Abstract
Thrombospondin (TSP)-1 has been reported to modulate T cell behavior both positively and negatively. We found that these opposing responses arise from interactions of TSP1 with two different T cell receptors. The integrin α4β1 recognizes an LDVP sequence in the NH2-terminal domain of TSP1 and was required for stimulation of T cell adhesion, chemotaxis, and matrix metalloproteinase gene expression by TSP1. Recognition of TSP1 by T cells depended on the activation state of α4β1 integrin, and TSP1 inhibited interaction of activated α4β1 integrin on T cells with its counter receptor vascular cell adhesion molecule-1. The α4β1 integrin recognition site is conserved in TSP2. A recombinant piece of TSP2 containing this sequence replicated the α4β1 integrin–dependent activities of TSP1. The β1 integrin recognition sites in TSP1, however, were neither necessary nor sufficient for inhibition of T cell proliferation and T cell antigen receptor signaling by TSP1. A second TSP1 receptor, CD47, was not required for some stimulatory responses to TSP1 but played a significant role in its T cell antigen receptor antagonist and antiproliferative activities. Modulating the relative expression or function of these two TSP receptors could therefore alter the direction or magnitude of T cell responses to TSPs.
Keywords: thrombospondins; integrins; chemotaxis; T cell antigen receptor; matrix metalloproteinases
Introduction
Thrombospondins (TSPs)* are a family of five extracellular matrix proteins that have cell- and context-specific effects on cell adhesion, growth, survival, differentiation, and motility (for reviews see Adams et al., 1995; Roberts, 1996; Bornstein et al., 2000; Lawler, 2000). TSP1 is the best-characterized protein in this family, because it is readily purified from platelet α-granules. Mice lacking TSP1 are viable but have several abnormalities, including a susceptibility to pneumonia during the neonatal period (Lawler et al., 1998). Based on the known role of the TSP1 receptor CD47 in immune function (for review see Brown and Frazier, 2001) and the in vitro responses to TSP1 of several cell types from the immune system (Savill et al., 1992; Yabkowitz et al., 1993; Mansfield and Suchard, 1994; Pierson et al., 1996; Vallejo et al., 2000; Li et al., 2001), regulation of immune responses by TSP1 may contribute to this TSP1-null phenotype.
TSP1 influences cell behavior by interacting with other extracellular matrix components, including latent TGFβ1, and with specific cell surface receptors. TSP1 receptors include αvβ3, α4β1, α5β1, and α3β1 integrins; CD36; CD47; low-density lipoprotein receptor–related protein; and heparan sulfate proteoglycans (HSPGs) (Roberts, 1996). Therefore, responses of cells to TSP1 probably reflect the integration of simultaneous signals from multiple TSP1 receptors as well as receptors for some extracellular ligands of TSP1. The expression levels and activation states of these receptors must be considered to understand the function of each receptor in mediating specific TSP1 responses.
Several TSP1 receptors are expressed in hematopoietic cells and have been implicated in the regulation of immune functions by TSP1. α5β1 and α4β1 integrins contributed to PMA-stimulated adhesion of CD4+ peripheral T lymphocytes on TSP1 (Yabkowitz et al., 1993). Based on global analysis of gene expression in T cells exposed to TSP1, however, a primary effect of TSP1 is to inhibit T cell antigen receptor (TCR) signaling (Li et al., 2001). Studies using a T cell line demonstrated roles for CD47 and HSPGs in mediating the inhibitory effects of TSP1 on T cell activation (Li et al., 2001). TSP1 also inhibits T cell proliferation (Beppu et al., 2001). Furthermore, a CD47-binding peptide from TSP1 inhibited the development of naive T cells into Th1 effectors (Avice et al., 2000). In contrast, interactions of TSP1 or TSP1 peptides with HSPG and β1 integrin receptors stimulated Ras–MAP kinase signaling in T cells (Wilson et al., 1999). Stimulatory effects of TSP1 or CD47-binding peptides derived from TSP1 have also been reported for the activation, infiltration, and clonal expansion of T cells (Reinhold et al., 1997; Ticchioni et al., 1997, 2001; Vallejo et al., 2000).
Taken together, these data indicate that TSP1 can both stimulate and inhibit specific signal transduction pathways in T cells. We have examined whether differential signaling through specific T cell TSP1 receptors could reconcile these apparently conflicting observations. We report here that the TSP1 receptors CD47 and α4β1 integrin are differentially required for modulation of TCR signaling, T cell proliferation, matrix metalloproteinase (MMP) expression, and T cell motility by TSP1. Furthermore, we demonstrate that the α4β1 integrin–dependent activities of TSP1 are replicated by recombinant NH2-terminal portions of TSP1 and TSP2 and identify peptide sequences in the NH2-terminal regions of both TSPs that are recognized by α4β1 integrin.
Results
The NH2-terminal heparin-binding domains of TSP1 and TSP2 contain an α4β1 integrin recognition site
Although α4β1 and α5β1 integrins are known to mediate adhesion of activated CD4+ T cells on a TSP1 substrate (Yabkowitz et al., 1993), the sites in TSP1 recognized by these integrins have not been defined. Recognition by α5β1 integrin has been predicted to involve the RGD sequence at residues 908–910, but no α4β1 integrin binding site was identified in TSP1. β1 integrin–mediated adhesion of both peripheral T cells (Yabkowitz et al., 1993) and Jurkat T lymphoma cells (Wilson et al., 1999) on TSP1 is activation dependent. We therefore identified β1 integrin binding sites by comparing adhesion of unstimulated Jurkat cells and cells treated with a β1 integrin–activating antibody on recombinant proteins spanning the entire TSP1 sequence (Fig. 1 A). β1 integrin–dependent adhesion was primarily mediated by the NH2-terminal region of TSP1, with a minor activity coinciding with the RGD sequence in the last type 3 repeat. Adhesion to a recombinant portion of TSP1 containing the NH2-terminal 175 amino acids was completely inhibited by a specific antagonist of α4β1 integrin (Fig. 1 B). Adhesion to recombinant proteins containing the type 3 repeats was mediated by α5β1 integrin, based on specific inhibition using both a specific function-blocking antibody and a peptide antagonist selective for α5β1 integrin (Fig. 1 B; unpublished data). T cells generally do not express αv integrins, and a specific antagonist of these integrins had no effect on adhesion to intact TSP1 or recombinant fragments containing the RGD sequence (unpublished data). Based on flow cytometry, we also confirmed that Jurkat cells lack α3β1 (unpublished data), the other β1 integrin known to recognize TSP1 (Krutzsch et al., 1999).
Figure 1.
α4β1 and α5β1 integrins mediate Jurkat cell adhesion to TSP1. (A) Resting Jurkat cells (closed bars) or cells stimulated by the β1 integrin– activating antibody TS2/16 (5 μg/ml, gray bars) were incubated on substrates coated with TSP1, GST, or the indicated recombinant portions of TSP1 for 20 min at 37°C. Some TSP1 sequences were fused to the COOH terminus of GST (G) or T7 10B capsid protein (T). Trimeric TSP1 was coated at 20 nM on a subunit basis, trimeric 1–356 (NoC1) was coated at 100 nM, and the remaining fragments were coated at 600 nM. Cells attached/mm2 are presented as mean ± SD, n = 3. (B) Differential roles of α4β1 and α5β1 integrins in mediating adhesion to two sites in TSP1 and to TSP1 versus FN. Substrates coated with intact TSP1 (10 μg/ml, solid bars), TSP1(1–175) (10 μg/ml, gray bars), GST–TSP1(877–1152) (30 μg/ml, striped bars), or FN (10 μg/ml, open bars) and blocked with BSA were incubated with TS2/16-activated Jurkat cells. Substrates blocked with BSA were used as a negative control. As indicated, cells were tested in the absence of inhibitors or in the presence of a specific α4β1 integrin antagonist ((4-((2- methylphenyl)aminocarbonyl)aminophenyl)acetyl-LDVP, 1 μM) or the selective α5β1 integrin blocking peptide GRGDNP (300 μM). Adhesion is presented normalized as a percent of the TS2/16-stimulated control for each substrate. (C) The NH2 termini of both TSP1 and TSP2 mediate activation-dependent T cell adhesion. Cell adhesion of resting Jurkat T cells (circles) or cells activated by TS2/16 (triangles) or PMA stimulation (squares) on recombinant NH2-terminal trimeric portions of TSP1 (NoC1, closed symbols) or TSP2 (NoC2, open symbols) was determined by assay of hexosaminidase activity and is presented as mean ± SD. (D) α4β1 integrin binding regions of TSP1 and TSP2 mediate adhesion of activated CD4+T cells. Resting T cells (solid bars) and T cells activated in the presence of 10 ng/ml PMA (gray bars) were incubated on the indicated substrates for 15 min. Adhesion is presented as mean ± SD, n = 3.
Because recombinant fragments of proteins may expose cryptic binding sites for integrins that are not functional in the intact proteins, we also compared the sensitivity to α4β1 and α5β1 antagonists of Jurkat cell adhesion on native platelet TSP1 and plasma fibronectin (FN), a known ligand for both integrins (Fig.1 B). Adhesion to TSP1 was more sensitive to the α4β1 integrin antagonist and less sensitive to the α5β1 antagonist than observed for adhesion of FN. Therefore, both integrin binding sites are functional in immobilized native TSP1, but Jurkat T cell adhesion to intact TSP1 is preferentially mediated by α4β1 integrin.
