Abstract
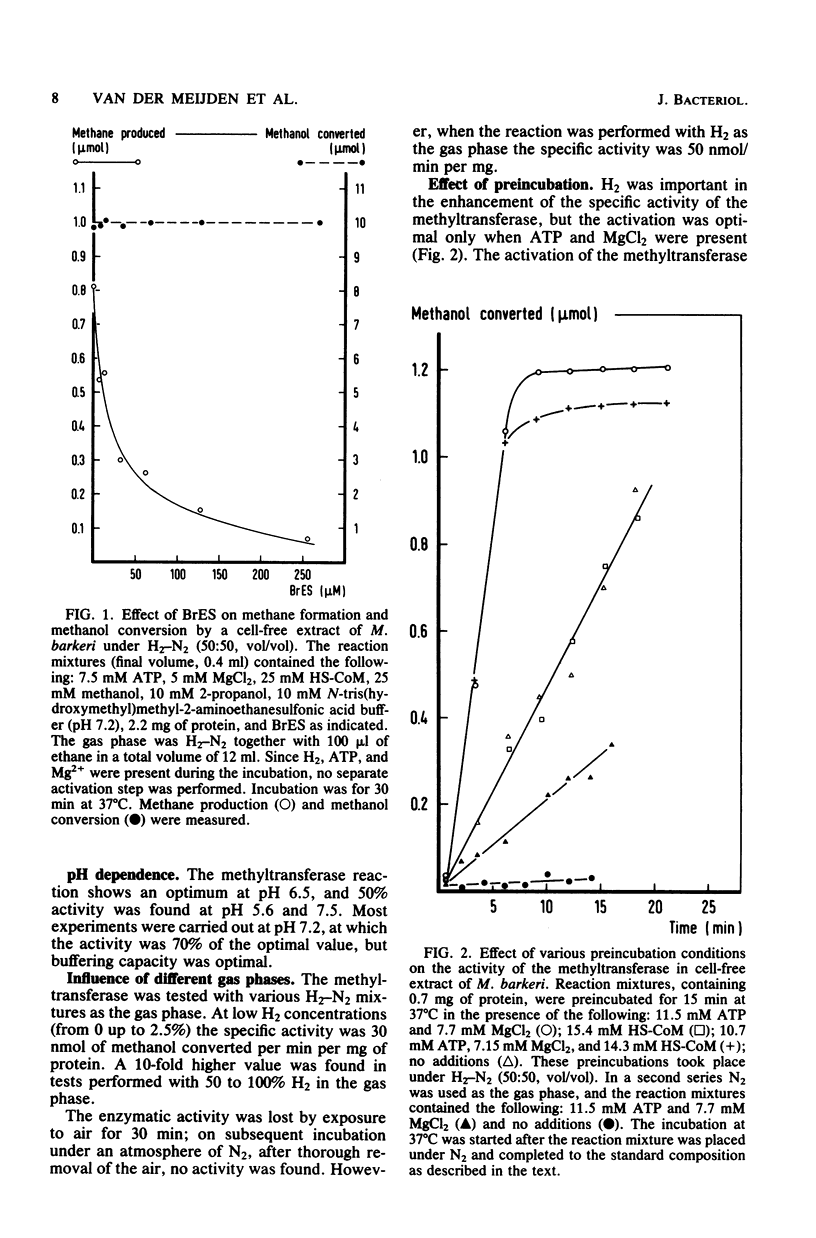
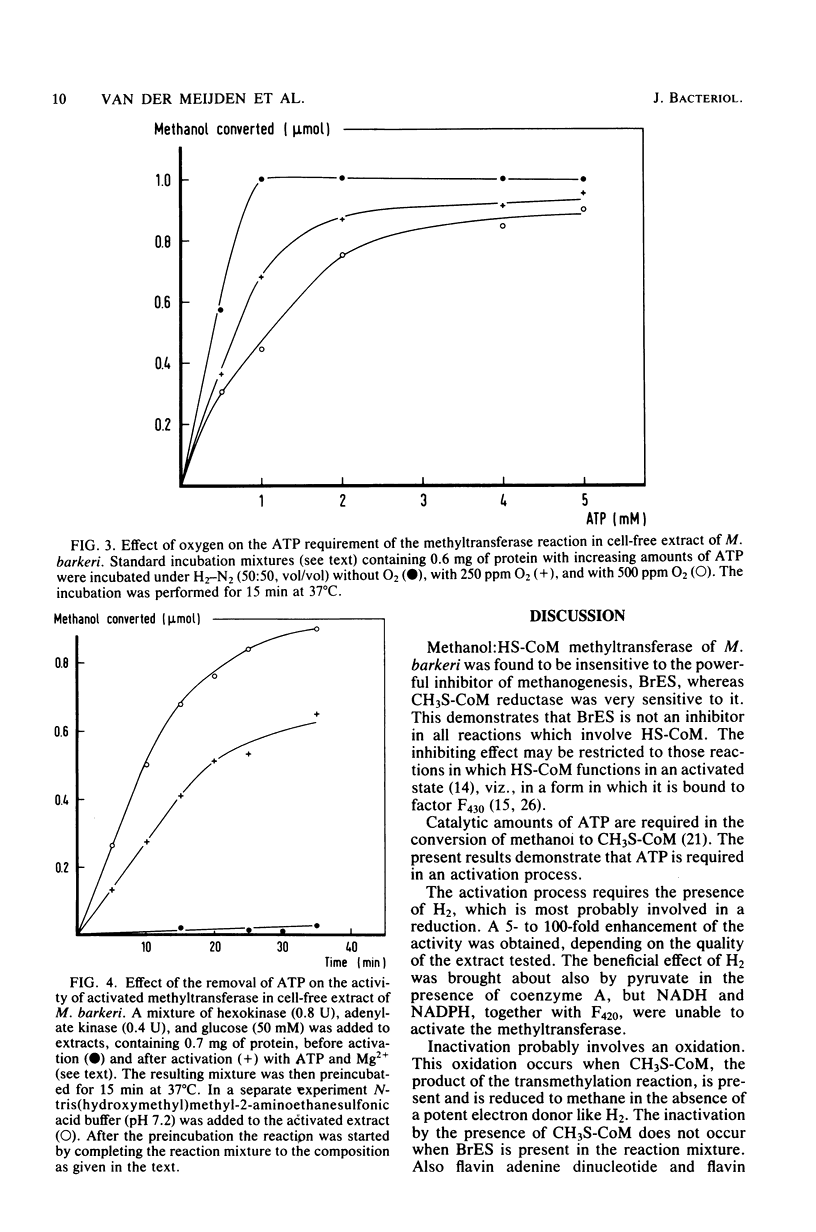
Methanol is converted to methane by crude extracts of Methanosarcina barkeri. The first reaction involved in this process, is catalyzed by methanol:2-mercaptoethanesulfonic acid methyltransferase (EC 2.1.1.-). The methyltransferase has an optimum at pH 6.5 and is not inhibited by 2-bromoethanesulfonic acid. Pyridoxal-5'-phosphate acts as an inhibitor (Ki = 0.30 mM). The methyltransferase was tested in the presence of 2-bromoethanesulfonic acid, which inhibits the conversion of 2-(methylthio)ethanesulfonic acid to methane. The reaction is subject to activation and inactivation. Inactivation is brought about by the presence of oxygen, flavin mononucleotide, flavin adenine dinucleotide, and 2-(methylthio)ethanesulfonic acid, the product of the reaction. Activation of the system requires the presence of ATP and Mg2+ and of hydrogen. Hydrogen can be replaced by enzymatic systems, such as pyruvate dehydrogenase, which deliver free hydrogen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAYLOCK B. A., STADTMAN T. C. Biosynthesis of methane from the methyl moiety of methylcobalamin. