Abstract
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid metabolite that regulates diverse biological processes by binding to a family of G protein–coupled receptors or as an intracellular second messenger. Mammalian S1P phosphatase (SPP-1), which degrades S1P to terminate its actions, was recently cloned based on homology to a lipid phosphohydrolase that regulates the levels of phosphorylated sphingoid bases in yeast. Confocal microscopy surprisingly revealed that epitope-tagged SPP-1 is intracellular and colocalized with the ER marker calnexin. Moreover, SPP-1 activity and protein appeared to be mainly enriched in the intracellular membranes with lower expression in the plasma membrane. Treatment of SPP-1 transfectants with S1P markedly increased ceramide levels, predominantly in the intracellular membranes, diminished survival, and enhanced apoptosis. Remarkably, dihydro-S1P, although a good substrate for SPP-1 in situ, did not cause significant ceramide accumulation or increase apoptosis. Ceramide accumulation induced by S1P was completely blocked by fumonisin B1, an inhibitor of ceramide synthase, but only partially reduced by myriocin, an inhibitor of serine palmitoyltransferase, the first committed step in de novo synthesis of ceramide. Furthermore, S1P, but not dihydro-S1P, stimulated incorporation of [3H]palmitate, a substrate for both serine palmitoyltransferase and ceramide synthase, into C16-ceramide. Collectively, our results suggest that SPP-1 functions in an unprecedented manner to regulate sphingolipid biosynthesis and is poised to influence cell fate.
Keywords: S1P phosphaxtase-1; sphingosine-1-phosphate; ceramide; sphingosine; apoptosis
Introduction
Sphingosine-1-phosphate (S1P),* a bioactive lipid produced from the metabolism of sphingolipids, regulates vital biological processes (Pyne and Pyne, 2000; Spiegel and Milstien, 2000a, 2002; Hla et al., 2001). S1P is the ligand of a family of specific cell surface G protein–coupled receptors (GPCRs), hereafter referred to as S1PRs. These receptors, which include EDG-1/S1P1, EDG-5/S1P2, EDG-3/S1P3, EDG-6/S1P4, and EDG-8/S1P5, couple to various G proteins to regulate cell migration, angiogenesis, vascular maturation, heart development, and neurite retraction (Pyne and Pyne, 2000; Spiegel and Milstien, 2000a, 2002; Hla et al., 2001). Sphinganine-1-phosphate (dihydro-S1P), which is identical to S1P and only lacks the trans 4,5 double bond, binds to all of the S1PRs and activates them, yet does not mimic all of the effects of S1P, especially those related to cell survival and protection against apoptosis (Van Brocklyn et al., 1998; Xia et al., 1998; Morita et al., 2000; Rosenfeldt et al., 2001). This led to the suggestion that S1P may have novel dual functions, acting not only as an extracellular mediator but possibly intracellularly through, as yet, unidentified targets (Spiegel and Milstien, 2002).
Many external stimuli, particularly growth and survival factors, activate sphingosine kinase, which phosphorylates sphingosine and increases cellular S1P levels. Intracellularly generated S1P can mobilize calcium from internal stores (Mattie et al., 1994; Meyer zu Heringdorf et al., 1998), suppress ceramide-mediated apoptosis (Cuvillier et al., 1996; Morita et al., 2000), or can act in a paracrine or autocrine fashion to activate S1P1 signaling, which is crucial for directed cell movement (Hobson et al., 2001). Thus, due to the pivotal role of S1P in regulating cellular processes, it is not surprising that its levels are low and tightly regulated in a spatial–temporal manner through its synthesis catalyzed by sphingosine kinase and degradation by an ER pyridoxal phosphate–dependent S1P lyase and still not well-characterized phosphohydrolase activities.
As a first approach to identify specific mammalian S1P phosphatases, two genes were cloned from Saccharomyces cerevisiae, LBP1/YSR2/LCB3 and LBP2/YSR3, that encode specific sphingoid base phosphate phosphatases (Mao et al., 1997; Qie et al., 1997; Mandala et al., 1998). These S1P phosphatases belong to the family of magnesium-independent, N-ethylmaleimide–insensitive type 2 lipid phosphate phosphohydrolases (LPPs) (Stukey and Carman, 1997; Brindley and Waggoner, 1998). However, except for their conserved residues within three domains present in all LPPs (Stukey and Carman, 1997), they have little overall homology to other known LPPs. Genetic manipulations demonstrated that these S1P phosphohydrolases regulate the levels of phosphorylated sphingoid bases and ceramide and influence growth and survival of yeast after nutrient deprivation and heat stress (Dickson et al., 1997; Jenkins et al., 1997; Mandala et al., 1998; Mao et al., 1999; Skrzypek et al., 1999).
On the basis of sequence homology with LBP1, the first mammalian homologue, murine S1P phosphatase 1 (mSPP-1), was recently cloned. This hydrophobic enzyme contains 430 amino acids, 8–10 membrane-spanning regions, and degrades S1P, but not lysophosphatidic acid (LPA), a structurally related lysophospholipid (Mandala et al., 2000). By contrast, the other LPPs have broad substrate specificities and degrade LPA, phosphatidic acid (PA), diacylglycerolpyrophosphate, S1P, and ceramide-1-phosphate (Brindley and Waggoner, 1998). Previous studies have demonstrated that LPP1 is localized on the plasma membrane, with an externally oriented catalytic site, and functions as an ectophosphatase to attenuate the actions of LPA as an agonist of its cell surface receptors (Jasinska et al., 1999). However, more recently, it has been shown that the ability of LPP1 and LPP2 to attenuate GPCR signaling correlates with reduced intracellular PA and not with ectolipid phosphate phosphatase activity (Alderton et al., 2001). Moreover, although LPA-induced mitogenesis is regulated by LPPs, in an analogous manner to the intracellular actions of S1P (Van Brocklyn et al., 1998), it is also LPA receptor independent (Hooks et al., 2001). Thus, we examined the subcellular localization of SPP-1 in order to define its biological function and to determine whether it might play a role in regulating extracellular or intracellular levels of S1P. The results of this study indicate that this novel lipid phosphohydrolase is located mainly in the ER and that it functions in an unprecedented manner to regulate biosynthesis of ceramide, which plays a critical role in apoptosis.
Results
mSPP-1 activity is membrane associated
Overexpression of mSPP-1 in human embryonic kidney (HEK) 293 cells, either transiently or stably, resulted in markedly increased S1P phosphohydrolase activity (Fig. 1 A), in agreement with previous studies (Le Stunff et al., 2002). This activity was almost exclusively membrane associated and the presence of the Myc epitope tag did not significantly alter the activity (Fig. 1 A). Western blot analysis with an anti-Myc mAb also revealed that mSPP-1 was only expressed in the membrane fraction (Fig. 1 B). Similar results were obtained in NIH 3T3 fibroblasts (unpublished data). Furthermore, fluorescence microscopy revealed a diffuse intracellular distribution of Myc-tagged mSPP-1 in these cells (Fig. 1 C).
Figure 1.
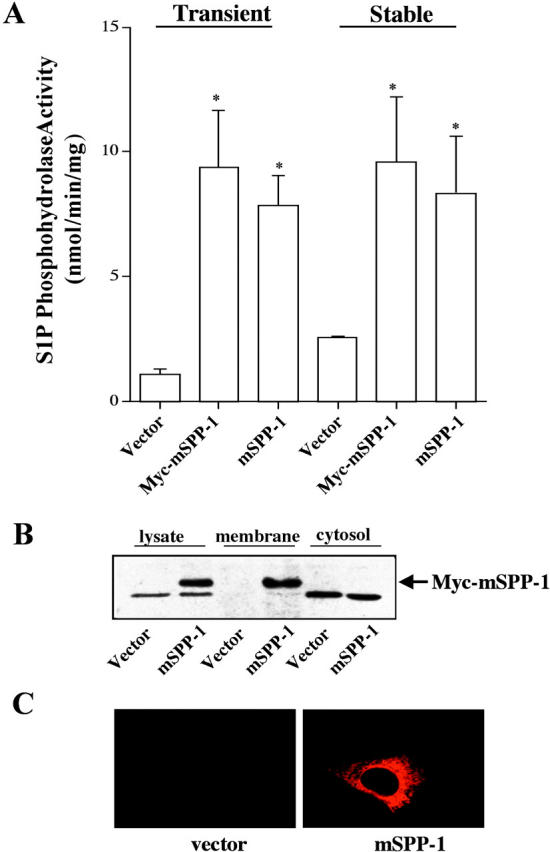
mSPP-1 activity is membrane associated in mammalian cells. (A) S1P phosphohydrolase activity was measured in membrane fractions from HEK 293 cells transiently and stably transfected with Myc-pcDNA3 (vector), mSPP-1–pcDNA3.1 (mSPP-1), or Myc-pcDNA–mSPP-1 (Myc–mSPP-1). S1P phosphohydrolase activity in cytosol fractions from these cells was <1 nmol/min/mg. Data are means ± SEM of five independent experiments, each performed in duplicate. (B) Western blot showing expression of mSPP-1. Triton X-100 extract (lysate) and membrane and cytosolic fractions (50 μg of proteins) from vector-transfected and Myc–mSPP-1–transfected HEK 293 cells were resolved by SDS-PAGE and then immunoblotted with a monoclonal Myc antibody. The lower nonspecific band was detected in both vector and mSPP-1–transfected cells. (C) Cellular localization of Myc–mSPP-1. NIH 3T3 fibroblasts transiently expressing vector or Myc-pcDNA–mSPP-1 were incubated with a monoclonal Myc antibody, stained with anti–mouse Texas red monoclonal IgG, and visualized by fluorescence microscopy.