Integrin-dependent adhesion of peripheral T cells to TSP1 is induced by phorbol esters (Yabkowitz et al., 1993), and we observed a similar induction of Jurkat cell adhesion on trimeric human thrombospondin-1 residues 1–356 (NoC1) using PMA (Fig. 1 C). The dose dependence for PMA-activated cells was similar to that for TS2/16-activated cells. Remarkably, the corresponding recombinant trimeric NH2-terminal region of TSP2 was even more active for promoting adhesion of Jurkat cells activated using either PMA or TS2/16 (Fig. 1 C). Therefore, the NH2-terminal regions of both TSP1 and TSP2 contain binding sites for β1 integrins.
Adhesion of CD4+ peripheral T cells showed a similar preference for the α4β1 integrin binding sites in TSPs (Fig. 1 D). PMA activation stimulated adhesion to NoC1 and thrombospondin-2 residues 1–359 (NoC2) to a similar extent as to TSP1, whereas adhesion to a fusion protein expressing the α5β1 integrin binding domain of TSP1 was not enhanced by PMA.
Identification of an α4β1 integrin recognition sequence in TSP1 and TSP2
The smallest portion of TSP1 tested that supported α4β1 integrin–dependent adhesion of T cells contained amino acid residues 1–175. Comparison of this sequence with known α4β1 binding sequences in FN and vascular cell adhesion molecule-1 (VCAM-1) (Vonderheide et al., 1994; Moyano et al., 1997) using MACAW version 2.0.5 (Schuler et al., 1991) identified a potential recognition site at residues 159–164, containing the sequence AELDVP. A synthetic peptide with this sequence inhibited Jurkat cell adhesion on substrates coated with NoC1 or NoC2 (Fig. 2 A). TSP2 contains a similar sequence at the same position (VALDEP) that conserves the Asp residue typically required for α4β1 integrin ligands (Wang and Springer, 1998). A synthetic peptide, VALDEP, inhibited adhesion on NoC1 and NoC2 (Fig. 2 A). Substitution of the Asp residue of this peptide with Ala (VALAEP) markedly diminished its inhibitory activity, indicating that this residue is important for binding of the TSP2 peptide to α4β1 integrin. Adhesion to TSP1(1–175) was also specifically inhibited by the TSP2 peptide VALDEP but not by the control peptide VALAEP (Fig. 2 B). The α4β1 integrin specificity of T cell adhesion on TSP1(1–175) and NoC1 was further confirmed using the function-blocking α4β1 antibody P4C2 (Fig. 2 B).
Figure 2.
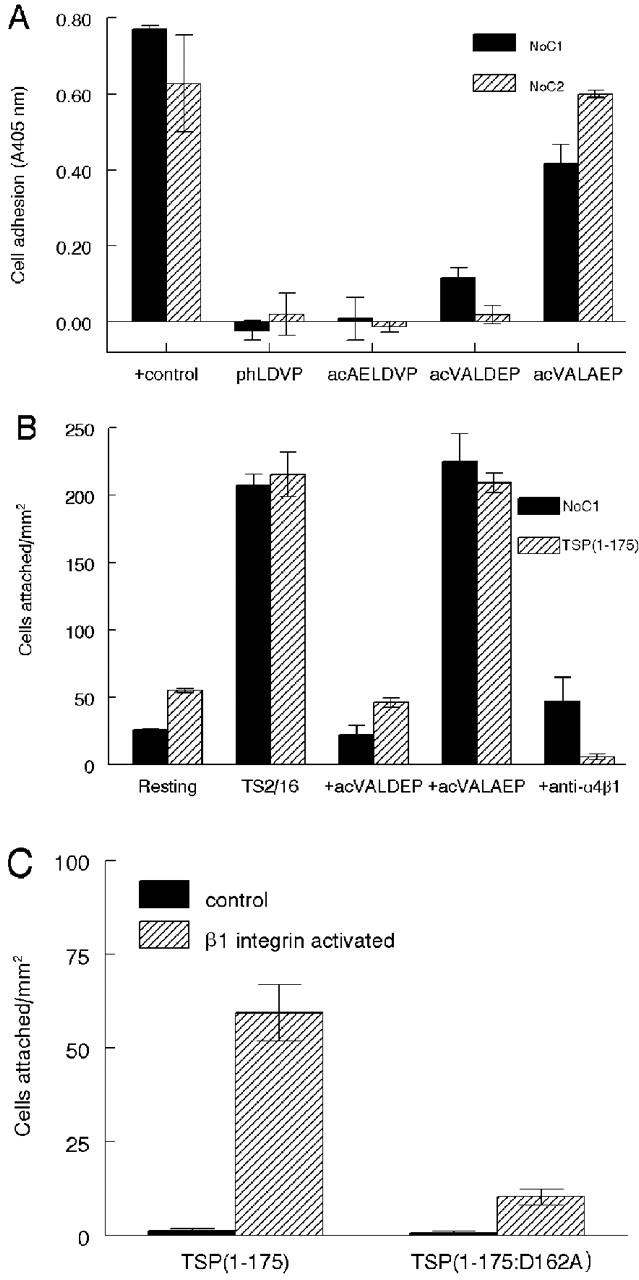
The NH2-terminal portions of both TSP1 and TSP1 contain α4β1 integrin recognition sequences. (A) Identification of α4β1 binding sites in the NH2 termini of TSP1 and TSP2. T cell adhesion was determined by the colorimetric assay. The peptide acetyl-AELDVP was derived from NoC1, acetyl-VALDEP was derived from NoC2, and the control peptide acetyl-VALAEP substituted Ala for Asp in the proposed α4β1 integrin binding site. The α4β1 integrin antagonist (4-((2-methylphenyl)aminocarbonyl)aminophenyl)acetyl-LDVP (phLDVP) was used as a positive blocking control. The solid bars represent adhesion on NoC1 (30 μg/ml) and the striped bars on NoC2 (30 μg/ml). (B) TSP2 peptide inhibits α4β1 integrin–dependent adhesion of Jurkat cells to NoC1 and TSP1(1–175). Substrates coated with 10 μg/ml NoC1 (solid bars) or TSP1(1–175) (striped bars) were incubated with resting or TS2/16-activated Jurkat cells or with activated cells in the presence of 150 μM of TSP2 peptide acetyl-VALDEP, 150 μM of the control peptide acetyl-VALAEP, or 5 μg/ml of the α4β1 integrin function–blocking antibody P4C2. (C) Substrates coated with 6.2 μg/ml of TSP1(1–175) or the mutated TSP1(1–175:D162A) were incubated with resting (solid bars) or TS2/16-stimulated Jurkat cells (striped bars). Cell adhesion was quantified microscopically.
To confirm the role of this sequence in α4β1 integrin–mediated T cell adhesion on TSP1, Asp(162) of TSP1(1–175) was mutated to an Ala residue, and adhesion was compared using the native and mutant recombinant proteins (Fig. 2 C). Activation-dependent adhesion was greatly diminished in the mutant protein, although some residual β1-dependent adhesion was observed in several independent experiments. Therefore, Asp(162) appears to play an important role in α4β1 integrin–mediated adhesion to this portion of TSP1, and the heparin binding sites in TSP1(1–175) are not sufficient to mediate adhesion of T cells. Residual activity of the mutant protein is consistent with the weak inhibitory activity of the peptide in which Asp was substituted by Ala (Fig. 2 A), but we cannot exclude the possibility that additional sequences participate in α4β1 integrin binding to this region of TSP1.
CD47 and HSPG are not sufficient to mediate T cell adhesion on TSP1
Although the preceding data establish that an α4β1 integrin binding site is required for activation-dependent adhesion to the NH2-terminal region of TSP1, they do not exclude roles of the two additional T cell receptors, HSPG and CD47, in mediating adhesion on intact TSP1. We therefore compared activation-dependent adhesion of Jurkat mutants deficient in β1 integrins (Romzek et al., 1998) or CD47 (Ticchioni et al., 2001). Flow cytometry confirmed deficiencies in the respective TSP1 receptors without a significant loss of the complementary receptor (Fig. 3 A). Neither the β1 integrin–activating antibody nor PMA stimulation significantly stimulated adhesion of β1 integrin–deficient T cells on intact TSP1 or its NH2-terminal region (Fig. 3 B). Therefore, the heparin and CD47 binding sites of intact TSP1 were not sufficient to mediate adhesion in the absence of β1 integrins. Conversely, adhesion of CD47-deficient T cells to the three proteins was induced by the β1 integrin–activating antibody and by PMA (Fig. 3 B). Although adhesion of the CD47-deficient mutant was slightly less than that of wild-type cells, the comparable decrease in adhesion to TSP1, which contains the CD47 binding domain, and two recombinant portions of TSP1 lacking this domain indicates that any contribution of CD47 to adhesion on TSP1 is indirect.
Figure 3.

CD47 is not required for activation-dependent T cell adhesion on TSP1. (A) Antibodies specific for α4 and β1 integrin subunits and CD47 were used for flow cytometry to compare expression of these TSP1 receptors in wild-type Jurkat cells and the β1-deficient (A1) and CD47-deficient mutant (JinB8). (B) Substrates coated with TSP1 (solid bars), NoC1 (striped bars), or TSP1(1–175) (open bars) were incubated with equal numbers of the indicated T cells alone or in the presence of 5 μg/ml TS2/16 antibody or 10 ng/ml PMA. Cell adhesion is presented as mean ± SD, n = 3.
