Abstract
p115 tethers coat protein (COP)I vesicles to Golgi membranes. The acidic COOH-terminal domain of p115 links the Golgins, Giantin on COPI vesicles, to GM130 on Golgi membranes. We now show that a SNARE motif-related domain within p115 stimulates the specific assembly of endogenous Golgi SNAREpins containing the t-SNARE, syntaxin 5. p115 catalyzes the construction of a cognate GOS-28–syntaxin-5 (v-/t-SNARE) complex by first linking the SNAREs to promote their direct interaction. These events are essential for NSF-catalyzed reassembly of postmitotic Golgi vesicles and tubules into mature cisternae. Staging experiments reveal that the linking of Golgins precedes SNAREpin assembly. Thus, p115 coordinates sequential tethering and docking of COPI vesicles by first using long tethers (Golgins) and then short tethers (SNAREs).
Keywords: Golgi apparatus; mitosis; p115; SNAREpin; Golgin
Introduction
High fidelity vesicle transfer is quintessential for the establishment and maintenance of organelle identity, biosynthetic transport, and synaptic transmission. Vesicle transfer can be deconstructed into a strict succession of highly interdependent subreactions that comprise vesicle biogenesis, tethering, docking, and fusion (Pfeffer, 1999). Once a vesicle has formed, targeting mechanisms are enforced which ensure cargo delivery to the correct destination. Compartmental identity is achieved in part by the inherent specificity of cognate interactions that occur between members of the SNARE superfamily (Scales et al., 2000). Whereby in the simplest sense, a unitary v-SNARE on a vesicle interacts uniquely with its cognate three-component t-SNARE on an acceptor membrane (McNew et al., 2000a; Parlati et al., 2000; Pelham, 2001). This interaction, termed a trans-SNARE complex or SNAREpin, docks a vesicle to its target membrane and either induces spontaneous bilayer mixing (Chen et al., 1999; McNew et al., 2000a) or signals to downstream components, which directly catalyze fusion (Ungermann et al., 1998; Peters et al., 2001). However, specificity is further predicated by a preceding layer of regulation termed vesicle tethering. Vesicle tethering is a SNARE-independent event and requires the activity of peripheral membrane proteins, which are often extended coiled-coil fibrous proteins or large multiprotein complexes, and seems to be coordinated by Rab-GTPases (Zerial and McBride, 2001; Short and Barr, 2002). Precisely how the protein interactions of vesicle tethering lead to the docking of cognate SNAREs and subsequent membrane fusion is unclear.
A well-characterized and highly conserved vesicle tethering protein is p115, which tethers coat protein (COP)*I vesicles to Golgi membranes (Sönnichsen et al., 1998). p115 functions include ER-Golgi transport (Cao et al., 1998), intra-Golgi transport (Waters et al., 1992; Seemann et al., 2000a), and stacking Golgi cisternae (Shorter and Warren, 1999). This myosin-shaped homodimer consists of an NH2-terminal globular head domain, a coiled-coil tail, and a short acidic COOH-terminal domain (Fig. 1 A) (Sapperstein et al., 1995). p115 juxtaposes membranes by simultaneously binding via its acidic COOH-terminal domain two Golgins, GM130 in one membrane and Giantin in the other (Sönnichsen et al., 1998; Shorter and Warren, 1999; Dirac-Svejstrup et al., 2000). GM130 and Giantin are long rod-like fibrous proteins due to an extensive coiled-coil domain structure typical of Golgins (Linstedt and Hauri, 1993; Nakamura et al., 1995). GM130 is restricted to Golgi cisternae, whereas Giantin is also present in COPI vesicles (Nakamura et al., 1995; Sönnichsen et al., 1998; Martinez-Menarguez et al., 2001). Thus, p115 may tether COPI vesicle to cisterna or cisterna to cisterna, depending on the topological restriction of Giantin, and so couple stacked Golgi structure to processive COPI vesicle flow (Linstedt, 1999; Shorter and Warren, 1999; Orci et al., 2000). The Giantin-p115-GM130 tether is mitotically regulated by cyclin B-CDK1, which directly phosphorylates GM130 and precludes p115 binding. This may help explain the accumulation of COPI vesicles that occurs during mitosis as part of the Golgi disassembly and inheritance process (Nakamura et al., 1997; Lowe et al., 1998). In addition, p115 in synergy with Rab1 tethers COPII vesicles to membranes, though the mechanism is obscure (Cao et al., 1998; Allan et al., 2000; Moyer et al., 2001).
Figure 1.
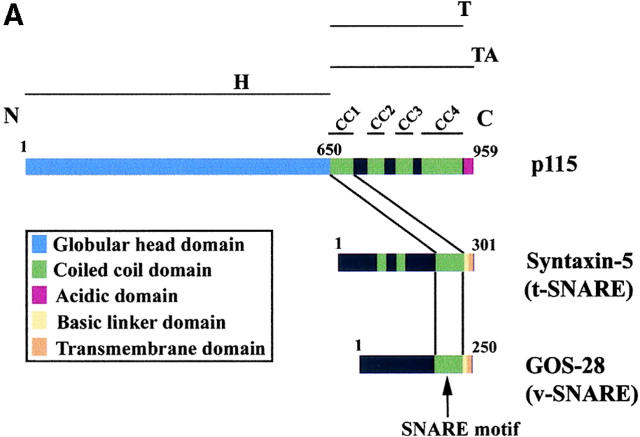
A SNARE motif-related region in p115 binds specific SNAREs. (A) The domain architecture of p115, syntaxin-5 (a t-SNARE), and GOS-28 (a v-SNARE). p115 consists of a globular head domain (H, blue), a tail domain (T) containing four coiled-coil domains (CC1-4, green), and an acidic COOH-terminal domain (A, red). SNAREs contain an ∼60 amino acid membrane proximal coiled-coil domain (green) termed the SNARE motif followed by a basic linker region (yellow) and a transmembrane domain (orange). t-SNAREs possess additional NH2-terminal coiled-coil regions. The first coiled-coil domain of p115 (CC1) displays weak homology to the SNARE motif (Weimbs et al., 1997). (B) RLGs (20 μg) were extracted with Triton X-100 buffer, clarified, and incubated with Neutravidin beads (mock) or beads bound to biotinylated p115 or CC1-4 peptides. Washed beads were eluted, and eluates were fractionated by SDS-PAGE and silver stained. Asterisks denote proteins selectively retained on p115 and CC1 beads but not others. Crosses denote proteins selectively retained on p115, CC1, and CC4 beads but not others. The triangle denotes p115 eluted from p115 beads. Squares denote proteins that correspond in size to Giantin (□, top) and GM130 (□, bottom), which are retained only on p115 beads. Arrows indicate Neutravidin breakdown products. (C) Immunoblot analyses of B. In addition, His-TA and His-T (0.5 μM) were incubated with clarified Golgi detergent extract, retrieved with Ni-NTA agarose, and processed as in B before immunoblot analysis.
How does Giantin-p115-GM130 tether formation facilitate SNAREpin assembly? One hint came when sophisticated profile-based sequence analyses defined the SNAREs as a superfamily by virtue of a homologous coiled-coil domain of ∼60 amino acids (aa) termed the SNARE motif (Weimbs et al., 1997; Jahn and Südhof, 1999). SNAREpins consist of an internal core of four SNARE motifs, one contributed by the v-SNARE and three by the t-SNARE, that are aligned in parallel to form an exceptionally stable helical bundle (Jahn and Südhof, 1999). Transduction of energy from this helical bundle via flexible linker regions to the transmembrane domains of SNAREs may forcibly drive bilayer mixing (McNew et al., 2000b). Furthermore, the precise topological restriction of individual components within an assembled SNAREpin may provide a universal syntax or code, which ultimately governs the specificity of membrane fusion events (McNew et al., 2000a; Parlati et al., 2000). The definition of the SNARE molecular clade has revealed that the first coiled-coil domain of p115 possesses weak homology to the SNARE motif (Weimbs et al., 1997). The homology is limited in that p115 has a leucine rather than the highly conserved central arginine or glutamine of the SNARE motif (Weimbs et al., 1998). This and the distinctive domain architecture of p115 compared with SNAREs (Fig. 1 A) means that p115 is not part of the SNARE superfamily. Although the homology is weak, it is intriguing, given the proximity of p115 and SNARE function in Golgi membrane fusion events and that p115 can bind to certain SNAREs during ER-Golgi transport (Allan et al., 2000). Furthermore, a recent fluorescence study might have discerned a p115 activity distinct from its interaction with Giantin and GM130 that might contribute to higher order Golgi structure (Puthenveedu and Linstedt, 2001). Although the lack of ultrastructural analysis precludes a definite conclusion, since vesiculated Golgi can still appear as a juxtanuclear ribbon by immunofluorescence (Seemann et al., 2000a,b), it raises the possibility of another p115 activity essential for Golgi membrane fusion. Could this be a direct role in SNAREpin assembly?
We have examined the significance of the putative p115–SNARE homology by using a cell-free system that reconstitutes many aspects of postmitotic Golgi reassembly. Isolated mitotic Golgi fragments (MGFs) will regenerate Golgi cisternae via one of two pathways controlled by the AAA ATPases NSF and p97. The NSF reaction requires Giantin-p115-GM130 tethers and the Golgi v-SNARE GOS-28 (Nagahama et al., 1996) and its cognate t-SNARE syntaxin-5 (Hay et al., 1997; Rabouille et al., 1998). Using this system, we now show that p115 physically couples COPI vesicle tethering (Golgin dependent) to COPI vesicle docking (SNARE dependent) by sequentially linking GM130 to Giantin, followed by GOS-28 to syntaxin-5, to actively catalyze SNAREpin assembly. This direct catalytic role of p115 in SNARE assembly is impelled via its SNARE motif-related region.
