Abstract
Spectacular examples of transdifferentiation—such as brain cells turning to blood and blood to brain—have given way to sneaking suspicions about artifacts in culture, fusion, and clonality. Could cell fates be relatively fixed after all?
The exact fate map of Caenorhabditis elegans is a dream for developmental biology control freaks. Stem cells, which can both self-renew and produce a particular set of differentiated progeny, seemed like the mammalian equivalent, albeit a little more malleable and messy.
But lately this view of development as a controlled series of fate maps, with each cell type performing one and only one function, has come under attack. Stem cell researchers have claimed that brain can be turned into blood (Bjornson et al., 1999), and blood into brain (Brazelton et al., 2000; Mezey et al., 2000; Priller et al., 2001), muscle (Ferrari et al., 1998), myocardium (Orlic et al., 2001) or liver (Lagasse et al., 2000). In the hands of some investigators, bone marrow (Krause et al., 2001) and neural stem cells (Clarke et al., 2000) can apparently turn into pretty much everything.
“I believe this phenomenon was missed for a very long time,” says Helen Blau (Stanford University, Stanford, CA), “because we didn't have the imagination to think that it could happen or the tools to prove it.” The orgy of plasticity has prompted Blau to propose a new way of thinking about cell fate determination (Blau et al., 2001), with stem cell capability existing as a switch that can be turned on and altered as easily as an apoptotic program.
But others see at least some of the reports of cell fate changes—also known as transdifferentiation—as simply sloppy science. “The emphasis,” says Sean Morrison (University of Michigan, Ann Arbor, MI), “has been on describing spectacular results rather than on describing it in an evenhanded way that would identify the real importance of the phenomenon.” Several dissenters have called for more stringent criteria for judging transdifferentiation experiments (Anderson, 2001; Anderson et al., 2001).
As follow-up studies emerge, questions have been raised about some of the earlier results. The verdict is not in yet, but the resolution of this debate will have implications on several fronts, determining our view of basic stem cell biology, affecting the strategy for treating patients with stem cell therapies, and influencing the steps used by the United States Congress to regulate stem cell research.
Political science and medical treatments
Transdifferentiation is of interest to Senate Select Committees because it could mean that embryonic stem cells (ESCs) are not needed for cellular therapies. If adult stem cells (ASCs) can turn into anything, why bother with the controversial ESCs? Unfortunately, says Darwin Prockop (MCP Hahnemann University, Philadelphia, PA), “the political debate over embryonic stem cells and adult stem cells has led both sides to overstate their cases.” In an effort to protect ESC research, “some of these [transdifferentiation] results have been criticized too heavily.”
The burgeoning literature supporting plasticity has made life harder for scientists seeking to protect the right to conduct ESC research. “Constantly thrown at us is ‘you can do everything with adult stem cells’—not by anyone in the scientific community, but it's picked up by politicians and lobbying groups,” says Austin Smith (University of Edinburgh, UK). “And it's quite difficult to argue against because they will just list all these published papers”—many of which Smith believes are incorrect.
Choosing one field over another is probably the wrong way to go in any case. “All the research should go on in parallel,” says Blau. “We don't know what will work best.”
Even if one remedy won't work for every disease, any specific treatment would still need to generate the desired cell type at a therapeutically meaningful frequency. Markus Grompe (Oregon Health Sciences University, Portland, OR) is not sure that this will always be possible with ASCs. “We think that transdifferentiation occurs, but the levels are three to four orders of magnitude less than has been reported by others.”
If Grompe is correct, many of the proposed therapies may not work. “If we're overstating the efficiency of replacement by one or two orders of magnitude,” says Morrison, “it won't be sufficient—we won't be able to cure people this way.”
The ins and outs of cell fate determination
And then there are the implications for basic stem cell biology. For Ron McKay (National Institutes of Health, Bethesda, MD), transdifferentiation results represented a much-needed correction from the excesses of molecular biology. “There has been a tendency to believe in the inviolability of how an organism is constructed,” he says, with cell fates seen as predetermined based on the particular set of transcription factors turned on in a certain stem cell. This view was bolstered by some earlier experiments in which transplanted cells failed to switch fates.
But some of this inflexibility may have been caused by a local microenvironment that, along with a particular set of factors, was transplanted with the cells (Trainor et al., 2002). When researchers tracked individual cells traveling into new tissues, they saw the unexpected fate switches, and the focus shifted to the importance of extracellular signals.
