Abstract
Tumor and viral antigens elicit a potent immune response by heat shock protein–dependent uptake of antigenic peptide with subsequent presentation by MHC I. Receptors on antigen-presenting cells that specifically bind and internalize a heat shock protein–peptide complex have not yet been identified. Here, we show that cells expressing CD40, a cell surface protein crucial for B cell function and autoimmunity, specifically bind and internalize human Hsp70 with bound peptide. Binding of Hsp70–peptide complex to the exoplasmic domain of CD40 is mediated by the NH2-terminal nucleotide–binding domain of Hsp70 in its ADP state. The Hsp70 cochaperone Hip, but not the bacterial Hsp70 homologue DnaK, competes formation of the Hsp70–CD40 complex. Binding of Hsp70-ADP to CD40 is strongly increased in the presence of Hsp70 peptide substrate, and induces signaling via p38. We suggest that CD40 is a cochaperone-like receptor mediating the uptake of exogenous Hsp70–peptide complexes by macrophages and dendritic cells.
Keywords: heat shock protein receptor; immune response; cross priming; co-chaperone; CD40; antigen-presenting cell
Introduction
Heat shock proteins and molecular chaperones, including Hsp70 and glucose-regulated protein 94 (Grp94),* have been established in recent years as immune adjuvants for cross priming with antigenic peptides (Srivastava et al., 1994). In this process, antigen-presenting cells (APCs) internalize exogenously administered heat shock proteins with bound peptides by receptor-mediated endocytosis (Arnold-Schild et al., 1999), resulting in antigen presentation via MHC I (Castellino et al., 2000). Tumor-bearing mice injected with Hsp70 (or Grp94) isolated from the tumor or with a complex of recombinant Hsp70 with bound, tumor-specific antigenic peptide have been shown to mount a potent CD8+ T cell response that can reduce or eliminate tumor progression (Srivastava et al., 1994). However, despite intensive research, the mechanistic details and the components involved in this pathway have remained obscure.
Various cell surface proteins on immune cells are thought to play a role in the induction of cellular responses to heat shock proteins. One group of proteins, including the toll-like receptors 2 and 4 with their cofactor CD14 (Asea et al., 2002), and CD36, are reported to induce cytokine secretion in an Hsp70-dependent manner. So far, only one surface protein (CD91) has been implicated in eliciting a CD8+ T cell response upon administration of peptide bound to heat shock protein (Grp94; Binder et al., 2000), but an essential role of CD91 in this process has been questioned (Berwin et al., 2002). A recent report by Wang et al. (2001) described a direct interaction between bacterial Hsp70 (DnaK) with CD40. However, this interaction is mediated by the substrate-binding domain of Hsp70, making a role in uptake of antigenic peptides unlikely.
The list of heat shock proteins and molecular chaperones implicated as immune adjuvants includes Grp94, Hsp60, calreticulin, and mammalian cytosolic Hsp90 and Hsp70 (Srivastava et al., 1986; Udono and Srivastava, 1993; Basu and Srivastava, 1999; Kol et al., 2000), as well as DnaK (Wang et al., 2001). In general, these chaperones bind to peptide segments of nonnative polypeptides either during synthesis or in conditions of cellular stress, preventing protein aggregation and mediating proper folding (Hartl and Hayer-Hartl, 2002). The highly conserved members of the Hsp70 family are the best studied among this class of components (Bukau and Horwich, 1998). Hsp70 consists of an NH2-terminal nucleotide-binding (ATPase) domain of ∼44 kD and a COOH-terminal 25-kD domain that binds peptide or polypeptide substrate. In its ATP-bound state, Hsp70 binds and releases peptide rapidly, whereas after hydrolysis, in the ADP state, bound peptide is held tightly (Flynn et al., 1989). Hsp70 recognizes heptapeptide segments with a broad specificity but with a preference for hydrophobic residues, such as leucine or isoleucine (Flynn et al., 1989; Blond-Elguindi et al., 1993; Rudiger et al., 1997). Based on this broad range of peptides recognized, Hsp70 would be especially suited to serve as a carrier of antigenic peptides for cross priming. Hsp70–peptide complexes may reach the extracellular space from necrotic cells or on viral cell lysis (Basu et al., 2000; Berwin and Nicchitta, 2001). Various cell lines have been investigated for their ability to bind heat shock proteins, including mostly professional APCs such as dendritic cells (Reed and Nicchitta, 2000), macrophages, and peripheral blood monocytes (Sondermann et al., 2000).
Here, we show that binding of Hsp70 to ANA-1 macrophages is markedly increased after stimulation with bacterial lipopolysaccharide (LPS). LPS treatment results in increased expression of CD40 (Tone et al., 2001), a member of the TNF receptor family with a crucial role in B cell function and autoimmunity (Bodmer et al., 2002). We find that human Hsp70 binds to the exoplasmic domain of CD40. Interestingly, this interaction is mediated by the NH2-terminal ATPase domain of human Hsp70 in its ADP-bound state. It is strongly enhanced by the presence of substrate peptide in the COOH-terminal domain of Hsp70 (C70) and is inhibited by Hip, a co-chaperone known to stabilize the Hsp70 ATPase domain in the ADP state. These surprising mechanistic features explain why the binding of human Hsp70 has remained undetected in a recent report describing the interaction of the COOH-terminal chaperone domain of bacterial Hsp70 (DnaK) with CD40 (Wang et al., 2001). We show further that binding of human Hsp70–peptide complex to cells that express CD40 leads to peptide uptake and induction of signaling via p38. Thus, CD40 is an extracellular receptor for peptide-loaded human Hsp70 and mediates the internalization of Hsp70-bound peptides.
Results
LPS stimulation increases expression of CD40 and binding of Hsp70 to ANA-I macrophages
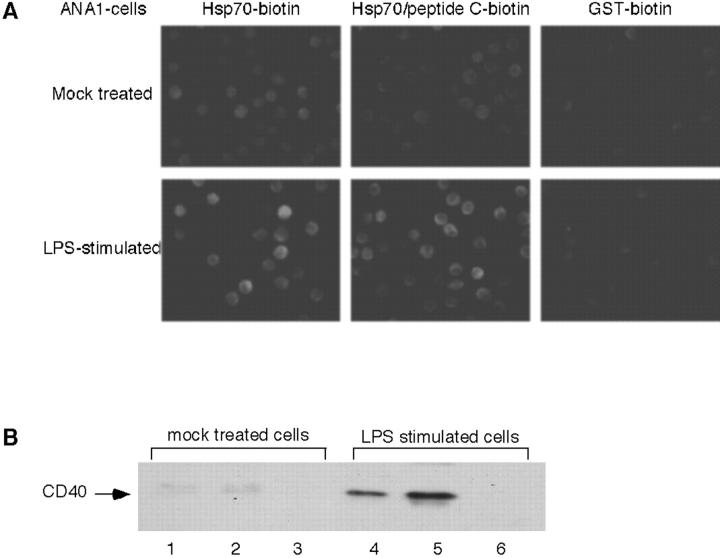
To determine the molecular basis for the binding of human Hsp70 to ANA-1 cells (Sondermann et al., 2001), cells were incubated either with biotinylated Hsp70 or with Hsp70 that had been loaded with biotinylated peptide C (GCEVFGLGWRSYKH; Flynn et al., 1989) in the presence of ADP. Bound protein was detected by fluorescence microscopy after reaction with fluorescent streptavidin (see Materials and methods). Unstimulated cells showed weak but clearly detectable binding with both Hsp70 and Hsp70–peptide complex, whereas no binding was observed with biotin-labeled GST as a control protein (Fig. 1 A, top). In contrast, LPS stimulation resulted in a significant increase in binding of Hsp70 and Hsp70 peptide, but not of GST (Fig. 1 A, bottom).