Subcellular localization of mSPP-1
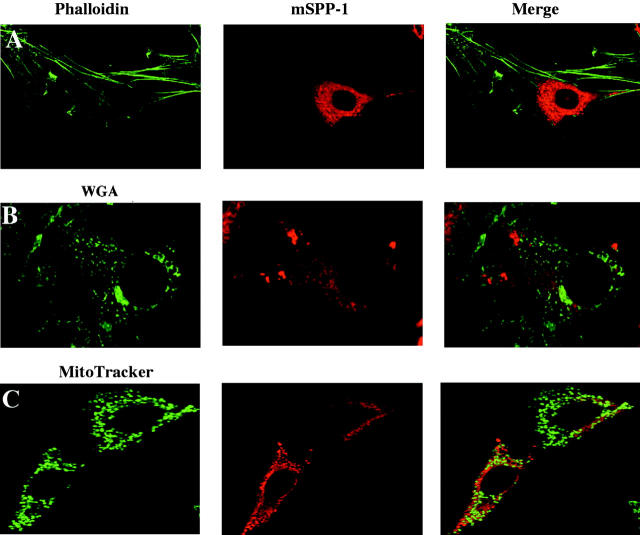
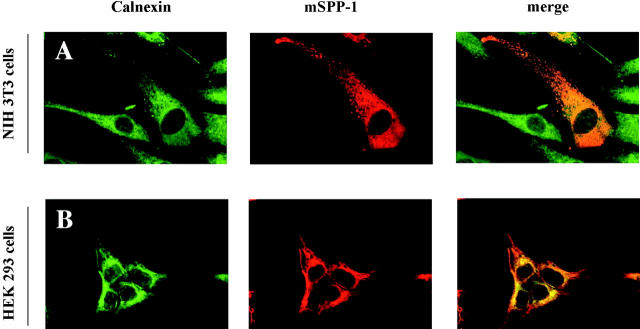
To better define the cellular localization of mSPP-1, we next used confocal immunofluorescence microscopy. In NIH 3T3 fibroblasts, Myc–mSPP-1 was distributed in a perinuclear and reticular pattern, reminiscent of an ER localization (Fig. 2). Indeed, there was no significant colocalization with cortical actin, stained with fluorescent phalloidin, the Golgi apparatus, stained with fluorescent WGA, or with MitoTracker-stained mitochondria, as demonstrated by the absence of yellow color in the merged images (Fig. 2). On the contrary, mSPP-1 expression was clearly colocalized with the ER marker calnexin (Fig. 3 A). Identical results were found with HEK 293 cells overexpressing Myc–mSPP-1 (Fig. 3 B), demonstrating that the subcellular distribution was not cell type specific. Thus, interestingly, in contrast to the well-defined plasma membrane localization of LPP1 (Jasinska et al., 1999; Zhang et al., 2000), mSPP-1 appeared to be expressed on intracellular membranes.
Figure 2.
mSPP-1 does not colocalize with the actin network, Golgi apparatus, or mitochondria. NIH 3T3 fibroblasts were transiently transfected with Myc–mSPP-1 and visualized by confocal fluorescence microscopy in the left panels after staining for cortical actin (A), Golgi network (B), or mitochondria (C) with Alexa Fluor®488–phalloidin, fluorescent WGA, or MitoTracker green, respectively. Center panels show the localization of mSPP-1 in the same cells visualized with anti-Myc antibody and Texas red–conjugated secondary antibody. Right panels show the superimposed pictures.
Figure 3.
mSPP-1 is localized to the ER. NIH 3T3 fibroblasts (A) and HEK 293 cells (B) were transiently transfected with Myc–mSPP-1, and stained for ER using anti-calnexin antibody (left panels) and for mSPP-1 using anti-Myc antibody (center panels). Cells were visualized by dual wavelength confocal microscopy. Right panels show the superimposed merged pictures, yellow color indicating colocalization.
Because LPP1 has been shown to have substantial ectophosphatase activity by virtue of its plasma membrane localization, it was of interest to also examine mSPP-1 activity in subcellular fractions. mSPP-1–expressing HEK 293 cells were subcellularly fractionated by differential centrifugation into P1, P2, and cytosol fractions. Specific enzyme markers were used to substantiate and define purity of the different compartments (Fig. 4 A). The P1 fraction contained intracellular organelles, as indicated by the presence of both cytochrome C oxidase II and phosphodisulfide isomerase, specific markers of the mitochondria and ER, respectively. Plasma membranes were associated with P2 as indicated by the expression of αv-integrin. mSPP-1 expression appeared to be enriched in the P1 intracellular membranes fraction (Fig. 4 A), in agreement with the ER localization shown by confocal microscopy. Very low levels of mSPP-1 expression were also detected in the P2 plasma membrane fraction. In concordance with the expression pattern of the protein, the majority of S1P phosphohydrolase activity was detected in P1, with much lower amounts in the P2 fraction (Fig. 4 B). Moreover, although mSPP-1–transfected cells had fivefold and threefold higher S1P phosphohydrolase activity in the P1 and P2 fractions, respectively, compared with the vector transfectants, the total activity in the P1 fraction was much greater, and mSPP-1 expression and activity were not detected in the cytosolic fraction (Fig. 4 B).
Figure 4.
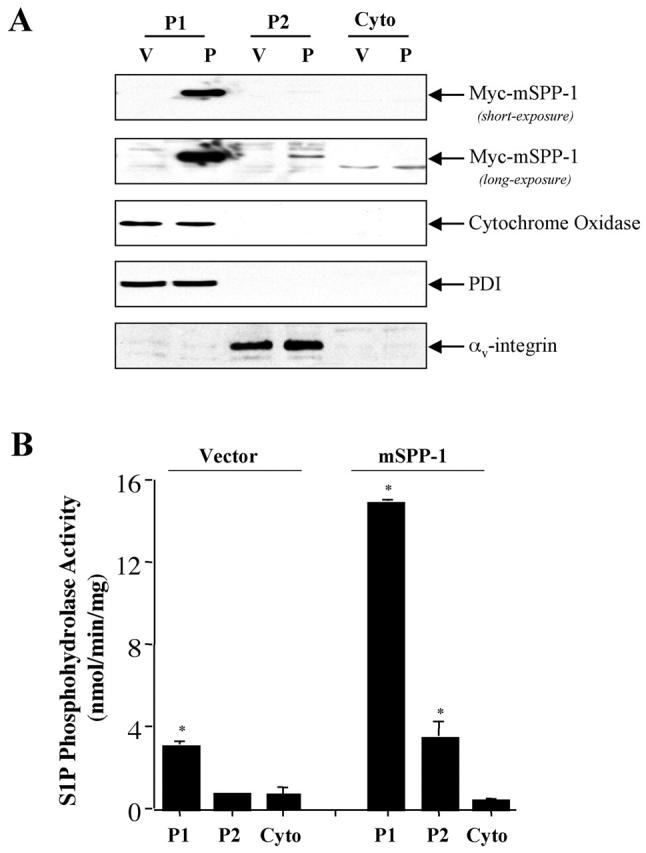
Subcellular fractionation of HEK 293 cells transfected with Myc–mSPP-1. (A) Lysates from HEK 293 cells stably transfected with vector or Myc–mSPP-1–pcDNA3 were subcellularly fractionated into P1 (intracellular membrane fraction containing mitochondria, ER, and Golgi), P2 (plasma membrane), and cytosol, as described in the Materials and methods. Proteins (25 μg) were resolved by SDS-PAGE and immunoblotted with anti-Myc, anti–cytochrome C oxidase, anti-PDI, or anti–αv-integrin as specific organelle markers. Similar results were obtained in two additional experiments. (B) S1P phosphohydrolase activity was determined in each subcellular fraction using [32P]S1P (10 μM) as substrate. Results are means ± SD of three different preparations.
Overexpression of mSPP-1 modulates ceramide levels
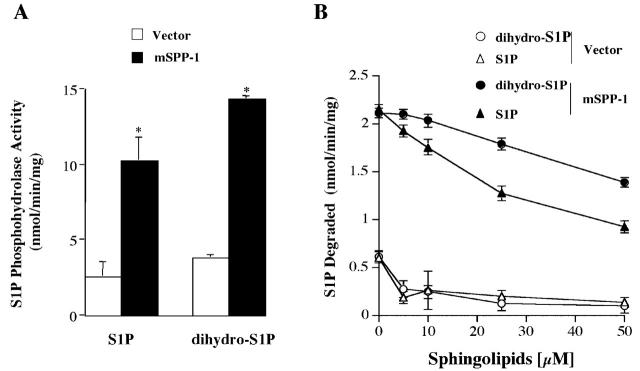
mSPP-1 is a highly specific sphingoid base phosphate phosphohydrolase (Mandala et al., 2000). In agreement with recent results in CHO and HEK 293 cells (Le Stunff et al., 2002), both S1P and dihydro-S1P, which has an identical structure except lacks the trans 4,5 double bond, are hydrolyzed similarly by mSPP-1 (Fig. 5 A). Moreover, the addition of unlabeled S1P and dihydro-S1P decreased hydrolysis of [32P]S1P in a dose-dependent manner, although S1P was more effective than dihydro-S1P (Fig. 5 B).