This debate between intrinsic and extrinsic determinants “is going to have to be sorted out on a case by case basis,” says David Anderson (California Institute of Technology, Pasadena, CA). In his view, “the idea that any cell type can make any other cell type given the correct extrinsic signal is unlikely to be right.” Such a scenario would, he points out, lead to extremely confused migratory cells, which would be unable to reach their destinations without becoming several different cell types while en route. Many embryonic differentiation signals or developmental gradients are no longer present in the adult, making Anderson wonder how proposed transdifferentiations in nonregenerating tissues might be directed.
New standards for a new field
These theoretical considerations are not the only ones troubling the field. The earliest examples of transdifferentiation were so unexpected and dramatic that only now is the community determining the criteria required to demonstrate bona fide fate changes. Earlier standards have been found wanting.
The new transdifferentiation manifesto has three elements. Ideally, introduced cells must be clonal, prospectively isolated (i.e, by sorting for marker proteins, without any in vitro culturing), and tracked until they can be shown to be functioning in their new environment.
Not all researchers are signing on to this program. Blau, for example, says that studies with hematopoietic (blood-forming) stem cells (HSCs) have only recently met these requirements after 20 years of research, whereas most ASC research is only a couple of years old. “The clonal experiments are incredibly hard to do,” she says. “Ultimately it would be nice to know, but it's something to work toward.”
Until clonality is accomplished, most existing studies have used mixed populations of cells, such as bone marrow. Thus it is possible or even probable that any neural cells arise not from HSCs, but from mesenchymal stem cells that are also present in bone marrow.
In at least one case, tracking the stem cell to its source has negated an earlier claim of transdifferentiation. Muscle can turn into blood, says Margaret Goodell (Baylor College of Medicine, Houston, TX), only because of the presence of roving HSCs interspersed in the muscle cells (McKinney-Freeman et al., 2002). “Many people are happy to see our results, because it casts some doubts on the results that are out there,” says Goodell. Even if a case of transdifferentiation holds up, she says, the clonal analysis is vital, because “you need to know the cell type so you can improve the efficiency.”
The cell type can be defined only with better isolation methods and cell surface markers. These markers, in turn, allow for prospective isolation of cells without intervening culture. Although neural stem cells have recently been isolated prospectively (Uchida et al., 2000; Rietze et al., 2001), most studies have used cells that have been extensively cultured. “You cannot be sure the same thing would happen if [the cells] hadn't been cultured,” says Jonas Frisén (Karolinska Institute, Stockholm, Sweden). “It's looking at what is possible rather than what is happening.”
One of the first and most spectacular examples of transdifferentiation has recently come under attack for just this reason. The demonstration that brain can be turned into blood (Bjornson et al., 1999) could not be replicated by Derek van der Kooy and colleagues (University of Toronto, Canada) when they used shorter passage times. As the cells were passaged for longer, however, they gradually accumulated changes in various growth properties, leading the authors to suggest that rare genetic or epigenetic events might trigger the neural-to-blood fate switch (Morshead et al., 2002). As a result, says van der Kooy, “I've completely reversed my view of what I thought was a very convincing piece of data.”
Others feel that additional studies will fall to a similar analysis. “My prediction would be that people will be much more hard-pressed to identify these transdifferentiation phenomena once they are using noncultured cells,” says Morrison.
Some of the most convincing clonal results have come from Diane Krause (Yale University, New Haven, CT), who showed that single marked cells that home to bone marrow can be transplanted to yield epithelial cells in various organs (Krause et al., 2001). This unorthodox method of cell isolation does, however, mean that other questions remain. As Krause herself says: “Whether that homing is necessary for plasticity, we just don't know.”
Culture-related changes may also taint the results of Catherine Verfaillie (University of Minnesota, Minneapolis, MN). She isolated a bone-marrow derived cell that has been dubbed the “ultimate stem cell” (Pagán-Westphal, 2002) because of its ability to generate cells of so many different tissues. Isolation through culture may skew ideas about basic biology, but changes to cells in culture may not prevent therapeutic applications, and Verfaillie's cells may well find important uses in medicine.
Technical difficulties
The third requirement—that researchers demonstrate evidence of functionality—has been at least partially answered in a recent study in the JCB. Priller et al. (2001) demonstrated that a marked bone marrow cell could end up as a highly differentiated Purkinje cell in the brain (Fig. 1 A). But this study raised an additional concern—this time about possible cell fusion events. “Stem cells are the most long-lived cells,” says van der Kooy, “and occasionally they will fuse with host cells.”