Figure 1.
LPS treatment of ANA-1 cells stimulates binding of Hsp70 and induces expression of CD40. Cells were incubated with LPS or mock-treated as outlined in Materials and methods. (A) Cells were incubated either with biotinylated Hsp70, Hsp70 loaded with biotinylated peptide C, or with biotinylated GST as a control. After 30 min at 4°C, cells were washed, incubated with TRITC-labeled streptavidin, washed again, and processed for fluorescence microscopy. (top) mock-treated ANA-1 cells; (bottom) ANA-1 cells after treatment with LPS. Note that Hsp70 carries on average five biotins, whereas peptide C contains a single biotin. (B) Cells were harvested, lysed, and centrifuged to obtain a total membrane pellet. Identical protein amounts of the samples were analyzed by immunoblotting with an antibody directed against CD40. (Lanes 1 and 4) total cell lysates; (lanes 2 and 5) membrane fractions; (lanes 3 and 6) supernatants.
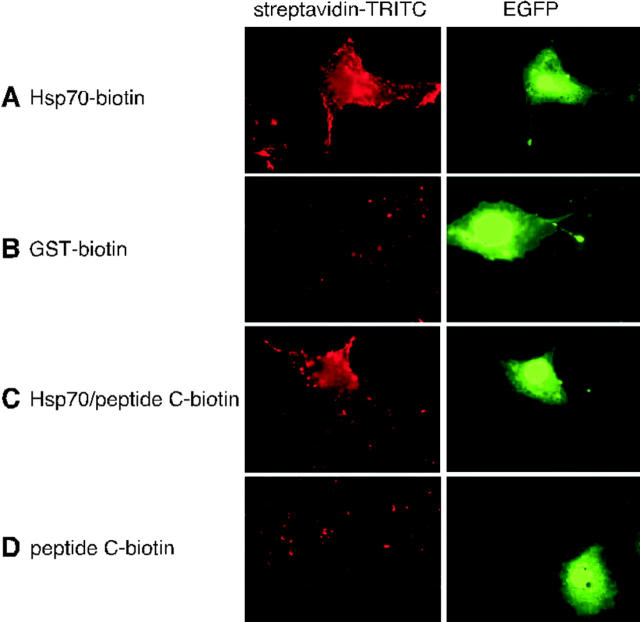
Mock-treated and LPS-stimulated ANA-1 cells were analyzed for their expression of CD40. As shown in Fig. 1 B, LPS-stimulated cells, but not control cells, express high levels of membrane-bound CD40, as detected by immunoblotting (Tone et al., 2001). To assess the possibility that CD40 itself may serve as an Hsp70 receptor, Cos-7 cells, which do not express CD40, were transfected with a cDNA construct containing the cDNA for human CD40, followed by an internal ribosomal entry site and the cDNA for EGFP. Transfected cells were identified by virtue of their GFP fluorescence, and were analyzed for their ability to bind biotin-labeled Hsp70 or Hsp70–biotin–peptide complex (Fig. 2). Indeed, Hsp70 and its peptide complex bound to the surface of transfected cells, but not to untransfected cells, whereas no binding above background was detected with biotinylated GST or biotinylated peptide alone (Fig. 2).
Figure 2.
Transfection with human CD40 cDNA renders Cos-7 cells active in binding human Hsp70. Cos-7 cells were transiently transfected with a fusion construct that contained human CD40 cDNA, followed by an internal ribosomal entry site and the cDNA for EGFP, giving rise to green fluorescence of transfected cells. Cells were incubated for 30 min at 0°C with biotinylated Hsp70 (A), biotinylated GST as a control (B), Hsp70–peptide complex containing biotinylated peptide C (C), or with biotinylated peptide C alone (D). Thereafter, cells were washed and incubated with TRITC-streptavidin and processed for fluorescence microscopy as described in Fig. 1. Left-hand panels show streptavidin fluorescence and right-hand panels show EGFP fluorescence.
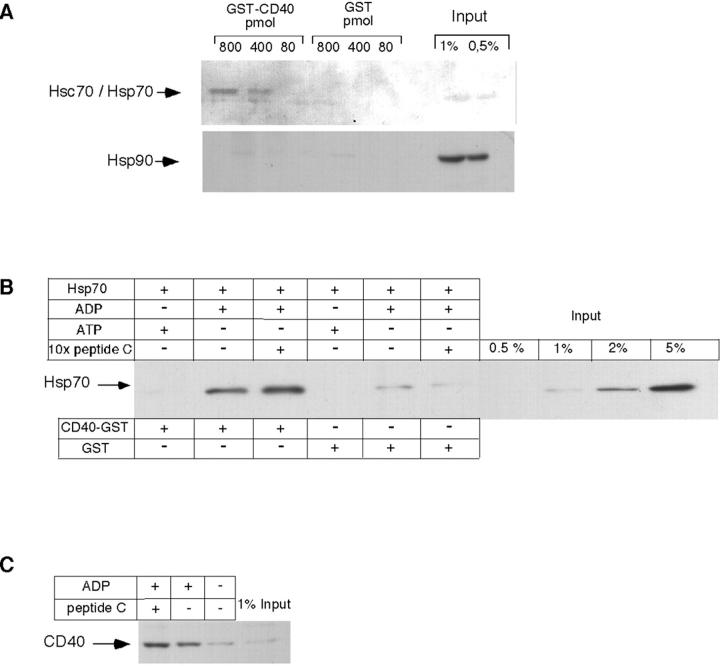
Binding experiments were performed in vitro to determine whether CD40 and Hsp70 interact directly. To this end, the exoplasmic domain of human CD40 (aa residues 20–212) was expressed in Escherichia coli as a soluble GST-fusion protein and used to study its interaction with heat shock proteins in HeLa cell lysates. Increasing amounts of GST-CD40 were incubated with the lysates and bound proteins were adsorbed to glutathione-Sepharose, followed by immunoblotting with antibodies directed against Hsc70, Hsp70, and Hsp90. As shown in Fig. 3 A, Hsc70 and Hsp70 bound to GST-CD40 in a concentration-dependent manner, whereas Hsp90 did not interact. As will be shown later, the interaction of Hsp70 with CD40 is enhanced in the presence of ADP. GST alone, used as a negative control, did not bind Hsc70/Hsp70. These experiments demonstrate a direct and specific interaction of CD40 with mammalian Hsp70s.
Figure 3.
Binding of Hsp70 to CD40 is direct and depends on ADP. (A) HeLa cell lysates were incubated with GST (control) or GST-CD40 in the amounts indicated. Thereafter, samples were affinity-purified on glutathione-sepharose as outlined in Materials and methods, subjected to SDS-PAGE, and analyzed by immunoblotting with antibodies directed against Hsc70 and Hsp70 (top lanes), and against Hsp90 (bottom lanes). (B) Human recombinant His6-tagged Hsp70 was incubated with ATP, ADP, or an excess of peptide C, followed by addition of CD40-GST or GST alone. After affinity purification, samples were analyzed by immunoblotting as described in A, using antibodies directed against the His6 tag. (C) Recombinant human His6-tagged Hsp70 was incubated with peptide C, either in the presence or absence of ADP. The reactions were then incubated with ANA-1 cell lysates as described in Materials and methods. Protein bound to Hsp70 was analyzed by affinity purification on Ni-NTA agarose and immunoblotting with antibodies directed against CD40. Input reflects the amount of protein subjected to affinity purification.
Human Hsp70 binds to CD40 via its ATPase domain
Next, we investigated the biochemical requirements for CD40–Hsp70 complex formation. Specifically, we addressed the question whether Hsp70 binds to CD40 via its COOH-terminal substrate-binding domain or via its NH2-terminal nucleotide-binding domain. In this context, it was of interest whether the association depended on a specific nucleotide-bound state of Hsp70. Recombinant Hsp70 was incubated with GST-CD40 in the presence of ADP or ATP, with or without substrate peptide (Fig. 3 B). Hsp70 binding to CD40 was barely detectable in the presence of ATP, and was only seen in the presence of ADP. Notably, addition of a molar excess of peptide C over Hsp70 did not compete with the interaction, as might have been expected if the COOH-terminal Hsp70 domain were to mediate binding of Hsp70 to CD40. Only background signals were observed when GST alone was analyzed as a control. Recombinant, His-tagged Hsp70 also interacted, in an ADP-dependent manner, with endogenous CD40 in extracts of LPS-stimulated ANA-1 cells, as detected by adsorption of the complex to Ni-NTA agarose and immunoblotting with CD40 antibody (Fig. 3 C). Together, these results suggested that Hsp70 binds to CD40 via its ATPase domain.
In light of recent findings that the bacterial Hsp70 homologue DnaK associates with CD40 via the COOH-terminal peptide–binding domain (Wang et al., 2001), experiments were performed to directly determine the specificity of CD40 for the domains of human Hsp70. Recombinant, full-length Hsp70 was incubated in the presence of ADP and a fivefold molar excess of either recombinant Hsp70 NH2-terminal domain (residues 1–381; Sondermann et al., 2001) or COOH-terminal domain (residues 382–641; Scheufler et al., 2000), or of recombinant, full-length DnaK. The NH2-terminal domain of Hsp70 (N70) efficiently competed for the binding of full-length Hsp70 to CD40, as detected with an antibody against the NH2-terminal His6 tag on recombinant Hsp70 (Fig. 4 A). The ATPase domain itself bound to CD40 in the presence of ADP but not ATP (Fig. 4 C). In contrast, neither the COOH-terminal Hsp70 domain (C70) nor DnaK had a significant effect on this binding (Fig. 4 A). To analyze whether under these conditions DnaK binds directly to CD40, a pulldown experiment was performed with DnaK. As shown in Fig. 4 B, DnaK indeed binds to CD40, and this interaction is enhanced in the presence of ADP when compared with ATP. This establishes that Hsp70 and DnaK bind to different sites on CD40, and confirms that DnaK binds via its COOH-terminal domain as reported earlier (Wang et al., 2001). This binding strongly suggested that it is the substrate-binding site of DnaK that mediates the interaction with CD40. To address this possibility, a competition experiment was performed with an excess of peptide C. As shown in Fig. 4 B, a 10-fold molar excess of the peptide almost completely abolishes the binding of DnaK to CD40. Thus, it is the substrate binding of DnaK that interacts with CD40. In summary, Hsp70 interacts with CD40 dependent on ADP, and via its ATPase domain, as shown with the endogenous proteins in cell extracts and the recombinant proteins in vitro. In contrast, DnaK binds to CD40 via its substrate binding site proper.
Figure 4.
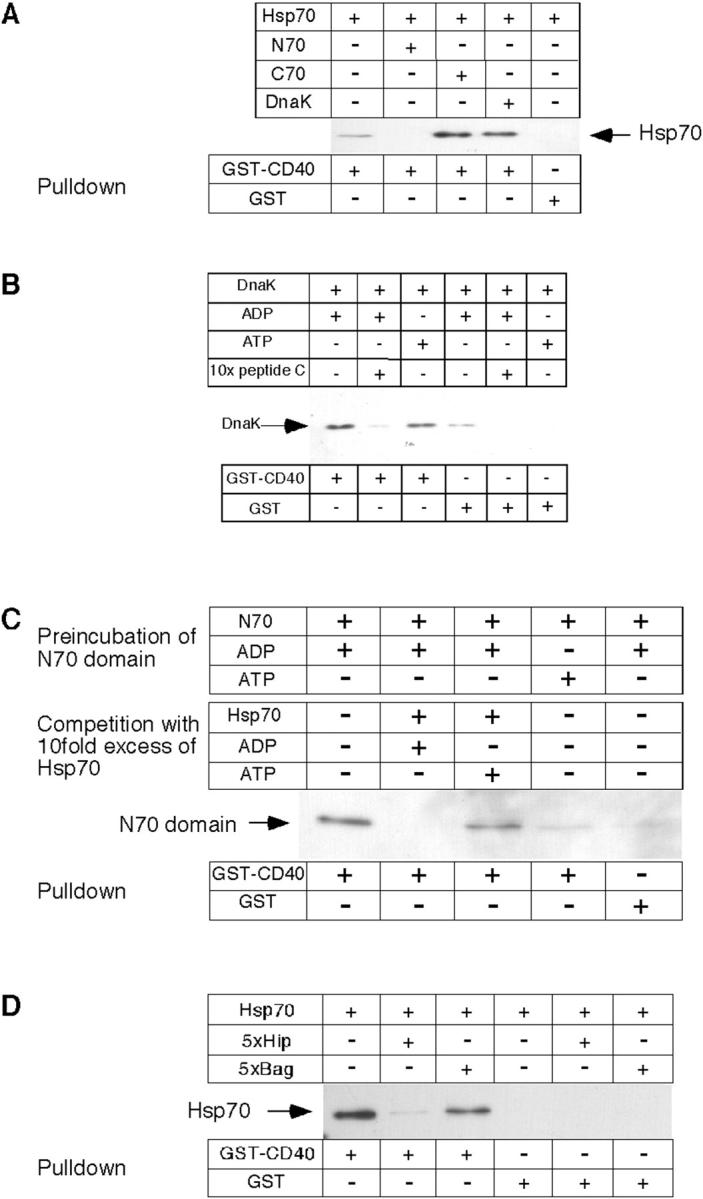
Hsp70 binding to CD40 is mediated by the NH2-terminal ATPase domain and is competed by Hip. (A) Human His6-tagged Hsp70, its NH2- or COOH-terminal domains, or recombinant bacterial DnaK was incubated either with GST-CD40 or with GST. (B) Recombinant DnaK was incubated with ADP, ATP, or an excess of peptide C, followed by addition of GST-CD40 or GST alone. (C) His6-tagged N70 was incubated in the presence of ADP or ATP, followed by incubation with a 10-fold molar excess of Hsp70 in the presence of ADP or ATP. (D) Recombinant human His6-tagged Hsp70 protein was incubated with a fivefold molar excess of either recombinant Hip protein or Bag-1, and with GST-CD40 or GST as a control. Bound protein was analyzed after affinity purification on glutathione-sepharose by immunoblotting with an antibody directed against the NH2-terminal His6 tags, or with an antibody directed against DnaK.