Recombinant portions of TSP1 and TSP2 inhibit binding of VCAM-1 to activated T cells
VCAM-1 is an important α4β1 integrin ligand for mediating leukocyte adhesion to endothelium at sites of inflammation (Wang and Springer, 1998). Because VCAM-1 binds to activated α4β1 integrin with high affinity (Jakubowski et al., 1995), we used radiolabeled S7D–VCAM-1 to examine whether NoC1 and NoC2 could competitively inhibit the binding of VCAM-1 to T cells (Fig. 4 A). The specificity of S7D–VCAM-1 binding in this assay was verified using the α4β1 antagonist (4-((2-methylphenyl)aminocarbonyl)aminophenyl)acetyl-LDVP (Lin et al., 1999), which inhibited binding of S7D–VCAM-1 to both resting and activated T cells. Both NoC1 and NoC2 inhibited S7D–VCAM-1 binding to the activated T cells (Fig. 4 A), confirming the binding of both proteins to α4β1 integrin and suggesting that both soluble TSP1 and TSP2 could antagonize α4β1/VCAM-1–mediated adhesion.
Figure 4.
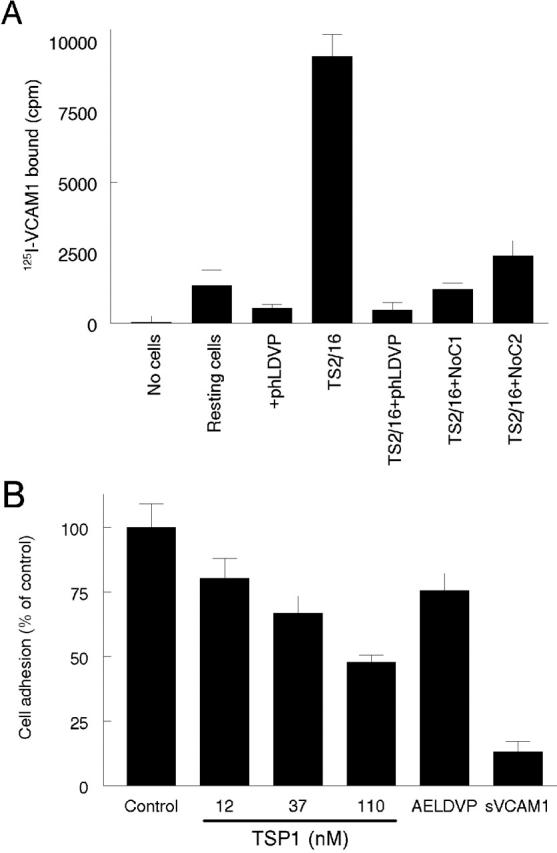
Thrombospondins compete with VCAM-1 for binding to α4β1 integrin. (A) NH2-terminal regions of TSP1 and TSP2 inhibit binding of soluble VCAM-1 to activated T cells. 125I-labeled S7D–VCAM-1 was used as a probe to bind either resting or TS2/16- (0.5 μg/ml) activated Jurkat T cells. NoC1 and NoC2 (10 μg/ml) were used to antagonize the binding of 125I-S7D–VCAM-1 (500,000 cpm) to α4β1 integrin on T cells. The α4-specific antagonist (4-((2-methylphenyl)aminocarbonyl)aminophenyl)acetyl-LDVP (phLDVP, 1 μM) was used as a positive control for α4β1 integrin blocking. S7D–VCAM-1 binding is presented as mean ± SD, n = 3. (B) Jurkat cell adhesion on wells coated using 1 μg/ml S7D–VCAM-1 was determined using the colorimetric assay in the presence of the indicated concentrations of TSP1, 100 μM of the TSP1 peptide acAELDVP, or 260 nM S7D–VCAM-1.
To test this hypothesis, TSP1 was tested as an inhibitor of Jurkat cell adhesion on immobilized S7D–VCAM-1 (Fig. 4 B). TSP1 was a dose-dependent inhibitor of adhesion on immobilized VCAM-1, and the α4β1-binding peptide from TSP1 also decreased T cell adhesion.
α4β1 integrin mediates chemotaxis of T cells to TSP1
A soluble form of VCAM-1 induced chemotaxis of Jurkat and synovial T cells by binding to α4β1 integrin (Kitani et al., 1998), suggesting that TSP1 might also be chemotactic for these cells. TSP1 induced a biphasic concentration-dependent chemotactic response in Jurkat cells that was maximal at 30 nM (Fig. 5 A). NoC1 and NoC2 also stimulated chemotaxis with similar dose response curves to platelet TSP1, indicating that the NH2-terminal region of TSP1 or TSP2 is sufficient to promote T cell chemotaxis. The same region was sufficient to stimulate chemotaxis of human primary CD4+ T cells, based on their responses to TSP1, NoC1, and NoC2 (Fig. 5 B).
Figure 5.
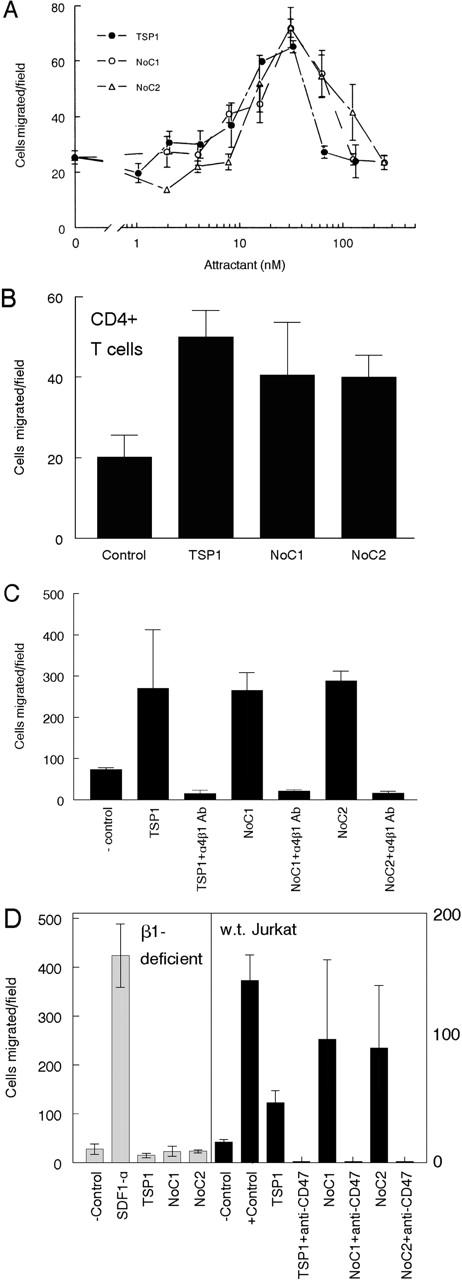
α4β1 integrin mediates chemotaxis of T cells to TSP1. (A) A modified Boyden chamber assay using polylysine-coated filters to support integrin-independent adhesion was used to characterize Jurkat T cell chemotaxis. The chemokine SDF1-α (25 ng/ml) was used as positive control (744 cells migrated/field). Results are represented as migrated cells per 20× field, mean ± SD, for the indicated concentrations of TSP1 (closed circles), NoC1 (open circles), and NoC2 (open triangles). (B) Chemotaxis of CD4+ T cells to optimal concentrations of TSP1, NoC1, and NoC2. (C) Cell migration assays stimulated by 30 μg/ml TSP1, 20 μg/ml NoC1, or 20 μg/ml NoC2 were performed in the presence or absence of an α4β1 integrin–specific blocking antibody (P4C2, 5 μg/ml) mixed with cells in the upper chamber. (D, left) α4β1 integrin is necessary for chemotaxis to NoC1 and NoC2 but not for chemotaxis to the chemokine SDF1. Chemotaxis of the β1-deficient Jurkat-derived A1 to TSP1 (20 μg/ml), NoC1 (5 μg/ml), and NoC2 (5 μg/ml) was assessed as described above. The chemokine SDF1-α was used as a positive control to confirm the migration capacity of the A1 cell line. (D, right) The CD47 function–blocking antibody B6H12 (20 μg/ml) added to the upper well with Jurkat cells inhibited chemotaxis stimulated by TSP1 as well as by NoC1 and NoC2, which lack the CD47 binding site.
Based on inhibition by an α4β1 integrin function–blocking antibody (P4C2; Fig. 5 C), α4β1 integrin is necessary for chemotaxis of Jurkat cells to TSP1, NoC1, and NoC2. To confirm the requirement for β1 integrins, we compared the chemotactic responses of wild-type and β1 integrin-deficient Jurkat cells (Fig. 5 D). The β1 integrin-deficient cells failed to migrate to TSP1 or the recombinant proteins, but migration to the CXCR4-binding chemokine SDF1α was normal in the β1 integrin–deficient cells. Although these cells could migrate in a chemotaxis assay without expressing β1 integrins, the NH2-terminal heparin-binding activities of NoC1 and NoC2 were not sufficient to promote chemotaxis in the absence of integrins.
We could not directly evaluate the role of CD47 in TSP1-stimulated chemotaxis using this approach, because CD47-deficient JinB8 cells did not migrate to any attractant tested (unpublished data). However, a function-blocking CD47 antibody (B6H12) inhibited motility stimulated by intact TSP1, NoC1, or NoC2 (Fig. 5 D). The latter do not contain the known TSP CD47 binding site, indicating that CD47 plays an essential but indirect role in these α4β1-mediated chemotactic responses.