Results
A SNARE motif-related region of p115 retains syntaxin-5 SNARE complexes from Golgi detergent extract
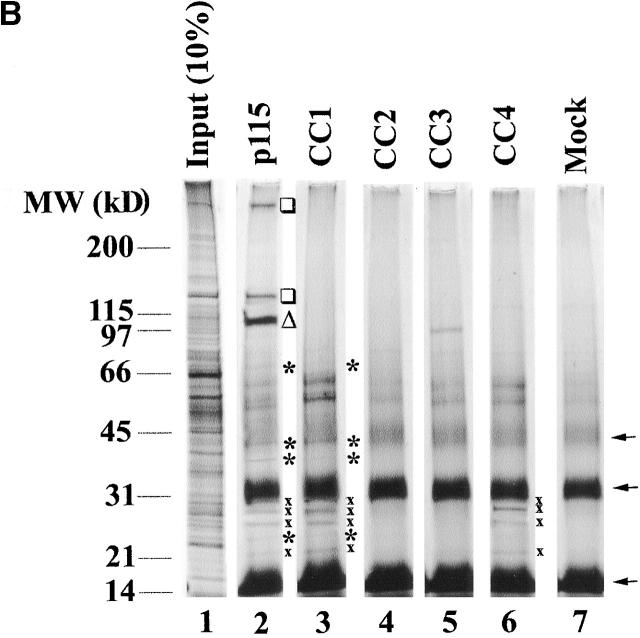
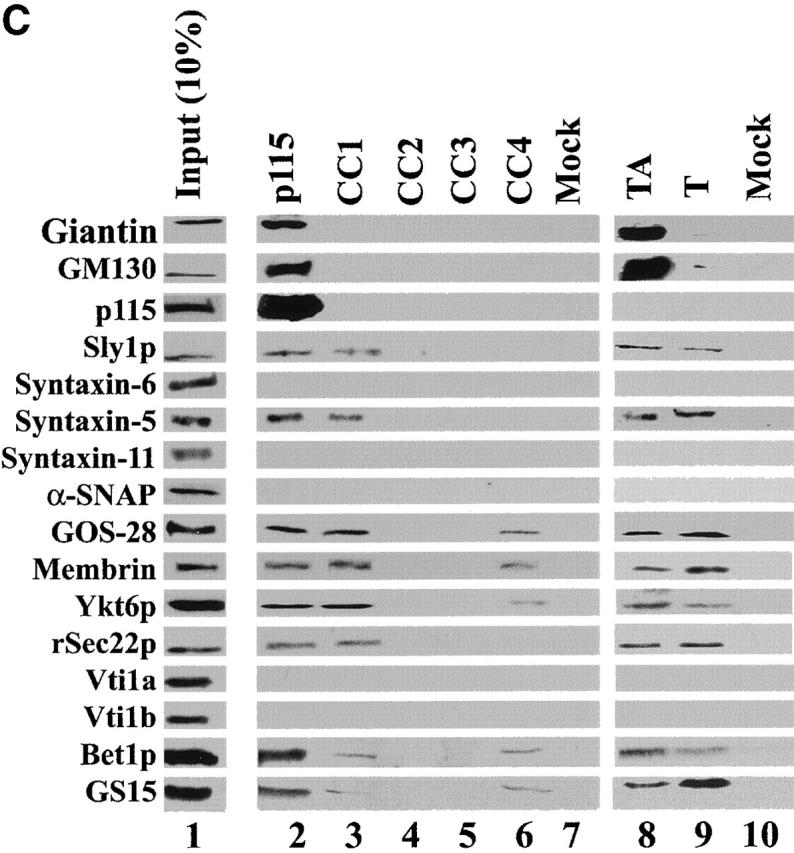
Affinity chromatography using the four coiled-coil domains of p115 (Fig. 1 A) was performed to test the functional significance of the homology between the first coiled-coil domain of p115 (CC1) and the SNARE motif. Biotinylated p115 and coiled-coil domain 1–4 of p115 (CC1-4) peptides were coupled to Neutravidin beads and used as affinity ligands to probe a Golgi detergent extract. Silver staining revealed that p115 specifically retained a subset of proteins that was not retained by mock beads (Fig. 1 B). A fraction of these proteins was also retained by CC1 (Fig. 1 B, asterisks), whereas a further subfraction was retained by CC1 and CC4 (Fig. 1 B, crosses). These proteins were mostly in the 20–40 kD molecular weight range, indicating that they might be SNAREs. Immunoblotting confirmed that syntaxin-5 (a Golgi t-SNARE) and a collection of its cognate v-SNAREs (GOS-28, membrin, rSec22p, Bet1p [Hay et al., 1997], and Ykt6p [Zhang and Hong, 2001]) were prominent proteins retained on p115 and CC1 beads but not on CC2, CC3, or mock beads (Fig. 1 C). GS15, another Golgi v-SNARE, whose cognate SNAREs are undefined, was also retained on p115 and CC1 beads but not on CC2, CC3, or mock beads (Xu et al., 1997). This retrieval was relatively efficient with typically 5–10% of input being retained (Fig. 1 C). This compares well with reported retrievals of SNARE complexes, directing distinct fusion events, especially since cross-linking was not required for their detection (compare with McBride et al., 1999; Allan et al., 2000). CC4 retained a further subset of these SNAREs (GOS-28, membrin, Ykt6p, Bet1p, and GS15) but with the exceptions of GS15 and Bet1p retained notably less than p115 or CC1. Since Golgi reassembly was unaffected by CC4 (see below), these CC4–SNARE interactions may not be functionally relevant.
The interactions between Golgi SNAREs and p115/CC1 were specific since the TGN/endosomal SNAREs syntaxin-6, syntaxin-11, Vti1a, and Vti1b were not retained (Fig. 1 C). Moreover, if a detergent extract of a rat liver postnuclear supernatant was probed with p115/CC1 beads neither syntaxin-1, SNAP-23, VAMP2 (plasma membrane SNAREs), nor SNAP-29 (multiple compartments) were retained (unpublished data). In contrast, syntaxin-5 and GOS-28 were still retrieved (unpublished data). Therefore, p115/CC1 does not interact indiscriminately with SNAREs but interacts specifically with a subset of Golgi SNAREs, which form SNAREpins that contain syntaxin-5 as their common component (Hay et al., 1997, 1998; Parlati et al., 2000; Zhang and Hong, 2001).
Sly1p (a syntaxin-5 binding Sec1/Munc18 protein [Jahn, 2000]) was also retained on p115/CC1 beads but not on CC2, CC3, CC4, or mock beads and may correspond to the ∼66-kD protein visible by silver stain (Fig. 1, B and C). Giantin, GM130, and p115 were not retained by CC1-4 beads, suggesting these peptides do not retrieve molecules from the extract simply because they contain coiled-coil domains. The fact that α-SNAP was not retained even though α-SNAP interacts with the SNARE motif of syntaxin-1 (Jahn and Südhof, 1999) further illustrates the specificity of p115/CC1–SNARE interactions.
His–tail and acidic domain of p115 (TA) and His–tail domain of p115 (T; Fig. 1 A) were also used as affinity ligands. In corroboration, both retrieved exactly the same subset of Golgi SNAREs as p115/CC1 (Fig. 1 C), indicating that the globular head domain of p115 is not required for these interactions. p115 and His-TA but not His-T retrieved Giantin and GM130, reinforcing the importance of the acidic domain of p115 for these interactions. Thus, p115 appears to be a multivalent scaffold for both Golgins (Giantin and GM130) and SNAREs.
p115 stimulates the assembly of syntaxin-5 SNARE complexes
Since a common feature of the specific subset of SNAREs retrieved by p115 is that they constitute syntaxin-5 SNAREpins, we tested whether p115 promoted their assembly. To this end, Golgi membranes were salt washed to remove endogenous p115 and incubated with NSF to disassemble preexisting cis-SNARE complexes (Otto et al., 1997). NSF was then inactivated (using NEM), and the membranes were solubilized in Triton X-100 buffer, clarified, and incubated with increasing amounts of p115. GOS-28 or syntaxin-5 was then immunoprecipitated, and the extent of coprecipitation of other Golgi SNAREs was determined by immunoblot (Fig. 2, A–D).
Figure 2.
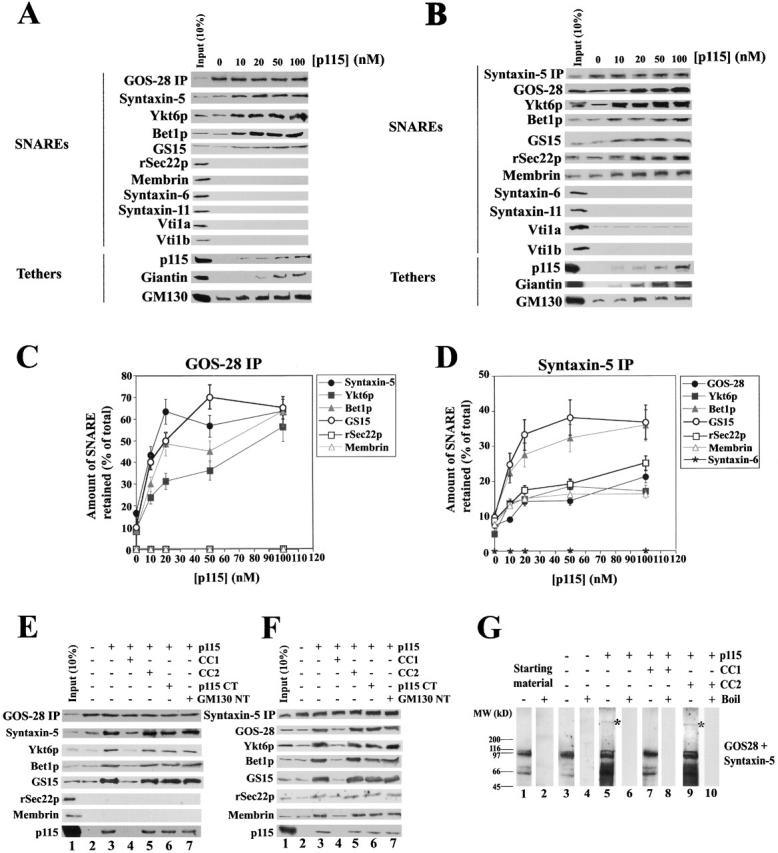
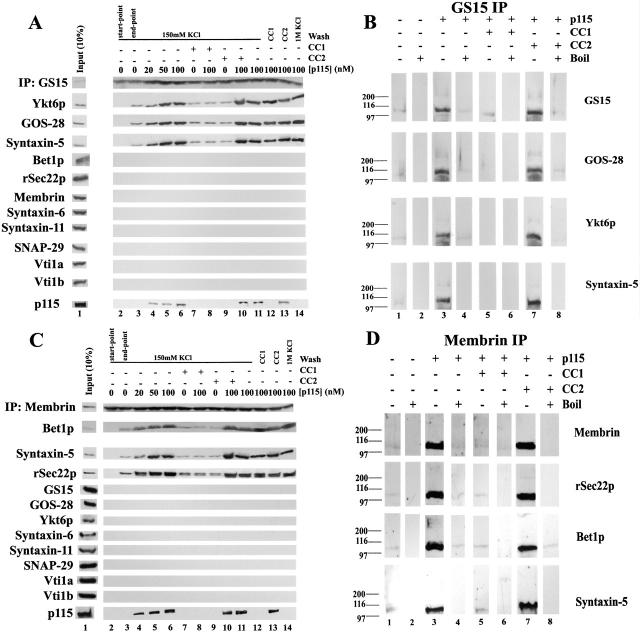
p115 stimulates specific SNARE complex formation in Golgi extracts. (A and B) Salt-washed RLGs that had been treated with NSF to disassemble cis-SNARE complexes were solubilized in Triton X-100 buffer and incubated with increasing concentrations of p115 (0–100 nM). GOS-28 (A) or syntaxin-5 (B) was immunoprecipitated, and the extent of coprecipitation of other Golgi SNAREs and tethers was determined by immunoblot. For the p115 immunoblot, the input (10%) lane reflects 10% of the maximum p115 concentration added (100 nM). (C and D) Quantitation of GOS-28 (C) and syntaxin-5 (D) immunoprecipitations. Amount of coprecipitated SNARE retained (% of total) as determined by densitometric scanning is plotted versus p115 (nM). Values represent means ± SEM (n = 3). (E and F) Golgi detergent extract (as in A) was incubated on ice with or without 100 nM p115 plus either buffer, 10 μM CC1 or CC2, 20 μM p115 CT (p115 COOH-terminal 75 aa), or GM130 NT (GM130 NH2-terminal 73 aa). GOS-28 (E) or syntaxin-5 (F) was immunoprecipitated, and the extent of coprecipitation of other Golgi SNAREs and p115 was determined by immunoblot. For the p115 immunoblot, the input (10%) lane reflects 10% of the maximum p115 concentration added (100 nM). (G) Salt-washed NSF-treated RLGs were incubated for 30 min at 37°C with or without 100 nM p115 plus or minus 10 μM CC1 or CC2. Reactions were stopped with SDS-PAGE sample buffer, incubated for 7 min at 25 or 95°C, and processed for immunoblot. Blots were probed with a mixture of anti–GOS-28 and anti–syntaxin-5 antibodies. Asterisk denotes a p115-induced high molecular weight species.
Increasing concentrations of p115 stimulated the coprecipitation of syntaxin-5, Ykt6p, Bet1p, and GS15 with GOS-28 from 10–15% up to 55–70% of total SNARE present (Fig. 2, A and C). In contrast, membrin, rSec22p, syntaxin-6, syntaxin-11, Vti1a, and Vti1b were excluded from these GOS-28–SNARE complexes (Fig. 2, A and C). Since four different SNAREs coprecipitate with GOS-28 and endomembrane SNAREpins are typically comprised of four SNAREs, this would suggest that p115 stimulates the formation of at least two different GOS-28 SNAREpins. Indeed, since only GOS-28, syntaxin-5, and Ykt6p coimmunoprecipitate with GS15 (see below) it may be that GS15–Ykt6p–GOS-28–syntaxin-5 form a SNAREpin. A potential Bet1p–Ykt6p–GOS-28–syntaxin-5 SNAREpin has also been observed (Zhang and Hong, 2001).
Similarly, increasing p115 concentrations enhanced the coprecipitation of GOS-28, Ykt6p, rSec22p, and membrin with syntaxin-5 from 5–10% to 15–20% of the total SNARE present, and GS15 and Bet1p from 10 to 35% (Fig. 2, B and D). The stimulated coprecipitation of rSec22p and membrin with syntaxin-5 suggests that p115 may also stimulate formation of the well-defined membrin–Bet1p–rSec22p–syntaxin-5 SNAREpin (Parlati et al., 2000; Xu et al., 2000). The specificity of this enhancement is reinforced by the lack of coprecipitation of syntaxin-6, syntaxin-11, or Vti1b (Fig. 2 B). Vti1a coprecipitated with syntaxin-5 at low levels (Fig. 2 B) consistent with a Vti1p–Sed5p complex at the cis-Golgi network in yeast that may function in retrograde vesicle transfer between vacuole and the cis-Golgi network (Fischer von Mollard and Stevens, 1999). However, Vti1a–syntaxin-5 complex formation was not enhanced by p115 (Fig. 2 B). This may suggest Vti1a–syntaxin-6 complexes are more prevalent in rat liver Golgi (Xu et al., 1998).
