Abstract
The neural cell adhesion molecule (NCAM) has been reported to stimulate neuritogenesis either via nonreceptor tyrosine kinases or fibroblast growth factor (FGF) receptor. Here we show that lipid raft association of NCAM is crucial for activation of the nonreceptor tyrosine kinase pathway and induction of neurite outgrowth. Transfection of hippocampal neurons of NCAM-deficient mice revealed that of the three major NCAM isoforms only NCAM140 can act as a homophilic receptor that induces neurite outgrowth. Disruption of NCAM140 raft association either by mutation of NCAM140 palmitoylation sites or by lipid raft destruction attenuates activation of the tyrosine focal adhesion kinase and extracellular signal–regulated kinase 1/2, completely blocking neurite outgrowth. Likewise, NCAM-triggered neurite outgrowth is also completely blocked by a specific FGF receptor inhibitor, indicating that cosignaling via raft-associated kinases and FGF receptor is essential for neuritogenesis.
Keywords: neurite outgrowth; NCAM; lipid rafts; palmitoylation; FGF receptor
Introduction
Neurite outgrowth is a major event in neural development being mediated by several members of the immunoglobulin superfamily of cell adhesion molecules, among them the neural cell adhesion molecule (NCAM).* NCAM is expressed on the surface of almost all neural cells throughout the central and peripheral nervous system. It plays a pivotal role in early brain development, synaptic plasticity, and memory consolidation (Schachner, 1997; Ronn et al., 1998) and promotes neurite outgrowth upon homophilic or heterophilic engagement with other molecules on adjacent cell surfaces and in the extracellular matrix (for review see Crossin and Krushel, 2000). This implicates NCAM both as a ligand and signal transducing receptor. Of the three major isoforms of NCAM (NCAM180, NCAM140, and NCAM120, named according to their apparent molecular masses), all have been found to serve as neuritogenic ligands (Doherty et al., 1989, 1990) due to their identical extracellular domains.
First attempts to elucidate the molecular events underlying NCAM-mediated neuritogenesis have attributed a fundamental role to the fibroblast growth factor (FGF) receptor (Williams et al., 1995). Overexpression of a truncated FGF receptor-1 with a deleted kinase domain inhibited neurite outgrowth of PC12 cells when cultured on NCAM-presenting fibroblasts (Saffell et al., 1997). The ability of NCAM to promote neurite outgrowth was suggested therefore to depend solely on the interaction between the extracellular domains of NCAM and the FGF receptor. According to this concept, the interaction of NCAM with the FGF receptor activates the receptor tyrosine kinase with subsequent activation of PLCγ. As a final consequence, Ca2+ influx into the neurons results in neurite growth.
The view that the FGF receptor may not be the only mediator of NCAM-dependent signal transduction was indicated by data showing that NCAM-dependent neurite outgrowth is impaired in cultured neurons from mice deficient in the nonreceptor tyrosine kinase fyn (Beggs et al., 1994). Moreover, immunoprecipitation studies revealed an association of NCAM140 but not NCAM180 with fyn (Beggs et al., 1997). According to this model, NCAM clustering at the cell surface induces fyn phosphorylation with further recruitment of the focal adhesion kinase (FAK) to the NCAM–fyn complex. Activation of downstream kinases by this complex is then thought to be the initial step in NCAM-mediated neurite outgrowth.
Although the two signaling mechanisms would appear distinct at first sight, it is conceivable that both pathways could be operant in cells expressing NCAM isoforms as receptors for neurite outgrowth. The possibility also needs to be considered that activation of these signaling pathways could depend on the particular localization of the individual NCAM isoforms in different membrane subcompartments at the cell surface. Based on the observation that the intracellular domains of NCAM140 can be palmitoylated at four cysteine residues adjacent to the transmembrane domain (Little et al., 1998), we asked whether localization to cholesterol-rich membrane microdomains, the so-called lipid rafts, or detergent-resistant microdomains could play a major role in signal transduction mediated by NCAM. These lipid rafts reveal high concentrations of cholesterol and sphingoglycolipids and thereby a higher resistance toward nonionic detergents such as Triton X-100 than other membrane compartments. Several studies have illuminated the involvement of lipid rafts in signaling of hematopoietic cells (Brown and London, 1998), and there is growing evidence that transition of cellular receptors into rafts can enhance downstream signaling (Cinek and Horejsi, 1992; Tansey et al., 2000). Since these microdomains are enriched in potent effectors of signal transduction (e.g., src family kinases and growth-associated protein [GAP]-43) (Simons and Ikonen, 1997), the accumulation of NCAM receptors in these microdomains might regulate downstream signaling in a particular manner. Therefore, we asked whether raft localization of particular NCAM isoforms was essential for downstream signaling leading to neurite outgrowth. In parallel, we intended to elucidate the relationship between NCAM-mediated signaling via lipid rafts and signal transduction via the FGF receptor that has been demonstrated to be present outside of rafts at the cell surface (Davy et al., 2000).
Results
All NCAM isoforms are present in lipid raft microdomains
Since NCAM140 and NCAM180 share the same palmitoylation sites in sequences located intracellularly close to the plasma membrane, we asked whether both isoforms can be present in lipid rafts. We used brain homogenates and two cell lines, neuroblastoma cells expressing the three major isoforms of NCAM and NCAM-negative CHO cells that were used for transfection with the NCAM isoforms.
Insolubility in cold nonionic detergents and flotation on sucrose density gradients are the well-established criteria for identification of lipid raft-associated proteins (Hooper, 1997). Using flotation gradients, we determined NCAM isoform localization in the Triton X-100–insoluble low density membrane fractions (raft associated) and in the insoluble high density fraction (probably cytoskeleton associated).
In the homogenate of forebrains of 2-d-old mice, all three main NCAM isoforms could be detected in the top fractions of the flotation gradient (Fig. 1 A, top, lanes 3 and 4), confirming an association of the NCAM isoforms with low density lipids in raft microdomains. A large fraction of NCAM molecules in the raft fractions appeared to be polysialylated, since a broad band could be detected extending from 180 kD to higher apparent molecular weights. The nonreceptor tyrosine kinase fyn (Fig. 1 A, middle), which has been described as raft associated (van't Hof and Resh, 1997) and the FAK (Fig. 1 A, bottom) were also found in the low density fractions of the gradient, thus codistributing NCAM with a potential signaling effector in the lipid raft fraction.
Figure 1.
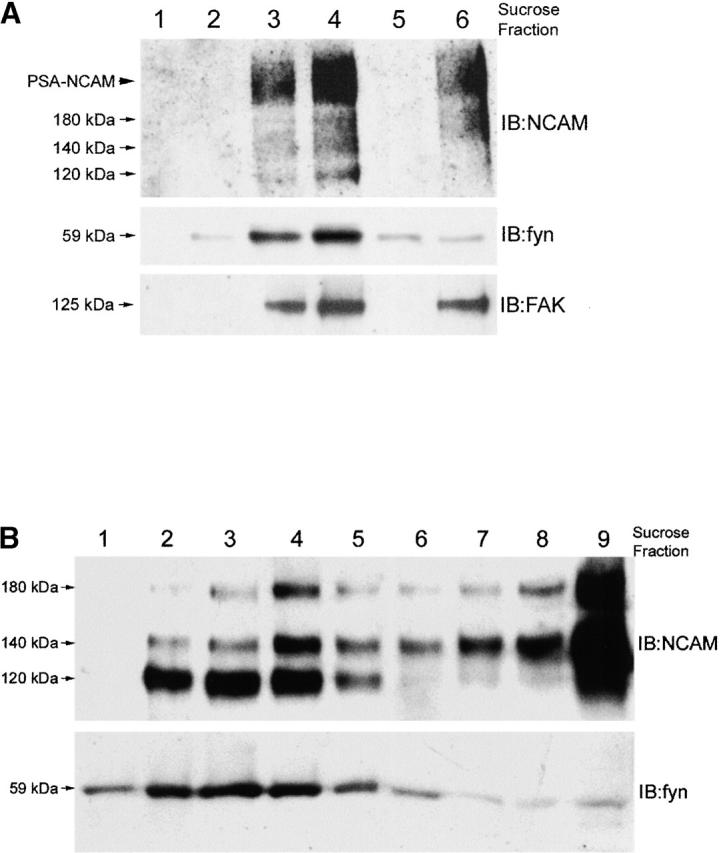
NCAM is present in detergent-resistant membrane fractions separation of Triton X-100–insoluble proteins by sucrose gradient fractionation. (A) Immunoblot analysis of homogenates of forebrain. NCAM isoforms are present in the Triton X-100–insoluble low density fractions (lanes 3 and 4) representing the lipid raft fraction and in the Triton X-100–insoluble high density fraction (lane 6), possibly containing cytoskeleton-associated proteins (top). The raft-associated nonreceptor tyrosine kinase fyn (middle) and the focal adhesion kinase FAK (bottom) colocalize with NCAM in the low density lipid raft fractions. (B) Immunoblot analysis of neuroblastoma cells. NCAM140 and NCAM180 are present in the Triton X-100–insoluble low density fractions (lanes 2–4) representing the lipid raft fraction and in the Triton X-100 insoluble high density fraction (lane 9). In contrast, the GPI-linked NCAM120 is mainly present in the raft fractions (lane 2–4) with only small amounts in the high density Triton X-100–insoluble fractions (lane 9). The raft-associated nonreceptor tyrosine kinase fyn is also exclusively present in the low density fraction. IB, immunoblot for the molecules indicated.