Figure 1.
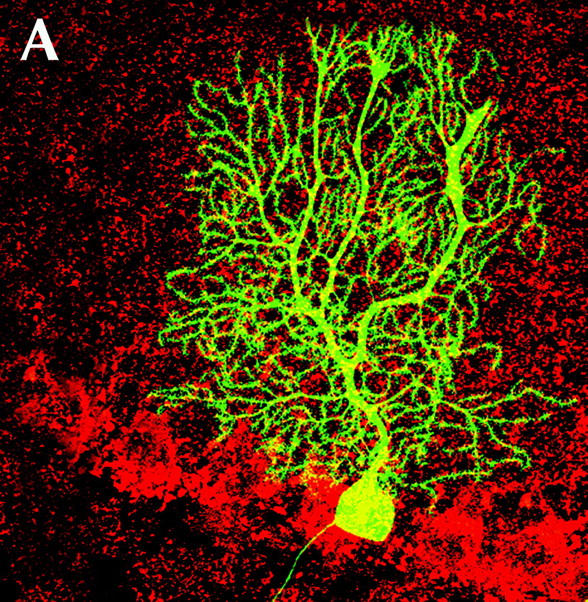
Plasticity or fusion? Marked bone marrow cells can become differentiated Purkinje cells (A), but another case of apparent plasticity—neural stem cells contributing to various tissues of a chimeric mouse (B)—occurs only after the neural cells have fused with cocultured embryonic stem cells.
“We haven't really ruled out fusion,” says Josef Priller (Humboldt University, Berlin, Germany). “It's really on probability grounds that we can argue—it's really not known at all that brain cells fuse.”
The cells introduced in Priller's experiments were genetically identical to the cells of the recipient (except for the GFP marker), so any possible fusion events are difficult to track. There is further concern given that Priller has not yet observed intermediate stages of Purkinje cell development with the introduced cells, although this could be explained by the limited time points studied. Blau has performed similar experiments and seen that some (but certainly not all) of the marked Purkinje cells have greater than 2N DNA. This may not be surprising, however, as greater than 2N DNA content can occur normally in Purkinje cells.
Krause is doing experiments to rule out the possibility of fusion in her experiments. For now, she says, “we haven't published any experiments to specifically rule [fusion] in or out.”
The possibility that fusion events might occur has been boosted by two recent reports one from Smith and another from Naohiro Terada (University of Florida, Gainesville, FL) and colleagues. Smith found that neural stem cells cocultured with embryonic stem (ES) cells could contribute to nonneural tissues not by dedifferentiation but via fusion with the ES cells (Fig. 1 B; Ying et al., 2002). Terada carried out similar coculture experiments with bone marrow cells and ES cells and found that the resulting ES-like cells, which could differentiate to many different cell types in vitro, were tetraploid or hexaploid (Terada et al., 2002). Smith points out that in vivo situations might be more or less permissive for cell fusion, but he believes that the frequency of reported transdifferentiation events is “not inconsistent” with the frequency of fusion events that he sees. “We cannot say that [fusion] explains the phenomenon that others have reported,” he says. “But in none of the other experiments can cell fusion be ruled out, so it's therefore possible that many of these reports could be explained by cell fusion.”
Whereas marked cells with complicated morphology could arise via fusion, marked cells with simpler morphology can be mistaken based on signal overlap. “Even when you are being careful it's still possible to incorrectly identify cells because nuclei can be so adjacent,” says Morrison. A similar problem has recently been seen in neurogenesis experiments—a follow-up study has shown that replicating cells in certain parts of the brain are not neurons but support cells that are very close to neurons (Kornack and Rakic, 2001). Although there is a trivial step that could rule out signal overlap—checking cells for continuing costaining after dispersal on slides—most transdifferentiation studies do not include this procedure.
Immunofluorescence studies can also lead to false positives when an arbitrary cut-off for positive staining is set too low. “From the liver angle, I know people have overestimated the extent of [transdifferentiation],” says Grompe, “so it makes me wonder about the other tissues.” Grompe's frequency estimates are based on the number of clonal patches rather than on absolute numbers of marked cells in the livers he has studied. The transdifferentiation events are extremely rare, but with selection present in these experiments the cells can become more numerous and functionally important. Grompe says that without selection (such as the use of hepatotoxic drugs), transdifferentiation is unlikely to translate into medically important treatments. Selection may be difficult to engineer for some proposed therapeutic sites, such as the brain.