Identical results were obtained if apyrase was added after quenching the NEM with DTT (unpublished data). Apyrase acts to deplete any remaining ATP in the system. Therefore, p115 was not stimulating SNARE complex assembly by simply inhibiting any residual NSF activity that might remain after NEM treatment.
In both immunoprecipitations, low levels of p115 were present in the retrieved complexes (Fig. 2, A and B), and increasing p115 concentration caused increasing amounts of GM130 and Giantin to be coprecipitated (Fig. 2, A and B). Very similar results were obtained if MGFs were used (unpublished data). This emphasizes that p115 may simultaneously bridge GM130 to Giantin, while coordinating syntaxin-5 SNAREpin assembly.
Addition of CC1 peptide abrogated this enhancement of syntaxin-5–GOS-28 SNARE complex formation and blocked the coprecipitation of p115 (Fig. 2, E and F), suggesting that the SNARE motif-related region of p115 mediates the observed stimulation. In contrast, CC2, p115 CT (COOH-terminal 75 aa of p115, which binds to Giantin and GM130) and GM130 NT (NH2-terminal 73 aa of GM130, which binds p115) had no effect (Fig. 2, E and F).
Could p115 also stimulate SNARE complex formation on intact Golgi membranes? This was tested by assaying the formation of SNARE complexes that are preserved in SDS at room temperature. cis-SNARE complexes were disassembled on salt-washed Golgi membranes (as above) and incubated for 30 min at 37°C with or without p115. The extent of GOS-28–syntaxin-5 SDS-resistant complex formation was then determined (Otto et al., 1997). Several distinct GOS-28–syntaxin-5 SDS-resistant complexes were apparent in the starting material and present at the same level after incubation with buffer (Fig. 2 G). These complexes were preserved at 25°C but disassembled at 95°C (Fig. 2 G). p115 greatly increased the formation of these SDS-resistant complexes, and densitometry revealed this was a fourfold stimulation that was inhibited by addition of CC1 but not CC2 (Fig. 2 G). These effects were also found when Bet1p and Ykt6p but not syntaxin-6 SDS-resistant complexes were analyzed (unpublished data). Intriguingly, p115 caused the formation of a very slow migrating GOS-28–syntaxin-5 species (Fig. 2 G, asterisks) that was not immunoreactive to anti-p115 antibodies (unpublished data) and may represent SNAREpin oligomers. Collectively, these data demonstrate that p115 assembles Golgi–SNARE complexes that contain syntaxin-5 in either detergent solution or on native membranes, and this assembly is mediated by the SNARE motif-related domain (CC1) of p115.
p115 stimulates assembly of GS15–Ykt6p–GOS-28–syntaxin-5 and membrin–Bet1p–rSec22p–syntaxin-5 SNAREpins
To determine precisely which SNAREpins were assembled by p115, reactions were performed as in the preceding section except that either GS15 or membrin was immunoprecipitated (Fig. 3). Without incubation before the immunoprecipitation, no SNAREs coprecipitated with GS15 (Fig. 3 A, lane 2). After incubation, only three SNAREs coprecipitated with GS15, namely Ykt6p, GOS-28, and syntaxin-5, strongly suggesting that they comprise a SNAREpin. Formation of this complex was enhanced approximately fivefold by p115 (Fig. 3 A, lane 3 compared with 6). This stimulation was abolished by inclusion of CC1 (Fig. 3 A, lane 8) but not CC2 (Fig. 3 A, lane 10) in the reaction, strongly suggesting that the SNARE-motif like region of p115 drives this assembly. CC1 was not acting to prevent direct SNARE–SNARE interactions, since SNARE complexes alone (no added p115) assembled at the same background level in the presence or absence of CC1 (Fig. 3 A, lane 3 compared with 7). Therefore, CC1 inhibited only the p115-mediated stimulation of SNAREpin assembly. p115 also coprecipitated with the SNAREs (Fig. 3 A, lanes 4–6) but was not acting to simply link the SNAREs together, since it could be removed at the end of the immunoprecipitation by either a wash with 10 μM CC1 (lane 12) or 1 M KCl (lane 14; 10 μM CC2 [lane 13] and 150 mM KCl [lane 11] had no effect), without affecting the integrity of the retrieved SNARE complexes. This strongly indicates that p115 assembles SNAREpins but is not required to maintain them, since it can be removed without affecting SNAREpin integrity.
Figure 3.
p115 stimulates assembly of SNAREpins containing either GS15–Ykt6p–GOS-28–syntaxin-5 or membrin–Bet1p–rSec22p–syntaxin-5. (A) Salt-washed RLGs that had been treated with NSF–α-SNAP to disassemble cis-SNARE complexes were solubilized in Triton X-100 buffer and incubated for 0 (lane 2) or 60 min (lanes 3–14) on ice with increasing concentrations of p115 (0–100 nM) in the presence or absence of 10 μM CC1 or CC2. GS15 was immunoprecipitated, and retrieved beads were then either washed with Triton X-100 buffer (containing 150 mM KCl; lanes 2–11) or Triton X-100 buffer supplemented with 10 μM CC1 (lane 12), 10 μM CC2 (lane 13), or 1 M KCl (lane 14). Beads were eluted with SDS-PAGE sample buffer, and the extent of coprecipitation of other Golgi SNAREs and p115 was determined by immunoblot. For the p115 immunoblot, the input (10%) lane reflects 10% of the maximum p115 concentration added (100 nM). (B) SDS resistance of retrieved immunocomplexes. Experiments were performed as in A (using 100 nM p115) except that at the end of the immunoprecipitation beads were eluted with SDS-PAGE sample buffer and incubated at either 25 (lanes 1, 3, 5, and 7) or 95°C for 7 min (lanes 2, 4, 6, and 8). Each panel reflects a different strip of nitrocellulose probed with a different antibody (which is denoted on the far right). (C and D) Reactions were performed as in A and B except that membrin was immunoprecipitated.
To determine whether bona fide SNAREpins had formed, the SDS resistance of the complexes retrieved by GS15 immunoprecipitation was assessed. p115 greatly enhanced the formation of SDS-resistant complexes (Fig. 3 B, lane 1 compared with 3). These were immunoreactive to antibodies against GS15, GOS-28, Ykt6p, and syntaxin-5 but not other SNAREs. The major SDS-resistant complex was between 97–116 kD in size, approximately the correct molecular weight for a SNAREpin composed of GS15, Ykt6p, GOS-28, and syntaxin-5. These complexes disappeared upon boiling the sample (Fig. 3 B, even lanes), and their formation was inhibited by the inclusion of 10 μM CC1 (Fig. 3 B, lane 5) but not 10 μM CC2 (Fig. 3 B, lane 7) in the reaction. Together these data strongly suggest that via its SNARE motif-related domain p115 mediates the formation of a bona fide GS15–Ykt6p–GOS-28–syntaxin-5 SNAREpin. In the absence of p115, very little SNAREpin assembly occurs.
Essentially identical effects were observed when membrin was immunoprecipitated except that the three SNAREs that coprecipitate with membrin are Bet1p, rSec22p, and syntaxin-5, the components of a well-characterized SNAREpin (Fig. 3 C) (Hay et al., 1997, 1998; Parlati et al., 2000; Xu et al., 2000). Their assembly into SDS-resistant complexes was also enhanced greatly by p115 (Fig. 3 D) in a manner dependent on the SNARE motif-like domain of p115. Thus, p115 assembles at least two distinct SNAREpins comprising GS15–Ykt6p–GOS-28–syntaxin-5 and membrin–Bet1p–rSec22p–syntaxin-5 via its SNARE motif-related domain.
The SNARE motif-related region of p115 specifically inhibits NSF-driven Golgi reassembly
We next asked if the SNARE motif-related region of p115 plays a role in Golgi membrane fusion. p115 is absolutely required for NSF-catalyzed cisternal regrowth from isolated MGFs, where it stimulates COPI vesicle fusion by linking Giantin on COPI vesicles to GM130 on acceptor tubular remnants. CC1 was added to this reaction and inhibited cisternal regrowth by >90% (Fig. 4 A). The inhibition was dose dependent with an IC50 of 1.9 μM, an ∼14-fold molar excess over p115 (Fig. 4 A, inset). Even when added at an equimolar concentration to p115, CC1 reduced cisternal regrowth by ∼30%. This biphasic response to CC1 is indicative of multiple binding sites for CC1 on Golgi membranes and is consistent with the ability of CC1 to bind several Golgi SNAREs and Sly1p (Fig. 1 C). This effect was specific to CC1, since CC2, CC3, and CC4 alone or in combination had little effect (Fig. 4 A). Thus, the CC1 inhibition is not due to nonspecific effects of coiled-coils. The lack of effect of CC4 implies that the weak interactions between CC4 and Golgi SNAREs (Fig. 1 C) are functionally irrelevant, at least in this assay.
Figure 4.
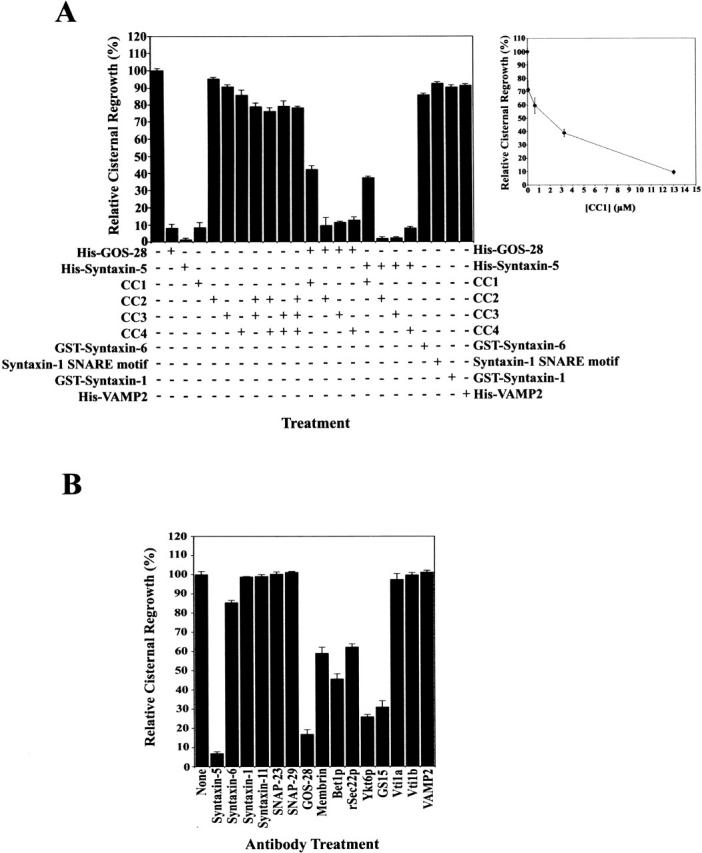
The SNARE motif-related region of p115 inhibits NSF-catalyzed Golgi reassembly. (A) MGFs were incubated at 37°C for 1 h with NSF, SNAPs, and p115 (130 nM) in the presence or absence of 13 μM of the indicated SNARE or peptide. CC2-4 peptides were also added in combination. CC1-4 were also preincubated with an equimolar amount of His–GOS-28 or His–syntaxin-5 and then added. Reactions were terminated by fixation, processed for EM, and the amount of relative cisternal regrowth was determined. 100% relative cisternal regrowth represents an increase from 25 to 75% of the total membrane present as cisternae. Values represent means ± SEM (n = 3–6). (Inset) Increasing amounts of CC1 were added to the NSF reaction. Values represent means ± SEM (n = 3). (B) MGFs were incubated at 37°C for 1 h with NSF, SNAPs, and p115 with or without various anti-SNARE antibodies. Reactions were processed as in A. Values represent means ± SEM (n = 3).