Also in neuroblastoma cells, all isoforms were present in the top fractions of the flotation gradient, confirming the association with lipids rafts (Fig. 1 B, lanes 2–4). The nonreceptor tyrosine kinase fyn also codistributed with NCAM in the upper fractions. However, whereas NCAM120 was mainly raft associated (Kramer et al., 1999) high amounts of NCAM140 and NCAM180 remained in the Triton X-100–insoluble bottom fractions (Fig. 1 B, lanes 8 and 9) of the flotation gradient, probably associated with cytoskeletal proteins (Montixi et al., 1998).
Additionally, fluorescence signals indicating NCAM antibody localization partially overlapped with the staining for the lipid raft markers ganglioside GM1 and phosphatidylinositolbisphosphate (PIP2) (Laux et al., 2000) in cultured hippocampal neurons (Fig. S1 available at http://www.jcb.org/cgi/content/full/jcb.200109059/DC1).
Mutation of the palmitoylation sites abolishes the presence of NCAM140 in rafts
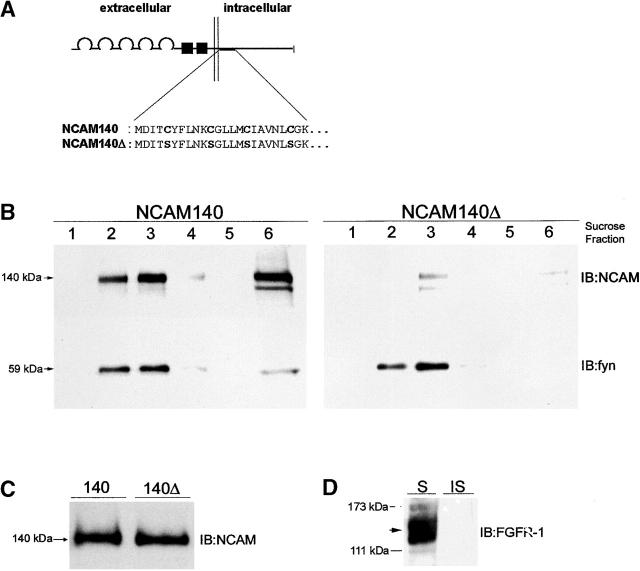
To investigate the importance of intracellular palmitoylation for the association of NCAM with lipid rafts, an NCAM140 mutant was constructed which lacks its four palmitoylation sites by replacing the four intracellular cysteines with serines (Fig. 2 A, NCAM140Δ). We then investigated whether NCAM localizes to rafts in transfected CHO cells. Analysis of the subcellular distribution of NCAM140 in transfected CHO cells revealed an association of NCAM140 with lipid rafts (Fig. 2 B, left, lanes 2 and 3). In contrast, NCAM140Δ was drastically reduced in lipid rafts when compared with NCAM140 (Fig. 2 B, right, lanes 2 and 3), indicating that palmitoylation is a major determinant in the localization of NCAM140 to lipid rafts. Mutation of NCAM140 palmitoylation sites did not significantly influenced the overall expression of the protein (Fig. 2 C) or its surface expression (Fig. 8 B). The amount of NCAM140Δ was also reduced in the bottom fraction of the sucrose gradient, indicating an overall reduced Triton X-100 insolubility (Fig. 2 B, right, lane 6) of the protein.
Figure 2.
Mutation of NCAM140 palmitoylation sites abolishes NCAM140 raft association. (A) Schematic diagram of the structure of NCAM140 and NCAM140Δ. The plasma membrane is indicated by the pair of vertical lines. The semicircles represent the five Ig-like domains. The two fibronectin type III–like domains are shown as black boxes. The expanded segment shows the NH2-terminal sequence of the cytoplasmic domain and the four cysteines that were mutated to serines in the NCAM140Δ construct to remove all sites for palmitoylation. (B) Immunoblot analysis of sucrose gradient fractions of NCAM140- (left) and NCAM140Δ- (right) transfected CHO cells. NCAM140 is present both in the Triton X-100–insoluble low density raft fractions (lanes 2 and 3) and high density fraction (lane 6). To estimate the percentage of raft-associated NCAM140 versus total NCAM140, we normalized the densitometrically determined immunoblot intensity of the NCAM140 bands in the total cell lysate (C) and in the raft fractions (B, left, lanes 2 and 3) to the total protein subjected to SDS-PAGE. Relating the amount of NCAM140 present in lipid rafts to the total amount of NCAM140 revealed that ∼2% of the total NCAM140 protein is present in the lipid raft fractions. Analysis of the sucrose gradient fractions of NCAM140Δ-transfected cells shows a drastically reduced amount of NCAM in the low density fractions (lanes 2 and 3) and the high density bottom fraction (lane 6), indicating that palmitoylation is essential for NCAM140 to be present in lipid rafts and in the cytoskeletal-associated fraction. The blots of the NCAM140 and NCAM140Δ gradient fractions were reprobed with fyn antibodies to confirm both equal protein loading and equal isolation of the lipid raft fractions. (C) Immunoblot analysis of the total cell lysate of NCAM140- and NCAM140Δ-transfected CHO cells using polyclonal NCAM antibodies. Comparison of expression levels in CHO cells transfected with both constructs indicate that mutation of the four cysteines did not significantly alter protein expression. (D) Immunoblot analysis of the Triton X-100–soluble (S) and the Triton X-100–insoluble fraction (IS) of a CHO cell lysate using a FGF receptor-1 antibody revealed that the FGF receptor (FGFR-1) is only present in the Triton X-100–soluble fraction. IB, immunoblot for the molecules indicated.
Figure 8.
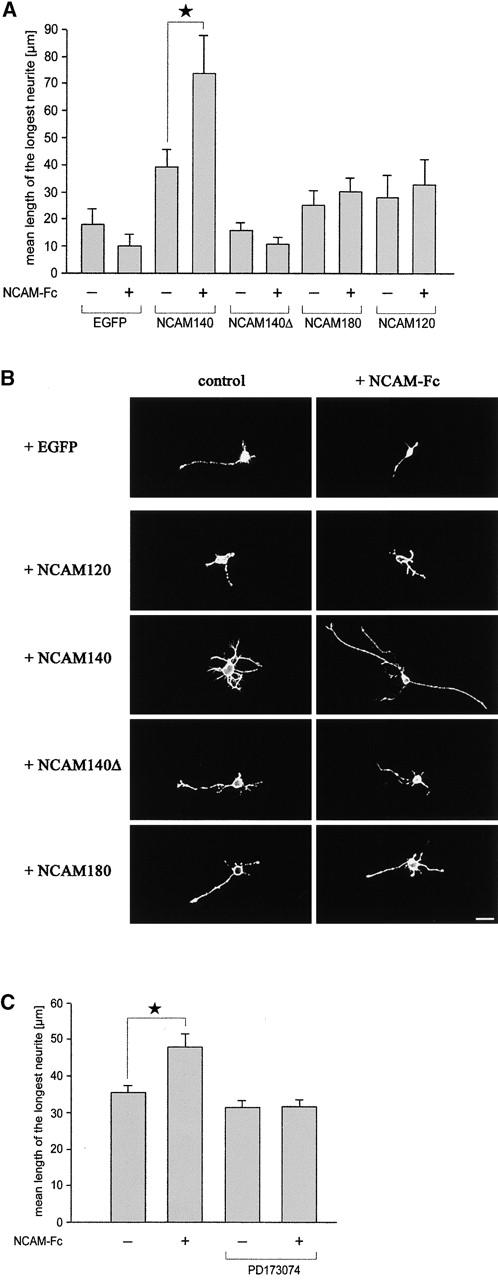
Exclusion of NCAM140 from lipid rafts or inhibition of the FGF receptor completely inhibits NCAM140-mediated neurite outgrowth. (A) Cultured hippocampal neurons from NCAM-deficient mice (NCAM−/−) were transfected with the indicated NCAM constructs and stimulated with NCAM-Fc. Means and SEM of the longest neurite are shown. Asterisks denote statistical significance of neuritogenesis after NCAM-Fc stimulation compared with unstimulated cultures (*p < 0.05). (B) Confocal images of hippocampal neurons from NCAM−/− mice transfected with the indicated NCAM isoform or EGFP and maintained in the presence (right) or absence (left) of NCAM-Fc. In case of EGFP transfection, the EGFP fluorescence signal is shown, whereas in case of transfection with the NCAM isoforms the cell surface immunoreactivity of NCAM is shown. Bar, 20 μm. (C) Cultured hippocampal neurons from NCAM+/+ mice were treated with the FGF receptor inhibitor and subsequently stimulated with NCAM-Fc. Inhibition of FGF receptor activity completely impairs NCAM-mediated neurite outgrowth. Means and SEM of the longest neurite are shown. Asterisks denote statistical significance of neuritogenesis after NCAM-Fc stimulation compared with unstimulated cultures (*p < 0.01).