TSP1 induces MMP expression through α4β1 integrin binding
Two previously known α4β1 integrin ligands, VCAM-1 and FN, induce MMP-2 and MMP-9 mRNA and protein expression in T cells (Esparza et al., 1999; Yakubenko et al., 2000). TSP1 also induces MMP-9 in endothelial cells, but this induction was attributed to a different TSP1 receptor (Qian et al., 1997). We used reverse transcriptase (RT)-PCR to examine expression of mRNAs for several MMPs in Jurkat cells exposed to soluble or immobilized TSP1 (Fig. 6 A). Both forms of TSP1 induced MMP-2, but the response was greater using immobilized TSP1. Therefore, all subsequent experiments were performed using immobilized proteins. NoC1 and NoC2 also induced MMP-2 expression. The responses were time dependent and maximal for both proteins at 4 h (Fig. 6 A). Similar results were observed using CD4+ T cells (Fig. 6 A). Both immobilized and soluble TSP1 induced MMP-2 mRNA, and induction was also observed using NoC2.
Figure 6.
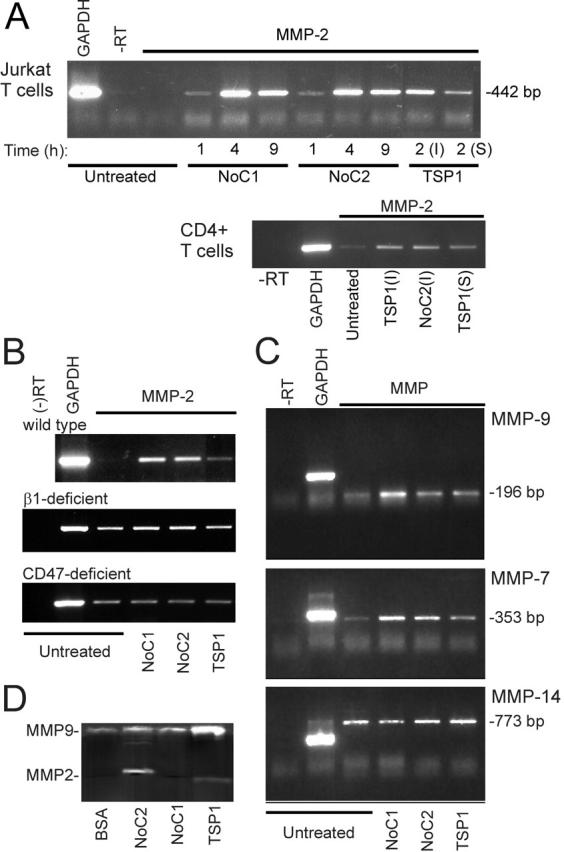
TSP1 and TSP2 induce MMP expression in T cells via α4β1 integrin binding. (A) Total mRNA was prepared from Jurkat cells (top) incubated for the indicated times on substrates coated with 10 μg/ml NoC1 or NoC2 or for 2 h with 20 μg/ml TSP1 immobilized (I) or in solution (S). Primary CD4+ T cells (bottom) were treated for 4 h in the presence of 1 mM Mn2+ as indicated. RT-PCR products using GAPDH or MMP-2 primers and a control reaction without reverse transcriptase (−RT) were analyzed after 31 (Jurkat) or 33 cycles of amplification (CD4+ cells) using a 1.5% agarose gel stained with ethidium bromide. (B) MMP-2 mRNA was analyzed using wild-type, β1-deficient, or CD47-deficient Jurkat cells after 4 h treatment with the indicated immobilized proteins. (C) Total mRNAs from Jurkat cells incubated for 4 h on uncoated plates or plates coated with 10 μg/ml NoC1, 10 μg/ml NoC2, or 20 μg/ml TSP1 were analyzed using the indicated primer sets (24 cycles for MMP-9 and 34 cycles for MMP-7 and MMP-14). (D) Gelatinase activity was assessed by zymography using 24 h–conditioned media from Jurkat cells plated on dishes coated with BSA, 10 μg/ml NoC1 or NoC2, or 30 μg/ml TSP1.
MMP-2 induction by intact TSP1 and the NoC constructs were β1 integrin–mediated, because no significant induction of MMP-2 mRNA was observed using β1 integrin–deficient T cells (Fig. 6 B). CD47 may play an indirect role in this response, because TSP1 did not induce MMP2 mRNA in CD47-deficient T cells, and the NoC fragments, which lack the CD47 binding site, were also inactive (Fig. 6 B). TSP1, NoC1, and NoC2 also induced mRNA expression of MMP-9 and MMP-7 (Fig. 6 C). In contrast, no induction was observed of the membrane-bound MMP-2 activator MMP-14 (Fig. 6 C), which was previously shown to be induced by FN (Esparza et al., 1999). These data indicate that the NH2-terminal regions of TSP1 and TSP2, which contain the α4β1 integrin binding sites, are sufficient to induce expression of MMP-2, MMP-7, and MMP-9 mRNAs in T cells, and CD47 may be indirectly required for the MMP-2 response to TSP1.
MMP induction at the protein level was confirmed by gelatin zymography (Fig. 6 D). TSP1, NoC1, and NoC2 increased MMP-9 activity. MMP-2 activity was induced by NoC2 and, apparently, was processed to a smaller activated form in cells exposed to TSP1 (Fig. 6 D).
α4β1 integrin binding is not sufficient for inhibition of TCR signaling by TSP1
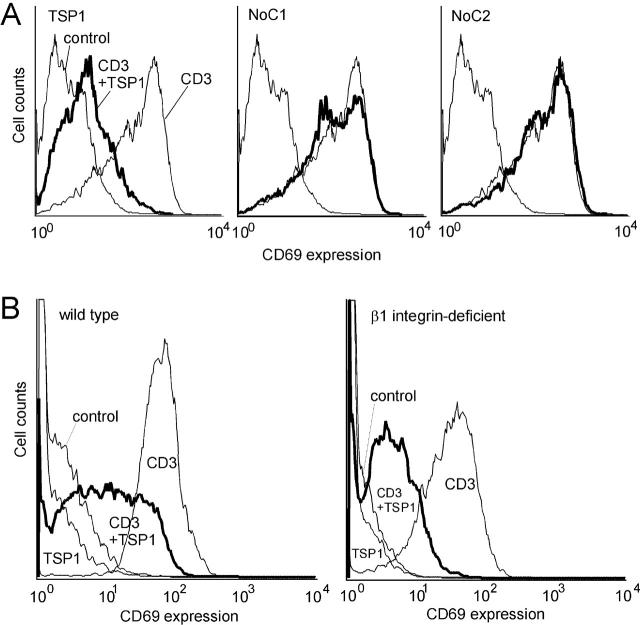
We previously demonstrated that TSP1 inhibits TCR-mediated T cell activation (Li et al., 2001). To determine whether α4β1 integrin is involved in this activity, we tested NoC1 and NoC2 for inhibition of TCR-mediated T cell activation (Fig. 7 A). TSP1 inhibited TCR-mediated T cell activation measured by induction of cell surface CD69 expression (90% inhibition in mean fluorescent intensity relative to the anti-CD3–stimulated positive control), but NoC1 (6% inhibition) and NoC2 (−12% inhibition) showed minimal or no inhibitory effects even using a fourfold higher molar concentration. Thus, the NH2-terminal regions of these proteins are not sufficient to mediate the inhibitory activity of TSP1 for T cell activation. TSP1 comparably inhibited CD69 expression in the wild-type and β1 integrin–deficient Jurkat cell lines (Fig. 7 B), demonstrating that β1 integrins are not required for the inhibitory effect of TSP1 on TCR signaling. Therefore binding of TSP1 to α4β1 integrin is neither necessary nor sufficient for the antagonist activity of TSP1 on TCR signaling. We previously demonstrated that two other T cell receptors for TSP1, CD47 and HSPG, mediate this inhibitory activity (Li et al., 2001).
Figure 7.
β1 integrins are not required for the inhibitory effect of TSP1 on TCR-mediated T cell activation. (A) The α4β1 integrin and NH2-terminal heparin binding sites of TSP1 and TSP2 are not sufficient to inhibit TCR-mediated T cell activation. Jurkat T cells with or without TCR stimulation by surface-bound anti-CD3 antibody, were treated with TSP1 (30 μg/ml), NoC1 (30 μg/ml), or NoC2 (30 μg/ml). Cells were stained with phycoerythrin-conjugated anti-CD69 antibody to measure T cell activation and analyzed by flow cytometry. (B) β1 integrins are not required for inhibition of TCR-mediated T cell activation by TSP1. The wild-type Jurkat cells and β1- deficient A1 cells were stimulated using surface-bound anti-CD3 antibody with or without TSP1. Cell surface CD69 was analyzed by flow cytometry as in panel A.