The cytoplasmic domains of GOS-28 and syntaxin-5 also greatly reduced cisternal regrowth (Fig. 4 A). In sharp contrast, the cytoplasmic domains of syntaxin-6, syntaxin-1, and VAMP2 had little effect, emphasizing the fidelity of fusion events in this system (Scales et al., 2000). Furthermore, the syntaxin-1 SNARE motif had no effect, suggesting that the CC1 inhibition is not simply due to its SNARE motif-related character. Rather, the effect of CC1 on Golgi fusion events was very specific as shown when soluble His–GOS-28 or His–syntaxin-5 was premixed before addition to the reaction with an equimolar amount of CC1. This pretreatment partially antagonized the soluble SNARE inhibition of cisternal regrowth, which recovered to ∼40% (Fig. 4 A). Contradistinctively, CC2-4 did not quench the inhibition (Fig. 4 A). Thus, the soluble SNAREs may inhibit the reaction in part by preventing p115 from binding to endogenous SNAREs. The fact that CC1 only partially counteracts this effect may indicate that the soluble SNAREs also sequester other essential factors such as endogenous SNAREs (Parlati et al., 2000). In total, these data demonstrate that the p115-mediated formation of syntaxin-5–GOS-28 SNAREpins is facilitated by the SNARE motif-related region of p115 (CC1) and is required for NSF-driven Golgi reassembly.
All of the Golgi SNAREs that interacted with p115 were required for NSF-driven Golgi reassembly as demonstrated by antibody inhibition (Fig. 4 B). Antibodies against GOS-28 and syntaxin-5 had the greatest effect, inhibiting cisternal regrowth by ≥85% (Fig. 4 B). Antibodies against membrin, Bet1p, rSec22p, Ykt6p, and GS15 inhibited by 40–75% (Fig. 4 B). Other anti-SNARE antibodies (against syntaxin-6, syntaxin-11, Vti1a, Vti1b, syntaxin-1, SNAP-23, SNAP-29, and VAMP2) had no effect on reassembly. Although antibodies rather than Fab fragments were used in these experiments, they provide corroborative evidence that those SNAREs interacting with p115 are also involved in Golgi reassembly (Fig. 4 B).
The SNARE motif-related domain of p115 does not disrupt Giantin-p115-GM130 tethers
How Giantin-p115-GM130 tether formation was related to the p115-mediated stimulation of syntaxin-5–GOS-28 SNAREpin formation was unclear. Was it possible that CC1 was blocking reassembly by disrupting Giantin-p115-GM130 tethers rather than p115–SNARE interactions? This seemed improbable, since CC1 beads retained neither GM130, Giantin, nor p115 from Golgi detergent extract (Fig. 1 C). CC1 did not bind to purified p115 directly either (unpublished data). However, to test this further Golgi membranes were solubilized and incubated with or without p115. Giantin was immunoprecipitated, and the extent of coprecipitation of GM130 and p115 was determined. GM130 only coprecipitated with Giantin in the presence of p115 (Fig. 5 A). This linking of Giantin to GM130 was effectively abolished by GM130 NT (Fig. 5 A) but was unaffected by CC1 (Fig. 5 A). This strongly suggests that CC1 does not inhibit reassembly by disrupting Giantin-p115-GM130 tethers.
Figure 5.
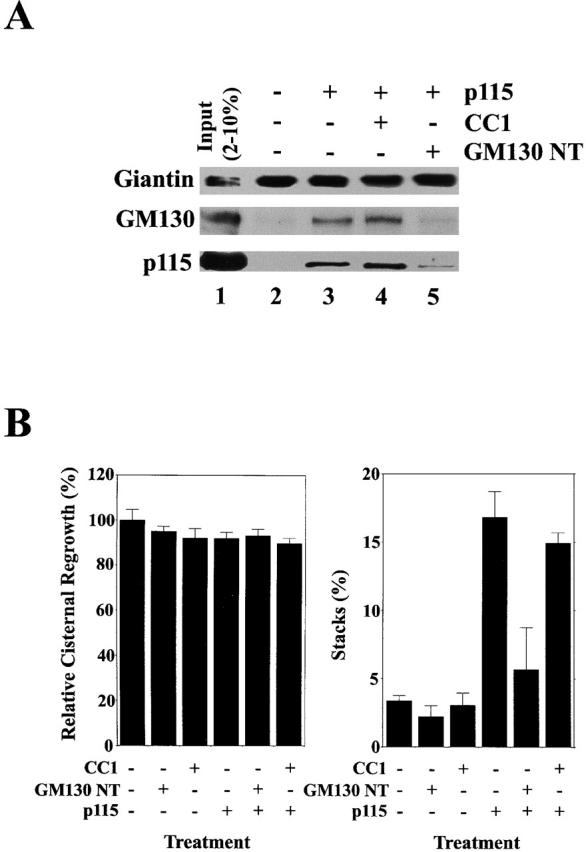
The SNARE motif-related domain of p115 does not disrupt Giantin-p115-GM130 tethers. (A) RLGs were dissolved in Triton X-100 buffer and incubated for 1 h on ice in either the presence or absence of p115 (250 nM) plus or minus 50 μM CC1 or GM130 NT. Giantin was then immunoprecipitated, and coprecipitation of GM130 and p115 was determined by immunoblot. The input lane reflects 2% total p115 input and 10% total GM130 and Giantin input. (B) MGFs were incubated at 37°C for 1 h with p97/p47 plus or minus p115 (130 nM). 13 μM CC1 or GM130 NT was added to some reactions. Reactions were terminated by fixation, processed for EM, and the amount of relative cisternal regrowth was determined. 100% relative cisternal regrowth represents an increase from 25 to 75% of the total membrane present as cisternae. The percentage total membrane present as stacks was also determined. Values represent means ± SEM (n = 2).
Giantin-p115-GM130 tethers are also required for the initial stacking of Golgi cisternae, but not for cisternal regrowth, during p97-catalyzed reassembly (Shorter and Warren, 1999). Therefore, we tested whether CC1 had any effect on p115-stimulated cisternal stacking in p97-driven reassembly. This would also control for whether CC1 was inhibiting reassembly due to some nonspecific effect on the membranes. Neither CC1 nor GM130 NT had any effect on p97-driven cisternal regrowth, whereas GM130 NT but not CC1 inhibited p115-induced stacking (Fig. 5 B). Thus, it seems likely that both Giantin-p115-GM130 tethers and p115-stimulated GOS-28–syntaxin-5 complexes are necessary for NSF-driven reassembly.
p115 assembles GOS-28–syntaxin-5 complexes directly via its SNARE motif-related domain
To resolve the role of p115 in SNAREpin formation more acutely, purified SNARE–p115 binding assays were established. Recombinant GST- or His-tagged SNAREs were expressed and purified from Escherichia coli. All SNAREs used were purified to ∼95% homogeneity as judged by Coomassie staining (unpublished data).
We focused on GOS-28 and syntaxin-5, since they were the most active SNAREs in Golgi reassembly (Fig. 4, A and B). The interaction between p115 and the purified cytoplasmic domains of syntaxin-5 or GOS-28 was direct and inhibited by ∼85% with CC1 but not with CC2 or CC3 (Fig. 6 A). This inhibition implies that p115 binds the two SNAREs via CC1, the SNARE motif-related region. Binding was specific for these SNAREs, since p115 did not bind His–VAMP-2, GST–syntaxin-1 (Fig. 6 B), or GST–syntaxin-6 (unpublished data). Furthermore, His-TA and His-T could bind His–GOS-28 and GST–syntaxin-5 (Fig. 6 B) as could CC1 (unpublished data). His–head domain of p115 (H; Fig. 6 B), CC2, and CC3 (unpublished data) did not bind purified SNAREs, suggesting they are not required for p115–SNARE interactions. In fact, p115 bound to GST–syntaxin-5, with an apparent K d of 1.8 μM (Fig. 6 C), and His–GOS-28, with an apparent K d of 1.5 μM (Fig. 6 D). Addition of CC1 at a fixed concentration of 13 μM greatly reduced the amount of p115 that bound to either SNARE (Fig. 6, C and D). Thus, CC1 may inhibit NSF-driven Golgi reassembly by reducing the probability of specific and productive p115–SNARE interactions.
Figure 6.
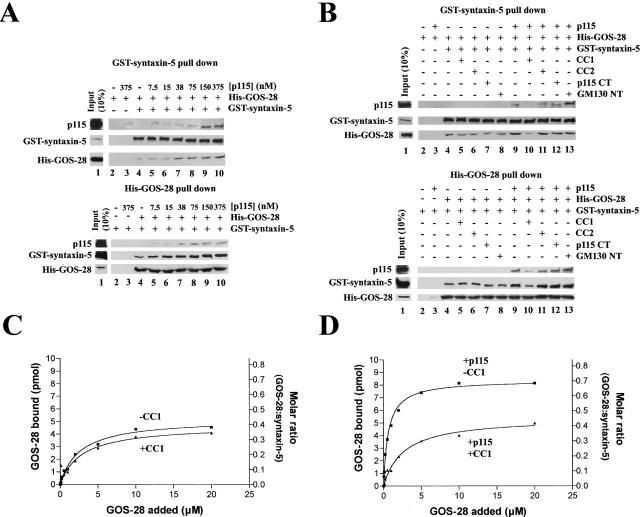
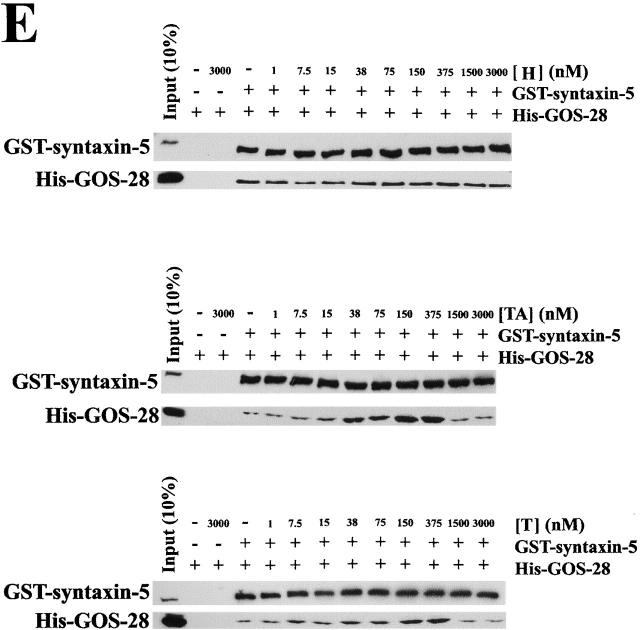
p115 binds GOS-28 and syntaxin-5 directly. (A) His–syntaxin-5 (0.4 μM; top) or His–GOS-28 (0.5 μM; bottom) was incubated for 1 h on ice with p115 (0.13 μM) plus or minus 13 μM CC1, CC2, or CC3. SNAREs were retrieved with Ni-NTA agarose. p115 alone was the control. Bound proteins were processed for immunoblot. (B) p115, H, TA, or T (0.38 μM) was incubated for 1 h on ice with either His–GOS-28, His-VAMP2, GST–syntaxin-5, or GST–syntaxin-1 (20 nM). His–GOS-28 and His-VAMP2 were then immunoprecipitated with specific antibodies. GST–syntaxin-5 and GST–syntaxin-1 were retrieved with glutathione-sepharose and processed as in A. (C and D) GST–syntaxin-5 (12 pmol; C) or His–GOS-28 (12 pmol; D) was incubated for 1 h on ice with increasing concentrations of p115 (0–20 μM) plus or minus CC1 (13 μM). SNAREs were retrieved, and bound proteins were processed for immunoblot. The amount of p115 bound was determined by densitometry. Datapoints represent means (n = 3). Binding isotherms were fitted to obtain apparent K d estimates.
To monitor the stimulation of GOS-28–syntaxin-5 complex formation by p115, His–GOS-28 was mixed with GST–syntaxin-5 at equimolar concentration and incubated with increasing concentrations of p115. At the end of the incubation, either GST–syntaxin-5 or His–GOS-28 was retrieved, and the extent of coprecipitation of the cognate SNARE and p115 was determined. Background binding was low (Fig. 7 A), and in the absence of p115 there was only a weak interaction between His–GOS-28 and GST–syntaxin-5. p115 stimulated the interaction fourfold and coprecipitated with the retrieved SNAREs (Fig. 7 A).