Figure 5.
S1P and dihydro-S1P are substrates of mSPP-1. (A) S1P phosphohydrolase activity was measured in membrane fractions from HEK 293 cells stably transfected with vector (open bars) or mSPP-1 (filled bars) with 10 μM 32P-labeled S1P or dihydro-S1P as substrates. (B) Effect of unlabeled S1P and dihydro-S1P on hydrolysis of [32P]S1P. Membrane fractions from the transfected cells were incubated with 1 μM [32P]S1P for 30 min at 37°C in the presence of increasing concentrations of unlabeled S1P or dihydro-S1P, and 32Pi release was measured. Results are the means ± SEM of three different experiments, each performed in duplicate.
Because both S1P and dihyro-S1P are substrates for mSPP-1 in vitro (Fig. 5), and the products sphingosine and dihydrosphingosine can be further metabolized by cells to ceramide and dihydroceramide, respectively, it was of interest to determine whether overexpression of mSPP-1 altered sphingolipid metabolism. When empty vector transfectants were incubated with either S1P or dihydro-S1P, there were no significant changes in ceramide levels (Fig. 6 A). However, treatment of mSPP-1–overexpressing cells with S1P, but surprisingly not with dihydro-S1P, resulted in a more than twofold increase in ceramide (Fig. 6 A). In apparent agreement with the localization of mSPP-1, most of the increase in ceramide after S1P treatment occurred in the internal membrane fraction, although there also was a small increase of ceramide in the plasma membrane fraction (Fig. 6 B). Not surprisingly, because the majority of ceramide is membrane associated, no changes in cytosolic ceramide levels were detected. Thus, it is most likely that as a consequence of overexpression of mSPP-1, the increased sphingosine generated by dephosphorylation of exogenous S1P has been diverted to ceramide generation by ceramide synthase–catalyzed N-acylation of sphingosine.
Figure 6.
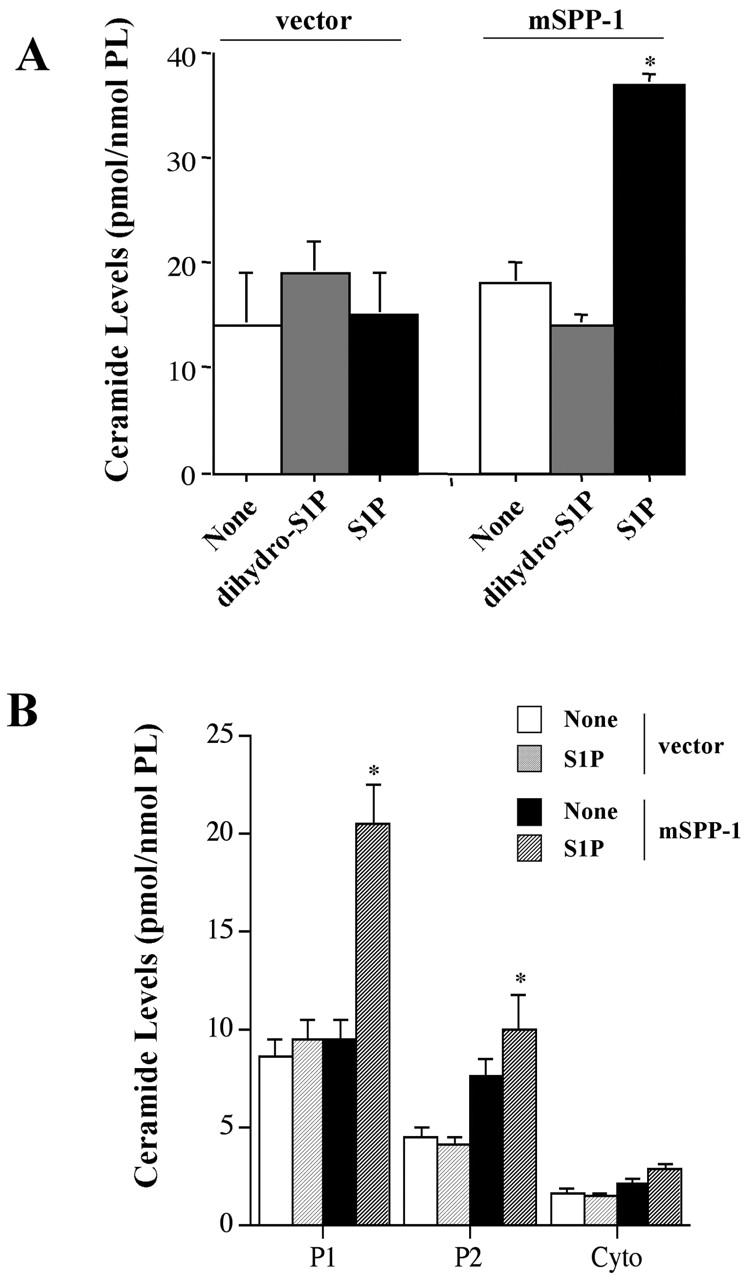
S1P, but not dihydro-S1P, increases ceramide levels in cells overexpressing mSPP-1. (A) Vector-transfected or mSPP-1– expressing HEK 293 cells were incubated in the absence (None) or presence of 5 μM dihydro-S1P or S1P for 48 h. Ceramide levels were then determined as described in the Materials and methods. (B) Ceramide levels are elevated predominantly in internal membranes. Lysates were prepared from cells treated with S1P, as described in Fig. 4 A, and ceramide levels in the indicated subcellular fractions (P1, P2, and cytosol) were determined. Data are the means ± SEM of three independent experiments, each performed in duplicate.
Ceramide accumulation is associated with apoptosis
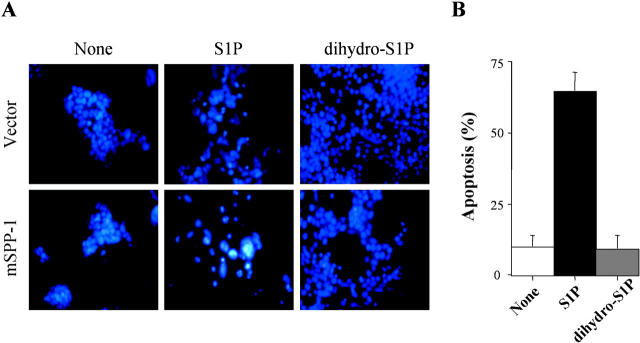
Ceramide has been implicated as a critical component of apoptosis (Kolesnick and Hannun, 1999; Hannun and Luberto, 2000), whereas elevation of S1P has been shown to play a cytoprotective role (Spiegel and Milstien, 2000b), particularly in opposing ceramide-mediated apoptosis (Cuvillier et al., 1996; Morita et al., 2000). Thus, we examined whether ceramide accumulation correlated with the induction of apoptosis. Similar to our results with NIH 3T3 fibroblasts transiently transfected with mSPP-1 (Mandala et al., 1998), treatment of HEK 293 cells stably expressing mSPP-1 with 5 μM S1P not only resulted in ceramide elevation (Fig. 6), it also markedly induced apoptosis (Fig. 7). By contrast, treatment with dihydro-S1P, which did not increase ceramide levels, had no significant effect on apoptosis.
Figure 7.
S1P, but not dihydro-S1P, induces apoptosis in cells overexpressing mSPP-1. (A) HEK 293 cells stably transfected with vector or mSPP-1 were incubated in the absence or the presence of 5 μM S1P or dihydro-S1P for 3 d. Note the typical condensed fragmented nuclei of apoptotic cells in mSPP-1 transfectants treated with S1P but not with dihydro-S1P. (B) Percentages of apoptotic cells overexpressing SPP-1 were determined by fluorescence microscopy as described in the Materials and methods. Apoptotic cells displaying fragmented nuclei indicative of apoptosis were counted and a minimum of 500 cells in each field was scored. Data are means ± SEM of three independent experiments, each performed in duplicate.
Ceramide biosynthesis: the role of ceramide synthase
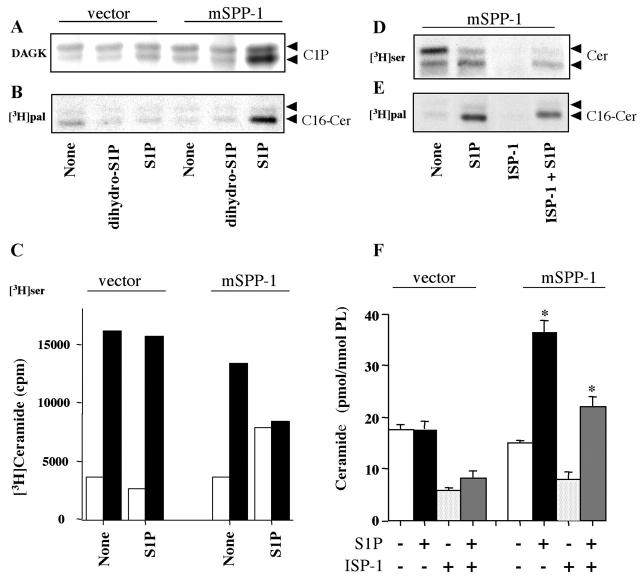
As S1P treatment increased ceramide levels, resulting in apoptosis, it was important to determine the source of this ceramide. Similar to previous studies (Kroesen et al., 2001), TLC resolved ceramide (after derivatization into ceramide phosphate) into two bands; long and very long chain ceramides migrate with the lower and upper bands, respectively (Kroesen et al., 2001). We noticed that after S1P addition to SPP-1–expressing cells, there was a marked increase in the lower band (Fig. 8 A). Indeed, when SPP-1 transfectants were labeled with [3H]palmitate, which serves as a substrate for both serine palmitoyltransferase and ceramide synthase, S1P induced a 3.8-fold increase in the incorporation of radioactivity into the lower ceramide band (Fig. 8 B), which comigrated with C16-ceramide. When cells were labeled with l-3-[3H]serine, a substrate for serine palmitoyltransferase, radioactive serine was incorporated predominantly into the upper ceramide band (Fig. 8, C and D). Surprisingly, S1P inhibited incorporation of serine into the upper ceramide band but increased incorporation into the lower ceramide band in mSPP-1 transfectants (Fig. 8, C and D).