To investigate whether NCAM140Δ would show similar features in neurons as in CHO cells, we transfected cultured hippocampal neurons of NCAM-deficient (NCAM−/−) mice with NCAM140 and NCAM140Δ and monitored the raft localization of the two proteins immunocytochemically by treating the cells with cold Triton X-100 followed by immunostaining for NCAM and the raft markers GM1 ganglioside and PIP2 (Fig. 3 A). In cold Triton X-100, nonraft proteins become solubilized, whereas raft-associated and cell cytoskeleton–associated proteins remain in the cell membrane (Ledesma et al., 1998). After extraction with cold Triton X-100, multiple clusters of NCAM140 and NCAM140Δ were still detected along neurites. Some of NCAM140 clusters colocalized with GM1 and PIP2 immunoreactivity (Fig. 3 A), indicating that NCAM140 is present in lipid rafts. In contrast, NCAM140Δ clusters did not significantly colocalize with GM1 and PIP2 (Fig. 3 A), supporting the view that palmitoylation is essential for NCAM140 localization to lipid rafts. Some NCAM140 and NCAM140Δ clusters not colocalizing with GM1 and PIP2 remained insoluble in cold Triton X-100, probably due to cytoskeletal association. Finally, we quantified differences in raft localization of NCAM140 and NCAM140Δ by measuring the intensity of GM1 immunofluorescence within clusters of NCAM staining. Measurement of fluorescence signals revealed that raft localization of NCAM140Δ was reduced by ∼80% compared with NCAM140 in hippocampal neurons (Fig. 3 B).
Figure 3.
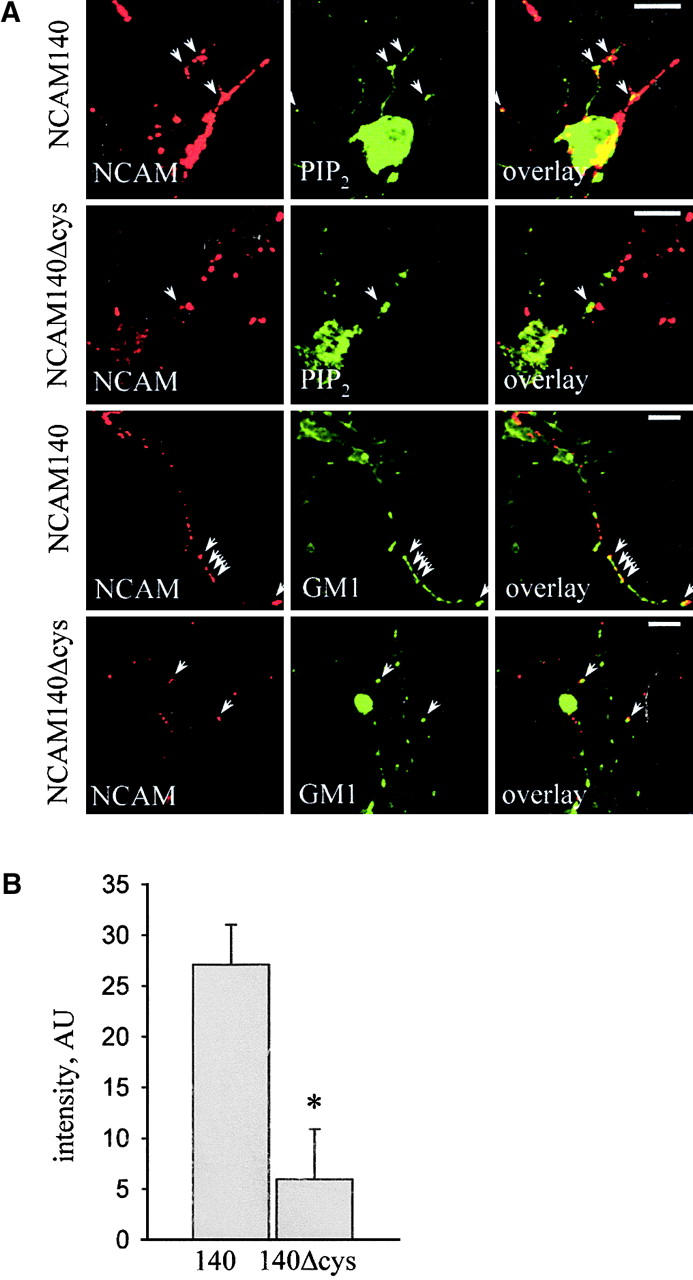
NCAM140Δ is excluded from lipid rafts in cultured hippocampal neurons. (A) Confocal images of immunofluorescence staining of Triton X-100–treated hippocampal neurons from NCAM−/− mice transfected with either NCAM140 or NCAM140Δ. Cells were stained for NCAM and GM1 or PIP2. Arrows show clusters of NCAM (red) colocalizing with GM1 or PIP2 (green). In NCAM140-transfected NCAM−/− neurons, multiple clusters of NCAM coincided with GM1 or PIP2 staining, whereas in NCAM140Δ-transfected NCAM−/− neurons the overlap of NCAM staining with GM1 or PIP2 staining is strongly reduced. Although not raft associated, a fraction of NCAM140Δ remains Triton X-100 insoluble, possibly due to interaction with the cytoskeleton. Bars, 10 μm. (B) Bar graph shows quantification of fluorescence signal of GM1 staining within clusters of NCAM staining. Intensity of GM1 staining within clusters of NCAM140Δ is reduced significantly compared with NCAM140 clusters (*p < 0.01).
As a next step, we addressed the question of the physiological relevance of the lipid raft localization of NCAM isoforms for activation of the fyn-FAK pathway and focused on the functional connection to the FGF receptor activation, which has been shown by us (Fig. 2 D) and others to be absent in the Triton X-100–insoluble fraction (Davy et al., 2000).
NCAM120 activates extracellular signal–regulated kinase 1/2 independently of the FGF receptor, whereas NCAM180 signaling is FGF receptor dependent
To determine the functional importance of NCAM localization to lipid rafts, we first investigated whether the three isoforms could activate the extracellular signal–regulated kinase (ERK)1/2 pathway in an FGF receptor–dependent manner and then asked how disruption of the lipid raft association may alter NCAM-mediated signaling. As described earlier, we used polyclonal NCAM antibodies to trigger NCAM-dependent signaling (Schuch et al., 1989; Schmid et al., 1999) and investigated ERK1/2 phosphorylation by immunoblot analysis using a phosphorylation state–specific antibody. To investigate a possible raft-dependent signaling of NCAM that is independent of the previously described FGF receptor–dependent signaling (Saffell et al., 1997; Kolkova et al., 2000a), endogenously expressed FGF receptors need to be blocked completely. The FGF receptor inhibitor PD173074 was used in this study, since it specifically inhibits signaling of the FGF receptor, leaving other tyrosine kinases unaffected (Fig. S2 available at http://www.jcb.org/cgi/content/full/jcb.200109059/DC1) (Skaper et al., 2000).
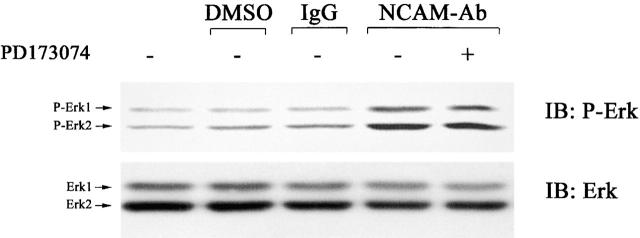
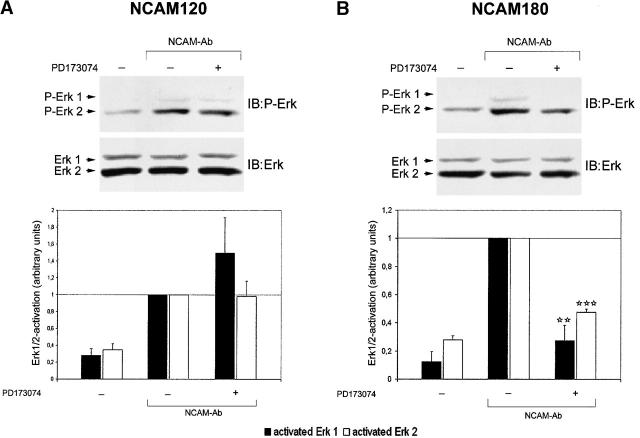
Stimulation of neuroblastoma cells with polyclonal NCAM antibodies showed an up-regulation of ERK1/2 phosphorylation 10 min after addition of antibodies (Fig. 4) as has also been reported in previous studies (Schmid et al., 1999). This activation was not attenuated by the FGF receptor inhibitor, indicating that stimulation of at least one of the NCAM isoforms expressed by neuroblastoma cells can activate ERK1/2 in an FGF-independent manner. Since neuroblastoma cells expressed all three major NCAM isoforms (Fig. 1 B), we studied whether NCAM signaling could be reconstituted with respect to the ERK1/2 activation in CHO cells transfected with one of the individual NCAM isoforms. For control, exposure of untransfected CHO cells to polyclonal NCAM antibodies did not result in mitogen-activated protein (MAP) kinase phosphorylation (Fig. S2 available at http://www.jcb.org/cgi/content/full/jcb.200109059/DC1). In NCAM120-, NCAM140-, and NCAM180-transfected CHO cells, ERK1/2 was phosphorylated 20 min after application of the NCAM antibodies as seen in neuroblastoma cells, indicating that all NCAM isoforms are capable to activate the MAP kinase in CHO cells, although possibly with different potency. We then compared the FGF receptor dependency of the individual NCAM isoforms with regard to ERK1/2 activation. Antibody stimulation of NCAM120-transfected CHO cells resulted in ERK1/2 phosphorylation that was not blocked by the FGF receptor inhibitor, indicating an FGF receptor–independent signaling of this isoform (Fig. 5 A). Comparison of NCAM180- and NCAM140-mediated signaling (Fig. 5 B and Fig. 6 A) demonstrated that NCAM180-mediated ERK1/2 phosphorylation was significantly attenuated by the FGF receptor inhibitor (∼50% reduction compared with maximal activation), whereas NCAM140-mediated signaling was not reduced. These data suggest that NCAM180 uses the FGF receptor for signaling, whereas NCAM140 does not act predominantly via this receptor.