Figure 7.
p115 stimulates complex formation between His–GOS-28 and GST–syntaxin-5. (A) GST–syntaxin-5 (75 nM) was incubated for 1 h on ice with His–GOS-28 (75 nM) and increasing concentrations of p115 (0–375 nM). GST–syntaxin-5 (top) or His–GOS-28 (bottom) was retrieved. Controls omitted the SNARE to be retrieved plus or minus p115. Bound proteins were processed for immunoblot. For the p115 immunoblot, the input (10%) lane reflects 10% of the maximum p115 concentration added (375 nM). (B) GST–syntaxin-5 (75 nM) was incubated for 1 h on ice with His–GOS-28 (75 nM) in the presence or absence of p115 (150 nM) with or without 15 μM CC1 or CC2, 30 μM p115 CT, or GM130 NT. GST–syntaxin-5 (top) or His–GOS-28 (bottom) was retrieved. Controls omitted the SNARE to be retrieved plus or minus p115. Reactions were processed as in A. Note the longer exposure time required to detect the coprecipitated SNARE (His–GOS-28, top, and GST–syntaxin-5, bottom). (C and D) GST–syntaxin-5 (12 pmol) was incubated for 1 h on ice with increasing concentrations of His–GOS-28 (0–20 μM) in the presence or absence of CC1 (20 μM), plus (D) or minus (C) p115 (0.2 μM). GST–syntaxin-5 was retrieved. Reactions were processed as in A. The amount of His–GOS-28 bound was determined by densitometry. Datapoints represent means (n = 3), and binding isotherms were fitted. (E) Reactions were performed as in A except p115 was replaced with His-H, His-TA, or His-T (0–3 μM).
CC1 specifically attenuated this stimulation (Fig. 7 B), whereas CC2, p115 CT, or GM130 NT had no effect (Fig. 7 B). CC1 also reduced p115 coprecipitation with the SNAREs (Fig. 7 B). CC1 was not acting to directly block the His–GOS-28–GST–syntaxin-5 interaction since equal amounts of cognate SNARE coprecipitated with or without CC1 in the absence of p115 (Fig. 7 B). These effects were specific for cognate SNARE pairs, since p115 did not promote nor was any interaction detected between noncognate pairs (GST–syntaxin-6–His–GOS-28, GST–syntaxin-6–His–syntaxin-5, GST–syntaxin-1–His–syntaxin-5, GST–syntaxin-1–His–GOS-28, His-VAMP2–biotinylated His–GOS-28, GST–syntaxin-5–His–VAMP-2, or GST/His–GOS-28 [unpublished data]). p115 linked neither His–GOS-28 to biotinylated His–GOS-28 nor GST–syntaxin-5 to His–syntaxin-5, implying that the p115–SNARE tether must be asymmetric (unpublished data).
Next, we added increasing concentrations of His–GOS-28 to a fixed amount of GST–syntaxin-5 in the presence or absence of CC1. Up to 0.4 moles of His–GOS-28 bound per mole of GST–syntaxin-5, and CC1 had very little effect on this amount, suggesting that CC1 did not interfere with GOS-28–syntaxin-5 binding directly (Fig. 7 C). Addition of p115 increased the amount of His–GOS-28 that bound to GST–syntaxin-5, especially at low GOS-28 concentrations and up to a maximum of 0.7 moles/mole, suggesting that it acts to increase the efficiency of GOS-28 binding to syntaxin-5 (Fig. 7 D). This effect was abolished by inclusion of CC1 (Fig. 7 D), illustrating that CC1 inhibited the stimulation of GOS-28–syntaxin-5 binding by p115 and not GOS-28–syntaxin-5 binding itself.
In corroboration, His-H did not stimulate His–GOS-28–GST–syntaxin-5 binding, whereas His-TA and His-T stimulated binding just as well as p115 (Fig. 7 E). Addition of His-TA or His-T to very high levels actually inhibited the binding of His–GOS-28 to GST–syntaxin-5 (Fig. 7 E). Such inhibition is diagnostic of a tethering event, since very high concentrations of the linking protein will simply suppress formation of any ternary complex at the expense of binary complexes.
p115 catalyzes SNARE assembly and is not required to maintain SNARE complexes
Was p115 simply linking His–GOS-28 to GST–syntaxin-5 or stimulating a direct interaction between them? To make this distinction, we adopted three independent approaches. First, we sought a condition that releases p115 from His–GOS-28–GST–syntaxin-5. If p115 simply links the two SNAREs, then such release should disrupt the His–GOS-28–GST–syntaxin-5 interaction. If His–GOS-28 and GST–syntaxin-5 were interacting directly, then p115 release should not disrupt the complex, providing the His–GOS-28–GST–syntaxin-5 interaction was resistant to the condition that released p115. Hence, binding experiments were performed (Fig. 7 A), except that after GST–syntaxin-5 retrieval complexes were challenged with different washes to try and release p115. p115–SNARE complexes were disrupted by 1 M KCl (Fig. 8 A) or 2 M urea (unpublished data). Strikingly, upon p115 release His–GOS-28 remained bound to GST–syntaxin-5 at the same level as in control washes (Fig. 8 A). Similarly, a 20 μM CC1 wash released p115 without affecting the level of His–GOS-28 retained (Fig. 8 A). This was specific for CC1, since CC2 did not release p115 (Fig. 8 A). Corroboratively, if endogenous GS15 or membrin-containing SNARE complexes were immunoprecipitated from Golgi detergent extracts and challenged with 1 M KCl or 10 μM CC1, p115 was released without affecting SNARE complex integrity (Fig. 3, A and C, compare lanes 11, 12, and 14). Moreover, p115 stimulated formation of multiple GOS-28–syntaxin-5 SDS-resistant complexes on Golgi membranes (Fig. 2 G and Fig. 3, B and D) that do not contain p115 (unpublished data). In total, these results imply that p115 stimulates the formation of GOS-28–syntaxin-5 complexes but is not required to maintain them.
Figure 8.
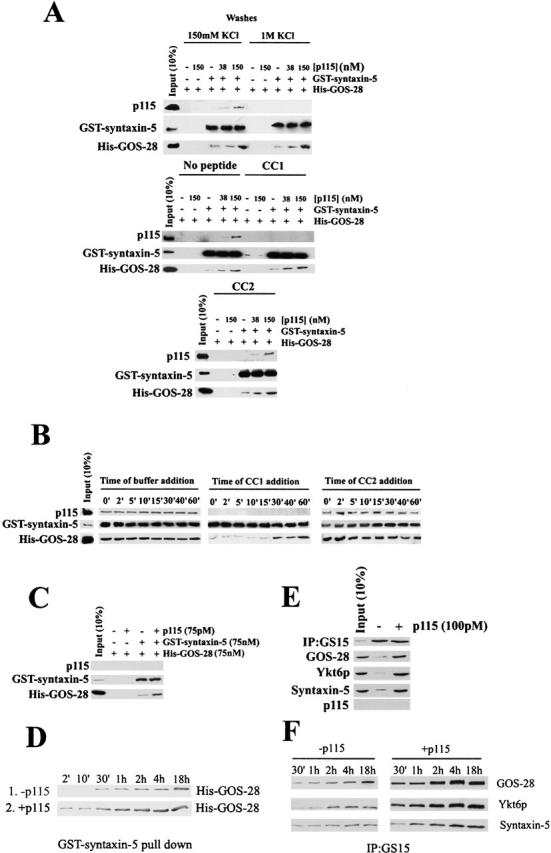
p115 catalyzes SNARE assembly and is not required to maintain SNARE complexes. (A) GST–syntaxin-5 (75 nM) was incubated for 1 h on ice with His–GOS-28 (75 nM) and p115 (0–150 nM). GST–syntaxin-5 was retrieved. His–GOS-28 alone or plus p115 served as controls. Beads were washed with either buffer containing 150 mM KCl, 1 M KCl, 20 μM CC1, or CC2. Bound proteins were processed for immunoblot. (B) GST–syntaxin-5 (75 nM) was incubated on ice with His–GOS-28 (75 nM) and p115 (150 nM). At various times during the incubation, the reaction was supplemented with buffer, 15 μM CC1, or CC2. After 1 h, GST–syntaxin-5 was retrieved, and bound proteins were processed for immunoblot. (C) GST–syntaxin-5 (75 nM) was incubated with His–GOS-28 (75 nM) and p115 (75 pM) for 4 h at 4°C with agitation. Reactions were processed as in B. (D) GST–syntaxin-5 (75 nM) was bound to glutathione beads and incubated with His–GOS-28 (75 nM) plus or minus p115 (75 pM) for various times ranging from 2 min to 18 h. Beads were recovered, and the amount of His–GOS-28 binding was determined by immunoblot. (E) Salt-washed RLGs that had been treated with NSF–α-SNAP to disassemble cis-SNARE complexes were solubilized in Triton X-100 buffer and incubated for 4 h on ice plus or minus p115 (100 pM). GS15 was immunoprecipitated, and retrieved beads were then washed with Triton X-100 buffer. Beads were eluted with SDS-PAGE sample buffer, and the extent of coprecipitation of other Golgi SNAREs and p115 was determined by immunoblot. (F) Reactions were performed as in E in the presence or absence of p115 (100 pM), and the incubation time varied from 30 min to 18 h.
Second, the temporal sensitivity of His–GOS-28–GST–syntaxin-5 binding to CC1 (Fig. 8 B) was determined. His–GOS-28–GST–syntaxin-5 binding assays with p115 were supplemented with buffer, 20 μM CC1, or CC2 at various times. After 1 h, GST–syntaxin-5 was retrieved, and the coprecipitation of His–GOS-28 and p115 was determined. His–GOS-28 and p115 coprecipitation was insensitive to buffer or CC2 at any time (Fig. 8 B). In contrast, both p115 and His–GOS-28 coprecipitation were inhibited if CC1 was added within the first 15 min. At time points later than 15 min, His–GOS-28 coprecipitation became insensitive to added CC1, whereas p115 was still released (Fig. 8 B). Supplementing reactions with 1 M KCl instead of 20 μM CC1 gave very similar results (unpublished data). Thus, one possibility is that a GOS-28–p115–syntaxin-5 interaction (0–15 min, CC1 sensitive) precedes and enhances a subsequent and direct GOS-28–syntaxin-5 interaction (15 min and later, CC1 insensitive) that does not require p115 to maintain it.
Finally, if p115 is a bona fide catalyst for His–GOS-28–GST–syntaxin-5 binding, then it should not be consumed by the reaction. Thus, very low substoichiometric levels of p115 should stimulate the reaction. Hence, His–GOS-28–GST–syntaxin-5 binding was conducted with a p115 concentration three orders of magnitude lower than the SNARE concentration. To detect any effect, the incubation time was increased to 4 h. Even at levels below detection by immunoblot, p115 catalyzed the formation of His–GOS-28–GST–syntaxin-5 complexes (Fig. 8 C). His–GOS-28 was incorporated into the complex in amounts that were at least 20-fold greater than the total amount of p115 present. Kinetic analysis of His–GOS-28–GST–syntaxin-5 binding revealed that this substoichiometric level of p115 greatly enhanced the initial rate of GOS-28–syntaxin-5 binding (Fig. 8 D). This suggests that p115 catalyzes SNARE assembly.
These findings were verified with endogenous Golgi SNAREs (Fig. 8 E). Golgi membranes were salt washed to remove endogenous p115 and incubated with NSF to disassemble preexisting cis-SNARE complexes (Otto et al., 1997). NSF was then inactivated (using NEM), and the membranes were solubilized in Triton X-100 buffer, clarified, and incubated with 100 pM p115. GS15 was immunoprecipitated, and the coprecipitation of cognate SNAREs was determined by immunoblot. With the use of recombinant SNAREs as standards, we estimated that rat liver Golgi membranes (RLGs) contain ∼2 ng/μg Golgi protein of GOS-28 and ∼4 ng/μg Golgi protein syntaxin-5 (unpublished data). Thus, the endogenous SNAREs are present in the reaction at nM concentrations (∼14 nM GOS-28 and 22 nM syntaxin-5) compared with at most pM p115 concentrations. This substoichiometric level of p115 was sufficient to stimulate the formation of the GS15–Ykt6p–GOS-28–syntaxin-5 SNARE complex (Fig. 8 E). Furthermore, kinetic analysis revealed that substoichiometric p115 enhanced the initial rate of GS15–Ykt6p–GOS-28–syntaxin-5 SNAREpin assembly (Fig. 8 F). Thus, p115 catalyzes SNARE assembly.
Sequence of events in NSF-driven Golgi reassembly
To determine the functional sequence of p115–Golgin and p115–SNARE interactions during reassembly, a range of inhibitors was screened (Fig. 9 B) and the morphology of the reaction products generated determined (Fig. 9, A and C). From the morphology, these inhibitors were grouped into two classes: those that inhibited fusion generating dispersed tubules/vesicles and those that inhibited fusion generating clustered tubules/vesicles.