Figure 8.
S1P, but not dihydro-S1P, induces biosynthesis of ceramide in mSPP-1–transfected cells. (A) Vector- and mSPP-1–transfected HEK 293 cells were incubated for 24 h without or with 5 μM S1P or 5 μM dihydro-S1P and ceramide determined after phosphorylation to ceramide-1-phosphate (C1P) in the DAG kinase assay. The upper and lower C1P bands are derived from very long and long chain ceramides, respectively. (B) Duplicate cultures were incubated with [3H]palmitic acid (10 μCi/ml), ceramide isolated, separated by TLC, and then visualized by autoradiography. (C) Similarly, labeled ceramide from cells incubated with l-3-[3H]serine (30 μCi/ml) was isolated, separated by TLC, and two ceramide bands were radioactivity quantitated with a radiochromatogram scanner (open bars, long chain ceramide; filled bars, very long chain ceramide). (D–F) Effect of ISP-1. Cells were incubated without or with 5 μM S1P in the absence or presence of 10 μM ISP-1 and labeled with l-[3H]serine (D) or [3H]palmitic acid (E). 24 h later, lipids were separated by TLC. Ceramide mass was measured in unlabeled duplicate cultures (F). Data are means ± SEM of three experiments, each performed in duplicate. For all panels, a representative result from three independent experiments is shown.
To examine the pathways involved in the generation of long chain ceramide, we used two inhibitors of de novo synthesis of ceramide: myriocin (ISP-1), a specific inhibitor of serine palmitoyltransferase; and fumonisin B1 (FB1), an inhibitor of CoA-dependent dihydroceramide/ceramide synthase. As expected, ISP-1 completely blocked [3H]serine incorporation into the ceramides (Fig. 8 D). However, ISP-1 only reduced the incorporation of labeled palmitic acid into C16-ceramide and ceramide accumulation induced by S1P in mSPP-1 transfectants by 30% (Fig. 8, E and F). ISP-1 also had no influence on the inability of dihydro-S1P to increase ceramide levels (unpublished data).
In contrast to ISP-1, FB1 completely prevented the increase in intracellular ceramide levels resulting from treatment of mSPP-1–expressing cells with S1P and did not affect the low levels of ceramide in dihydro-S1P–treated cells (Fig. 9 A). In agreement, there was a concomitant large increase in sphingosine levels (Fig. 9 B). These results indicate that the recombinant mSPP-1 was indeed active in vivo, converting S1P to sphingosine that was then readily metabolized to ceramide via ceramide synthase. Remarkably, although no changes in the levels of ceramides could be detected when mSPP-1–expressing cells were treated with dihydro-S1P, when added in the presence of FB1, there was a large accumulation of dihydrosphingosine (Fig. 9 B). These results confirm that dihydro-S1P is indeed cleaved by mSPP-1 intracellularly, yet there is no accompanying accumulation of ceramide, suggesting rapid metabolism of dihydroceramide to sphingomyelin or complex sphingolipids. Moreover, dihydro-S1P, which has no effect on the survival of vector- or mSPP-1–transfected cells, induces apoptosis of the mSPP-1 transfectants, but not vector cells, only when added in the presence of FB1 (Fig. 9 B, inset). This is in agreement with previous studies showing that the accumulation of long chain sphingoid bases causes apoptosis (Sakakura et al., 1998; Perry et al., 2000; Cuvillier et al., 2001; Kagedal et al., 2001). It should be noted that FB1 caused a large increase in basal sphingosine levels in mSPP-1 compared with vector transfectants (Fig. 9 B). This result suggests that normal turnover of intracellular S1P is a dynamic process that can contribute to the regulation of ceramide levels.
Figure 9.
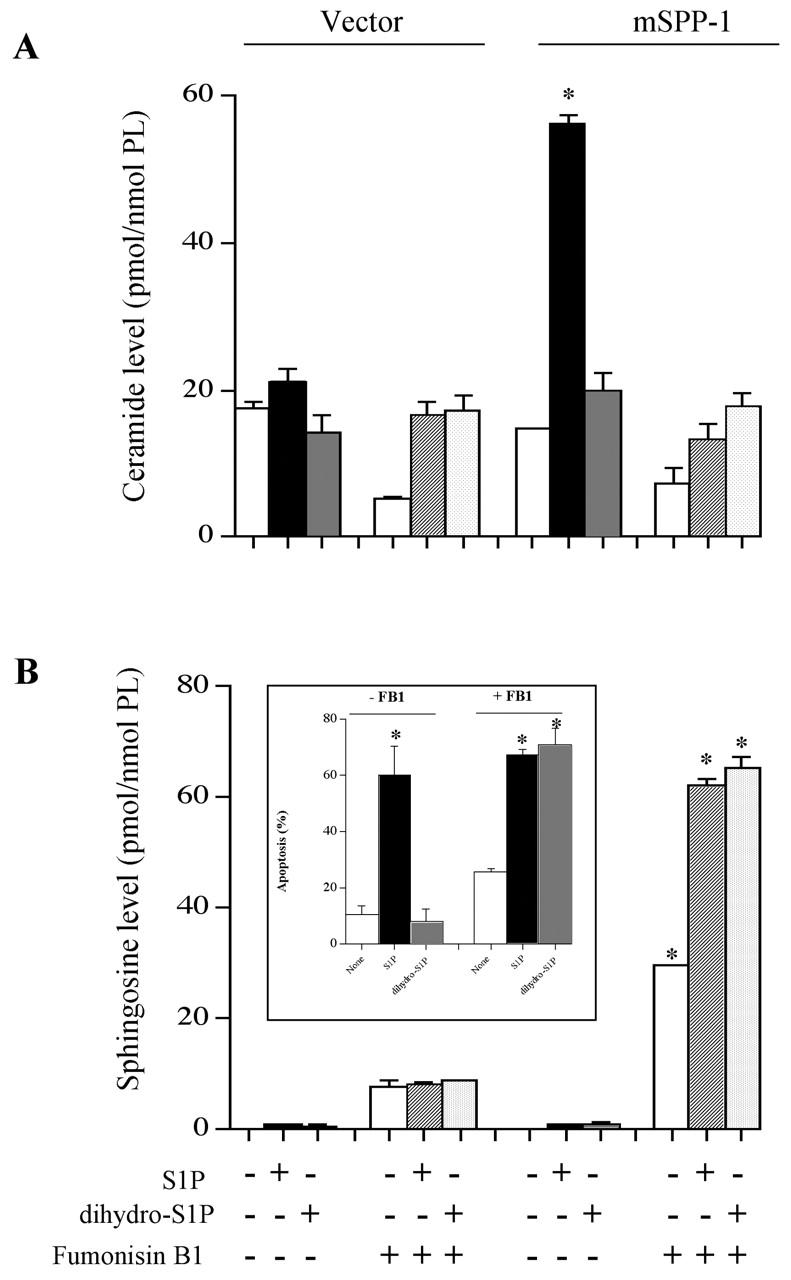
Fumonisin B1 prevents ceramide elevation induced by S1P in mSPP-1 transfectants. Vector- or mSPP-1–transfected HEK 293 cells were treated with 5 μM S1P or dihydro-S1P in the absence or presence of 25 μM fumonisin B1 for 48 h as indicated. Ceramide (A) and sphingosine levels (B) were determined as described in the Materials and methods. Sphingosine levels in vector and mSPP-1 transfectants treated with vehicle, S1P, or dihydro-S1P were 0.1 ± 0.02 and 0.2 ± 0.02, 0.9 ± 0.3 and 1.1 ± 0.3, 1 ± 0.4 and 1 ± 0.4 pmol/nmol phospholipid, respectively. Data are the means ± SEM of three independent experiments, each performed in duplicate. (Inset) mSPP-1–transfected HEK 293 cells were treated with vehicle, S1P (5 μM), or dihydro-S1P (5 μM) in the absence (−) or presence (+) of 25 μM fumonisin B1 for 48 h. Percentages of apoptotic cells were determined by Hoechst staining. A minimum of 500 cells in each field was scored.
The (R,R)-(d-threo) isomer of 1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP), an analogue of glucosylceramide and a potent inhibitor of glucosylceramide synthase (Shayman et al., 2000), was used to examine whether the increased ceramide in mSPP-1–expressing cells might arise partly due to a lower rate of its conversion to glucosylceramide and other complex glycosphingolipids. PDMP at 10 μM, a concentration that potently inhibits glucosylceramide synthase activity in vitro (Shayman et al., 2000), increased ceramide by 2.5-fold over basal levels in untreated and dihydro-S1P–treated mSPP-1–expressing cells, and markedly augmented the elevated ceramide accumulation induced by S1P (Fig. 10 A) without significantly affecting sphingosine levels (Fig. 10 B). Thus, it is unlikely that decreased utilization of ceramide for the formation of glucosylceramide is responsible for its accumulation.
Figure 10.

PDMP enhances ceramide production induced by S1P in mSPP-1 transfectants. Vector- or mSPP-1–transfected HEK 293 cells were incubated without or with 5 μM S1P or dihydro-S1P in the absence or presence of 10 μM PDMP as indicated. 24 h later, ceramide (A) and sphingosine (B) levels were determined. Data are means ± SD of three independent experiments, each performed in duplicate.
Discussion
mSPP-1 is localized to the ER
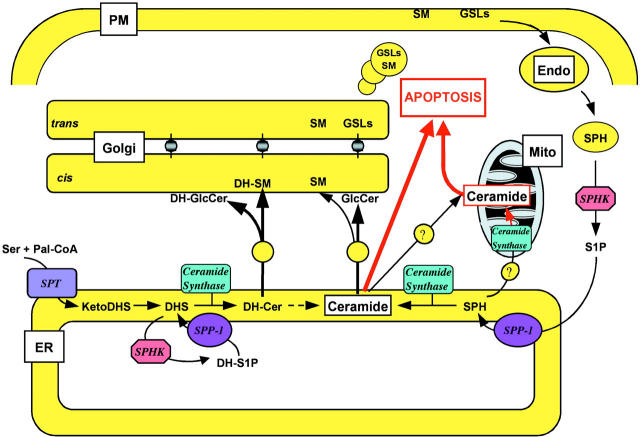
The bioactive lipids S1P and LPA are both intracellular and extracellular mediators. To better understand their functions, it is important to determine how their levels are regulated. Whereas much has been learned about the phosphatases that degrade LPA, less is known about degradation of S1P. It has been suggested that LPPs function as ectophosphohydrolases to degrade LPA and thus attenuate extracellular cell signaling by decreasing the availability of this GPCR agonist (Waggoner et al., 1999). We have now demonstrated that SPP-1 resides predominantly in the ER both by confocal microscopy and subcellular fractionation. Thus, it is likely that SPP-1 is one of the enzymes responsible for the regulation of the intracellular levels of S1P and dihydro-S1P and, concomitantly, sphingosine and dihydrosphingosine. Similarly, the yeast homologues of SPP-1, LBP1/YSR2/LCB3 and LBP2/YSR3, are also located in the ER (Mao et al., 1999). Because the ER contains the other enzymes of ceramide biosynthesis, localization of SPP-1 to this compartment suggests that it could play an important role in the regulation of sphingolipid metabolism, particularly ceramide biosynthesis.
SPP-1 regulates ceramide levels
Ceramide biosynthesis at the cytoplasmic face of the ER is initiated by condensation of l-serine with palmitoyl CoA, catalyzed by serine palmitoyltransferase (Futerman et al., 1990; Merrill et al., 1993). In two rapid reactions, the product, 3-ketosphinganine, is reduced to sphinganine (dihydrosphingosine) and subsequently acylated by ceramide synthase to form dihydroceramide (Fig. 11). Dihydroceramide is then converted to ceramide by a desaturase (Michel and van Echten-Deckert, 1997). Ceramide, or in some cases, dihydroceramide, is translocated from the ER to the Golgi apparatus by not well-defined mechanisms, and then converted to sphingomyelin by the enzyme phosphatidylcholine:ceramide cholinephosphotransferase (sphingomyelin synthase) on the lumenal side of the Golgi apparatus or to glucosylceramide on the cytosolic surface of the Golgi apparatus (for reviews see van Meer and Holthuis, 2000; Funato and Riezman, 2001). After translocation into the Golgi lumen, glucosylceramide is further converted to lactosylceramide and more complex glycosphingolipids.
Figure 11.
Role of SPP-1 in biosynthesis and trafficking of sphingolipids and apoptosis. Scheme depicting the bioactive intermediates of sphingolipid biosynthesis. In brief, dihydroceramide and ceramide biosynthesis takes place at the cytosolic surface of the ER. In the membrane recycling/salvage pathway, sphingosine produced from sphingolipids in the lysosome is phosphorylated in the cytosol to S1P and dephosphorylated in the ER by SPP-1, where it is reutilized for synthesis of ceramide. Dihydroceramide and ceramide are transported to the cis-Golgi by vesicular transport, and nonvesicular transport to the TGN where they are converted to sphingomyelin and glucosylceramides. See text for more information.
In line with a significant role for SPP-1 in regulating the balance of sphingolipid metabolites, we found that overexpressing SPP-1 in mammalian cells increased ceramide levels, particularly in the presence of exogenous S1P. Similarly, yeast overexpressing LBP1 accumulate ceramide and, conversely, deletion of LBP1 results in the accumulation of phosphorylated long chain sphingoid bases and reduced ceramide levels (Mao et al., 1997, 1999; Mandala et al., 1998). Surprisingly, however, dihydro-S1P, which is also readily dephosphorylated by SPP-1 in situ, did not cause an elevation of ceramide levels. Yet, FB1, an inhibitor of ceramide synthase, induced accumulation of sphingoid bases when cells were treated with either S1P or dihydro-S1P, suggesting that the sphingosine and sphinganine produced in these cells are indeed substrates for ceramide synthase. So why does ceramide and not dihydroceramide accumulate and what is the source of this ceramide?
ISP-1, an inhibitor of serine palmitoyltransferase, slightly reduced the elevation of ceramide levels, suggesting that the de novo pathway is not a major contributor. Moreover, both S1P and dihydro-S1P decreased [3H]serine incorporation into very long chain ceramides, whereas only S1P enhanced incorporation into long chain ceramides that comigrated with C16-ceramide. In agreement, S1P, and not dihydro-S1P, also increased the incorporation of [3H]palmitate into long chain ceramides. In addition, it appears that there is not a major defect in the transport of ceramide from its site of production at the ER to the Golgi apparatus, the site of synthesis of glycosphingolipids, because PDMP, an inhibitor of glucosylceramide synthase, enhanced ceramide levels induced by S1P. Collectively, our data suggest that the presence or absence of the trans double bond in the sphingoid bases dictates their function in the biosynthesis of ceramide.
Why does S1P, and not dihydro-S1P, regulate ceramide biosynthesis?
The generation of sphingosine, and not sphinganine, from the hydrolysis of sphingolipids is a consequence of the constitutive turnover of plasma membrane components through the endocytic pathway. Even if a very small fraction of membrane lipids are metabolized (Thilo, 1994), the absolute amount of sphingosine generated would be quite large relative to the small intracellular pools. Although sphingosine is solely a product of sphingolipid catabolism, it is also a substrate for ceramide synthase (Merrill et al., 1993). In view of the constitutive nature of the endocytic process/salvage pathway, cells can conserve energy by reutilizing sphingosine, minimizing the de novo synthesis of sphinganine. Indeed, it has been shown that slowly dividing cells rely on this recycling hydrolytic pathway for synthesis of the majority of glycosphingolipids and sphingomyelin (Gillard et al., 1998). Hence, sphingosine produced in late endosomes and lysosomes is phosphorylated on the cytosolic surface by sphingosine kinase (Fig. 11) and S1P thus formed, due its more hydrophilic nature, is readily transported to the ER and dephosphorylated by SPP-1 to sphingosine, where it is converted to ceramide, predominantly N-palmitoyl-sphingosine. It is tempting to speculate that S1P may play a critical role in the downregulation of the de novo pathway by affecting serine palmitoyltransferase or ceramide synthase (Fig. 11). Thus, when the salvage pathway is active, sphingosine, and concomitantly S1P, levels increase, leading to inhibition of the de novo pathway. Ectopic expression of SPP-1 and degradation of S1P results in the relief of this inhibition and, concomitantly, formation of sphingosine, the substrate of ceramide synthase. In support of this provocative idea, suppression of sphingoid base synthesis was observed after the addition of lipoproteins or free sphingoid bases, as a consequence of the downregulation of serine palmitoyltransferase by sphingoid base-1-phosphates (van Echten-Deckert et al., 1997). Moreover, we and others have convincingly demonstrated that mutations or deletions of LBP1, the yeast phosphatase, completely prevented the incorporation of exogenous dihydrosphingosine into sphingolipids (Mao et al., 1997; Qie et al., 1997; Mandala et al., 1998; Zanolari et al., 2000). This defect in sphingolipid synthesis supports the intriguing possibility that externally supplied sphingoid bases are phosphorylated after their entry into the cell and require dephosphorylation before they can be used for ceramide biosynthesis (Zanolari et al., 2000). Likewise, disruption of LCB4 and LCB5, the genes encoding the sphingosine kinases in yeast, markedly reduced the incorporation of exogenous dihydrosphingosine into sphingolipids (Zanolari et al., 2000).
SPP-1 and cell fate decisions
If S1P indeed plays a crucial role in regulating the key step in biosynthesis of ceramide, this might be the explanation for the opposing actions of S1P and ceramide on apoptosis and why their dynamic balance is tightly coupled and conversely regulated. Whereas ceramide levels increase in response to stress stimuli, suppression of apoptosis is associated with increases in S1P levels and decreases in ceramide (for reviews see Hannun, 1996; Kolesnick and Hannun, 1999; Spiegel and Milstien, 2000b). Likewise, the balance between the levels of ceramide and S1P, regulated by LBP1, is also critical for survival and resistance to environmental stress in yeast (Mao et al., 1997, 1999; Mandala et al., 1998; Skrzypek et al., 1999), suggesting that the sphingolipid rheostat is an evolutionarily conserved stress regulatory mechanism.
Insertion of the trans 4,5 double bond into ceramide by the desaturase is an important step because ceramide, and not dihydroceramide, induces apoptosis (Hannun, 1996; Kolesnick and Hannun, 1999; Scaffidi et al., 1999). Remarkably, although dihydro-S1P is also a substrate for recombinant SPP-1 in situ, forming dihydrosphingosine (60 pmol/nmol phospholipid), which is then converted by an FB1-sensitive ceramide synthase to dihydroceramide, our finding that it did not accumulate suggests that dihydroceramide may be more efficiently used for sphingomyelin and/or glycosphingolipid biosynthesis than ceramide. Alternatively, its translocation to the Golgi apparatus may be more rapid. In this regard, whereas glucosylceramide synthase in the cis-Golgi receives ceramide via vesicular transport, nonvesicular transport from the ER to the Golgi apparatus has been described in mammalian cells and yeast (Kok et al., 1998; Funato and Riezman, 2001). Hence, ceramide, or more likely, dihydroceramide, can be translocated from the ER to the trans-Golgi via membrane contact sites to sphingomyelin and glucosylceramide synthases (Funato and Riezman, 2001). Thus, we are suggesting that ceramide and dihydroceramide may have different biosynthetic trafficking and that vesicular and nonvesicular pathways of ceramide versus dihydroceramide transport may serve special functions (Fig. 11). Because membrane contact sites have often been observed between the ER and mitochondria (Marsh et al., 2001), it is also possible that these could be responsible for the delivery of apoptotic ceramide, but not dihydroceramide, to the mitochondria. Because dihydroceramides are much less potent than ceramides in the induction of apoptosis (Hannun, 1996; Kolesnick and Hannun, 1999; Scaffidi et al., 1999), our suggestion of divergence in the regulation of ceramide and dihydroceramide synthesis has important implications, not only for sphingolipid metabolism, but also for their distinct roles in apoptosis.
Ceramide generation and apoptosis
Ceramide is now emerging as an important component of mitochondrial-dependent apoptosis and in the regulation of stress responses (Hannun, 1996; Kolesnick and Hannun, 1999; Scaffidi et al., 1999). Although it was originally proposed that ceramide was generated from sphingomyelin by the activation of one or more sphingomyelinases, a flurry of recent studies have begun to implicate ceramide generated from de novo sphingolipid biosynthesis in apoptosis (Bose et al., 1995; Kolesnick and Hannun, 1999; Perry et al., 2000; Kroesen et al., 2001). There is convincing evidence that ceramide synthase as well as serine palmitoyltransferase are activated during apoptosis (Bose et al., 1995; Perry et al., 2000; Kroesen et al., 2001). In agreement, we found that whereas S1P induced ceramide biosynthesis, particularly C16-ceramide, and apoptosis in mSPP-1 transfectants, dihydro-S1P had no effect on ceramide levels nor did it induce apoptosis. Interestingly, induction of apoptosis through B cell receptor cross-linking occurs via de novo–generated C16-ceramide (Kroesen et al., 2001). In addition, in the presence of FB1, both S1P and dihydro-S1P increased levels of sphingoid bases and induced cell death. These results are in concordance with several recent reports suggesting that sphingoid bases are also apoptotic signaling molecules (Gudz et al., 1997; Cuvillier et al., 2000; Kagedal et al., 2001).
Recent studies suggest a crucial role for de novo–generated C16-ceramide in mitochondrial damage leading to downstream activation of caspases and apoptosis (Kroesen et al., 2001). However, to date, how de novo–generated ceramide is transported from the ER to mitochondria is not known, although as discussed above, close membrane contact sites (Marsh et al., 2001) could be involved. Alternatively, ceramide might be generated from sphingosine in mitochondria via a mitochondrial ceramide synthase (Shimeno et al., 1995). More recently, a novel mitochondrial ceramidase with reciprocal (ceramide synthase) activity that shows strong preference for sphingosine over dihydrosphingosine as substrate has been cloned (El Bawab et al., 2001). Thus, sphingosine, but not sphinganine, formed at the ER by mSPP-1 might be transported to the mitochondria and serve as substrate for either of these ceramide synthase activities (Fig. 11). This ceramide may induce apoptosis due to mitochondria damage, generation of reactive oxygen species, release of cytochrome C, and subsequent activation of caspase activity. Interestingly, C16-ceramide, but not dihydroceramide, can form stable channels in membranes that are large enough to allow cytochrome C permeation and could be involved in initiating mitochondrial-dependent apoptosis (Siskind and Colombini, 2000). In agreement, elevation of endogenous ceramide specifically in mitochondria, but not in other subcellular compartments, resulted in the induction of apoptosis (Birbes et al., 2001), further supporting a role of endogenous mitochondrial ceramide in regulating apoptosis.
Materials and methods
Materials
[γ-32P]ATP, [3H]palmitic acid, and l-3-[3H]serine were purchased from Amersham Biosciences. Dihydro-S1P, S1P, FB1, myriocin (ISP-1), sphingosine, and PDMP were obtained from Biomol Research Laboratory Inc. Serum and medium were obtained from Biofluids, Inc. G418 was from Invitrogen. Recombinant diacylglycerol kinase was from Calbiochem. mAb against Myc was from Zymed Laboratories, and anti–mouse Texas red–conjugated goat antibody was from Jackson ImmunoResearch Laboratories. Alexa Fluor®488–phalloidin, MitoTracker green, anti–cytochrome c oxidase subunit II mAb, polyclonal rabbit anti-calnexin antibody, and the Anti-Fade kit were from Molecular Probes. Anti–protein disulfide isomerase (PDI) was from StressGen Biotechnologies. Other chemicals were from Sigma-Aldrich.
Cell culture
NIH 3T3 fibroblasts (American Type Culture Collection [ATCC] accession No. CRL-1658) and human embryonic kidney cells (HEK 293; ATCC CRL-1573) were cultured in high glucose DME containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine supplemented with 10% calf serum or FBS, respectively (Olivera et al., 1999). Cells were transfected with Myc-pcDNA3 alone or with Myc-pcDNA3 containing mSPP-1 construct using Lipofectamine Plus (Life Technologies) as previously described (Mandala et al., 2000). Transfection efficiencies were typically 30% and 60% for NIH 3T3 and HEK 293 cells, respectively. Stable transfectants of HEK 293 cells were selected in medium containing 1 g/liter G418.
S1P phosphohydrolase activity
HEK 293 cells (2 × 106) were seeded in 100-mm poly-d-lysine–coated plates and cultured to confluency. Cells were washed twice with ice-cold PBS and scraped on ice with 1 ml of buffer A (100 mM Hepes, pH 7.5, containing 10 mM EDTA, 1 mM DTT, and 10 μg/ml each leupeptin, aprotinin, and soybean trypsin inhibitor). Cells were freeze–thawed seven times and then centrifuged at 100,000 g for 1 h. Crude membranes were resuspended in buffer A and protein concentrations were determined by the Bradford assay (Bio-Rad Laboratories). 32P-labeled S1P was prepared with recombinant SPKH1a as previously described (Olivera et al., 2000). mSPP-1 activity was determined by adding 32P-labeled S1P (2 nmol, 100,000 cpm, 0.3% BSA complex) to membrane fractions (4 μg) in 200 μl buffer A, and S1P phosphohydrolase activity was determined as recently described (Le Stunff et al., 2002). Similar results were obtained by measurement of disappearance of labeled S1P or by formation of sphingosine (Le Stunff et al., 2002). Where indicated, 32P-labeled dihydro-S1P was used as substrate.
Immunofluorescence and confocal microscopy
Vector or Myc–SPP-1 transfectants grown on glass coverslips coated with collagen I were incubated overnight in DME supplemented with 2 μg/ml insulin, 2 μg/ml transferrin, and 20 μg/ml BSA. Cells were washed with PBS, fixed for 20 min at room temperature with 4% paraformaldehyde in 5% sucrose, and permeabilized with 0.1% Triton X-100 in PBS for 5 min. After washing, cells were incubated for 45 min with primary antibodies for Myc and calnexin in PBS containing 0.1% BSA, and then for 45 min with the corresponding secondary antibodies conjugated with Texas red. In some experiments, Alexa Fluor®488 fluorescent phalloidin or Texas red WGA were also included in the secondary antibody incubation. To visualize mitochondria, cells were incubated with 500 nM MitoTracker green for 30 min at 37°C before fixation. Coverslips were mounted on glass slides using an Anti-Fade kit and examined by confocal microscopy. Images were collected by an Olympus Fluoview laser scanning microscope equipped with argon (488 nm) and krypton (568 nm and 647 nm) lasers and a 60X/1.4 NA PlanApo lens. Quantitative image analysis was performed using Metamorph image processing software. To avoid fluorescence crossover between the channels, FITC and Texas red images were collected separately using the appropriate laser excitation (488 nm and 568 nm, respectively) and then merged.