Figure 4.
The FGF receptor inhibitor does not impair NCAM-mediated ERK1/2 activation. Neuroblastoma cells were assayed for ERK1/2 kinase activation using a phosphorylation state-specific ERK1/2 antibody by immunoblot analysis. Polyclonal NCAM antibodies induce phosphorylation of ERK1/2 10 min after antibody application. PD173074 did not impair NCAM-mediated ERK1/2 phosphorylation, indicating a FGF receptor–independent signaling of NCAM in neuroblastoma cells. Control experiments show that neither carrier (DMSO) nor normal rabbit IgG activate ERK1/2. IB, immunoblot for the molecules indicated.
Figure 5.
In transfected CHO cells, NCAM120 activates ERK1/2 in a FGF receptor–independent manner, whereas NCAM180-mediated ERK1/2 activation is FGF receptor dependent. CHO cells were transfected with NCAM120 or NCAM180 and stimulated with polyclonal NCAM antibodies. (A) Immunoblot of NCAM120-transfected CHO cell lysates analyzed for phosphorylated ERK1/2 (top). Bar graph shows ERK1/2 phosphorylation by densitometric analysis of immunoblots (bottom). Stimulation of NCAM120-transfected CHO cells result in phosphorylation of ERK1/2, which is independent of the presence of PD173074, indicating that activation of ERK1/2 by NCAM120 is FGF receptor independent. (B) Immunoblot of NCAM180-transfected CHO cell lysates analyzed for phosphorylated ERK1/2 (top). Bar graph shows ERK1/2 phosphorylation by densitometric analysis of immunoblots (bottom). NCAM180-mediated ERK1/2 activation is attenuated by the FGF receptor inhibitor, showing FGF receptor dependency of NCAM180 signaling with respect to ERK1/2 activation. The bars show means and SEM of four independent experiments. Asterisks denote statistical significance (paired t test) of attenuation of ERK1/2 phosphorylation due to treatment with the FGF receptor inhibitor compared with NCAM-mediated ERK1/2 activation in transfected CHO cells, which were not treated with PD173074 (**p < 0.005; ***p < 0.0005). IB, immunoblot for the molecules indicated.
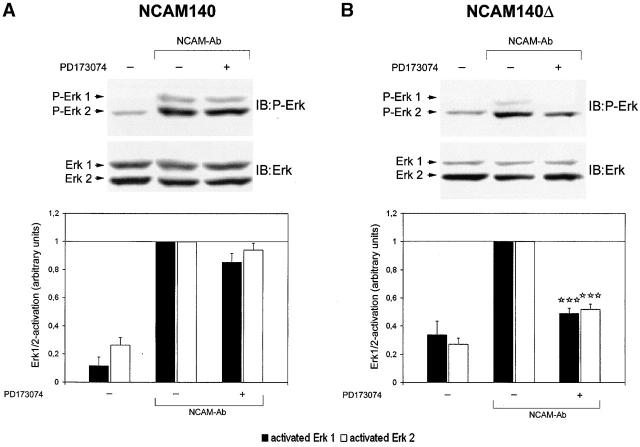
Figure 6.
Deletion of NCAM140 palmitoylation sites results in a shift of NCAM140-mediated signal transduction from FGF receptor–independent toward FGF receptor–dependent signaling. CHO cells were transfected with NCAM140 or NCAM140Δ and assayed for ERK1/2 activation by immunoblot analysis. (A) Immunoblot of NCAM140-transfected CHO cell lysates analyzed for phosphorylated ERK1/2 (top). Bar graph shows ERK1/2 phosphorylation by densitometric analysis of immunoblots (bottom). Stimulation of NCAM140-transfected CHO cells results in phosphorylation of ERK1/2, which is not attenuated by the FGF receptor inhibitor PD173074, indicating an FGF receptor–independent signaling of NCAM140. (B) Immunoblot of NCAM140Δ-transfected CHO cell lysates analyzed for phosphorylated ERK1/2 (top). Bar graph shows ERK1/2 phosphorylation revealed by densitometric analysis of immunoblots (bottom). Stimulation of NCAM140Δ also results in phosphorylation of ERK1/2, which in contrast to NCAM140 is impaired by PD173074. The bars show mean and SEM of 8–12 independent experiments. Asterisks denote statistical significance (paired t test) of attenuation of ERK1/2 phosphorylation due to treatment with the FGF receptor inhibitor compared with NCAM-mediated ERK1/2 activation in transfected CHO cells, which were not treated with PD173074 (***p < 0.0005). IB, immunoblot for the molecules indicated.
Disruption of NCAM140 localization in lipid rafts attenuates FGF receptor–independent signaling
We next addressed the question whether the FGF receptor independency of NCAM140 signaling is related to its presence in lipid rafts. Since NCAM140Δ is excluded from lipid rafts (Fig. 2 B, right, and Fig. 3 A), we first investigated NCAM140Δ signaling in CHO cells. Stimulation of NCAM140Δ-expressing CHO cells with polyclonal NCAM antibodies resulted in ERK1/2 phosphorylation levels comparable to those in NCAM140-transfected and -stimulated cells (Fig. 6, A and B). However, in NCAM140Δ-transfected CHO cells phosphorylation of ERK1/2 was significantly attenuated by the FGF receptor inhibitor (∼50% of the maximal phosphorylation observed without FGF receptor inhibitor), indicating a dependence of NCAM140Δ signaling on the FGF receptor.
The importance of lipid rafts for NCAM140 signaling was further characterized by treatment of NCAM140-expressing CHO cells with methyl-β-cyclodextrin (MCD) that binds to cholesterol, thereby destroying the integrity of lipid rafts. It has been reported that this treatment inhibits signaling of receptor complexes that contain glycosyl phosphatidylinositol (GPI)-linked components (Bruckner et al., 1999) but does not impair signaling of nonraft-associated receptors (Peiro et al., 2000). As shown in Fig. 7 A, NCAM140-expressing CHO cells depleted of cholesterol and stimulated with polyclonal NCAM antibodies were reduced in ERK1/2 phosphorylation by ∼50% compared with control cells, indicating an involvement of lipid rafts in NCAM140 signaling.
Figure 7.
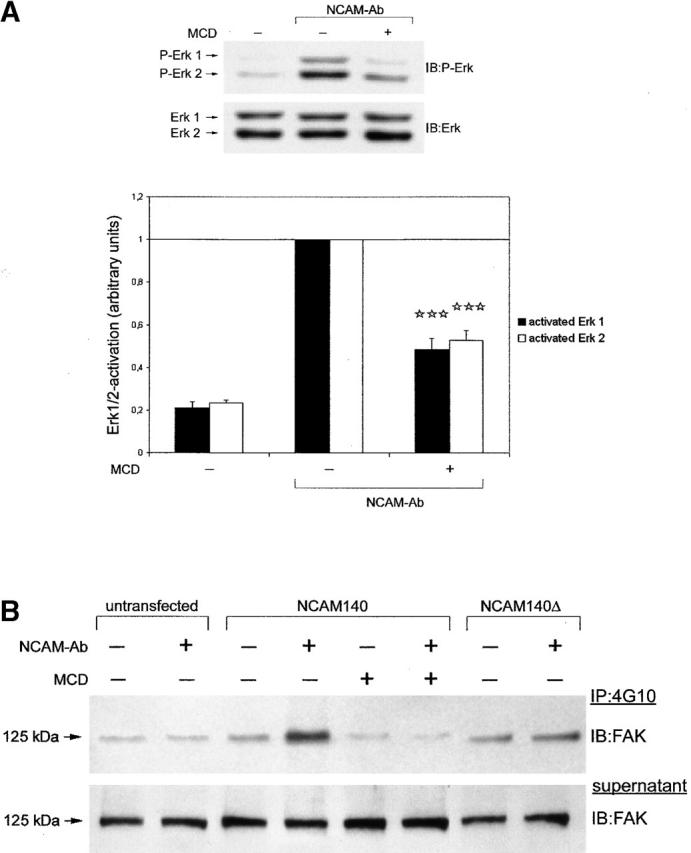
Lipid raft integrity is critical for NCAM140-mediated signal transduction. NCAM140-transfected CHO cells were treated with 10 mM MCD to disperse lipid rafts before NCAM stimulation with polyclonal antibodies. (A) Cholesterol-depleted cells (+MCD) display attenuated ERK1/2 activation in response to NCAM stimulation compared with control cells (−MCD). The bar graph (bottom) shows the mean and SEM of six independent experiments. Asterisks denote statistical significance (paired t test) of attenuation of ERK1/2 phosphorylation in response to MCD treatment compared with antibody stimulation without MCD treatment (***p < 0.0005). (B) NCAM140- and NCAM140Δ-transfected CHO cells were stimulated with polyclonal NCAM antibodies, and tyrosine- phosphorylated proteins were immunoprecipitated (IP) from the cell lysates. Immunoblot analysis with a FAK protein antibody detects tyrosine-phosphorylated FAK in immunoprecipitates. NCAM140 stimulation induces phosphorylation of FAK in control (−MCD) cells. Exclusion of NCAM140 from lipid rafts either by cholesterol depletion (+MCD) or by mutation of NCAM140 palmitoylation sites (NCAM140Δ) attenuates phosphorylation of FAK. IB, immunoblot for the molecules indicated.