Picking through the rubble
In theory, transdifferentiation could be detected as a complication of bone marrow transplant procedures. But a 1–2% replacement rate—as claimed in many transdifferentiation reports—was never noted by transplantation pioneers. This reassures Grompe that plasticity is limited. “I'm glad we don't have cells differentiating everywhere after transplantation,” he says.
Smith also feels that plasticity reports had gone too far. “I find it a little surprising that people had so readily discarded everything that developmental biology had established over the past 20 years—that cells are generally lineage restricted,” he says. Now he feels that the balance is beginning to swing back.
But even transdifferentiation's critics are far from outright pessimists. The spirit of many of these researchers is captured by van der Kooy. “Right now is a very exciting time,” he says. “There are so many reports of plasticity that not all of them can be wrong due to these problems. We just have to look at all of them very carefully.” Blau is concerned that current criticisms should not dampen the enthusiasm of those in the field and says that careful analysis using confocal microscopy and genetic marking of transplants should improve the results in more recent studies. “We should be as rigorous as possible, but we shouldn't turn our backs on this,” she says. “To slam a field because it's new and you haven't proven everything is also bad science.”
Rossi feels that the reliability of the studies depends in part on the direction of the claimed transformation. “Most of the reports of other cell types making blood have been put in doubt in the last year or so,” he says, “but the [work showing] blood making other cells is pretty robust.”
A physiological function
Plasticity has extraordinary consequences for cell biology theory and medical treatment, but what is the physiological significance for the organism? Are transdifferentiation events used for any purpose in living animals?
There are at least two trivial explanations for transdifferentiation. First, brain cells might normally have a restricted potential simply because they are stuck within the brain. Only when this geographical restraint is relaxed (e.g., because stem cells are injected intravenously) are other options open to them. Second, transdifferentiation may reflect a certain error rate in cell specification that was not detectable before sensitive molecular biological tools were available.
Then come the more interesting explanations. In Prockop's opinion, bone marrow cells “look as though they are part of a normal repair system.” Consistent with this, Krause has seen increased engraftment efficiency after tissue damage. The bone marrow might be a good place to store such a repair team, as the cells are highly mobile.
Grompe, however, has looked at the issue of repair more closely and found that transdifferentiation occurs at a low frequency that is not dependent on tissue injury. Only with more chronic liver damage, over nine months, does he see significant bone-marrow–derived replacement. “It's possible that the precursor pool in the liver gets replaced by stem cells from the bone marrow in a stochastic process all the time,” he says. “If there is a physiological relevance for this I think it may be in very long term replenishment. The replacement rate does not appear to be injury driven.”
Future experiments
As part of studying the normal function of transdifferentiation, several researchers have their eyes on the types of parabiosis experiments used to study HSC trafficking. In these experiments the blood systems of two mice are joined artificially, so that cells can traffic from one marked mouse to another unmarked mouse, without the interference of cell injections or radiation treatments.
Along with these in vivo studies will come in vitro work to define the local signals that drive transdifferentiations. Rossi speculates that these signals are perturbed in disease. “Once you isolate these cells you can identify the mechanisms that drive the transdifferentiation,” he says. “If you can supply the signals in vitro and prime the cells to do something, there is a possibility to bypass the messed up microenvironment. That is clearly the main goal of the field.”
It should be possible, says McKay, to derive the equivalent of thermodynamic models for each of these transitions. Although the models might be generalizable, McKay suspects that the rate constants will vary widely depending on the cell type involved. “Not all fates are equally probable,” he says. “If you imagine a landscape with 200 fates on it, a given cell may not have equal access to all fates.”
Thus the debate over transdifferentiation may be cast not in black and white—does it happen or does it not happen—but more in shades of gray. “People want completely simple answers,” says McKay, “but I think one has to be careful not to generalize.”