Hsp70 binding to CD40 is competed by the Hsp70 cochaperone Hip, and is enhanced by peptide substrate
N70 is known to specifically interact with the regulatory cochaperone proteins Hip and Bag-1. Although Hip stabilizes the ADP state of Hsp70, which binds substrate tightly (Höhfeld et al., 1995), Bag-1 functions as an ADP–ATP exchange factor and causes substrate release from Hsp70 (Höhfeld and Jentsch, 1997; Sondermann et al., 2001). In light of the observed ADP dependence, it seemed possible that CD40 binding to Hsp70 has certain features in common with the interaction between Hip and Hsp70. Binding of Hsp70 to CD40 was analyzed in the presence of a fivefold molar excess of recombinant Hip or Bag-1 over Hsp70 (Fig. 4 D). Hip acted as an effective competitor of the interaction, in contrast to Bag-1, which interacts only relatively weakly with the ADP state of Hsp70 (Höhfeld, 1998; Sondermann et al., 2001). Thus, CD40 and Hip may share similar binding regions on the ATPase domain of Hsp70.
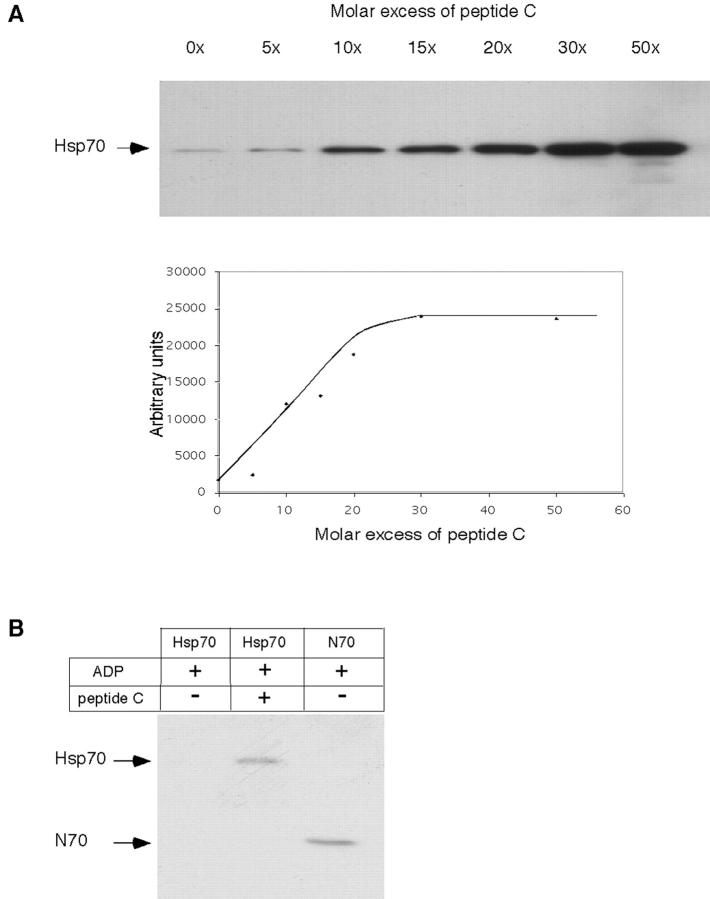
The known cochaperone function of Hip in stabilizing Hsp70 in its substrate-bound ADP state (Höhfeld et al., 1995) raised the interesting possibility that the interaction between CD40 and Hsp70 may not only be ADP-regulated, but may also depend on Hsp70 substrate. Strikingly, binding of Hsp70-ADP to CD40 increased dramatically in the presence of peptide C (Fig. 5, A and B). The effect of peptide was saturable (ka ≈ 30 μM) in a range corresponding to the affinity of peptide C for Hsp70 (5–10 μM; Greene et al., 1995). These results demonstrate that it is the peptide- and ADP-bound state of Hsp70 that is recognized preferentially by CD40 and suggest that, similar to Hip, CD40 has a regulatory function in stabilizing the Hsp70–substrate complex. This effect would ensure that CD40 binds Hsp70 predominantly when it is in complex with peptide.
Figure 5.
Peptide substrate stimulates Hsp70 binding to CD40. (A) His6-tagged Hsp70 was incubated with increasing concentrations of peptide C as indicated, and binding to GST-CD40 was analyzed by immunoblotting with antibodies directed against the His6-tag. The bottom panel is a quantitation of the data. (B) Equivalent concentrations (3 μM) of His6-tagged Hsp70 (in the presence of 2 mol ADP and in the absence or presence of a 30-fold molar excess of peptide C) or His6-tagged N70 were incubated with GST-CD40, and bound protein was analyzed as described for panel A.
The striking increase in CD40 binding of ADP-loaded Hsp70 observed in the presence of peptide substrate raised the question of whether the free C70 has an inhibitory effect on the ATPase domain, reducing the ability of the latter to recognize CD40. This possibility was addressed by comparing the binding efficiency of substrate-saturated Hsp70 to that of the NH2-terminal domain, both in their ADP states. As shown in Fig. 5 C, substrate-loaded Hsp70 and the NH2-terminal domain bound to CD40 with similar efficiencies, indicating that the peptide-free C70 masks the NH2-domain for binding to CD40, either directly or by causing an allosteric conformational change.