Activation of the focal adhesion kinase FAK by NCAM140 is impaired when NCAM140 is excluded from lipid rafts
The nonreceptor tyrosine kinase fyn and the focal adhesion kinase FAK have been implicated in NCAM-mediated signaling (Beggs et al., 1997) and NCAM-mediated neurite outgrowth using dominant negative constructs of the two kinases (Kolkova et al., 2000a). We reasoned that the FGF receptor–independent signaling of NCAM140 could be linked to raft-associated kinases, such as fyn, and phosphorylation of downstream effectors of fyn, namely FAK. Given that FAK can activate the ERK1/2 pathway (Chen et al., 1998) and that it plays a pivotal role in NCAM signaling (Beggs et al., 1997), we investigated whether FAK phosphorylation is altered either when lipid rafts are disrupted by MCD in NCAM140-transfected CHO cells or when the NCAM molecule is excluded from lipid rafts by mutation of palmitoylation sites (transfection with NCAM140Δ). Tyrosine-phosphorylated proteins were immunoprecipitated from lysates of control or NCAM-stimulated cells transfected with NCAM140 or NCAM140Δ. Immune complexes were then subjected to immunoblot analysis with FAK antibody. Up-regulation of FAK phosphorylation was readily detectable after stimulation of NCAM140-transfected CHO cells with polyclonal NCAM antibodies (Fig. 7 B). In contrast, only low levels of FAK immunoreactivity could be detected in immunoprecipitates of NCAM140Δ-transfected cells or in MCD-treated cholesterol-depleted NCAM140-transfected cells. Furthermore, FAK activation by NCAM140 was not reduced by the FGF receptor inhibitor PD173074 (unpublished data). These observations provide evidence that NCAM140-mediated signaling via FAK depends on the presence of NCAM140 in lipid rafts. Disruption of the integrity of the lipid raft matrix or exclusion of NCAM140 from lipid rafts impairs the ability of NCAM140 to activate the FAK kinase.
Among the three NCAM isoforms, only NCAM140 can function as a neuritogenic receptor in hippocampal neurons
We then addressed the question whether lipid raft association of NCAM is important for one of its biological functions, such as promotion of neurite outgrowth. We transfected cultured hippocampal neurons of NCAM-deficient (NCAM−/−) mice with one of the NCAM isoforms together with the fluorescent marker enhanced green fluorescent protein (EGFP). This assay provides the advantage of reintroducing the NCAM isoforms or the NCAM140Δ mutant into their “natural” environment without NCAM background expression. The NCAM-transfected EGFP marked neurons were stimulated with NCAM-Fc to induce neurite outgrowth.
We first investigated which NCAM isoform would promote neurite outgrowth. Transfected neurons were stained with polyclonal NCAM antibodies to ensure that all NCAM isoforms and NCAM140Δ were expressed at the cell surface at similar levels (Fig. 8 B). Quantification of neurite length revealed that only NCAM140-transfected neurons extended their neurites approximately twofold better upon homophilic stimulation with NCAM-Fact than unstimulated neurons, whereas stimulation of NCAM120- or NCAM180-transfected neurons did not enhance neurite outgrowth in comparison with cells transfected to express only EGFP (Fig. 8 A). These observations indicate that only NCAM140 can serve as a receptor for neuritogenesis, whereas NCAM180 and NCAM120 are ineffective. It needs to be mentioned that neurite length was also enhanced in NCAM140-transfected cells in the absence of NCAM-Fc, probably due to basal stimulation of NCAM140 by soluble NCAM fragments known to be generated by proteolytic processing of all NCAM isoforms (Probstmeier et al., 1989). However, only NCAM140 can promote neurite outgrowth upon homophilic triggering by NCAM fragments. The fact that NCAM−/− cells transfected with NCAM140 showed a more pronounced neuritogenesis than wild-type neurons is most likely due to the possibility that this isoform is the most neuritogenic one of all three major isoforms and may thus predominate over the neurite outgrowth signal transduction machinery in the absence of nonneuritogenic isoforms. Furthermore, the exclusive expression of the neuritogenic NCAM140 isoform on the cell surface may also account for the longer neurite length after NCAM-Fc stimulation compared with wild-type neurons expressing all NCAM isoforms endogenously (Fig. 8 C), since NCAM120 and NCAM180 are ineffective in promoting neurite outgrowth and might thus act as NCAM-Fc scavengers with regard to neuritogenesis. Finally, it needs to be emphasized that activation of the FGF receptor does not appear to be the only prerequisite for NCAM-mediated neuritogenesis, since NCAM180, which can also activate the FGF receptor (Fig. 5 B), is ineffective for neuritogenesis.
Exclusion from rafts or block of the FGF receptor diminishes the ability of NCAM140 to promote neurite outgrowth
We then asked if NCAM140 facilitates cosignaling for effective neurite outgrowth by activating two distinct pathways: the raft- and the FGF receptor–dependent pathway. We blocked either of the two pathways and investigated the capability of NCAM140 to promote neurite outgrowth. First, hippocampal neurons of NCAM−/− mice were transfected with NCAM140Δ, since it can activate the FGF receptor but lacks the ability to activate raft-associated signaling cascades, such as the fyn-FAK pathway. If our hypothesis is valid, stimulation of NCAM140Δ should not promote neuritogenesis. Indeed, NCAM140Δ did not promote neurite outgrowth upon NCAM-Fc stimulation (Fig. 8 A) with neurite lengths being in the same range as for NCAM120-, NCAM180-, or only EGFP-transfected neurons.
Finally, we confirmed the influence of the FGF receptor inhibitor on NCAM-stimulated neuritogenesis (Fig. 8 C). For this purpose, we stimulated hippocampal neurons with NCAM-Fc in the presence of PD173074. NCAM-Fc induced neurite outgrowth of hippocampal neurons in a reproducible manner at concentrations used in previous publications (Meiri et al., 1998). PD173074 blocked NCAM-dependent neurite outgrowth completely. It is noteworthy that neuron survival and neurite outgrowth was not influenced by PD173074 in the absence of NCAM-Fc, since number of neurons and neurite lengths maintained in the absence or presence of PD173074 were similar, underscoring the specificity of PD173074 for the FGF receptor and supporting the importance of the FGF receptor for NCAM-mediated neuritogenesis.
Discussion
Two membrane subcompartments contain NCAM isoforms
In this study, we have shown that the three major isoforms of NCAM are present in two membrane subcompartments. One compartment comprises the Triton X-100–soluble fraction containing the majority of membrane-associated glycoproteins. The other compartment is the low density Triton X-100–insoluble fraction of proteins, also named lipid rafts or detergent resistant microdomains, which are characterized by their accumulation of cholesterol and sphingolipids. Typically, this fraction is enriched in GPI-linked glycoproteins, among them molecules of the immunoglobulin superfamily such as F3–F11–contactin, TAG-1–axonin-1, and Thy-1. Of the three major NCAM isoforms, the GPI-linked NCAM120 was found to be highly enriched in lipid rafts of neuroblastoma cells. This localization of NCAM120 to rafts has also been reported previously for a permanent oligodendrocyte cell line (Kramer et al., 1999). The other two NCAM isoforms, NCAM140 and NCAM180, were shown to be located both in the Triton X-100–soluble and –insoluble compartments. From these observations it seemed reasonable to assume that, as is the case for some receptor molecules (Tansey et al., 2000), a partitioning of NCAM isoforms into topologically distinct compartments could contribute to a biologically meaningful diversity of functions.
Selective localization of the different NCAM isoforms in membrane subcompartments contributes to distinct signal transduction pathways
In view of the idea that the FGF receptor mediates the common signal transduction pathway for all NCAM isoforms, it appeared surprising at first sight that NCAM120 activates ERK1/2 in an FGF receptor–independent way. It has been demonstrated by us (this study) and others (Davy et al., 2000) that the FGF receptor is absent in the low density Triton X-100–insoluble fraction where NCAM120 is enriched. It is therefore unlikely that NCAM120 can “meet” the FGF receptor for activation by a direct interaction. These results are in line with previous observations showing that the intracellular domain of NCAM140 is necessary to promote neurite outgrowth (Saffell et al., 1995). Since several other signal molecules are enriched in lipid rafts, including members of the src kinase family, heteromeric G-proteins, and the neurite outgrowth-associated protein GAP-43 (Simons and Ikonen, 1997; Arni et al., 1998; Aarts et al., 1999), it is more likely that GPI-linked molecules, such as NCAM120 or F3–F11–contactin, cause productive interactions with fyn by clustering in rafts, thereby activating ERK1/2 as has been discussed elsewhere (Kramer et al., 1999; Crossin and Krushel, 2000). Alternatively, activation of intracellular kinases by GPI-anchored molecules may depend on cis interactions with transmembrane molecules (Zeng et al., 1999).