The antiproliferative activity of TSP1 requires CD47 but not α4β1 integrin
As recently reported for in vitro cocultures of peripheral T cells with antigen-presenting cells (Beppu et al., 2001), soluble TSP1 was a dose-dependent inhibitor of Jurkat T cell proliferation (Fig. 8 A). In contrast, soluble NoC1 had no effect on proliferation (Fig. 8 A), indicating that the α4β1 integrin and heparin binding sites contained in this trimeric portion of TSP1 are not sufficient to mediate its antiproliferative activity. Furthermore, immobilized TSP1 or NoC1 did not significantly inhibit proliferation after coating at the same concentrations (unpublished data), indicating that inhibition of T cell proliferation is a specific function of soluble TSP1. To further examine whether α4β1 integrin is necessary for the antiproliferative activity of intact TSP1, we used β1 integrin–deficient Jurkat cells. Soluble TSP1 inhibited proliferation of the β1-deficient T cells with a similar dose response as that obtained using the wild-type cells (Fig. 8 B). As expected, NoC1 did not inhibit proliferation of the β1-deficient cells. In contrast, proliferation of the CD47-deficient Jurkat mutant was only weakly inhibited by TSP1 (23% inhibition at 40 μg/ml; Fig. 8 C). The weak inhibition appeared to be specific, inasmuch as intact TSP1 was significantly more inhibitory than the NoC1 portion of TSP1. Nevertheless, these data demonstrate that CD47 expression on the T cells is necessary for the full antiproliferative activity of soluble TSP1.
Figure 8.
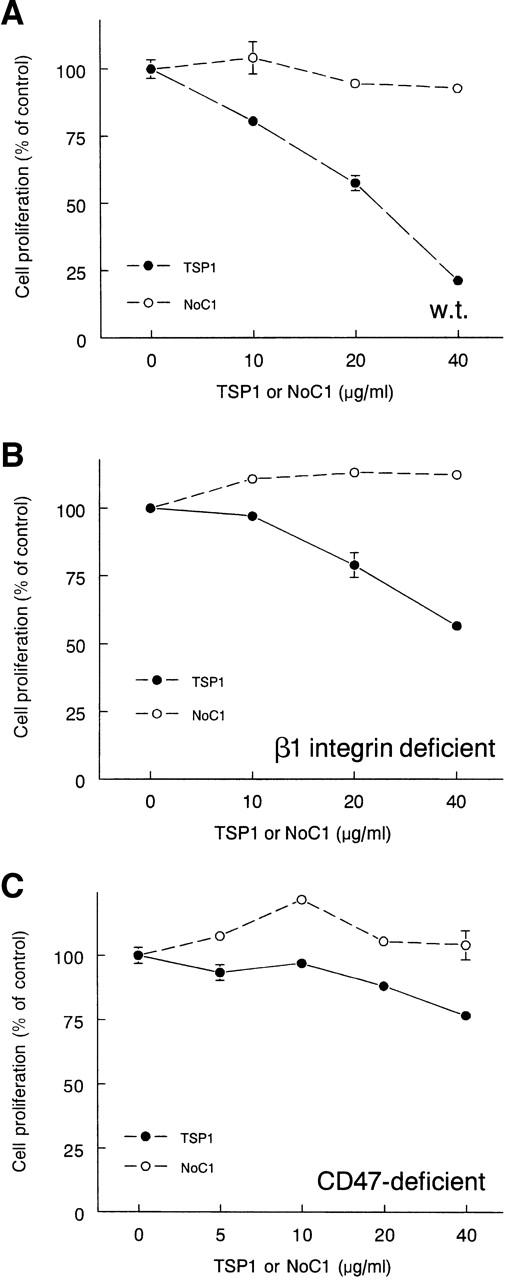
The antiproliferative activity of TSP1 for T cells requires CD47 but not β1 integrin expression. Wild-type Jurkat cells (A) and β1 integrin– (B) and CD47-deficient clones (C) were grown for 72 h in the presence of the indicated concentrations of TSP1 (closed circles) or NoC1 (open circles). Net proliferation was assessed from colorimetric tetrazolium assays at times 0 and 72 h. Results are presented as mean ± SD, n = 3, normalized to the proliferation response of untreated cells for each cell line.
Discussion
Our results demonstrate that interactions of TSP1 with two of its receptors on T cells elicit different biological responses. A sequence in the NH2-terminal pentraxin modules of TSP1 and TSP2 binds to α4β1 integrin and stimulates chemotaxis, MMP gene expression, and activation-dependent adhesion of T cells. The NH2-terminal heparin binding sites may contribute to some of these activities but are not sufficient, based on integrin blocking studies, mutagenesis of the integrin binding sequence, and loss of activity in β1 integrin–deficient Jurkat cells. These activities of TSP1 are retained in a recombinant portion of TSP1 that lacks binding sites for α5β1 integrin, CD36, and CD47. Therefore, interactions of TSP1 with the latter receptors are not required for these biological activities, although CD47 expression appears to play an indirect role in T cell chemotaxis and MMP induction. In contrast, the same recombinant proteins failed to replicate the antiproliferative and TCR antagonist activities of TSP1. These activities are mediated, at least in part, by interactions of TSP1 with CD47 and HSPGs on T cells (this study; Li et al., 2001).
Cellular responses to TSP1 can be modulated by altering the expression of specific TSP1 receptors, the activation state of these receptors, or conformation states of TSP1 that regulate its binding to specific receptors (Rodrigues et al., 2001). T cells express at least three TSP1 receptors, and of these, α4β1 is known to be regulated in its affinity and avidity for binding to ligands. α4β1 integrin is inactive in resting T cells but has low activity in resting Jurkat cells (Jakubowski et al., 1995). Activation of protein kinase C by phorbol esters, which increases α4β1 avidity but not its affinity for ligands (for review see Woods and Shimizu, 2001), induced adhesion of T cells on immobilized TSP1 and the NH2-terminal region of TSP2. Soluble TSP1 also inhibited interactions of activated α4β1 integrin with its high-affinity ligand VCAM-1. Therefore both stimulatory and inhibitory activities of TSPs may be modulated by signals that alter α4β1 integrin activation. α4β1 integrin activation is modulated by signals resulting from ligation of the TCR (Jakubowski et al., 1995), CD47 (Ticchioni et al., 2001), and some chemokine receptors (Jakubowski et al., 1995). Notably, CD47 itself is a TSP1 receptor, and binding of TSP1 peptides to CD47 activates several integrins (for review see Brown and Frazier, 2001). Therefore, binding of soluble TSP1 to CD47 could potentially enhance adhesion of T cells to immobilized TSP1 mediated by α4β1 integrin. This cross-talk between CD47 and α4β1 integrin may regulate arrest of T cells on inflammatory endothelium (Ticchioni et al., 2001).
Our data define a new α4β1 integrin recognition sequence in the NH2-terminal pentraxin-related domain of TSP1. This site is conserved in TSP2 and is distinct from the previously identified α3β1 integrin binding site in the same domain of TSP1 (Krutzsch et al., 1999). Thus, the NH2-terminal domains of TSPs functionally resemble the paralogous G domains of laminins (Beckmann et al., 1998) in that they contain multiple β1 integrin recognition sites. Although the LDVP sequence we identified in TSP1 is conserved at the corresponding position in some laminin G domain modules (unpublished data), recognition of laminins by α4β1 integrin has not been reported. Based on alignment with other members of the pentraxin family of known structure (Beckmann et al., 1998), the α4β1 integrin binding sites of TSP1 and TSP2 are located in a loop connecting the predicted β strands J and K. This is consistent with the location of the α4β1 integrin binding sequence IDSP of VCAM-1 in a loop between its C and D β strands (Jones et al., 1995).
TSP1 regulates the expression of MMP-9 in endothelial cells (Qian et al., 1997), and TSP2 also regulates extracellular MMP-2 through direct interactions (Yang et al., 2001). Previously identified ligands of α4β1 integrin, including VCAM1 and FN, induced expression of MMPs in T cells and fibroblasts (Huhtala et al., 1995; Xia et al., 1996b; Yakubenko et al., 2000). Our data demonstrate that recombinant portions of TSP1 and TSP2 containing α4β1 integrin binding sites are sufficient to induce MMP-2, MMP-7, and MMP-9 expression in T cells. Notably, these recombinant proteins lack the TSP type 1 repeat sequence implicated in MMP-9 induction by TSP1 in endothelial cells (Qian et al., 1997). Our data suggest that MMP induction is a general T cell response to all α4β1 integrin ligands and that this integrin may mediate the observed effects of TSPs on MMP expression in other cell types.
Inhibition of TCR signaling by TSP1 in Jurkat cells is independent of α4β1 integrin ligation, but this result does not eliminate the possibility that TSP1 and TSP2 may positively modulate T cell activation through this receptor. Integrins are well-documented costimulators of TCR signaling in peripheral T cells (for review see Epler et al., 2000). However, Jurkat cells lack CasL, which is required for β1 integrin costimulation of TCR signaling (Kamiguchi et al., 1999). Therefore, we can observe only an inhibitory effect of TSP1 on TCR signaling in Jurkat T cells. In normal T cells, a second positive signal from TSP1 interacting with α4β1 integrin may offset this inhibition. Further investigation is needed to define the net effect of TSP1 on TCR signaling and T cell activation in various physiological and pathological contexts.
Both TSP1 and FN recognize α4β1 and α5β1 integrins on T cells. Our data show that the relative contributions of these two integrins differ, however, for mediating T cell adhesion to each protein. α5β1 integrin is the predominant FN receptor, whereas α4β1 is the major TSP1 receptor. FN is a constitutive component of extracellular matrix, but in most tissues TSP1 is only present in matrix at sites of injury and tissue remodeling (Adams et al., 1995). The different relative strengths of TSP1 and FN interactions with these two integrins may therefore indicate to a T cell whether the tissue microenvironment it transverses is undergoing these processes, and this may in turn modulate each of the T cell behaviors we have examined here.
Although FN and TSP1 share these two integrin receptors on T cells, their effects on T cell behavior differ. FN stimulates proliferation of T cells and TCR signaling (Davis et al., 1990; Shimizu et al., 1990), whereas TSP1 inhibits these responses. α4β1 and α5β1 were implicated in the stimulation of T cell proliferation by FN (Davis et al., 1990; Shimizu et al., 1990), which is consistent with our evidence that these integrins mediate some positive effects of TSP1 on T cells but not its antiproliferative activity. CD47, which is required for the inhibitory activities, is a receptor for TSP1 but not for FN.
Our data suggest that TSP1 and TSP2 could modulate recirculation of T cells and diapedesis of other leukocytes. We demonstrated that TSPs inhibit VCAM-1 binding to T cells and T cell adhesion mediated by α4β1 integrin binding to VCAM-1. Because α4 integrin function is essential for lymphocyte recruitment to sites of inflammation (Arroyo et al., 2000), TSP1 and TSP2 could potentially inhibit this process. Conversely, we found that TSP1 and the NH2-terminal portion of TSP2 stimulate chemotaxis of T cells by engagement of the same integrin and stimulate expression of several MMPs that may be necessary for passage of T cells through basement membranes (Xia et al., 1996a; Faveeuw et al., 2001) or tissue invasion (Lynch and McDonnell, 2000). Thus, expression of TSP1 or TSP2 in tissues could enhance recruitment of T cells to these sites by facilitating cell motility and invasion. Combined with the higher potency we observed for stimulating chemotaxis versus inhibiting α4β1 integrin–VCAM-1 binding, the net effect of TSP1 and TSP2 expression in tissue may be to enhance recruitment. In this context, it is notable that increased leukocyte recruitment was observed in breast tumors overexpressing TSP1 (Weinstat-Saslow et al., 1994).
Materials and methods
Proteins and peptides
TSP1 was purified from human platelets obtained from the NIH blood bank (Roberts et al., 1994). A recombinant trimeric portion of TSP1 (NoC1, residues 1–356 of the mature protein) was prepared as previously described in insect cells using the pAcGP67.coco transplacement plasmid to generate recombinant baculovirus (Misenheimer et al., 2000). The corresponding recombinant portion of TSP2 (NoC2, residues 1–359 of the mature protein; GenBank/EMBL/DDBJ accession no. L12350) was expressed and purified by a similar method using pAcGP67.coco and was also trimeric. The sequence of NoC2 at residues 153–155 is DSF rather than GPV as reported in GenBank/EMBL/DDBJ. MG63 human osteosarcoma cells were the source of the 5′ portion of the full-length TSP2 cDNA used in preparing pAcGP67.coco for the expression of recombinant NoC2. A recombinant piece of TSP1 (Met residues 1–175; provided by Tikva Vogel, BioTechnology General, Rehovot, Israel), a T7 10B capsid protein fusion expressing residues 879–947 (Guo et al., 1998), and glutathione-S-transferase (GST) fusion proteins expressing various regions of TSP1 (provided by Jack Lawler, Harvard University, Boston, MA) were prepared as previously described (Vogel et al., 1993; Guo et al., 1998). Synthetic peptides derived from TSP1 and TSP2 were synthesized and characterized as previously described (Krutzsch et al., 1999).
Recombinant soluble 7 domain VCAM-1 (S7D–VCAM-1, residues 1–674 of the mature protein; GenBank/EMBL/DDBJ accession no. X53051) was expressed in insect cells after insertion into pAcGP67.coco and generation of recombinant baculovirus. S7D–VCAM-1 was purified on a Ni+-chelate resin as previously described for NoC1 (Misenheimer et al., 2000).
Site-directed mutagenesis of Asp(162) to Ala in TSP1(1–175) was performed using the Stratagene QuickChange mutagenesis kit. The forward and reverse primer sequences were 5′-GAG AAT GCT GAG TTG GCC GTC CCC ATC CAA AGC G-3′ and 5′-GCT TTG GAT GGG GAC GGC CAA CTC AGC ATT CTC C-3′, respectively. After transformation into Escherichia coli XL1- blue, mutant clones were verified by DNA sequencing. The mutated plasmid was then transformed into E. coli A4255F−. E. coli A4255F− transformants were grown overnight at 28°C in Superbroth plus 50 μg/ml carbenicillin and induced by adding 10 g/liter of glucose and incubating at 42°C for 2 h. Inclusion bodies were isolated, and the mutant recombinant protein was purified as previously described for the wild-type recombinant protein (Vogel et al., 1993).
Cell culture
Jurkat T cells were maintained in RPMI 1640 medium supplemented with 10% FBS, 2 mM glutamine, penicillin, and streptomycin. β1 integrin–deficient (A1) and CD47-deficient (JinB8) T cell lines derived from Jurkat T cells (Romzek et al., 1998; Ticchioni et al., 2001) were provided by Yoji Shimizu (University of Minnesota Medical School, Minneapolis, MN) and Eric Brown (University of California San Francisco, San Francisco, CA), respectively. All cell cultures were grown at 37°C with 5% CO2.
Human primary CD4+ T cells were isolated by magnetic sorting using CD4 positive selection MicroBeads and autoMACS magnetic isolation system (Miltenyi Biotec). Human peripheral blood mononuclear cells were isolated by Ficoll gradient centrifugation from buffy coats obtained from normal donors (protocol no. 99-CC-0168), and CD4+ cells were purified according to the manufacturer's instructions. Cells were resuspended in complete RPMI medium and rested at 4°C overnight. FACS® analysis demonstrated >95% purity of the isolated CD4+ T cells (unpublished data).
Antibodies and reagents
Anti-CD3 antibody (clone HIT3a) was obtained from BD PharMingen. Anti-CD47 antibody (clone C1Km) was from ICN Biomedicals. A function-blocking antibody for α4β1 integrin (clone P4C2) was from GIBCO BRL. A CD47 function–blocking antibody, B6H12, and the β1 integrin function–stimulating antibody, TS2/16 (Hemler et al., 1984), were produced from hybridoma cell lines (American Type Culture Collection). A human integrin β1 blocking antibody (mAb13) was provided by Ken Yamada (National Institute of Dental and Craniofacial Research, Bethesda, MD). An α5β1 integrin peptide antagonist (GRGDNP; Pierschbacher and Ruoslahti, 1987) and an α4β1 integrin antagonist (4-((2-methylphenyl)aminocarbonyl)aminophenyl)acetyl-LDVP (Lin et al., 1999) were obtained from Bachem. SDF1α was obtained from Sigma-Aldrich.
Cell adhesion assays
Matrix protein–mediated cell adhesion was measured as previously described (Wilson et al., 1999). TSP1 and the NoC proteins were coated in PBS, and the other recombinant portions of TSP1 were coated in 25 mM NaHCO3 buffer, pH 8.2. Overnight cell cultures (<5 × 105 cells/ml) were resuspended in RPMI with 0.1% BSA (Sigma-Aldrich) at 2 × 105 cells/ml. The plates were chilled in a 4°C bath, and 100 μl of cell suspension with the indicated treatment was added into each well. The plates were then incubated in a 37°C bath for 15 min. Unbound cells were removed by washing, and adherent cells were quantified by hexosaminidase assay (Wilson et al., 1999).
Adhesion was also assessed using a microscopic assay. TSP1, recombinant proteins, or S7D–VCAM-1 diluted in Dulbecco's PBS or NaHCO3 buffer were adsorbed on bacteriological polystyrene dishes by incubation overnight at 4°C. After blocking with 1% BSA in Dulbecco's PBS, adhesion assays were performed by adding cells to prewarmed dishes containing RPMI with 1 mg/ml BSA. Cell attachment and spreading were quantified after 15 min by washing to remove nonadherent cells, fixing the adherent cells with 1% glutaraldehyde in PBS, and staining with Diff-Quik (Dade International).
Cell migration assay
A 48-well microwell modified Boyden chamber was used with 8-μm (Jurkat) or 5-μm (CD4+ T cells) pore polyvinylpyrrolidone-free polycarbonate membranes (Neuro Probe, Inc.). Wells in the lower chamber contained assay medium (RPMI with 0.1% BSA) and attractants as indicated. Polycarbonate membranes were coated overnight at 4°C with either 100 μg/ml of gelatin in 0.1% acetic acid or 20 μg/ml of polylysine in PBS and air dried. Jurkat cultures (<5 × 105 cells/ml) were resuspended in assay medium at 2 × 106 cells/ml with the indicated additions, added into wells of the upper chamber, and incubated at 37°C for 2–5 h. The membranes were fixed and stained, cells on the upper face were removed, and cells migrated to the lower face of the membrane were counted microscopically.
Flow cytometry analysis
To analyze T cell activation, expression of CD69 was measured by flow cytometry as previously described (Li et al., 2001). Cells were activated by incubating with surface-bound anti-CD3 antibody (BD PharMingen) in RPMI plus 0.1% BSA. After a 24-h stimulation at 37°C, the cells were washed and stained with PE-conjugated anti-CD69 antibody (BD PharMingen). CD69 expression was quantified using a FACS®Caliber flow cytometer (Becton Dickinson).
VCAM-1 cell binding assay
S7D–VCAM-1 was labeled with 125I using Iodogen (Pierce Chemical Co.). Jurkat T cells were washed with 4°C chilled Dulbecco's PBS (without Ca2+ or Mg2+) and resuspended in chilled binding buffer (RPMI with 0.1% BSA) at 3 × 106 cells/ml. On ice, 100 μl of the cell suspension was premixed with the indicated concentrations of TSP1, NoC1, or NoC2. 125I-S7D–VCAM-1, diluted in chilled binding buffer, was added into each tube to bring the final volume to 200 μl. The tubes were mixed by vortexing and transferred to a 37°C water bath for 15 min. The cell suspensions were then transferred to plastic tubes containing 100 μl of Nyosil oil (William F. Nye, Inc.), centrifuged for 1 min, and washed with 200 μl of cell binding buffer. The pellets were collected, and the bound radioactivity was quantified.
Proliferation
Effects of soluble or immobilized TSP1 reagents on Jurkat cell proliferation were quantified using a tetrazolium dye proliferation assay (Cell Titer Assay; Promega). The indicated cell lines (7.5 × 103 cells/well) were seeded in 96-well Nunc tissue culture plates for treatment with soluble proteins or in 96-well Nunc Maxisorp plates precoated with TSP1 or NoC1 and blocked with 1% BSA. Proliferation was assessed after growth for 72 h in RPMI medium containing 2% FCS.
MMP expression
Semiquantitative RT-PCR was used to analyze effects of TSPs on expression of mRNAs for MMP-2, MMP-7, MMP-9, and MMP-14 by Jurkat and CD4+ T cells. Cells were placed in untreated Falcon 1008 dishes or dishes previously coated overnight with TSP1, NoC1, or NoC2. Total RNA was isolated using Trizol reagent (GIBCO BRL) according to the instructions of the manufacturer. First strand cDNA synthesis was performed with Superscript II reverse transcriptase (GIBCO BRL) and 16 μg/ml oligo(dT) using 2 μg of total RNA. The enzyme was inactivated at 70°C for 15 min. The cDNA was amplified using Platinum Taq DNA polymerase (GIBCO BRL) and specific primer pairs to amplify glyceraldehyde phosphate dehydrogenase, MMP-2, MMP-7, MMP-9, or MMP-14 sequences (Table I). Amplification was conducted using two cycles of 1 min at 95°C and 4 min at 55°C followed by the indicated number of cycles of 1 min at 95°C, 2.5 min at 55°C, and 10 min at 70°C.
Table I. Primer sequences for MMPs.
| MMP | Primer sequences | PCR product size |
|---|---|---|
| MMP-2 | ||
| sense | 5′CCTGAGCTCCCGGAAAAGATTGAT3′ | |
| antisense | 5′AGCAGCCTAGCCAGTCGGATTTGA3′ | 442 bp |
| MMP-7 | ||
| sense | 5′CGGAATTCCACCTACAGGATCGTATCATA3′ | |
| antisense | 5'GCTCTAGATCAGAGGAATGTCCCATACCC3' | 353 bp |
| MMP-9 | ||
| sense | 5′AGTTCCCGGAGTGAGTTGAA3′ | |
| antisense | 5′CTCCACTCCTCCCTTTCCTC3′ | 196 bp |
| MMP-14 | ||
| sense | 5′AAACCCCAAAAACCCCACCTATG3′ | |
| antisense | 5′GGCGTCTGAAGAAGAAGACTGCAAG3′ | 773 bp |
For gelatin zymography (Yakubenko et al., 2000), 0.02% Brij 35 was added to 24 h serum-free conditioned media, which were then concentrated 50- to 100-fold using Centricon YM10. Samples were analyzed using 10% acrylamide, 0.1% gelatin SDS gels without reduction. Gels were washed and developed for 24 h at room temperature in 50 mM Tris, 200 mM NaCl, 5 mM CaCl2, 0.05% Tween 20, 0.02% NaN3, pH 7.2.
Acknowledgments
We thank Drs. Jack Lawler, Tikva Vogel, Ken Yamada, Yoji Shimizu, and Eric Brown for providing reagents. We thank Paul Tooney for the original preparation of full-length cDNA for TSP2.
This work was supported in part by NIH grants HL54462 and 56396 (to D.F. Mosher).
Footnotes
Abbreviations used in this paper: FN, fibronectin; GST, glutathione-S-transferase; HSPG, heparan sulfate proteoglycan; MMP, matrix metalloproteinase; NoC1, trimeric human thrombospondin-1 residues 1–356; NoC2, thrombospondin-2 residues 1-359; RT-PCR, reverse transcriptase-PCR; TCR, T cell antigen receptor; TSP, thrombospondin; VCAM-1, vascular cell adhesion molecule-1.
References
- Adams, J.C., R.P. Tucker, and J. Lawler. 1995. The Thrombospondin Gene Family. R.G. Landes Company, Austin, TX. 200 pp.
- Arroyo, A.G., D. Taverna, C.A. Whittaker, U.G. Strauch, B.L. Bader, H. Rayburn, D. Crowley, C.M. Parker, and R.O. Hynes. 2000. In vivo roles of integrins during leukocyte development and traffic: insights from the analysis of mice chimeric for alpha 5, alpha v, and alpha 4 integrins. J. Immunol. 165:4667–4675. [DOI] [PubMed] [Google Scholar]
- Avice, M.N., M. Rubio, M. Sergerie, G. Delespesse, and M. Sarfati. 2000. CD47 ligation selectively inhibits the development of human naive T cells into Th1 effectors. J. Immunol. 165:4624–4631. [DOI] [PubMed] [Google Scholar]
- Beckmann, G., J. Hanke, P. Bork, and J.G. Reich. 1998. Merging extracellular domains: fold prediction for laminin G-like and amino-terminal thrombospondin-like modules based on homology to pentraxins. J. Mol. Biol. 275:725–730. [DOI] [PubMed] [Google Scholar]
- Beppu, R., K. Nakamura, H. Miyajima-Uchida, M. Kuroki, P.D. Khare, Y. Yamauchi, Y. Yamashita, and T. Shirakusa. 2001. Soluble thrombospondin-1 suppresses T cell proliferation and enhances IL-10 secretion by antigen presenting cells stimulated with phytohemagglutinin. Immunol. Invest. 30:143–156. [DOI] [PubMed] [Google Scholar]
- Bornstein, P., L.C. Armstrong, K.D. Hankenson, T.R. Kyriakides, and Z. Yang. 2000. Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol. 19:557–568. [DOI] [PubMed] [Google Scholar]
- Brown, E.J., and W.A. Frazier. 2001. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 11:130–135. [DOI] [PubMed] [Google Scholar]
- Davis, L.S., N. Oppenheimer-Marks, J.L. Bednarczyk, B.W. McIntyre, and P.E. Lipsky. 1990. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J. Immunol. 145:785–793. [PubMed] [Google Scholar]
- Epler, J.A., R. Liu, and Y. Shimizu. 2000. From the ECM to the cytoskeleton and back: how integrins orchestrate T cell action. Dev. Immunol. 7:155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza, J., C. Vilardell, J. Calvo, M. Juan, J. Vives, A. Urbano-Marquez, J. Yague, and M.C. Cid. 1999. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood. 94:2754–2766. [PubMed] [Google Scholar]
- Faveeuw, C., G. Preece, and A. Ager. 2001. Transendothelial migration of lymphocytes across high endothelial venules into lymph nodes is affected by metalloproteinases. Blood. 98:688–695. [DOI] [PubMed] [Google Scholar]
- Guo, N., V.S. Zabrenetzky, L. Chandrasekaran, J.M. Sipes, J. Lawler, H.C. Krutzsch, and D.D. Roberts. 1998. Differential roles of protein kinase C and pertussis toxin-sensitive G-binding proteins in modulation of melanoma cell proliferation and motility by thrombospondin-1. Cancer Res. 58:3154–3162. [PubMed] [Google Scholar]
- Hemler, M.E., F. Sanchez-Madrid, T.J. Flotte, A.M. Krensky, S.J. Burakoff, A.K. Bhan, T.A. Springer, and J.L. Strominger. 1984. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J. Immunol. 132:3011–3018. [PubMed] [Google Scholar]
- Huhtala, P., M.J. Humphries, J.B. McCarthy, P.M. Tremble, Z. Werb, and C.H. Damsky. 1995. Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J. Cell Biol. 129:867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski, A., M.D. Rosa, S. Bixler, R. Lobb, and L.C. Burkly. 1995. Vascular cell adhesion molecule (VCAM)-Ig fusion protein defines distinct affinity states of the very late antigen-4 (VLA-4) receptor. Cell Adhes. Commun. 3:131–142. [DOI] [PubMed] [Google Scholar]
- Jones, E.Y., K. Harlos, M.J. Bottomley, R.C. Robinson, P.C. Driscoll, R.M. Edwards, J.M. Clements, T.J. Dudgeon, and D.I. Stuart. 1995. Crystal structure of an integrin-binding fragment of vascular cell adhesion molecule-1 at 1.8 A resolution. Nature. 373:539–544. [DOI] [PubMed] [Google Scholar]
- Kamiguchi, K., K. Tachibana, S. Iwata, Y. Ohashi, and C. Morimoto. 1999. Cas-L is required for beta 1 integrin-mediated costimulation in human T cells. J. Immunol. 163:563–568. [PubMed] [Google Scholar]
- Kitani, A., N. Nakashima, T. Izumihara, M. Inagaki, X. Baoui, S. Yu, T. Matsuda, and T. Matsuyama. 1998. Soluble VCAM-1 induces chemotaxis of Jurkat and synovial fluid T cells bearing high affinity very late antigen-4. J. Immunol. 161:4931–4938. [PubMed] [Google Scholar]
- Krutzsch, H.C., B. Choe, J.M. Sipes, N. Guo, and D.D. Roberts. 1999. Identification of an alpha(3)beta(1) integrin recognition sequence in thrombospondin-1. J. Biol. Chem. 274:24080–24086. [DOI] [PubMed] [Google Scholar]
- Lawler, J. 2000. The functions of thrombospondin-1 and -2. Curr. Opin. Cell Biol. 12:634–640. [DOI] [PubMed] [Google Scholar]
- Lawler, J., M. Sunday, V. Thibert, M. Duquette, E.L. George, H. Rayburn, and R.O. Hynes. 1998. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J. Clin. Invest. 101:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., L. He, K.E. Wilson, and D.D. Roberts. 2001. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J. Immunol. 166:2427–2436. [DOI] [PubMed] [Google Scholar]
- Lin, K., H.S. Ateeq, S.H. Hsiung, L.T. Chong, C.N. Zimmerman, A. Castro, W.C. Lee, C.E. Hammond, S. Kalkunte, L.L. Chen, et al. 1999. Selective, tight-binding inhibitors of integrin alpha4beta1 that inhibit allergic airway responses. J. Med. Chem. 42:920–934. [DOI] [PubMed] [Google Scholar]
- Lynch, C.C., and S. McDonnell. 2000. The role of matrilysin (MMP-7) in leukaemia cell invasion. Clin. Exp. Metastasis. 18:401–406. [DOI] [PubMed] [Google Scholar]
- Mansfield, P.J., and S.J. Suchard. 1994. Thrombospondin promotes chemotaxis and haptotaxis of human peripheral blood monocytes. J. Immunol. 153:4219–4229. [PubMed] [Google Scholar]
- Misenheimer, T.M., K.G. Huwiler, D.S. Annis, and D.F. Mosher. 2000. Physical characterization of the procollagen module of human thrombospondin 1 expressed in insect cells. J. Biol. Chem. 275:40938–40945. [DOI] [PubMed] [Google Scholar]
- Moyano, J.V., B. Carnemolla, C. Dominguez-Jimenez, M. Garcia-Gila, J.P. Albar, P. Sanchez-Aparicio, A. Leprini, G. Querze, L. Zardi, and A. Garcia-Pardo. 1997. Fibronectin type III5 repeat contains a novel cell adhesion sequence, KLDAPT, which binds activated alpha4beta1 and alpha4beta7 integrins. J. Biol. Chem. 272:24832–24836. [DOI] [PubMed] [Google Scholar]
- Pierschbacher, M.D., and E. Ruoslahti. 1987. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J. Biol. Chem. 262:17294–17298. [PubMed] [Google Scholar]
- Pierson, B.A., K. Gupta, W.-S. Hu, and J.S. Miller. 1996. Human natural killer cell expansion is regulated by thrombospondin-mediated activation of transforming growth factor-beta1 and independent accessory cell-derived contact and soluble factors. Blood. 87:180–189. [PubMed] [Google Scholar]
- Qian, X., T.N. Wang, V.L. Rothman, R.F. Nicosia, and G.P. Tuszynski. 1997. Thrombospondin-1 modulates angiogenesis in vitro by up-regulation of matrix metalloproteinase-9 in endothelial cells. Exp. Cell Res. 235:403–412. [DOI] [PubMed] [Google Scholar]
- Reinhold, M.I., F.P. Lindberg, G.J. Kersh, P.M. Allen, and E.J. Brown. 1997. Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway. J. Exp. Med. 185:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, D.D. 1996. Regulation of tumor growth and metastasis by thrombospondin-1. FASEB J. 10:1183–1191. [PubMed] [Google Scholar]
- Roberts, D.D., J. Cashel, and N. Guo. 1994. Purification of thrombospondin from human platelets. J. Tissue Cult. Methods. 16:217–222. [Google Scholar]
- Rodrigues, R.G., N. Guo, L. Zhou, J.M. Sipes, S.B. Williams, N.S. Templeton, H.R. Gralnick, and D.D. Roberts. 2001. Conformational regulation of the fibronectin binding and alpha 3beta 1 integrin-mediated adhesive activities of thrombospondin-1. J. Biol. Chem. 276:27913–27922. [DOI] [PubMed] [Google Scholar]
- Romzek, N.C., E.S. Harris, C.L. Dell, J. Skronek, E. Hasse, P.J. Reynolds, S.W. Hunt III, and Y. Shimizu. 1998. Use of a beta1 integrin-deficient human T cell to identify beta1 integrin cytoplasmic domain sequences critical for integrin function. Mol. Biol. Cell. 9:2715–2727. [PMC free article] [PubMed] [Google Scholar]
- Savill, J., N. Hogg, Y. Ren, and C. Haslett. 1992. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 90:1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler, G.D., S.F. Altschul, and D.J. Lipman. 1991. A workbench for multiple alignment construction and analysis. Proteins. 9:180–190. [DOI] [PubMed] [Google Scholar]
- Shimizu, Y., G.A. van Seventer, K.J. Horgan, and S. Shaw. 1990. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J. Immunol. 145:59–67. [PubMed] [Google Scholar]
- Ticchioni, M., M. Deckert, F. Mary, G. Bernard, E.J. Brown, and A. Bernard. 1997. Integrin-associated protein (CD47) is a comitogenic molecule on CD3-activated human T cells. J. Immunol. 158:677–684. [PubMed] [Google Scholar]
- Ticchioni, M., V. Raimondi, L. Lamy, J. Wijdenes, F.P. Lindberg, E.J. Brown, and A. Bernard. 2001. Integrin-associated protein (CD47/IAP) contributes to T cell arrest on inflammatory vascular endothelium under flow. FASEB J. 15:341–350. [DOI] [PubMed] [Google Scholar]
- Vallejo, A.N., L.O. Mugge, P.A. Klimiuk, C.M. Weyand, and J.J. Goronzy. 2000. Central role of thrombospondin-1 in the activation and clonal expansion of inflammatory T cells. J. Immunol. 164:2947–2954. [DOI] [PubMed] [Google Scholar]
- Vogel, T., N.H. Guo, H.C. Krutzsch, D.A. Blake, J. Hartman, S. Mendelovitz, A. Panet, and D.D. Roberts. 1993. Modulation of endothelial cell proliferation, adhesion, and motility by recombinant heparin-binding domain and synthetic peptides from the type I repeats of thrombospondin. J. Cell. Biochem. 53:74–84. [DOI] [PubMed] [Google Scholar]
- Vonderheide, R.H., T.F. Tedder, T.A. Springer, and D.E. Staunton. 1994. Residues within a conserved amino acid motif of domains 1 and 4 of VCAM-1 are required for binding to VLA-4. J. Cell Biol. 125:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., and T.A. Springer. 1998. Structural specializations of immunoglobulin superfamily members for adhesion to integrins and viruses. Immunol. Rev. 163:197–215. [DOI] [PubMed] [Google Scholar]
- Weinstat-Saslow, D.L., V.S. Zabrenetzky, K. VanHoutte, W.A. Frazier, D.D. Roberts, and P.S. Steeg. 1994. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res. 54:6504–6511. [PubMed] [Google Scholar]
- Wilson, K.E., Z. Li, M. Kara, K.L. Gardner, and D.D. Roberts. 1999. Beta 1 integrin- and proteoglycan-mediated stimulation of T lymphoma cell adhesion and mitogen-activated protein kinase signaling by thrombospondin-1 and thrombospondin-1 peptides. J. Immunol. 163:3621–3628. [PubMed] [Google Scholar]
- Woods, M.L., and Y. Shimizu. 2001. Signaling networks regulating beta 1 integrin-mediated adhesion of T lymphocytes to extracellular matrix. J. Leukoc. Biol. 69:874–880. [PubMed] [Google Scholar]
- Xia, M., D. Leppert, S.L. Hauser, S.P. Sreedharan, P.J. Nelson, A.M. Krensky, and E.J. Goetzl. 1996. a. Stimulus specificity of matrix metalloproteinase dependence of human T cell migration through a model basement membrane. J. Immunol. 156:160–167. [PubMed] [Google Scholar]
- Xia, M., S.P. Sreedharan, P. Dazin, C.H. Damsky, and E.J. Goetzl. 1996. b. Integrin-dependent role of human T cell matrix metalloproteinase activity in chemotaxis through a model basement membrane. J. Cell. Biochem. 61:452–458. [DOI] [PubMed] [Google Scholar]
- Yabkowitz, R., V.M. Dixit, N. Guo, D.D. Roberts, and Y. Shimizu. 1993. Activated T-cell adhesion to thrombospondin is mediated by the alpha 4 beta 1 (VLA-4) and alpha 5 beta 1 (VLA-5) integrins. J. Immunol. 151:149–158. [PubMed] [Google Scholar]
- Yakubenko, V.P., R.R. Lobb, E.F. Plow, and T.P. Ugarova. 2000. Differential induction of gelatinase B (MMP-9) and gelatinase A (MMP-2) in T lymphocytes upon alpha(4)beta(1)-mediated adhesion to VCAM-1 and the CS-1 peptide of fibronectin. Exp. Cell Res. 260:73–84. [DOI] [PubMed] [Google Scholar]
- Yang, Z., D.K. Strickland, and P. Bornstein. 2001. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J. Biol. Chem. 276:8403–8408. [DOI] [PubMed] [Google Scholar]