Binding of Hsp70–peptide complex to CD40 results in intracellular signaling and peptide uptake
Binding of CD40 ligand to CD40 induces signal transduction via phosphorylation of p38, a component of the signal cascade between activated CD40 and NFκB, which eventually results in the release of TNFα and subsequent secretion of interferon-γ (Pullen et al., 1999). Binding of the COOH-terminal domain of DnaK to CD40 was reported to have a similar effect (Wang et al., 2001). Therefore, we investigated whether binding of human Hsp70–peptide complex to CD40 also stimulates this signaling pathway. These experiments were performed in HEK293T cells stably transfected with human CD40 cDNA. After incubation with either Hsp70–peptide complex, recombinant Hsp70 domains, or DnaK, in the presence of ADP or the nonhydrolysable ATP analogue AMPPNP, cells were lysed and lysates were analyzed by immunoblotting with an antibody directed against active (i.e., phosphorylated) p38. Indeed, human Hsp70 and its ATPase domain caused an increase in phosphorylated p38 to an extent comparable to that observed with DnaK (Fig. 6 A, top panel). Although modest when compared with the signal induced by the same molar concentration of CD40 ligand, activation of p38 by Hsp70 was significant, and depended on the presence of ADP. As a control, HEK293T cells stably transfected with the same vector, but containing the cDNA for an unrelated membrane protein, murine cationic amino acid transporter (MCAT), did not show a detectable response to the various stimuli (Fig. 6 A, bottom panel). Thus, Hsp70–peptide complex and the Hsp70 ATPase domain activate signaling via CD40, dependent on the presence of ADP and in a manner comparable to the effect of DnaK, although the latter binds to CD40 via its COOH-terminal domain (Wang et al., 2001).
Figure 6.
Binding of Hsp70 complex to CD40-expressing HEK293T cells induces signaling via p38 and causes peptide uptake. (A) HEK293T cells, stably transfected with cDNA encoding human CD40 or the unrelated membrane protein (MCAT) were incubated for 20 min at 37°C with CD40L or with Hsp70, N70, C70, or bacterial DnaK in the presence of peptide C and ADP or AMPPNP, or buffer alone. Thereafter, cells were washed, solubilized in SDS-sample buffer, and analyzed by immunoblotting with antibodies directed against phosphorylated (active) p38. Blots were also developed with an antibody against tubulin in order to control for equal loading. (B) Cells were incubated at 0°C for 30 min with recombinant human Hsp70, N70, or C70, all in the presence FITC-labeled peptide, or with the FITC-labeled peptide alone. Thereafter, cells were washed, incubated for 15 min at 37°C, and processed for fluorescence microscopy. (1) Hsp70, (2) FITC-labeled peptide alone, (3) C70, and (4) N70. Only cells stably transfected with CD40 are shown. Cell boundaries are emphasized with broken lines. MCAT-expressing control cells did not show fluorescence above background.
Next, we examined whether binding of Hsp70–peptide complex to CD40 results in the uptake of peptide. To this end, HEK293T-CD40 cells and HE293T-MCAT cells (not expressing CD40) were incubated with a fluorescent (FITC) derivative of peptide C for 30 min at 0°C in the presence of ADP, with or without Hsp70 or its two domains. After removal of excess material, the cells were incubated at 37°C for 15 min, fixed, and then analyzed by fluorescence microscopy. Representative images are presented in Fig. 6 B. After 15-min incubation at 37°C, fluorescent peptide was observed in punctuate intracellular structures only in cells expressing CD40, and only when Hsp70 was present during the incubation for binding at 0°C (Fig. 6 B, panel 1). No staining above background was detected when Hsp70 was omitted (Fig. 6 B, panel 2). Likewise, incubation of HEK293T-CD40 cells with either recombinant C70 or N70 and peptide did not result in a peptide signal above background (Fig. 6 B, panels 3 and 4). HEK293T-MCAT control cells did not give rise to a signal under any of the conditions tested (unpublished data). We conclude that the specific interaction of CD40 with Hsp70–peptide complex mediates the uptake of peptide.
Discussion
Our results establish the cell surface protein CD40 as a receptor for exogenously added human Hsp70–peptide complexes. The functional properties of the CD40–Hsp70 interaction ensure surface binding and uptake of Hsp70-associated peptide (Fig. 7). Binding to CD40 is mediated by the ATPase domain of Hsp70, and depends critically on the ADP-loaded state of the chaperone, which binds peptide tightly. Moreover, complex formation between CD40 and Hsp70 is strongly enhanced by the presence of Hsp70 peptide substrate. We suggest that the CD40–Hsp70 interaction shares important functional features with the interaction between Hsp70 and certain intracellular cochaperones, such as Hip.
Figure 7.
Model for the release of Hsp70–peptide complex from a necrotic tumor cell, followed by binding and uptake by an APC. (1) Necrosis leads to cell swelling and a concomitant drop in the concentration ratio of ATP/ADP. Intracellular Hsp70 is bound to unfolded protein or peptide. (2) Release of Hsp70–peptide complexes upon cell lysis. At the low nucleotide concentrations prevailing in the extracellular space, the half-life of the Hsp70-ADP state determines the stability of the Hsp70–peptide complex. (3) Hsp70–peptide complex binds to the surface of an APC. (4) Cell surface binding is mediated by the extracytoplasmic domain of CD40. (5) Hsp70-peptide binding alters the trimeric structure of CD40 (Chan et al., 2000), followed by activation of signaling via p38. (6), Hsp70–peptide complex is internalized by receptor-mediated endocytosis.
Although a variety of cell surface proteins have been reported to stimulate the immune system upon binding of molecular chaperones in model cell systems (Introduction), only in the case of CD40 has such an interaction been established directly. Wang et al. (2001) have shown recently that the bacterial homologue of mammalian Hsp70 (DnaK) is able to bind to HEK293T cells transfected with CD40 cDNA, and can be coimmunoprecipitated with CD40 from monocytic THP1 cells. However, this interaction is mediated by the COOH-terminal substrate-binding domain of DnaK, as opposed to the ATPase domain. In fact, Wang et al. (2001) concluded that human Hsp70 does not bind to CD40, apparently because binding was examined in the absence of nucleotide and peptide substrate. Our observations are fully consistent with those of Wang et al. (2001), as we find that human Hsp70 and DnaK both bind to independent sites on CD40. Nevertheless, both chaperones are able to elicit signaling via the CD40 receptor to a similar extent. This signaling is an established function of the interaction of CD40 with CD40 ligand, which induces a signal transduction cascade that activates NFκB, leads to secretion of TNFα and interferon-γ, and involves uptake of the receptor–ligand complex (Manning et al., 2002). The presence of a dileucine motif in the cytoplasmic domain of CD40 suggests a mechanism of uptake by endocytosis via the adaptor/clathrin system (Kirchhausen et al., 1997). Thus, in contrast to bacterial DnaK, binding of human Hsp70 has a dual role. In addition to stimulating activation of p38, human Hsp70 mediates the uptake of peptide bound to its substrate-binding domain. Evidently, this latter function cannot be served by DnaK, considering that its substrate-binding site is occupied by CD40. The less pronounced dependence on ADP of DnaK binding to CD40 may be explained by the fact that DnaK has a higher intrinsic ATPase activity than Hsp70, generating ADP-bound DnaK in the incubation.