NCAM180 is localized in both membrane subcompartments. However, NCAM180 signals predominantly through the FGF receptor pathway as demonstrated by using the FGF receptor inhibitor. Although NCAM180 is localized in lipid rafts, it apparently lacks the ability to activate FGF receptor–independent pathways, possibly due to the additional 268 amino acids contributed by exon 18, which are inserted into the intracellular domain of NCAM140. Amino acids derived from exon 18 are particularly rich in proline, and it is conceivable that these contribute in a yet unknown manner to a particular conformation of the intracellular domain of NCAM180 that prevents it from activation of the fyn-FAK kinase pathway (Beggs et al., 1997; Kolkova et al., 2000b).
Like NCAM180, NCAM140 is localized in both membrane subcompartments but in contrast to NCAM180 is able to activate the fyn-FAK kinase pathway. Although NCAM140 lacks a classical fyn binding motif, it is conceivable that NCAM140 associates with other molecules in rafts that are able to signal transduce. It is also possible that rafts can promote low affinity “kiss-and-run” interactions due to locally enriched concentrations of interacting molecules and thus serve as a matrix for the orchestrated activation of signaling molecules. Beside its SH3 binding domain, fyn can undergo low affinity interactions via its NH2 terminus as reported for the T cell receptor (Gauen et al., 1994). Outside of lipid rafts NCAM140 triggers the FGF receptor, which activates ERK1/2 and other pathways. Whether NCAM140 interacts in this compartment in a tight association with the FGF receptor as has been postulated by Doherty, Walsh, and coworkers (Doherty et al., 1996; Doherty and Walsh, 1996) and suggested by Cavallaro et al. (2001) or whether it indirectly modulates the activity of the FGF receptor (Kamiguchi and Lemmon, 2000) cannot be determined by our present experiments.
Palmitoylation of NCAM140 is necessary for its localization in lipid rafts
Our data raise the question about the regulatory mechanisms determining the localization of NCAM140 in the two different compartments. We could show that ablation of the four cysteine residues representing the palmitoylation sites in the membrane proximal domain of NCAM140 reduces its capacity to associate with lipid rafts. Replacement of cysteines by serines has been proven to be a useful tool to inhibit raft localization of proteins (Kabouridis et al., 1997; Zhang et al., 1998). Abrogation of palmitoylation sites not only destroys raft localization of NCAM140 but also reduces the ability of NCAM140 to activate the fyn-FAK kinase pathway, which was monitored by phosphorylation of FAK, a direct downstream effector of fyn (Schlaepfer et al., 1994). The view that raft association of NCAM140 is necessary for activation of the fyn-FAK pathway is supported by the observation that destruction of lipid rafts by removal of cholesterol with MDC reduces NCAM140-triggered activation of the fyn-FAK kinase pathway. Our study underscores the importance of NCAM140 recruitment to lipid rafts and discloses the possibility that NCAM140 signaling can be regulated by localization of NCAM140 in lipid rafts. The mechanisms controlling enzymatic acylation of the cysteine residues in NCAM are unknown presently but will be necessary to study for the characterization of functional consequences of NCAM140 accumulation in rafts. Also, the mechanisms removing palmitoic acid from cysteine residues to shift NCAM140 localization from lipid rafts to the nonlipid raft compartment will need to be investigated.
Signaling pathways within the two membrane subcompartments need to be activated for NCAM140-dependent neurite outgrowth
In our study, we blocked one of the two signal transduction pathways, either by inhibition of the FGF receptor by PD173074 or by preventing NCAM140 to accumulate in lipid rafts. No residual neurite outgrowth promoting activity could be seen either by blocking the initiation of the fyn-FAK kinase pathway or by inhibiting activation of the FGF receptor signaling pathway. Thus, NCAM140 has to be activated in both compartments in order to signal transduce effectively for induction of neurite outgrowth. It is not surprising that NCAM120 or NCAM180 are not neuritogenic when transfected into NCAM-deficient neurons: NCAM120 localizes predominantly to lipid rafts and may thus not be able to activate the FGF receptor even when present in the same cell. Since NCAM120 is not highly expressed in neurons, this scenario may not have biological relevance for neurite outgrowth but may be important for signaling in glial cells where NCAM120 is highly expressed. The observation that NCAM180 does not activate the fyn-FAK kinase pathway (Beggs et al., 1997) is in agreement with our experiments, since NCAM180 does not mediate neurite outgrowth when transfected into NCAM-deficient neurons.
However, our arguments should not imply that convergence of the two pathways on ERK1/2, thereby enhancing its activity by synergistic mechanisms and going beyond the threshold necessary for effective neurite outgrowth, is the only way of cosignaling. Other effectors signaling in the two compartments that are not converging on the ERK1/2 pathway may also achieve cosignaling and may thus be important for neuritogenesis (hypothetical scheme can be seen in Fig. 9). Our observations also indicate that besides the FGF receptor there may be other yet unidentified receptors involved in NCAM-mediated signaling, since activation of the MAP kinase is not completely blocked by the FGF receptor inhibitor when NCAM140Δ and NCAM180 are stimulated. Nevertheless, our observations appear to reconcile two divergent views on the activation of neurite outgrowth through homophilic binding of NCAM: one favoring the predominance of the fyn-FAK kinase pathway and the other favoring a predominant action of the FGF receptor. Thus, a biologically meaningful concept emerges in that an important morphogenic event, such as neurite outgrowth, is not controlled by only one signal transduction mechanism but depends on the concomitant and coincident activation of at least two signal transduction pathways for neuritogenesis. Our results are in agreement with observations on a switch in the palmitoylation of proteins early in the critical period, which includes a decrease in the palmitoylation of GAP-43 and other major substrates characteristic of growth cones (Patterson and Skene, 1999). Previous studies have also shown that a similar reduction in palmitoylation of growth cone proteins is sufficient to stop neurite extension, suggesting that a developmental regulation of protein palmitoylation serves to down-regulate the molecular machinery for neurite outgrowth (Hess et al., 1993; Patterson and Skene, 1994). It remains to be seen whether other physiological functions that are regulated by recognition molecules, such as neuronal survival, synaptogenesis, and synaptic plasticity, also depend on their presence in lipid rafts and similar cosignaling mechanisms.
Figure 9.
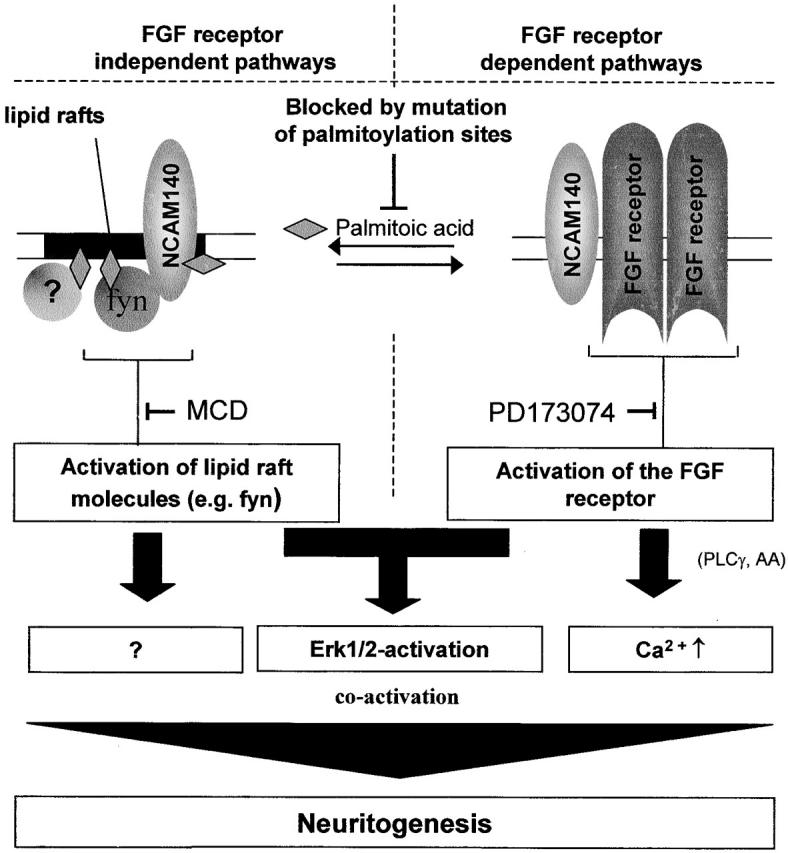
Hypothetical diagram of NCAM140 cosignaling cascades. Within the plasma membrane, NCAM140 is located both in lipid rafts and outside of rafts, thus defining two membrane subcompartments in which NCAM140 is present. The presence of NCAM140 in lipid rafts is regulated by palmitoylation of intracellular cysteines by yet unknown mechanisms. Upon homophilic activation, NCAM triggers distinct signal transduction pathways in the two compartments: in lipid rafts NCAM140 phosphorylates FAK most likely via raft-associated fyn kinase, whereas in the nonraft compartment NCAM140 facilitates FGF receptor–activated downstream signaling. Both pathways merge in the activation of ERK1/2 but can branch upstream of ERK1/2 into other pathways, such as the PLCγ activation or other yet unknown effectors. Activation of both pathways is necessary for NCAM140 to function as a neuritogenic receptor. AA, arachidonic acid.