Subcellular fractionation
HEK 293 cells overexpressing Myc-tagged mSPP-1 were rinsed twice in ice-cold PBS, resuspended in buffer A containing 0.25 M sucrose, and homogenized in a Dounce homogenizer at 4°C. Subcellular fractionation was performed by sequential centrifugation: lysates were centrifuged at 1,000 g for 5 min at 4°C to remove unbroken cells and nuclei, postnuclear supernatants were further centrifuged for 15 min at 17,000 g at 4°C, and the pellet P1 (intracellular membrane fraction containing mitochondria, ER, and Golgi) was resuspended in the same buffer. The remaining supernatant was centrifuged at 100,000 g for 1 h at 4°C to obtain cytosol and pellet P2 containing the plasma membranes.
Western blot analysis
Equal amounts of protein were separated on 7.5 or 10% SDS-PAGE and electroblotted onto nitrocellulose membranes for 1 h at 100 V and 4°C. Blots were blocked in 5% nonfat dry milk in TBS containing 0.1% Tween-20 (TBST) for 2 h at room temperature and probed with anti-Myc mAb or with anti–cytochrome oxidase II, anti-PDI, or anti–αv-integrin antibodies as markers for mitochondria, ER, and plasma membrane, respectively. After washing three times with TBST, blots were incubated with secondary antibodies for 1 h at room temperature. Protein bands were visualized by ECL using Super Signal (Pierce Chemical Co.).
Measurement of mass levels of sphingosine and ceramide
HEK 293 cells (5 × 105) were seeded in six-well poly-l-lysine–coated plates. Cells were incubated with 5 μM S1P or dihydro-S1P for the indicated time periods. Mass levels of sphingosine and ceramide and total phospholipids in cellular lipid extracts were measured essentially as previously described (Edsall et al., 1997).
Labeling with l-3-[3H]serine and [3H]palmitic acid
Vector- or mSPP-1–transfected HEK 293 cells were incubated without or with 5 μM S1P or dihydro-S1P in the absence or presence of [3H]palmitic acid (10 μCi/ml) or l-3-[3H]serine (30 μCi/ml). 24 h later, cells were scraped twice in 600 μl of methanol/concentrated HCl (200:2). Extracts were sonicated and 600 μl of chloroform and 500 μl of H2O were added, mixed, and phases were separated by the addition of 600 μl 2 M KCL and 600 μl chloroform and vigorous vortexing. Aliquots of the organic phases (corresponding to 5 × 106 cpm) were spotted on silica gel plates and developed in chloroform/acetic acid (9:1). Labeled sphingolipids were visualized by autoradiography and [3H]ceramide was quantified by a radiochromatogram scanner (Bioscan).
Staining of apoptotic nuclei
Apoptosis was assessed by staining cells with 8 μg/ml bisbenzimide trihydrochloride (Hoechst 33258) in 30% glycerol/PBS for 10 min at room temperature. The percentage of apoptotic cells, distinguished by condensed, fragmented nuclear regions, was determined as previously described (Olivera et al., 1999). A minimum of 500 cells were scored in a double blinded manner.
Reproducibility of data
Experiments were repeated at least three times with consistent results and statistical differences were determined by ANOVA. Statistically different groups are indicated by an asterisk (P ≤ 0.05).
Acknowledgments
This work was supported by National Institutes of Health grant GM43880 to S. Spiegel. H. Le Stunff received a postdoctoral fellowship from the Association de Recherche contre le Cancer (France).
Footnotes
Abbreviations used in this paper: GPCR, G protein–coupled receptor, mSPP-1, murine S1P phosphatase 1; FB1, fumonisin B1; HEK, human embryonic kidney; LPA, lysophosphatidic acid; LPP, lipid phosphate phosphohydrolase; PDI, protein disulfide isomerase; PDMP, 1-phenyl-2-decanoylamino-3-morpholino-1-propanol; S1P, sphingosine-1-phosphate.
References
- Alderton, F., P. Darroch, B. Sambi, A. McKie, I.S. Ahmed, N. Pyne, and S. Pyne. 2001. G-protein-coupled receptor stimulation of the p42/p44 mitogen-activated protein kinase pathway is attenuated by lipid phosphate phosphatases 1, 1a, and 2 in human embryonic kidney 293 cells. J. Biol. Chem. 276:13452–13460. [DOI] [PubMed] [Google Scholar]
- Birbes, H., S. El Bawab, Y.A. Hannun, and L.M. Obeid. 2001. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J. 15:2669–2679. [DOI] [PubMed] [Google Scholar]
- Bose, R., M. Verheij, A. Haimovitz-Friedman, K. Scotto, Z. Fuks, and R. Kolesnick. 1995. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 82:405–414. [DOI] [PubMed] [Google Scholar]
- Brindley, D.N., and D.W. Waggoner. 1998. Mammalian lipid phosphate phosphohydrolases. J. Biol. Chem. 273:24281–24284. [DOI] [PubMed] [Google Scholar]
- Cuvillier, O., G. Pirianov, B. Kleuser, P.G. Vanek, O.A. Coso, S. Gutkind, and S. Spiegel. 1996. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 381:800–803. [DOI] [PubMed] [Google Scholar]
- Cuvillier, O., L. Edsall, and S. Spiegel. 2000. Involvement of sphingosine in mitochondria-dependent fas-induced apoptosis of type II jurkat T cells. J. Biol. Chem. 275:15691–15700. [DOI] [PubMed] [Google Scholar]
- Cuvillier, O., V.E. Nava, S.K. Murthy, L.C. Edsall, T. Levade, S. Milstien, and S. Spiegel. 2001. Sphingosine generation, cytochrome c release, and activation of caspase-7 in doxorubicin-induced apoptosis of MCF7 breast adenocarcinoma cells. Cell Death Differ. 8:162–171. [DOI] [PubMed] [Google Scholar]
- Dickson, R.C., E.E. Nagiec, M. Skrzypek, P. Tillman, G.B. Wells, and R.L. Lester. 1997. Sphingolipids are potential heat stress signals in Saccharomyces. J. Biol. Chem. 272:30196–30200. [DOI] [PubMed] [Google Scholar]
- Edsall, L.C., G.G. Pirianov, and S. Spiegel. 1997. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J. Neurosci. 17:6952–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bawab, S., H. Birbes, P. Roddy, Z.M. Szulc, A. Bielawska, and Y.A. Hannun. 2001. Biochemical characterization of the reverse activity of rat brain ceramidase. A CoA-independent and fumonisin B1-insensitive ceramide synthase. J. Biol. Chem. 276:16758–16766. [DOI] [PubMed] [Google Scholar]
- Funato, K., and H. Riezman. 2001. Vesicular and nonvesicular transport of ceramide from the ER to the Golgi apparatus in yeast. J. Cell Biol. 155:949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman, A.H., B. Stieger, A.L. Hubbard, and R.E. Pagano. 1990. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J. Biol. Chem. 265:8650–8657. [PubMed] [Google Scholar]
- Gillard, B.K., R.G. Clement, and D.M. Marcus. 1998. Variations among cell lines in the synthesis of sphingolipids in de novo and recycling pathways. Glycobiology. 8:885–890. [DOI] [PubMed] [Google Scholar]
- Gudz, T.I., K.Y. Tserng, and C.L. Hoppel. 1997. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J. Biol. Chem. 272:24154–24158. [DOI] [PubMed] [Google Scholar]
- Hannun, Y. 1996. Functions of ceramide in coordinating cellular responses to stress. Science. 274:1855–1859. [DOI] [PubMed] [Google Scholar]
- Hannun, Y.A., and C. Luberto. 2000. Ceramide in the eukaryotic stress response. Trends Cell Biol. 10:73–80. [DOI] [PubMed] [Google Scholar]
- Hla, T., M.J. Lee, N. Ancellin, J.H. Paik, and M.J. Kluk. 2001. Lysophospholipids-receptor revelations. Science. 294:1875–1878. [DOI] [PubMed] [Google Scholar]
- Hobson, J.P., H.M. Rosenfeldt, L.S. Barak, A. Olivera, S. Poulton, M.G. Caron, S. Milstien, and S. Spiegel. 2001. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 291:1800–1803. [DOI] [PubMed] [Google Scholar]
- Hooks, S.B., W.L. Santos, D.S. Im, C.E. Heise, T.L. Macdonald, and K.R. Lynch. 2001. Lysophosphatidic acid induced mitogenesis is regulated by lipid phosphate phosphatases and is Edg-receptor independent. J. Biol. Chem. 276:4611–4621. [DOI] [PubMed] [Google Scholar]
- Jasinska, R., Q.X. Zhang, C. Pilquil, I. Singh, J. Xu, J. Dewald, D.A. Dillon, L.G. Berthiaume, G.M. Carman, D.W. Waggoner, and D.N. Brindley. 1999. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem. J. 340:677–686. [PMC free article] [PubMed] [Google Scholar]
- Jenkins, G.M., A. Richards, T. Wahl, C. Mao, L. Obeid, and Y. Hannun. 1997. Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. J. Biol. Chem. 272:32566–32572. [DOI] [PubMed] [Google Scholar]
- Kagedal, K., M. Zhao, I. Svensson, and U.T. Brunk. 2001. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 359:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok, J.W., T. Babia, K. Klappe, G. Egea, and D. Hoekstra. 1998. Ceramide transport from endoplasmic reticulum to Golgi apparatus is not vesicle-mediated. Biochem. J. 333:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick, R., and Y.A. Hannun. 1999. Ceramide and apoptosis. Trends Biochem. Sci. 24:224–225. [DOI] [PubMed] [Google Scholar]