Figure 9.
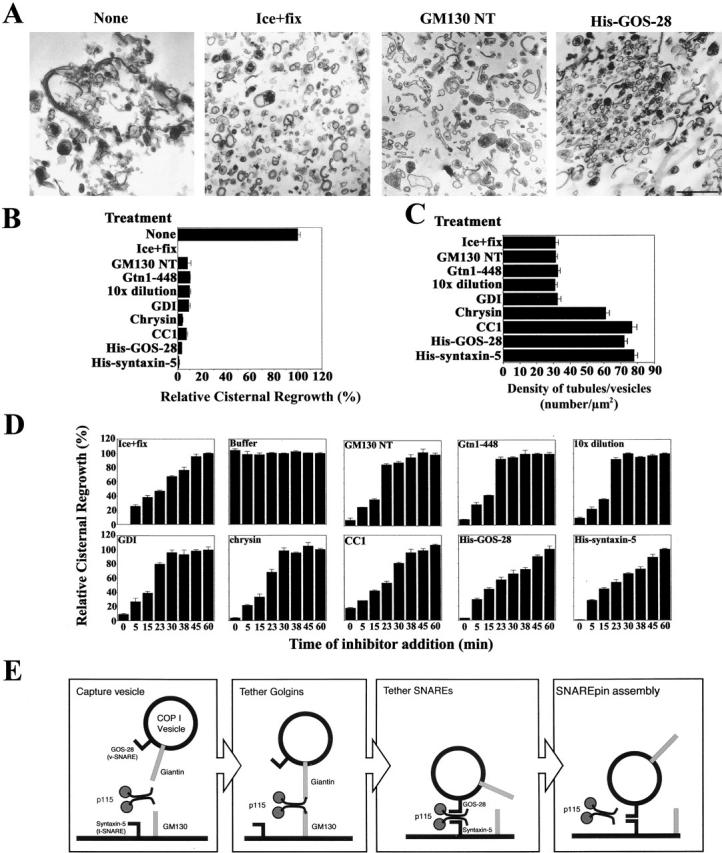
Resolution of vesicle tethering and SNARE assembly during NSF-driven Golgi reassembly. (A) Morphology of NSF reaction products in the presence of various inhibitors. Note the presence of stacks of cisternae in the absence of inhibitor compared with dispersed tubules and vesicles in the MGFs (ice + fix) or in the presence of GM130 NT, and the highly clustered tubules and vesicles in the presence of His–GOS-28. Bar, 0.5 μm. (B) MGFs were incubated at 37°C for 1 h with NSF, SNAPs, and p115 (0.13 μM). Various inhibitors were added: GM130 NT (13 μM), Gtn1-448 (13 μM), GDI (14 μM), chrysin (120 μM), CC1 (13 μM), His–GOS-28 (6 μM), or His–syntaxin-5 (6 μM). Dilution (10×) was with reaction buffer containing NSF, SNAPs, and p115. Reactions were terminated by fixation, processed for EM, and the amount of relative cisternal regrowth was determined. 100% relative cisternal regrowth represents an increase from 25 to 75% of the total membrane present as cisternae. Values represent means ± SEM (n = 3). (C) Quantitation of density of tubules/vesicles per μm2 for the reactions described in B. (D) Kinetic sensitivity of NSF reaction to various inhibitors. NSF reactions were performed as in B except that at the indicated times reactions were either stopped by fixation or treated as indicated and incubated for a total of 1 h at 37°C. Reactions were processed as in B. Values represent means ± SEM (n = 4). (E) A model depicting the proposed sequence of events during GOS-28–syntaxin-5 complex assembly in NSF-driven Golgi reassembly (see Discussion).
The first class of inhibitor included reagents that disrupt the Giantin-p115-GM130 tether, such as GM130 NT and Gtn1-448 (Giantin NH2-terminal 448 aa), which compete for p115 binding to GM130 and Giantin (Nakamura et al., 1997; Lesa et al., 2000). The morphology generated by these inhibitors was highly congruent to that of MGFs (Fig. 9, A and C, Ice + fix), where the Giantin-p115-GM130 tether is mitotically disrupted. Similarly, a 10-fold dilution and GDI induced this morphology (Fig. 9 C). GDI extracts Rab-GDP and the Rab effector p115 from membranes (unpublished data; Cao et al., 1998), implying that a Rab-GTPase controls Giantin-p115-GM130 tether formation. Collectively, these data suggest that failure to form Giantin-p115-GM130 tethers results in a population of dispersed tubules and vesicles.
The second class of inhibitor induced a population of clustered tubules/vesicles. These inhibitors increased the density of tubules/vesicles more than twofold (Fig. 9 C) and includes chrysin (Fig. 9 B), an inhibitor of a Golgi-associated CKII-like kinase that phosphorylates p115. p115 phosphorylation is required for reassembly and strengthens the Giantin-p115-GM130 tether (Dirac-Svejstrup et al., 2000). This may elicit a transition from tethering to SNAREpin formation. CC1, soluble GOS-28 (Fig. 9 A), and syntaxin-5 also had this effect (Fig. 9 C) as did anti–GOS-28 and anti–syntaxin-5 antibodies (unpublished data). Since these reagents do not disrupt Giantin-p115-GM130 tethers, it would seem that the fragments are aligned in preparation for fusion by this tether. However, fusion may not occur because productive SNAREpins do not form.
To examine this possibility, the kinetic sensitivity of NSF-driven reassembly to each inhibitor was determined. Thus, various inhibitors were added to NSF-driven reassembly at designated times and allowed to proceed for a total time of 1 h (Fig. 9 D). Alternatively, reactions were terminated by fixation or treated with buffer. This mode of enquiry determines the latest stage of the reaction that is sensitive to each inhibitor. Once the reaction acquires resistance to an inhibitor, this implies the target of the inhibitor has completed its function. For example, an inhibitor which blocks only at early time points affects a target required at early stages of the process. In contrast, an inhibitor that blocks at all time points affects a target required at a terminal phase of the process. Thus, a putative sequence of events can be discerned (Ungermann et al., 1998).
Termination by fixation revealed that reassembly proceeds with approximately linear kinetics for the first 45 min (Fig. 9 D). The reaction was completely insensitive to added buffer (Fig. 9 D). GM130 NT, Gtn1-448 (Giantin NH2-terminal 448 aa), 10-fold dilution, and GDI only inhibited the reaction if added within the incipient 15 min (Fig. 9 D). At later time points, these inhibitors were impotent, implying the reaction had moved to a stage beyond the initial formation of Giantin-p115-GM130 tethers. This is consistent with the kinetic sensitivity of cisternal stacking to GM130 NT (Shorter and Warren, 1999). The reaction remained sensitive to chrysin for slightly longer, as appreciable inhibition occurred at 23 min (Fig. 9 D). CC1 inhibited the reaction up to 30 min (Fig. 9 D), implying that p115-catalyzed GOS-28–syntaxin-5 pairing is required up to this stage. In contrast, soluble GOS-28 and syntaxin-5 effectively stopped reassembly whenever they were added (Fig. 9 D), implicating the SNAREs in a terminal phase of membrane fusion. Ultimately, these data suggest that p115 consecutively links Golgins then SNAREs, which leads to SNAREpin assembly and membrane fusion during Golgi reassembly.
Discussion
Herein we provide the first biochemical evidence that p115 catalyzes the specific assembly of cognate SNARE complexes during NSF-driven Golgi reassembly and by extension during interphase Golgi vesicle transport. A SNARE motif-related domain of p115 (CC1) in isolation or in the context of p115, TA, or T specifically and efficiently retrieves a subset of SNAREs from Golgi detergent extract, namely syntaxin-5, GOS-28, membrin, Ykt6p, rSec22p, Bet1p, and GS15, and the syntaxin-5 binding Sec1/Munc18 protein, Sly1p. A common feature of this subset is that they form SNAREpins that contain syntaxin-5 as their common determinant. In addition, these p115-interacting SNAREs are necessary for NSF-driven Golgi reassembly. GOS-28 and syntaxin-5 immunoprecipitation patterns suggested that p115 catalyzes the assembly of at least three distinct endogenous Golgi SNAREpins likely containing the following: (a) GS15–Ykt6p–GOS-28–syntaxin-5, (b) Bet1p–Ykt6p–GOS-28–syntaxin-5 (Zhang and Hong, 2001), and (c) membrin–Bet1p–rSec22p–syntaxin-5 (Sapperstein et al., 1996; Parlati et al., 2000). Immunoretrieval of GS15 or membrin confirmed that p115 catalyzed the assembly of two distinct SDS-resistant SNAREpins composed of GS15–Ykt6p–GOS-28–syntaxin-5 and membrin– Bet1p–rSec22p–syntaxin-5. This facility may help explain p115 involvement in multiple transport steps between the ER and medial Golgi (Waters et al., 1992; Cao et al., 1998). p115 stimulated assembly of GOS-28–syntaxin-5 SDS-resistant complexes on native Golgi membranes, a property that correlates with the membrane fusion activity of SNAREs (Chen et al., 1999). The SNARE assembly activity of p115 was essential for NSF-driven Golgi reassembly, since a synthetic CC1 peptide potently inhibited p115-mediated SNARE assembly and cisternal regrowth. This inhibition of reassembly could be antagonized if CC1 was preincubated with syntaxin-5 or GOS-28. This vividly suggests that interactions between the SNARE motif-related region of p115 and the SNAREs, GOS-28 and syntaxin-5, participate in NSF-mediated Golgi reassembly.
The assembly of cognate topologically correct SNAREpins (Parlati et al., 2000) is likely a highly orchestrated process entailing the interdependent sequential assembly of SNARE monomers or oligomers to form a four-helical bundle (Xu et al., 2000; Chen et al., 2001; Fasshauer et al., 2002). Using a minimal system, we focused on the interaction between the well-defined cognate SNARE pair GOS-28 and syntaxin-5 (Hay et al., 1997, 1998; Zhang and Hong, 2001), a likely step in the assembly of GOS-28–syntaxin-5 SNAREpins. These were the two most active SNAREs in Golgi reassembly, emphasizing the physiological significance of their interaction. p115 stimulated GOS-28–syntaxin-5 binding, and this effect was abolished by CC1 peptide. In the absence of p115, CC1 had no effect on the amount of GOS-28–syntaxin-5 binding, despite its ability to bind to both SNAREs individually. Thus, CC1 was not affecting Golgi reassembly by blocking SNARE pairing but by inhibiting specific and productive p115–SNARE interactions.
p115 was not required to maintain endogenous or purified SNARE complexes, since it could be released at the end of the reaction without affecting SNARE complex integrity. Thus, the endpoint of the reaction is not p115 tethering the SNAREs together. The assembly of GOS-28–syntaxin-5 complexes by p115 traverses two kinetic phases. The initial phase is sensitive to CC1 and 1 M KCl, whereas the terminal phase is not. One possibility is that p115 stimulates GOS-28–syntaxin-5 binding by first linking the SNAREs together and then allowing them to interact directly. In this way, p115 may enhance GOS-28–syntaxin-5 binding by stabilizing an early and otherwise labile reaction intermediate. This putative SNARE tethering role for p115 may help explain why very high concentrations of TA and T actually inhibited GOS-28–syntaxin-5 complex formation. Such a response is symptomatic of tethering because a large excess of the linking protein will inhibit ternary complex formation by sequestering the proteins to be linked in binary complexes. In addition, it may help explain why CC1 alone did not stimulate GOS-28–syntaxin-5 interactions. This is because CC1 is largely monomeric in contrast to p115, TA, and T that as dimers can link the two SNAREs together. Finally, p115 stimulated the rate of assembly of GOS-28–syntaxin-5 complexes at concentrations three orders of magnitude lower than the SNAREs, making it highly improbable that p115 was linking the SNAREs together in a final complex. Since p115 enhances the rate of SNAREpin assembly and is not consumed by the process, it is a catalyst in the purest sense.