Clearly, the most remarkable feature of Hsp70 binding to CD40 is the dramatic dependence of the interaction on substrate peptide bound to C70. Our results suggest that CD40 interacts exclusively with the ATPase domain of Hsp70, in the presence of ADP. Thus, the free COOH-terminal domain apparently masks the ATPase domain, either directly or by an allosteric conformational change, preventing recognition by CD40. This inhibitory effect is released when peptide is bound (Fig. 5, A and B). Conversely, it follows that CD40 stabilizes the ATPase domain in the ADP state that holds bound peptide stably. A similar effect in stabilizing Hsp70 in the ADP state has previously been described for the Hsp70 cochaperone Hip (Höhfeld et al., 1995). Indeed, binding of Hip to the ATPase domain of Hsp70 blocks the interaction of Hsp70 with CD40, suggesting that Hip and CD40 recognize overlapping regions on the ATPase domain.
The functional features of the CD40–Hsp70 interaction may be adapted to a role in the uptake of Hsp70–peptide complexes into APCs for cross priming. Fig. 7 describes a model for the binding of peptide antigen to Hsp70 in a tumor cell, followed by release after necrotic cell lysis and CD40-mediated uptake of the Hsp70–peptide complex by an APC. Peptide binding to Hsp70 would be facilitated by the high intracellular concentration of ATP and the activity of the Hsp70 cochaperone Hsp40 in catalyzing peptide loading (Minami and Minami, 1999). During cell necrosis, the internal concentration of ATP relative to ADP drops markedly (Bradbury et al., 2000). A further dilution of ATP (and of Hsp70 cochaperones) would occur upon lysis and release of cytosol content into the extracellular medium. As a result, peptide-bound Hsp70 remains in its ADP state, the stability of which determines the half-life of the Hsp70–peptide complex. Importantly, although peptide loading onto Hsp70 is possible in the absence of nucleotide with low efficiency (Minami et al., 1996), low nucleotide concentration would prohibit the reformation of an Hsp70–ADP–peptide complex in the extracellular space. Thus, the strong preference of CD40 for Hsp70–ADP–peptide ensures not only the binding of peptide-loaded Hsp70, but would also guarantee that intracellular peptide antigen is made available for cross priming. Thus, the uptake of circulating extracellular peptides, potentially triggering autoimmune reactions, would be avoided. Future work will be directed toward testing this model, with a focus on the fate of Hsp70-bound peptide after uptake into APCs, including possible peptide representation on the cell surface via MHC I.
Materials and methods
Plasmids and protein preparation
Human Hsp70, N70 and C70, Hip, and Bag-1 were expressed and purified as His-tagged proteins (Höhfeld et al., 1995; Scheufler et al., 2000; Sondermann et al., 2000, 2001). DnaK was expressed as described previously (Szabo et al., 1994). After ATP-sepharose affinity chromatography as described previously (Sondermann et al., 2000) Hsp70, N70, and DnaK were extensively dialyzed (twice against a 200-fold volume of PBS) in order to obtain essentially nucleotide-free proteins. Equine GST was purchased from Sigma-Aldrich. Biotinylated recombinant Hsp70 and equine GST were prepared by labeling with the FluoReporter® Biotin-XX Protein Labeling Kit (Molecular Probes, Inc.). Human CD40 cDNA was amplified by PCR from a human primary macrophage cDNA library (Invitrogen), and inserted into pcDNA3.1/Zeo and pIRES2-EGFP vector (Invitrogen). The cDNA for the exoplasmic domain of CD40 (aa 20–212) was amplified by PCR from the human CD40 cDNA described above, and inserted into pGEX-2T vector (Amersham Biosciences). GST and GST-CD40 fusion protein was purified by glutathione affinity chromatography (Glutathione Sepharose 4 Fast Flow; Amersham Biosciences). Peptide C (GCEVFGLGWRSYKH), biotinylated peptide C (biotin-GCEVFGLGWRSYKH), and peptide C-FITC (FITC-GCEVFGLGWRSYKH) were custom synthesized by R. Piepkorn (Deutsches Krebsforschungs Zentrum, Heidelberg).
For Hsp70–peptide complex formation, Hsp70 was incubated with a fivefold excess of peptide C, biotinylated peptide C, or peptide C-FITC for 30 min at 37°C in binding buffer consisting of the following (mM): 10 MOPS-KOH, pH 7.2, 150 KCl, 2 ADP, and 3 MgCl2. Excess of unbound peptide C–biotin was removed by gel filtration on Sephadex G-50 (Amersham Biosciences). Quantification of Hsp70 complex formation by ELISA using a streptavidin–peroxidase conjugate (Molecular Probes, Inc.) indicated yields of 20–30%. All proteins were centrifuged at 100,000 g at 4°C for 1 h before the experiments in order to remove aggregates.
Cell culture and fluorescence microscopy
The murine macrophage cell line ANA-11 was provided by H. Wagner (Technische Universität München, Munich, Germany). ANA-1 cells were cultured in VLE-RPMI 1640 (Biochrom Ltd.), supplemented with 10% FCS, 2 mM l-glutamine, and antibiotics. For fluorescence microscopy, ANA-1 cells were either stimulated with LPS (20 μg/ml; Sigma-Aldrich), or kept in an LPS-free medium (mock treatment) for 7 h. After stimulation, cells were harvested and incubated with 100 nM biotinylated Hsp70, biotinylated GST, or Hsp70–peptide C–biotin complex for 30 min at 0°C in VLE-RPMI 1640. After incubation, cells were processed for staining with streptavidin-TRITC (Sigma-Aldrich), fixed with PFA, and analyzed by fluorescence microscopy (Axiovert 35; Carl Zeiss MicroImaging, Inc.) as described previously (Sondermann et al., 2000). Cos-7 cells were cultured in DME supplemented with 10% FCS, 2 mM l-glutamine, and antibiotics (Biochrom Ltd.), grown on coverslips in 24-well plates, and transiently transfected with human CD40 cDNA (inserted into the pIRES2-EGFP vector; Invitrogen) by the calcium phosphate method (Chen and Okayama, 1987). 24 h after transfection, cells were incubated with 250 nM biotinylated Hsp70, biotinylated GST, or Hsp70–peptide C–biotin complex for 30 min at 0°C in DME, fixed, stained with streptavidin-TRITC, and analyzed by fluorescence microscopy as described previously (Sondermann et al., 2000). HEK293T cells were grown on collagen-coated dishes or coverslips, and cultured in DME supplemented with 10% FCS, 2 mM l-glutamine, and antibiotics. HEK293T cells were stably transfected (Chen and Okayama, 1987) with CD40 cDNA cloned into pcDNA3.1/Zeo (Invitrogen), and selected with Zeocin™ (Invitrogen). Stably transfected cell lines were analyzed by immunoblotting for CD40 expression using an anti-CD40 antibody (CSA180; StressGen Biotechnologies). HEK293T-MCAT cells expressing the MCAT cloned into pcDNA3.1/Zeo vector were provided by W. Nickel (Biochemie Zentrum Heidelberg, Heidelberg, Germany). S-HeLa cells were cultured in α-MEM supplemented with 8% FCS, l-glutamine, and antibiotics, and grown in spinner flasks.
