. 1997. Intragenic duplication and divergence in the spectrin superfamily of proteins. Mol. Biol. Evol. 14:1285–1295. [DOI] [PubMed] [Google Scholar]
- Thomas, G.H., D.C. Zarnescu, A.E. Juedes, M.A. Bales, A. Londergan, C.C. Korte, and D.P. Kiehart. 1998. Drosophila βHeavy-spectrin is essential for development and contributes to specific cell fates in the eye. Development. 125:2125–2134. [DOI] [PubMed] [Google Scholar]
- Tsukita, S., and S. Yonemura. 1999. Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J. Biol. Chem. 274:34507–34510. [DOI] [PubMed] [Google Scholar]
- Wodarz, A., F. Grawe, and E. Knust. 1993. CRUMBS is involved in the control of apical protein targeting during Drosophila epithelial development. Mech. Dev. 44:175–187. [DOI] [PubMed] [Google Scholar]
- Wodarz, A., U. Hinz, M. Engelbert, and E. Knust. 1995. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 82:67–76. [DOI] [PubMed] [Google Scholar]
- Yeaman, C., K.K. Grindstaff, and W.J. Nelson. 1999. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 79:73–98. [DOI] [PubMed] [Google Scholar]
- Zarnescu, D.C., and G.H. Thomas. 1999. Apical spectrin is essential for epithelial morphogenesis but not apicobasal polarity in Drosophila. J. Cell Biol. 146:1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]