Materials and methods
DNA constructs
Rat NCAM140 and rat NCAM180/pcDNA3 were a gift of P. Maness (University of North Carolina, NC). Rat NCAM120 (a gift of E. Bock, University of Copenhagen, Denmark) was subcloned into the pcDNA3 vector (Invitrogen). The plasmid coding for EGFP was from CLONTECH Laboratories, Inc. The NCAM140Δ construct was generated as described previously (Little et al., 1998) with the exception that mutations were performed using an EcoRI-XhoI subfragment of NCAM140.
CHO and neuroblastoma cell culture, transfection of CHO cells, and NCAM or growth factor activation
Mouse neuroblastoma (Neuro2A) cells were maintained in DME containing 10% FCS and 1 mM sodium pyruvate. CHO were maintained in Glasgow modified Eagle's medium (GMEM) containing 10% FCS. Cells were transfected and stimulated as described in Online supplemental material.
Analysis of ERK1/2 and FAK phosphorylation
Cells were lysed in modified RIPA buffer consisting of 50 mM Tris, pH 7.6, 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, and complete protease inhibitor cocktail (Roche Diagnostics) using 250 μl per well. Lysates were cleared by centrifugation at 12,000 g for 10 min at 4°C. After determination of protein concentration using the BCA protein assay kit (Pierce Chemical Co.), small aliquots of supernatants were removed and resuspended in 5× SDS buffer for SDS-PAGE and immunoblot analysis with an antiphospho-p42/p44 ERK1/2 antibody (1:10,000; Sigma-Aldrich). Blots were stripped and reprobed with an antibody against ERK1/2 protein (1:1,000, Santa Cruz Biotechnology, Inc.) to confirm equal protein loading of samples. Phosphorylation of ERK1/2 was quantified by densitometric analysis of immunoblot phospho-ERK1/2 signals using Scion Image (Scion Corporation). For analysis of phosphorylated FAK, tyrosine-phosphorylated proteins were immunoprecipitated from supernatants adjusted to equal protein concentrations using agarose-coupled 4G10 antibodies (Upstate Biotechnology). Agarose beads were resuspended in 50 μl of 2× SDS buffer and subjected to SDS-PAGE and immunoblot analysis using a polyclonal antibody against FAK protein (Santa Cruz Biotechnology, Inc.). Total protein levels were monitored by subjecting aliquots of the above supernatants to SDS-PAGE followed by immunoblotting for FAK.
Isolation of detergent-resistant membrane fractions
Lipid rafts were isolated (Arni et al., 1998; Melkonian et al., 1999) with slight modifications as described in Online supplemental material.
Cultures of hippocampal neurons
Cultures of hippocampal neurons were prepared by a combination of enzymatic and mechanical dissociation from 1- to 3-d-old age-matched C57BL/6J or NCAM-deficient (NCAM−/−) mice (Cremer et al., 1994) inbred for at least nine generations onto the C57BL/6J background. During the first 20 h after plating on a poly-l-lysine substrate, cells were maintained in culture medium containing 10% specifically tested horse serum (Dityatev et al., 2000). Afterwards, hormonally supplemented serum-free culture medium containing Neurobasal-A medium, 2% B-27, 5 μg/ml gentamycin (all from Life Technologies), 0.5 mM l-glutamine, and 10 ng/ml β-FGF (Sigma-Aldrich) was applied.
Calcium phosphate transfection of hippocampal neurons
Cells were transfected 24 h after seeding by the calcium phosphate method (Ethell and Yamaguchi, 1999) using the Mammalian Transfection Kit (Stratagene).
Treatment of hippocampal neurons with NCAM-Fc and FGF receptor inhibitor
Mouse NCAM-Fc (2.5 μg/ml) (Chen et al., 1999) and/or the FGF receptor inhibitor PD173074 (50 nM) were added to the cultures 28 h after seeding the cells. 10 h after the first application of NCAM-Fc and PD173074, the medium was replaced with the fresh medium containing the two compounds and incubated for another 4 h before fixation of cells.
Detergent extraction of hippocampal neurons
Detergent extraction of hippocampal neurons was performed as described by Ledesma et al. (1998).
Indirect immunocytochemistry and neurite outgrowth measurement
Hippocampal neurons were fixed and stained with antibodies as previously described (Dityatev et al., 2000). For PIP2 (Laux et al., 2000) staining, cells were permeabilized with 0.25% Triton X-100 in PBS and then blocked with 3% BSA in PBS. Images of stained neurons were acquired using a confocal laser-scanning microscope (LSM510; ZEISS) with a thickness of the optical slice of 5 μm. Only neurites of isolated neurons were traced, and their lengths were measured using Scion Image (Scion Corporation). Since the length of the longest neurites always correlated with length of all neurites, only one parameter, namely the length of the longest neurite, is documented in the figures. Immunofluorescence profiles were calculated using LSM510 (ZEISS) software.
Fluorescence labeling of GM1
To visualize cholesterol-enriched lipid rafts, FITC-labeled cholera toxin B subunit (Sigma-Aldrich) diluted in PBS containing 1% BSA to a final concentration of 8 μg/ml was applied to fixed hippocampal neurons for 30 min at room temperature. GM1 immunofluorescence overlapping with NCAM staining was quantified using Scion image (Scion Corporation).
Statistical analysis
Comparison of data was performed with the Student's t test. Differences were considered significant at p < 0.05. Data are from at least three independent experiments if not indicated otherwise.
Online supplemental material
Included in the supplemental materials are details of the lipid raft isolation and images showing immunohistochemically the lipid raft association of NCAM (Fig. S1) and Western blots showing the specificity of the FGF receptor inhibitor PD173074 (Fig. S2). Online supplemental materials are available at http://www.jcb.org/cgi/content/full/jcb.200109059/DC1.
Supplemental Material
Acknowledgments
We thank Dr. Harold Cremer for his gift of NCAM-deficient mice, Galina Dityateva for hippocampal cultures and production of NCAM-Fc, Melanie Richter for purification of NCAM-Fc, and Drs. Patricia Maness and Elisabeth Bock for the gift of NCAM cDNAs. The FGF inhibitor PD173074 was a gift from Parke-Davis.
This work was supported by Deutsche Forschungsgemeinschaft Scha 185/17 (to M. Schachner). M. Delling is a scholar of the Studienstiftung des Deutschen Volkes.
The online version of this article contains supplemental material.
P. Niethammer and M. Delling contributed equally to this work.
Footnotes
Abbreviations used in this paper: EGFP, enhanced green fluorescent protein; ERK, extracellular signal–regulated kinase; FAK, focal adhesion kinase; FGF, fibroblast growth factor; GAP, growth-associated protein; GPI, glycosyl phosphatidylinositol; MAP, mitogen-activated protein; MCD, methyl-β-cyclodextrin; NCAM, neural cell adhesion molecule; PIP2, phosphatidylinositolbisphosphate.