For experiments to study uptake of the Hsp70–peptide C–FITC complex, HEK293T-CD40 and HEK293T-MCAT cells were seeded on collagen-coated coverslips 24 h before the experiment. For complex formation Hsp70, N70, or C70 were preincubated with a 10-fold molar excess of peptide C-FITC for 30 min at 37°C, as described above for the biotinylated proteins. HEK293T-CD40 and HEK293T-MCAT were incubated with 0.5 μM of Hsp70, N70, or C70, and preincubated with 2 mM ADP and 5 μM peptide C-FITC, or 5 μM peptide C-FITC alone for 30 min on ice. Thereafter, the cells were washed three times with medium, incubated for 15 min at 37°C, fixed, embedded in Fluoromount-G, and analyzed by confocal microscopy (LSM 510; Carl Zeiss MicroImaging, Inc.).
Analysis of CD40 expression
For immunoblot analysis, ANA-1 cells were stimulated with LPS or mock treated as described under Cell culture and fluorescence microscopy, harvested, lysed by repeated passage through a needle (0.4–0.8 mm; Braun) in the presence of protease inhibitors (complete, EDTA free; Roche), and fractionated by centrifugation at 100,000 g at 4°C for 1 h. The pellet was resuspended in 0.1% Triton X-100/PBS, and protein concentrations were determined in total lysate; supernatant and pellet fractions were determined by the Bradford assay (Bio-Rad Laboratories). Equal amounts of protein were subjected to SDS-PAGE, and were analyzed by immunoblotting with an anti-CD40 antibody (CSA180; StressGen Biotechnologies).
Binding assays with GST-CD40
For binding of endogenous Hsp70 and Hsc70 from HeLa cell lysate, HeLa cells were lysed as described above and centrifuged at 100,000 g at 4°C for 1 h. 20-μl bed volume of Glutathione Sepharose 4 Fast Flow (Amersham Biosciences) per sample was preincubated with 80 or 400 pmol of GST or GST–CD40 fusion protein in 50 μl binding buffer. Unbound protein was removed by washing three times with PBS. The beads were treated with 1% BSA-PBS to avoid nonspecific binding, and then incubated with 150 μl of HeLa cell lysate in the presence of 2 mM DTT for 20 min at 16°C. The immobilized proteins were washed three times with 1 ml PBS and eluted with 20 μl elution buffer (50 mM Tris-HCl, pH 8.0, 10 mM reduced glutathione) for 10 min at 37°C. 10 μl of the eluates was subjected to SDS-PAGE and analyzed by immunoblotting using an anti-Hsc/Hsp70 antibody and an anti-Hsp90 antibody (SPA822 and SPA835; StressGen Biotechnologies).
For binding assays with recombinant His6-tagged Hsp70 and its His6-tagged domains or DnaK, 2.8 μM of each protein was incubated with 2 mM ATP, 2 mM ADP, or 2 mM ADP and 28 μM peptide C in 50 μl binding buffer consisting of the following (mM): 10 MOPS-KOH, pH 7.2, 150 KCl, and 3 MgCl2 for 30 min at 37°C. In competition experiments, binding was probed with either a 10-fold excess of Hsp70, the two domains, DnaK, or with a fivefold excess of Hip or Bag-1 for 10 min at 0°C. To this end, Hsp70, N70, C70, and DnaK were preincubated with ADP, ATP, and peptide C as described under Plasmids and protein preparation. For comparison of CD40 binding of Hsp70 and its NH2-terminal domain, Hsp70 was preincubated in the presence or absence (mock treatment) of a 30-fold molar excess of peptide C. 3 μM GST or GST-CD40 was added to the samples and incubated for 20 min at 16°C. For titration of peptide C, 5–50-fold excess of peptide C (15–150 μM) was added to the preincubation of Hsp70. Thereafter, the samples were combined with 20 μl of 1% BSA-treated Glutathione Sepharose 4 Fast Flow and incubated in the presence of 2 mM DTT for another 20 min at 16°C. Unbound proteins were removed by washing the beads three times with 1 ml PBS, and immobilized proteins were eluted with 30 μl elution buffer for 20 min at 22°C. 10 μl of the eluates were subjected to SDS-PAGE and analyzed by immunoblotting using a Penta-His antibody (QIAGEN). Blot signals were quantified by Quantity One software (Bio-Rad Laboratories).
Binding assay with His6-tagged Hsp70 and Ni-NTA agarose
ANA-1 cells were stimulated with LPS as described above and lysed in lysis buffer (150 mM Tris-HCl, pH 7.5, 1% CHAPS) for 45 min at 4°C. Cell lysate was centrifuged at 100,000 g for 15 min at 4°C. 500 μl of the supernatant was incubated with 10 μg His6-tagged Hsp70, preincubated with ADP and a 30-fold excess of peptide C, or mock-treated, as described above for biotinylated proteins, for 30 min at 4°C. Thereafter, the samples were added to 20 μl of 1% BSA-treated Ni-NTA agarose (QIAGEN) and incubated for 30 min at 4°C. Unbound protein was removed by washing the beads three times with 1 ml lysis buffer, and immobilized proteins were eluted with 10 μl SDS-PAGE–sample buffer by incubation for 5 min at 95°C. Eluates were analyzed by immunoblotting using an anti-CD40 antibody (CSA-180; StressGen Biotechnologies).
p38 kinase assay
24 h before p38 kinase assays, HEK293T-CD40 and HEK293T-MCAT cells were seeded into a 24-well plate coated with collagen and incubated with 100 nM CD40L (Alexis Biochemicals Corp.), HSP70, N70-domain, C70-domain, or DnaK for 20 min at 37°C. DnaK, Hsp70, N70, and C70 were preincubated with either 2 mM ADP and 30 μM peptide C or 40 μM AMPPNP (Sigma-Aldrich). On stimulation, cells were washed twice with ice-cold PBS and lysed in SDS–sample buffer. Equal amounts of protein were analyzed by immunoblotting with antibodies directed against phosphorylated p38 (Promega), and with a monoclonal antitubulin antibody (J. Wehland, German Research Centre for Biotechnology, Braunschweig, Germany).
Acknowledgments
We thank Sabine Wegehingel for support with the cell culture, Dr. Ed Hurt Heidelberg for critically reading the manuscript, and Drs. Walter Nickel Heidelberg and Holger Sondermann Berkeley for helpful discussion.
Footnotes
Abbreviations used in this paper: APC, antigen-presenting cell; C70, COOH-terminal domain of Hsp70; Grp94, glucose-regulated protein 94; LPS, lipopolysaccharide; MCAT, murine cationic amino acid transporter; N70, NH2-terminal domain of Hsp70.