It may be that p115 helps to relieve a hysteresis associated with SNARE complex assembly (Barrick and Hughson, 2002; Fasshauer et al., 2002). That is, p115 may relieve a kinetic barrier between the disassembled and assembled states (Baker and Agard, 1994). In the absence of p115, the SNAREs may be kinetically trapped and cannot assemble into SNAREpins on a biologically relevant time scale. Thus, we were unable to derive a dissociation constant for the His–GOS-28–GST–syntaxin-5 interaction in the absence of p115, since the reaction was likely not at equilibrium (Fig. 7 C). This was only revealed by the fact that p115 stimulated the amount of GOS-28–syntaxin-5 complex that formed in this time frame (Fig. 7 D) and that p115 was acting catalytically, since it was not required to maintain the final GOS-28–syntaxin-5 complex. p115 may help reduce the activation energy required for SNAREpin assembly and therefore enhance assembly rates, perhaps by stabilizing an early and otherwise unstable reaction intermediate. A similar function may be performed by complexin in the assembly of syntaxin-1–SNAP-25–VAMP SNAREpins (Tokumaru et al., 2001).
Both the SNAREpin assembly activity of p115 and Giantin-p115-GM130 tethers are required for NSF-driven reassembly. Agents that selectively disrupt either p115 activity prevent reassembly. Importantly, CC1 had no effect on Giantin-p115-GM130 tether formation, and GM130 NT had no effect on p115-induced SNARE assembly, thus providing a means to discriminate between these two events. The differential kinetic sensitivity of reassembly to various inhibitors resolved these two consecutive functions of p115. Based on these kinetic data, we propose a sequence of events during NSF-driven Golgi reassembly (Fig. 9 E). First, a COPI vesicle is attached to its acceptor membrane via Giantin-p115-GM130 tethers (GM130 NT, Giantin NH2-terminal 448 aa, 10-fold dilution sensitive). This event is coordinated by a Rab-GTPase (GDI sensitive), possibly Rab1, which interacts with p115 and GM130 (Allan et al., 2000; Moyer et al., 2001; Weide et al., 2001) or Rab2, Rab6, or Rab33b, which interact with GM130 (Short et al., 2001; Valsdottir et al., 2001). Second, p115 phosphorylation is required (chrysin sensitive). Thereafter, p115 may tether GOS-28 to syntaxin-5 (CC1 sensitive) and promote their direct tight interaction during SNAREpin assembly that initiates bilayer mixing (soluble SNARE sensitive).
p115 might promote assembly by incorporating GOS-28 as the unitary v-SNARE into the SNAREpin (Fig. 9 E). Alternatively, this may be in the construction of the three-component t-SNARE on the acceptor membrane, which may be the rate-limiting step in SNAREpin assembly (Fasshauer et al., 2002). Since no membranes are present in many of the binding reactions we performed, the configuration of the complexes may be more akin to cis-SNARE complexes. However, we prefer the former model because syntaxin-5 is enriched on mitotic cisternal/tubular remnants and GOS-28 on COPI vesicles (unpublished data; Orci et al., 2000). Whether other factors are required downstream of SNAREs for bilayer mixing remains unresolved (Peters et al., 2001). The next step is to test the predictions of this model in vivo.
When viewed in this light, SNAREs may be seen as simply short tethers that once assembled into SNAREpins catalyze or signal for membrane fusion. Conversely, one may view the Golgins as extended SNAREs that evolved for the specialized function of long range vesicle capture. We envision p115 to play a pivotal role in membrane docking by gradually bringing the COPI vesicle closer to its target via these successive interactions. First, Giantin-p115-GM130 tethers mediate long range COPI vesicle capture. Upon capture, p115 phosphorylation may fasten the Giantin-p115-GM130 tether (Dirac-Svejstrup et al., 2000). This would enable p115 to engage the cognate SNAREs GOS-28 and syntaxin-5, thus catalyzing SNAREpin assembly and membrane fusion. The fact that p115, GM130, and Giantin coimmunoprecipitate with both GOS-28 and syntaxin-5 (Fig. 2, A and B) suggests they may be components of a large tethering complex.
p115 also contributes to the specificity of vesicle transfer, since it did not promote noncognate SNARE interactions and only interacted with SNAREs required for NSF-driven Golgi reassembly. Furthermore, p115 did not link GOS-28 or syntaxin-5 to themselves. It may be that p115 bound to GOS-28 is restricted to a conformation that is only able to bind syntaxin-5 and not another GOS-28 molecule. Therefore, the p115–SNARE tether must be asymmetric in nature. A similar situation may exist for Giantin–p115–GM130 interactions. Thus, binding of one SNARE to p115 transmits or encodes specificity to any subsequent p115–SNARE interaction. In this way, p115 may form part of the syntax that ensures that only cognate topologically correct SNAREpins will assemble and so enhances vesicle transfer specificity.
The characteristics of CC1 that are related to the SNARE motif appear to be maintained in the Drosophila and yeast p115 homologues. Thus, this may be a conserved function of p115. Intriguingly, the globular heads of p115 seem to play no direct role in p115-catalyzed SNAREpin assembly or Giantin-p115-GM130 tether formation (Dirac-Svejstrup et al., 2000), despite containing some of the most conserved parts of the molecule (Sapperstein et al., 1995). Corroboratively, His-TA and His-T retrieve the entire complement of p115-interacting SNAREs from Golgi detergent extract, bind to GOS-28 and syntaxin-5 directly, and can promote GOS-28–syntaxin-5 complex formation. Furthermore, His-TA can replace p115 in NSF-driven Golgi reassembly, but reassembly only proceeds with ∼60% efficiency (Dirac-Svejstrup et al., 2000). Thus, the head domains of p115 may regulate or enhance tail function, perhaps by binding to a Rab-GTPase. In contrast, His-T cannot support NSF-driven Golgi reassembly, reinforcing the importance of the Giantin-p115-GM130 tether in this process (unpublished data).
We can now perceive a sophisticated regulatory network that physically couples the successive phenomena of vesicle tethering, docking, and fusion in the mammalian Golgi apparatus. Central to this network is p115, which executes consecutive linkages, joining first the long tethers, Giantin, and GM130, and then the short tethers, GOS-28 and syntaxin-5, as part of cognate SNAREpin assembly. Thus, p115 ineluctably guides COPI vesicles into contact with their correct final destination for cargo delivery.
Materials and methods
Proteins and peptides
Recombinant proteins were expressed in E. coli and purified with Ni-NTA agarose or glutathione-sepharose. Recombinant SNAREs lacked their transmembrane domain. p115 and p97 were purified from rat liver (Rabouille et al., 1998). p115 and His–GOS-28 were biotinylated with NHS-LC-biotin (Pierce Chemical Co.).
p115 CT, GM130 NT (Dirac-Svejstrup et al., 2000), CC1 (aa 637–699 of rat p115), CC2 (aa 728–765 of rat p115), CC3 (aa 783–827 of rat p115), and CC4 (aa 843–930 of rat p115) were synthesized as NH2-terminally biotinylated peptides (Lovering et al., 1993). Superdex-75 gel filtration, laser light scattering (Dirac-Svejstrup et al., 2000), and native gel analyses revealed that CC1, CC2, CC3, and CC4 were mostly monomeric (∼70%) but with dimeric (∼20%) and tetrameric (∼10%) subpopulations.
Antibodies
Monoclonal antibodies used in this study were against p115 (G. Waters, Princeton University, Princeton, NJ), Giantin (H.P. Hauri, University of Basel, Basel, Switzerland), GOS-28, α-SNAP, syntaxin-6, syntaxin-11, GM130, GS15, Vti1a, Vti1b, Bet1p (Transduction Labs), membrin (Stressgen), syntaxin-1 (G. Schiavo, Cancer Research UK), and hexahistidine (Amersham Pharmacia Biotech). Rabbit polyclonals used in this study were against Sly1p (W. Balch, The Scripps Research Institute, La Jolla, CA), syntaxin-6 (F. Wendler, Cancer Research UK), Rab1, Rab6 (Santa Cruz Biotechnology, Inc.), GOS-28, membrin, rSec22p, Bet1p (J. Rothman, Memorial Sloan Kettering Cancer Center, New York, NY), Ykt6p (W. Hong, Institute of Molecular and Cell Biology, Singapore), SNAP-23, SNAP-29, VAMP2 (Synaptic Systems), GST (Sigma-Aldrich), p115 (M. Lowe, University of Manchester, Manchester, UK), Giantin (L. Pelletier, Cancer Research, UK), and syntaxin-5 (A. Price, Cancer Research UK).
Affinity chromatography from Golgi detergent extracts
Biotinylated p115, CC1-4 were coupled to Neutravidin beads (Pierce Chemical Co.) at 10 μM. 20 μg RLGs (Shorter and Warren, 1999) were extracted for 15 min at 4°C with 200 μl Triton X-100 buffer (20 mM Hepes-KOH, pH 7.3, 200 mM KCl, 5 mM magnesium acetate, 0.1 mM DTT, 0.5% Triton X-100). A Triton X-100 extract of rat liver postnuclear supernatant was also used. Extracts were clarified by centrifugation (14,000 rpm, 10 min, 4°C) and incubated for 1 h at 4°C with 10 μl p115, CC1-4, or mock (no protein) beads, His-TA (0.5 μM), or His-T (0.5 μM). His-TA/T were retrieved at the end of the incubation with Ni-NTA agarose. Recovered beads were washed with Triton X-100 buffer and eluted with SDS-PAGE sample buffer. Eluates were fractionated by SDS-PAGE and silver stained or processed for immunoblot.
Immunoprecipitations
Anti–GOS-28, antimembrin, or anti-GS15 monoclonal antibodies were covalently coupled to Affigel-10 (Bio-Rad Laboratories), and anti–syntaxin-5 polyclonals were covalently coupled to protein A–sepharose with DMP (Pierce Chemical Co.). RLGs/MGFs were resuspended at 0.2 mg/ml in 1 M KCl buffer (25 mM Hepes-KOH, pH 7.3, 1 M KCl, 5 mM magnesium acetate, 0.2 M sucrose, 0.1 mM DTT) for 2 min at 4°C and recovered by centrifugation (10,000 rpm, 30 min, 4°C). Membranes (1 mg/ml) were incubated for 30 min at 37°C in the same buffer (except with 60 mM KCl, 2 mM ATP) with NSF (1.3 μM), α-SNAP (0.7 μM), γ-SNAP (0.7 μM), and an ATP regeneration system (Rabouille et al., 1998). NEM (2.5 mM) was added for 5 min at 4°C followed by DTT (5 mM) for 5 min at 4°C. In some experiments, apyrase (5 U/ml; Sigma-Aldrich) was then added. Membranes (0.2 mg/ml) were extracted in Triton X-100 buffer for 15 min at 4°C and incubated for 30 min at 4°C with 0–100 nM p115 plus or minus 10 μM CC1, CC2, 20 μM p115 CT, or GM130 NT. In kinetic experiments, this incubation time was varied from 30 min to 18 h. 10 μl anti–GOS-28-Affigel, antimembrin-Affigel, anti–GS15-Affigel, or anti–syntaxin-5 protein A–sepharose was then applied for 30 min at 4°C. Washed beads were eluted with SDS-PAGE sample buffer minus reducing agents. Eluates were separated from beads, supplemented with 3.3% (vol/vol) β-mercaptoethanol and processed for immunoblot. Giantin was immunoprecipitated as in Dirac-Svejstrup et al. (2000).
SDS-resistant complexes
1 M KCl-extracted RLGs were treated with NSF and SNAPs as above. After NEM treatment, membranes were incubated for 30 min at 37°C plus or minus p115 (100 nM) with or without 10 μM CC1 or CC2. SDS-resistant complex formation was monitored as in Otto et al. (1997).
Golgi reassembly assay
Golgi reassembly was performed as in Shorter and Warren (1999). Treatments were as indicated in the figure legends. Tubule/vesicle density was determined as in Nagahama et al. (1996).
SNARE–p115 binding reactions
p115 was incubated for 1 h on ice with SNARE(s) in binding buffer (BB; 20 mM Hepes-KOH, pH 7.3, 150 mM KCl, 2 mM MgCl2, 30 mM histidine, 5% glycerol, 0.5% Triton X-100, 0.1 mM DTT, 0.5 mg/ml STI) as indicated in the figure legends. SNAREs were recovered via their tags, and beads were washed with BB and processed for immunoblot. In some reactions, beads were washed with BB plus either 1 M KCl, 2 M urea, or 20 μM CC1 or CC2. In kinetic experiments, GST–syntaxin-5 was coupled to glutathione-sepharose before addition to the reaction. GST–syntaxin-5 beads were incubated with His–GOS-28 and retrieved at various times (2 min to 18 h).