- Kroesen, B.J., B. Pettus, C. Luberto, M. Busman, H. Sietsma, L. de Leij, and Y.A. Hannun. 2001. Induction of apoptosis through B-cell receptor cross-linking occurs via de novo generated C16-ceramide and involves mitochondria. J. Biol. Chem. 276:13606–13614. [DOI] [PubMed] [Google Scholar]
- Le Stunff, H., C. Peterson, R. Thornton, S. Milstien, S.M. Mandala, and S. Spiegel. 2002. Characterization of murine sphingosine-1-phosphate phosphohydrolase. J. Biol. Chem. 277:8920–8927. [DOI] [PubMed] [Google Scholar]
- Mandala, S.M., R. Thornton, Z. Tu, M.B. Kurtz, J. Nickels, J. Broach, R. Menzeleev, and S. Spiegel. 1998. Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proc. Natl. Acad. Sci. USA. 95:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala, S.M., R. Thornton, I. Galve-Roperh, S. Poulton, C. Peterson, A. Olivera, J. Bergstrom, M.B. Kurtz, and S. Spiegel. 2000. Molecular cloning and characterization of a lipid phosphohydrolase that degrades sphingosine-1-phosphate and induces cell death. Proc. Natl. Acad. Sci. USA. 97:7859–7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, C., M. Wadleigh, G.M. Jenkins, Y.A. Hannun, and L.M. Obeid. 1997. Identification and characterization of Saccharomyces cerevisiae dihydrosphingosine-1-phosphate phosphatase. J. Biol. Chem. 272:28690–28694. [DOI] [PubMed] [Google Scholar]
- Mao, C., J.D. Saba, and L.M. Obeid. 1999. The dihydrosphingosine-1-phosphate phosphatases of Saccharomyces cerevisiae are important regulators of cell proliferation and heat stress responses. Biochem. J. 342:667–675. [PMC free article] [PubMed] [Google Scholar]
- Marsh, B.J., D.N. Mastronarde, K.F. Buttle, K.E. Howell, and J.R. McIntosh. 2001. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc. Natl. Acad. Sci. USA. 98:2399–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattie, M., G. Brooker, and S. Spiegel. 1994. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J. Biol. Chem. 269:3181–3188. [PubMed] [Google Scholar]
- Merrill, A.H., Jr., Y.A. Hannun, and R.M. Bell. 1993. Introduction: sphingolipids and their metabolites in cell regulation. Adv. Lipid Res. 25:1–24. [PubMed] [Google Scholar]
- Meyer zu Heringdorf, D., H. Lass, R. Alemany, K.T. Laser, E. Neumann, C. Zhang, M. Schmidt, U. Rauen, K.H. Jakobs, and C.J. van Koppen. 1998. Sphingosine kinase-mediated Ca2+ signalling by G-protein-coupled receptors. EMBO J. 17:2830–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, C., and G. van Echten-Deckert. 1997. Conversion of dihydroceramide to ceramide occurs at the cytosolic face of the endoplasmic reticulum. FEBS Lett. 416:153–155. [DOI] [PubMed] [Google Scholar]
- Morita, Y., G.I. Perez, F. Paris, S.R. Miranda, D. Ehleiter, A. Haimovitz-Friedman, Z. Fuks, Z. Xie, J.C. Reed, E.H. Schuchman, et al. 2000. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat. Med. 6:1109–1114. [DOI] [PubMed] [Google Scholar]
- Olivera, A., T. Kohama, L.C. Edsall, V. Nava, O. Cuvillier, S. Poulton, and S. Spiegel. 1999. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J. Cell Biol. 147:545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera, A., K.D. Barlow, and S. Spiegel. 2000. Assaying sphingosine kinase activity. Methods Enzymol. 311:215–223. [DOI] [PubMed] [Google Scholar]
- Perry, D.K., J. Carton, A.K. Shah, F. Meredith, D.J. Uhlinger, and Y.A. Hannun. 2000. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J. Biol. Chem. 275:9078–9084. [DOI] [PubMed] [Google Scholar]
- Pyne, S., and N.J. Pyne. 2000. Sphingosine 1-phosphate signalling in mammalian cells. Biochem. J. 349:385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qie, L., M.M. Nagiec, J.A. Baltisberger, R.L. Lester, and R.C. Dickson. 1997. Identification of a Saccharomyces gene, LCB3, necessary for incorporation of exogenous long chain bases into sphingolipids. J. Biol. Chem. 272:16110–16117. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt, H.M., J.P. Hobson, M. Maceyka, A. Olivera, V.E. Nava, S. Milstien, and S. Spiegel. 2001. EDG-1 links the PDGF receptor to Src and focal adhesion kinase activation leading to lamellipodia formation and cell migration. FASEB J. 15:2649–2659. [DOI] [PubMed] [Google Scholar]
- Sakakura, C., E.A. Sweeney, T. Shirahama, A. Hagiwara, T. Yamaguchi, T. Takahashi, S. Hakomori, and Y. Igarashi. 1998. Selectivity of sphingosine-induced apoptosis. Lack of activity of DL-erythyro-dihydrosphingosine. Biochem. Biophys. Res. Commun. 246:827–830. [DOI] [PubMed] [Google Scholar]
- Scaffidi, C., I. Schmitz, J. Zha, S.J. Korsmeyer, P.H. Krammer, and M.E. Peter. 1999. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J. Biol. Chem. 274:22532–22538. [DOI] [PubMed] [Google Scholar]
- Shayman, J.A., L. Lee, A. Abe, and L. Shu. 2000. Inhibitors of glucosylceramide synthase. Methods Enzymol. 311:373–387. [DOI] [PubMed] [Google Scholar]
- Shimeno, H., S. Soeda, M. Yasukouchi, N. Okamura, and A. Nagamatsu. 1995. Fatty acyl-Co A: sphingosine acyltransferase in bovine brain mitochondria: its solubilization and reconstitution onto the membrane lipid liposomes. Biol. Pharm. Bull. 18:1335–1339. [DOI] [PubMed] [Google Scholar]
- Siskind, L.J., and M. Colombini. 2000. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J. Biol. Chem. 275:38640–38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypek, M.S., M.M. Nagiec, R.L. Lester, and R.C. Dickson. 1999. Analysis of phosphorylated sphingolipid long-chain bases reveals potential roles in heat stress and growth control in Saccharomyces. J. Bacteriol. 181:1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel, S., and S. Milstien. 2000. a. Functions of a new family of sphingosine-1-phosphate receptors. Biochim. Biophys. Acta. 1484:107–116. [DOI] [PubMed] [Google Scholar]
- Spiegel, S., and S. Milstien. 2000. b. Sphingosine-1-phosphate: signaling inside and out. FEBS Lett. 476:55–67. [DOI] [PubMed] [Google Scholar]
- Spiegel, S., and S. Milstien. 2002. Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 277:25851–25854. [DOI] [PubMed] [Google Scholar]
- Stukey, J., and G.M. Carman. 1997. Identification of a novel phosphatase sequence motif. Protein Sci. 6:469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilo, L. 1994. Endocytosis: aspects of organellar processing. Ann. NY Acad. Sci. 710:209–216. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn, J.R., M.J. Lee, R. Menzeleev, A. Olivera, L. Edsall, O. Cuvillier, D.M. Thomas, P.J.P. Coopman, S. Thangada, T. Hla, and S. Spiegel. 1998. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled orphan receptor edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 142:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Echten-Deckert, G., A. Zschoche, T. Bar, R.R. Schmidt, A. Raths, T. Heinemann, and K. Sandhoff. 1997. cis-4-Methylsphingosine decreases sphingolipid biosynthesis by specifically interfering with serine palmitoyltransferase activity in primary cultured neurons. J. Biol. Chem. 272:15825–15833. [DOI] [PubMed] [Google Scholar]
- van Meer, G., and J.C. Holthuis. 2000. Sphingolipid transport in eukaryotic cells. Biochim. Biophys. Acta. 1486:145–170. [DOI] [PubMed] [Google Scholar]
- Waggoner, D.W., J. Xu, I. Singh, R. Jasinska, Q.X. Zhang, and D.N. Brindley. 1999. Structural organization of mammalian lipid phosphate phosphatases: implications for signal transduction. Biochim. Biophys. Acta. 1439:299–316. [DOI] [PubMed] [Google Scholar]
- Xia, P., J.R. Gamble, K.A. Rye, L. Wang, C.S. Hii, P. Cockerill, Y. Khew-Goodall, A.G. Bert, P.J. Barter, and M.A. Vadas. 1998. Tumor necrosis factor-α induces adhesion molecule expression through the sphingosine kinase pathway. Proc. Natl. Acad. Sci. USA. 95:14196–14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanolari, B., S. Friant, K. Funato, C. Sutterlin, B.J. Stevenson, and H. Riezman. 2000. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J. 19:2824–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q.X., C.S. Pilquil, J. Dewald, L.G. Berthiaume, and D.N. Brindley. 2000. Identification of structurally important domains of lipid phosphate phosphatase-1: implications for its sites of action. Biochem. J. 345:181–184. [PMC free article] [PubMed] [Google Scholar]