References
- Arnold-Schild, D., D. Hanau, D. Spehner, C. Schmid, H.G. Rammensee, H. de la Salle, and H. Schild. 1999. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J. Immunol. 162:3757–3760. [PubMed] [Google Scholar]
- Asea, A., M. Rehli, E. Kabingu, J.A. Boch, O. Bare, P.E. Auron, M.A. Stevenson, and S.K. Calderwood. 2002. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277:15028–15034. [DOI] [PubMed] [Google Scholar]
- Basu, S., and P.K. Srivastava. 1999. Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J. Exp. Med. 189:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, S., R.J. Binder, R. Suto, K.M. Anderson, and P.K. Srivastava. 2000. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol. 12:1539–1546. [DOI] [PubMed] [Google Scholar]
- Berwin, B., and C.V. Nicchitta. 2001. To find the road traveled to tumor immunity: the trafficking itineraries of molecular chaperones in antigen-presenting cells. Traffic. 2:690–697. [DOI] [PubMed] [Google Scholar]
- Berwin, B., J.P. Hart, S.V. Pizzo, and C.V. Nicchitta. 2002. Cutting edge: CD91-independent cross-presentation of GRP94(gp96)-associated peptides. J. Immunol. 168:4282–4286. [DOI] [PubMed] [Google Scholar]
- Binder, R.J., D.K. Han, and P.K. Srivastava. 2000. CD91: a receptor for heat shock protein gp96. Nat. Immunol. 1:151–155. [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi, S., S.E. Cwirla, W.J. Dower, R.J. Lipshutz, S.R. Sprang, J.F. Sambrook, and M.J. Gething. 1993. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 75:717–728. [DOI] [PubMed] [Google Scholar]
- Bodmer, J.L., P. Schneider, and J. Tschopp. 2002. The molecular architecture of theTNF superfamily. Trends Biochem. Sci. 27:19–26. [DOI] [PubMed] [Google Scholar]
- Bradbury, D.A., T.D. Simmons, K.J. Slater, and S.P. Crouch. 2000. Measurement of the ADP:ATP ratio in human leukaemic cell lines can be used as an indicator of cell viability, necrosis and apoptosis. J. Immunol. Methods. 240:79–92. [DOI] [PubMed] [Google Scholar]
- Bukau, B., and A.L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell. 92:351–366. [DOI] [PubMed] [Google Scholar]
- Castellino, F., P.E. Boucher, K. Eichelberg, M. Mayhew, J.E. Rothman, A.N. Houghton, and R.N. Germain. 2000. Receptor-mediated uptake of antigen–heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J. Exp. Med. 191:1957–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, F.K., H.J. Chun, L. Zheng, R.M. Siegel, K.L. Bui, and M.J. Lenardo. 2000. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 288:2351–2354. [DOI] [PubMed] [Google Scholar]
- Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, G.C., T.G. Chappell, and J.E. Rothman. 1989. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 245:385–390. [DOI] [PubMed] [Google Scholar]
- Greene, L.E., R. Zinner, S. Naficy, and E. Eisenberg. 1995. Effect of nucleotide on the binding of peptides to 70-kDa heat shock protein. J. Biol. Chem. 270:2967–2973. [DOI] [PubMed] [Google Scholar]
- Hartl, F.U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 295:1852–1858. [DOI] [PubMed] [Google Scholar]
- Höhfeld, J. 1998. Regulation of the heat shock conjugate Hsc70 in the mammalian cell: the characterization of the anti-apoptotic protein BAG-1 provides novel insights. Biol. Chem. 379:269–274. [PubMed] [Google Scholar]
- Höhfeld, J., and S. Jentsch. 1997. GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 16:6209–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhfeld, J., Y. Minami, and F.U. Hartl. 1995. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 83:589–598. [DOI] [PubMed] [Google Scholar]
- Kirchhausen, T., J.S. Bonifacino, and H. Riezman. 1997. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr. Opin. Cell Biol. 9:488–495. [DOI] [PubMed] [Google Scholar]
- Kol, A., A.H. Lichtman, R.W. Finberg, P. Libby, and E.A. Kurt-Jones. 2000. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 164:13–17. [DOI] [PubMed] [Google Scholar]
- Manning, E., S.S. Pullen, D.J. Souza, M. Kehry, and R.J. Noelle. 2002. Cellular responses to murine CD40 in a mouse B cell line may be TRAF dependent or independent. Eur. J. Immunol. 32:39–49. [DOI] [PubMed] [Google Scholar]
- Minami, Y., and M. Minami. 1999. Hsc70/Hsp40 chaperone system mediates the Hsp90-dependent refolding of firefly luciferase. Genes Cells. 4:721–729. [DOI] [PubMed] [Google Scholar]
- Minami, Y., J. Höhfeld, K. Ohtsuka, and F.U. Hartl. 1996. Regulation of the heat shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J. Biol. Chem. 271:19617–19624. [DOI] [PubMed] [Google Scholar]
- Pullen, S.S., T.T. Dang, J.J. Crute, and M.R. Kehry. 1999. CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs). Binding site specificity and activation of downstream pathways by distinct TRAFs. J. Biol. Chem. 274:14246–14254. [DOI] [PubMed] [Google Scholar]
- Reed, R.C., and C.V. Nicchitta. 2000. Chaperone-mediated cross-priming: a hitchhiker's guide to vesicle transport (review). Int. J. Mol. Med. 6:259–264. [DOI] [PubMed] [Google Scholar]
- Rudiger, S., L. Germeroth, J. Schneider-Mergener, and B. Bukau. 1997. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 16:1501–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheufler, C., A. Brinker, G. Bourenkov, S. Pegoraro, L. Moroder, H. Bartunik, F.U. Hartl, and I. Moarefi. 2000. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 101:199–210. [DOI] [PubMed] [Google Scholar]
- Sondermann, H., T. Becker, M. Mayhew, F. Wieland, and F.U. Hartl. 2000. Characterization of a receptor for heat shock protein 70 on macrophages and monocytes. Biol. Chem. 381:1165–1174. [DOI] [PubMed] [Google Scholar]
- Sondermann, H., C. Scheufler, C. Schneider, J. Höhfeld, F.U. Hartl, and I. Moarefi. 2001. Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science. 291:1553–1557. [DOI] [PubMed] [Google Scholar]
- Srivastava, P.K., A.B. DeLeo, and L.J. Old. 1986. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc. Natl. Acad. Sci. USA. 83:3407–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, P.K., H. Udono, N.E. Blachere, and Z. Li. 1994. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 39:93–98. [DOI] [PubMed] [Google Scholar]
- Szabo, A., T. Langer, H. Schroder, J. Flanagan, B. Bukau, and F.U. Hartl. 1994. The ATP hydrolysis-dependent reaction cycle of the Escherichia coli Hsp70 system DnaK, DnaJ, and GrpE. Proc. Natl. Acad. Sci. USA. 91:10345–10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone, M., Y. Tone, P.J. Fairchild, M. Wykes, and H. Waldmann. 2001. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc. Natl. Acad. Sci. USA. 98:1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udono, H., and P.K. Srivastava. 1993. Heat shock protein 70-associated peptides elicit specific cancer immunity. J. Exp. Med. 178:1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., C.G. Kelly, J.T. Karttunen, T. Whittall, P.J. Lehner, L. Duncan, P. MacAry, J.S. Younson, M. Singh, W. Oehlmann, et al. 2001. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity. 15:971–983. [DOI] [PubMed] [Google Scholar]