To estimate the apparent K d of p115–SNARE interactions, the amounts of p115 or His–GOS-28 bound (pmol) were determined by densitometry with reference to p115 or His–GOS-28 standard curves (0.1–20 pmol) using NIH image. Means (n = 3) were fitted with binding isotherms to obtain apparent K d estimates using Prism 3 software (Graphpad).
Acknowledgments
We are indebted to H. Meyer, S. Maday, J. Malsam, A. Price, L. Pelletier, A. Satoh, D. Sheff, I. Mellman, and the entire Warren/Mellman group for discussion and support; J. Müller, G. Lesa, F. Wendler, M. Lowe, G. Schiavo, N. O'Reilly, J. Rothman, G. Waters, W. Hong, W. Balch, and H.P. Hauri for generous provision of reagents; R. Watson, M. Pypaert, and K. Murphy for help with EM; and E. Folta-Stogniew for light scattering.
This work was funded by National Institutes of Health grant GM60478-01.
Footnotes
Abbreviations used in this paper: aa, amino acids; CC1-4, coiled-coil domain 1–4 of p115; COP, coat protein; Gtn1-448, Giantin NH2-terminal 448 aa; H, head domain of p115; MGF, mitotic Golgi fragment; RLG, rat liver Golgi membrane; T, tail domain of p115; TA, tail and acidic domain of p115.
References
- Allan, B.B., B.D. Moyer, and W.E. Balch. 2000. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 289:444–448. [DOI] [PubMed] [Google Scholar]
- Baker, D., and D.A. Agard. 1994. Kinetics versus thermodynamics in protein folding. Biochemistry. 33:7505–7509. [DOI] [PubMed] [Google Scholar]
- Barrick, D., and F.M. Hughson. 2002. Irreversible assembly of membrane fusion machines. Nat. Struct. Biol. 9:78–80. [DOI] [PubMed] [Google Scholar]
- Cao, X., N. Ballew, and C. Barlowe. 1998. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 17:2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.A., S.J. Scales, S.M. Patel, Y.C. Doung, and R.H. Scheller. 1999. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 97:165–174. [DOI] [PubMed] [Google Scholar]
- Chen, Y.A., S.J. Scales, and R.H. Scheller. 2001. Sequential SNARE assembly underlies priming and triggering of exocytosis. Neuron. 30:161–170. [DOI] [PubMed] [Google Scholar]
- Dirac-Svejstrup, A.B., J. Shorter, M.G. Waters, and G. Warren. 2000. Phosphorylation of the vesicle-tethering protein p115 by a casein kinase II–like enzyme is required for Golgi reassembly from isolated mitotic fragments. J. Cell Biol. 150:475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer, D., W. Antonin, V. Subramaniam, and R. Jahn. 2002. SNARE assembly and disassembly exhibit a pronounced hysteresis. Nat. Struct. Biol. 9:144–151. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard, G., and T.H. Stevens. 1999. The S. cerevisiae v-SNARE Vti1p is required for multiple membrane transport pathways to the vacuole. Mol. Biol. Cell. 10:1719–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, J.C., D.S. Chao, C.S. Kuo, and R.H. Scheller. 1997. Protein interactions regulating vesicle transport between the ER and Golgi apparatus in mammalian cells. Cell. 89:149–158. [DOI] [PubMed] [Google Scholar]
- Hay, J.C., J. Klumperman, V. Oorschot, M. Steegmaier, C.S. Kuo, and R.H. Scheller. 1998. Localization, dynamics, and protein interactions reveal distinct roles for ER and Golgi SNAREs. J. Cell Biol. 141:1489–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn, R. 2000. Sec1/Munc18 proteins: mediators of membrane fusion moving to center stage. Neuron. 27:201–204. [DOI] [PubMed] [Google Scholar]
- Jahn, R., and T.C. Südhof. 1999. Membrane fusion and exocytosis. Annu. Rev. Biochem. 68:863–911. [DOI] [PubMed] [Google Scholar]
- Lesa, G.M., J. Seemann, J. Shorter, J. Vandekerckhove, and G. Warren. 2000. The amino-terminal domain of the Golgi protein Giantin interacts directly with the vesicle-tethering protein p115. J. Biol. Chem. 275:2831–2836. [DOI] [PubMed] [Google Scholar]
- Linstedt, A.D. 1999. Stacking the cisternae. Curr. Biol. 9:R893–R896. [DOI] [PubMed] [Google Scholar]
- Linstedt, A.D., and H.P. Hauri. 1993. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell. 4:679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering, R., I.M. Hanson, K.L. Borden, S. Martin, N.J. O'Reilly, G.I. Evan, D. Rahman, D.J. Pappin, J. Trowsdale, and P.S. Freemont. 1993. Identification and preliminary characterization of a protein motif related to the zinc finger. Proc. Natl. Acad. Sci. USA. 90:2112–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, M., C. Rabouille, N. Nakamura, R. Watson, M. Jackman, E. Jamsa, D. Rahman, D.J. Pappin, and G. Warren. 1998. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 94:783–793. [DOI] [PubMed] [Google Scholar]
- Martinez-Menarguez, J.A., R. Prekeris, V.M. Oorschot, R. Scheller, J.W. Slot, H.J. Geuze, and J. Klumperman. 2001. Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport. J. Cell Biol. 155:1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride, H.M., V. Rybin, C. Murphy, A. Giner, R. Teasdale, and M. Zerial. 1999. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 98:377–386. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., F. Parlati, R. Fukuda, R.J. Johnston, K. Paz, F. Paumet, T.H. Söllner, and J.E. Rothman. 2000. a. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 407:153–159. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., T. Weber, F. Parlati, R.J. Johnston, T.J. Melia, T.H. Söllner, and J.E. Rothman. 2000. b. Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J. Cell Biol. 150:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer, B.D., B.B. Allan, and W.E. Balch. 2001. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic. 2:268–276. [DOI] [PubMed] [Google Scholar]
- Nagahama, M., L. Orci, M. Ravazzola, M. Amherdt, L. Lacomis, P. Tempst, J.E. Rothman, and T.H. Söllner. 1996. A v-SNARE implicated in intra-Golgi transport. J. Cell Biol. 133:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, N., C. Rabouille, R. Watson, T. Nilsson, N. Hui, P. Slusarewicz, T.E. Kreis, and G. Warren. 1995. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131:1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, N., M. Lowe, T.P. Levine, C. Rabouille, and G. Warren. 1997. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell. 89:445–455. [DOI] [PubMed] [Google Scholar]
- Orci, L., M. Ravazzola, A. Volchuk, T. Engel, M. Gmachl, M. Amherdt, A. Perrelet, T.H. Söllner, and J.E. Rothman. 2000. Anterograde flow of cargo across the Golgi stack potentially mediated via bidirectional “percolating” COPI vesicles. Proc. Natl. Acad. Sci. USA. 97:10400–10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto, H., P.I. Hanson, and R. Jahn. 1997. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc. Natl. Acad. Sci. USA. 94:6197–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati, F., J.A. McNew, R. Fukuda, R. Miller, T.H. Söllner, and J.E. Rothman. 2000. Topological restriction of SNARE-dependent membrane fusion. Nature. 407:194–198. [DOI] [PubMed] [Google Scholar]
- Pelham, H. 2001. SNAREs and the specificity of membrane fusion. Trends Cell Biol. 11:99–101. [DOI] [PubMed] [Google Scholar]
- Peters, C., M.J. Bayer, S. Buhler, J.S. Andersen, M. Mann, and A. Mayer. 2001. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 409:581–588. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S.R. 1999. Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1:E17–E22. [DOI] [PubMed] [Google Scholar]
- Puthenveedu, M.A., and A.D. Linstedt. 2001. Evidence that Golgi structure depends on a p115 activity that is independent of the vesicle tether components Giantin and GM130. J. Cell Biol. 155:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille, C., H. Kondo, R. Newman, N. Hui, P. Freemont, and G. Warren. 1998. Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell. 92:603–610. [DOI] [PubMed] [Google Scholar]
- Sapperstein, S.K., D.M. Walter, A.R. Grosvenor, J.E. Heuser, and M.G. Waters. 1995. p115 is a general vesicular transport factor related to the yeast ER to Golgi transport factor Uso1p. Proc. Natl. Acad. Sci. USA. 92:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein, S.K., V.V. Lupashin, H.D. Schmitt, and M.G. Waters. 1996. Assembly of the ER to Golgi SNARE complex requires Uso1p. J. Cell Biol. 132:755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales, S.J., Y.A. Chen, B.Y. Yoo, S.M. Patel, Y.C. Doung, and R.H. Scheller. 2000. SNAREs contribute to the specificity of membrane fusion. Neuron. 26:457–464. [DOI] [PubMed] [Google Scholar]
- Seemann, J., E.J. Jokitalo, and G. Warren. 2000. a. The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol. Biol. Cell. 11:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann, J., E. Jokitalo, M. Pypaert, and G. Warren. 2000. b. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature. 407:1022–1026. [DOI] [PubMed] [Google Scholar]
- Short, B., and F.A. Barr. 2002. Membrane traffic: exocyst III makes a family. Curr. Biol. 12:R18–R20. [DOI] [PubMed] [Google Scholar]
- Short, B., C. Preisinger, R. Korner, R. Kopajtich, O. Byron, and F.A. Barr. 2001. A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J. Cell Biol. 155:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter, J., and G. Warren. 1999. A role for the vesicle tethering protein, p115, in the post-mitotic stacking of reassembling Golgi cisternae in a cell-free system. J. Cell Biol. 146:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen, B., M. Lowe, T. Levine, E. Jamsa, B. Dirac-Svejstrup, and G. Warren. 1998. A role for giantin in docking COPI vesicles to Golgi membranes. J. Cell Biol. 140:1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumaru, H., K. Umayahara, L.L. Pellegrini, T. Ishizuka, H. Saisu, H. Betz, G.J. Augustine, and T. Abe. 2001. SNARE complex oligomerization by synaphin/complexin is essential for synaptic vesicle exocytosis. Cell. 104:421–432. [DOI] [PubMed] [Google Scholar]
- Ungermann, C., K. Sato, and W. Wickner. 1998. Defining the functions of trans-SNARE pairs. Nature. 396:543–548. [DOI] [PubMed] [Google Scholar]
- Valsdottir, R., H. Hashimoto, K. Ashman, T. Koda, B. Storrie, and T. Nilsson. 2001. Identification of rabaptin-5, rabex-5, and GM130 as putative effectors of rab33b, a regulator of retrograde traffic between the Golgi apparatus and ER. FEBS Lett. 508:201–209. [DOI] [PubMed] [Google Scholar]
- Waters, M.G., D.O. Clary, and J.E. Rothman. 1992. A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J. Cell Biol. 118:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide, T., M. Bayer, M. Koster, J.P. Siebrasse, R. Peters, and A. Barnekow. 2001. The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep. 2:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimbs, T., K. Mostov, S.H. Low, and K. Hofmann. 1998. A model for structural similarity between different SNARE complexes based on sequence relationships. Trends Cell Biol. 8:260–262. [DOI] [PubMed] [Google Scholar]
- Weimbs, T., S.H. Low, S.J. Chapin, K.E. Mostov, P. Bucher, and K. Hofmann. 1997. A conserved domain is present in different families of vesicular fusion proteins: a new superfamily. Proc. Natl. Acad. Sci. USA. 94:3046–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, D., A.P. Joglekar, A.L. Williams, and J.C. Hay. 2000. Subunit structure of a mammalian ER/Golgi SNARE complex. J. Biol. Chem. 275:39631–39639. [DOI] [PubMed] [Google Scholar]
- Xu, Y., S.H. Wong, T. Zhang, V.N. Subramaniam, and W. Hong. 1997. GS15, a 15-kilodalton Golgi SNARE homologous to rbet1. J. Biol. Chem. 272:20162–20166. [DOI] [PubMed] [Google Scholar]
- Xu, Y., S.H. Wong, B.L. Tang, V.N. Subramaniam, T. Zhang, and W. Hong. 1998. A 29-kilodalton Golgi SNARE (Vti1-rp2) implicated in protein trafficking in the secretory pathway. J. Biol. Chem. 273:21783–21789. [DOI] [PubMed] [Google Scholar]
- Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107–117. [DOI] [PubMed] [Google Scholar]
- Zhang, T., and W. Hong. 2001. Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in ER-Golgi transport. J. Biol. Chem. 276:27480–27487. [DOI] [PubMed] [Google Scholar]