References
- Anderson, D.J. 2001. Stem cells and pattern formation in the nervous system: the possible versus the actual. Neuron. 30:19–35. [DOI] [PubMed] [Google Scholar]
- Anderson, D.J., F.H. Gage, and I.L. Weissman. 2001. Can stem cells cross lineage boundaries? Nat. Med. 7:393–395. [DOI] [PubMed] [Google Scholar]
- Bjornson, C.R., R.L. Rietze, B.A. Reynolds, M.C. Magli, and A.L. Vescovi. 1999. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 283:534–537. [DOI] [PubMed] [Google Scholar]
- Blau, H.M., T.R. Brazelton, and J.M. Weimann. 2001. The evolving concept of a stem cell: entity or function? Cell. 105:829–841. [DOI] [PubMed] [Google Scholar]
- Brazelton, T.R., F.M. Rossi, G.I. Keshet, and H.M. Blau. 2000. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 290:1775–1779. [DOI] [PubMed] [Google Scholar]
- Clarke, D.L., C.B. Johansson, J. Wilbertz, B. Veress, E. Nilsson, H. Karlstrom, U. Lendahl, and J. Frisén. 2000. Generalized potential of adult neural stem cells. Science. 288:1660–1663. [DOI] [PubMed] [Google Scholar]
- Ferrari, G., G. Cusella-De Angelis, M. Coletta, E. Paolucci, A. Stornaiuolo, G. Cossu, and F. Mavilio. 1998. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 279:1528–1530. [DOI] [PubMed] [Google Scholar]
- Kornack, D.R., and P. Rakic. 2001. Cell proliferation without neurogenesis in adult primate neocortex. Science. 294:2127–2130. [DOI] [PubMed] [Google Scholar]
- Krause, D.S., N.D. Theise, M.I. Collector, O. Henegariu, S. Hwang, R. Gardner, S. Neutzel, and S.J. Sharkis. 2001. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 105:369–377. [DOI] [PubMed] [Google Scholar]
- Lagasse, E., H. Connors, M. Al-Dhalimy, M. Reitsma, M. Dohse, L. Osborne, X. Wang, M. Finegold, I.L. Weissman, and M. Grompe. 2000. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 6:1229–1234. [DOI] [PubMed] [Google Scholar]
- McKinney-Freeman, S.L., K.A. Jackson, F.D. Camargo, G. Ferrari, F. Mavilio, and M.A. Goodell. 2002. Muscle-derived hematopoietic stem cells are hematopoietic in origin. Proc. Natl. Acad. Sci. USA. 99:1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey, E., K.J. Chandross, G. Harta, R.A. Maki, and S.R. McKercher. 2000. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 290:1779–1782. [DOI] [PubMed] [Google Scholar]
- Morshead, C.M., P. Benveniste, N.N. Iscove, and D. van der Kooy. 2002. Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nat. Med. 8:268–273. [DOI] [PubMed] [Google Scholar]
- Orlic, D., J. Kajstura, S. Chimenti, I. Jakoniuk, S.M. Anderson, B. Li, J. Pickel, R. McKay, B. Nadal-Ginard, D.M. Bodine, et al. 2001. Bone marrow cells regenerate infarcted myocardium. Nature. 410:701–705. [DOI] [PubMed] [Google Scholar]
- Pagán-Westphal, S. 2002. Ultimate stem cell discovered. New Sci. 173:4. 11995738 [Google Scholar]
- Priller, J., D.A. Persons, F.F. Klett, G. Kempermann, G.W. Kreutzberg, and U. Dirnagl. 2001. Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J. Cell Biol. 155:733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietze, R.L., H. Valcanis, G.F. Brooker, T. Thomas, A.K. Voss, and P.F. Bartlett. 2001. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 412:736–739. [DOI] [PubMed] [Google Scholar]
- Terada, N., T. Hamazaki, M. Oka, M. Hoki, D.M. Mastalerz, Y. Nakano, E.M. Meyer, L. Morel, B.E. Petersen, and E.W. Scott. 2002. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 10.1038/nature730. [DOI] [PubMed]
- Trainor, P.A., L. Ariza-McNaughton, and R. Krumlauf. 2002. Role of the isthmus and FGFs in resolving the paradox of neural crest plasticity and prepatterning. Science. 295:1288–1291. [DOI] [PubMed] [Google Scholar]
- Uchida, N., D.W. Buck, D. He, M.J. Reitsma, M. Masek, T.V. Phan, A.S. Tsukamoto, F.H. Gage, and I.L. Weissman. 2000. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. USA. 97:14720–14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying, Q.-L., J. Nichols, and A.G. Smith. 2002. Changing potency by spontaneous fusion. Nature. 10.1038/nature729. [DOI] [PubMed]