References
- Aarts, L.H., P. Verkade, J.J. van Dalen, A.J. van Rozen, W.H. Gispen, L.H. Schrama, and P. Schotman. 1999. B-50/GAP-43 potentiates cytoskeletal reorganization in raft domains. Mol. Cell Neurosci. 14:85–97. [DOI] [PubMed] [Google Scholar]
- Arni, S., S.A. Keilbaugh, A.G. Ostermeyer, and D.A. Brown. 1998. Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J. Biol. Chem. 273:28478–28485. [DOI] [PubMed] [Google Scholar]
- Beggs, H.E., P. Soriano, and P.F. Maness. 1994. NCAM-dependent neurite outgrowth is inhibited in neurons from Fyn-minus mice. J. Cell Biol. 127:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs, H.E., S.C. Baragona, J.J. Hemperly, and P.F. Maness. 1997. NCAM140 interacts with the focal adhesion kinase p125(fak) and the SRC-related tyrosine kinase p59(fyn). J. Biol. Chem. 272:8310–8319. [DOI] [PubMed] [Google Scholar]
- Brown, D.A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136. [DOI] [PubMed] [Google Scholar]
- Bruckner, K., L.J. Pablo, P. Scheiffele, A. Herb, P.H. Seeburg, and R. Klein. 1999. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 22:511–524. [DOI] [PubMed] [Google Scholar]
- Cavallaro, U., J. Niedermeyer, M. Fuxa, and G. Christofori. 2001. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat. Cell Biol. 3:650–657. [DOI] [PubMed] [Google Scholar]
- Chen, H.C., P.C. Chan, M.J. Tang, C.H. Cheng, and T.J. Chang. 1998. Tyrosine phosphorylation of focal adhesion kinase stimulated by hepatocyte growth factor leads to mitogen-activated protein kinase activation. J. Biol. Chem. 273:25777–25782. [DOI] [PubMed] [Google Scholar]
- Chen, S., N. Mantei, L. Dong, and M. Schachner. 1999. Prevention of neuronal cell death by neural adhesion molecules L1 and CHL1. J. Neurobiol. 38:428–439. [DOI] [PubMed] [Google Scholar]
- Cinek, T., and V. Horejsi. 1992. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J. Immunol. 149:2262–2270. [PubMed] [Google Scholar]
- Cremer, H., R. Lange, A. Christoph, M. Plomann, G. Vopper, J. Roes, R. Brown, S. Baldwin, P. Kraemer, and S. Scheff. 1994. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 367:455–459. [DOI] [PubMed] [Google Scholar]
- Crossin, K.L., and L.A. Krushel. 2000. Cellular signaling by neural cell adhesion molecules of the immunoglobulin superfamily. Dev. Dyn. 218:260–279. [DOI] [PubMed] [Google Scholar]
- Davy, A., C. Feuerstein, and S.M. Robbins. 2000. Signaling within a caveolae-like membrane microdomain in human neuroblastoma cells in response to fibroblast growth factor. J. Neurochem. 74:676–683. [DOI] [PubMed] [Google Scholar]
- Dityatev, A., G. Dityateva, and M. Schachner. 2000. Synaptic strength as a function of post- versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron. 26:207–217. [DOI] [PubMed] [Google Scholar]
- Doherty, P., and F.S. Walsh. 1996. CAM-FGF receptor interactions: a model for axonal growth. Mol. Cell. Neurosci. 8:99–111. [DOI] [PubMed] [Google Scholar]
- Doherty, P., C.H. Barton, G. Dickson, P. Seaton, L.H. Rowett, S.E. Moore, H.J. Gower, and F.S. Walsh. 1989. Neuronal process outgrowth of human sensory neurons on monolayers of cells transfected with cDNAs for five human N-CAM isoforms. J. Cell Biol. 109:789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, P., M. Fruns, P. Seaton, G. Dickson, C.H. Barton, T.A. Sears, and F.S. Walsh. 1990. A threshold effect of the major isoforms of NCAM on neurite outgrowth. Nature. 343:464–466. [DOI] [PubMed] [Google Scholar]
- Doherty, P., P. Smith, and F.S. Walsh. 1996. Shared cell adhesion molecule (CAM) homology domains point to CAMs signalling via FGF receptors. Perspect. Dev. Neurobiol. 4:157–168. [PubMed] [Google Scholar]
- Ethell, I.M., and Y. Yamaguchi. 1999. Cell surface heparan sulfate proteoglycan syndecan-2 induces the maturation of dendritic spines in rat hippocampal neurons. J. Cell Biol. 144:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauen, L.K., Y. Zhu, F. Letourneur, Q. Hu, J.B. Bolen, L.A. Matis, R.D. Klausner, and A.S. Shaw. 1994. Interactions of p59fyn and ZAP-70 with T-cell receptor activation motifs: defining the nature of a signaling motif. Mol. Cell. Biol. 14:3729–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, D.T., S.I. Patterson, D.S. Smith, and J.H. Skene. 1993. Neuronal growth cone collapse and inhibition of protein fatty acylation by nitric oxide. Nature. 366:562–565. [DOI] [PubMed] [Google Scholar]
- Hooper, N.M. 1997. Glycosyl-phosphatidylinositol anchored membrane enzymes. Clin. Chim. Acta. 266:3–12. [DOI] [PubMed] [Google Scholar]
- Kabouridis, P.S., A.I. Magee, and S.C. Ley. 1997. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J. 16:4983–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi, H., and V. Lemmon. 2000. IgCAMs: bidirectional signals underlying neurite growth. Curr. Opin. Cell Biol. 12:598–605. [DOI] [PubMed] [Google Scholar]
- Kolkova, K., V. Novitskaya, N. Pedersen, V. Berezin, and E. Bock. 2000. a. Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway. J. Neurosci. 20:2238–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolkova, K., N. Pedersen, V. Berezin, and E. Bock. 2000. b. Identification of an amino acid sequence motif in the cytoplasmic domain of the NCAM-140 kDa isoform essential for its neuritogenic activity. J. Neurochem. 75:1274–1282. [DOI] [PubMed] [Google Scholar]
- Kramer, E.M., C. Klein, T. Koch, M. Boytinck, and J. Trotter. 1999. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J. Biol. Chem. 274:29042–29049. [DOI] [PubMed] [Google Scholar]
- Laux, T., K. Fukami, M. Thelen, T. Golub, D. Frey, and P. Caroni. 2000. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J. Cell Biol. 149:1455–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma, M.D., K. Simons, and C.G. Dotti. 1998. Neuronal polarity: essential role of protein-lipid complexes in axonal sorting. Proc. Natl. Acad. Sci. USA. 95:3966–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, E.B., G.M. Edelman, and B.A. Cunningham. 1998. Palmitoylation of the cytoplasmic domain of the neural cell adhesion molecule N-CAM serves as an anchor to cellular membranes. Cell Adhes. Commun. 6:415–430. [DOI] [PubMed] [Google Scholar]
- Meiri, K.F., J.L. Saffell, F.S. Walsh, and P. Doherty. 1998. Neurite outgrowth stimulated by neural cell adhesion molecules requires growth-associated protein-43 (GAP-43) function and is associated with GAP-43 phosphorylation in growth cones. J. Neurosci. 18:10429–10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkonian, K.A., A.G. Ostermeyer, J.Z. Chen, M.G. Roth, and D.A. Brown. 1999. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274:3910–3917. [DOI] [PubMed] [Google Scholar]
- Montixi, C., C. Langlet, A.M. Bernard, J. Thimonier, C. Dubois, M.A. Wurbel, J.P. Chauvin, M. Pierres, and H.T. He. 1998. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 17:5334–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, S.I., and J.H. Skene. 1994. Novel inhibitory action of tunicamycin homologues suggests a role for dynamic protein fatty acylation in growth cone-mediated neurite extension. J. Cell Biol. 124:521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, S.I., and J.H. Skene. 1999. A shift in protein S-palmitoylation, with persistence of growth-associated substrates, marks a critical period for synaptic plasticity in developing brain. J. Neurobiol. 39:423–437. [DOI] [PubMed] [Google Scholar]
- Peiro, S., J.X. Comella, C. Enrich, D. Martin-Zanca, and N. Rocamora. 2000. PC12 cells have caveolae that contain TrkA. J. Biol. Chem. 275:37846–37852. [DOI] [PubMed] [Google Scholar]
- Ronn, L.C., B.P. Hartz, and E. Bock. 1998. The neural cell adhesion molecule (NCAM) in development and plasticity of the nervous system. Exp. Gerontol. 33:853–864. [DOI] [PubMed] [Google Scholar]
- Probstmeier, R., K. Kuhn, and M. Schachner. 1989. Binding properties of the neural cell adhesion molecule to different components of the extracellular matrix. J. Neurochem. 53:1794–1801. [DOI] [PubMed] [Google Scholar]
- Saffell, J.L., P. Doherty, M.C. Tiveron, R.J. Morris, and F.S. Walsh. 1995. NCAM requires a cytoplasmic domain to function as a neurite outgrowth-promoting neuronal receptor. Mol. Cell. Neurosci. 6:521–531. [DOI] [PubMed] [Google Scholar]
- Saffell, J.L., E.J. Williams, I.J. Mason, F.S. Walsh, and P. Doherty. 1997. Expression of a dominant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron. 18:231–242. [DOI] [PubMed] [Google Scholar]
- Schachner, M. 1997. Neural recognition molecules and synaptic plasticity. Curr. Opin. Cell Biol. 9:627–634. [DOI] [PubMed] [Google Scholar]
- Schlaepfer, D.D., S.K. Hanks, T. Hunter, and P. van der Geer. 1994. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 372:786–791. [DOI] [PubMed] [Google Scholar]
- Schmid, R.S., R.D. Graff, M.D. Schaller, S. Chen, M. Schachner, J.J. Hemperly, and P.F. Maness. 1999. NCAM stimulates the Ras-MAPK pathway and CREB phosphorylation in neuronal cells. J. Neurobiol. 38:542–558. [PubMed] [Google Scholar]
- Schuch, U., M.J. Lohse, and M. Schachner. 1989. Neural cell adhesion molecules influence second messenger systems. Neuron. 3:13–20. [DOI] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Skaper, S.D., W.J. Kee, L. Facci, G. Macdonald, P. Doherty, and F.S. Walsh. 2000. The FGFR1 inhibitor PD 173074 selectively and potently antagonizes FGF-2 neurotrophic and neurotropic effects. J. Neurochem. 75:1520–1527. [DOI] [PubMed] [Google Scholar]
- Tansey, M.G., R.H. Baloh, J. Milbrandt, and E.M. Johnson, Jr. 2000. GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron. 25:611–623. [DOI] [PubMed] [Google Scholar]
- van't Hof, W., and M.D. Resh. 1997. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J. Cell Biol. 136:1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, E.J., B. Mittal, F.S. Walsh, and P. Doherty. 1995. FGF inhibits neurite outgrowth over monolayers of astrocytes and fibroblasts expressing transfected cell adhesion molecules. J. Cell Sci. 108:3523–3530. [DOI] [PubMed] [Google Scholar]
- Zeng, L., L. D'Alessandri, M.B. Kalousek, L. Vaughan, and C.J. Pallen. 1999. Protein tyrosine phosphatase alpha (PTPalpha) and contactin form a novel neuronal receptor complex linked to the intracellular tyrosine kinase fyn. J. Cell Biol. 147:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., R.P. Trible, and L.E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 9:239–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.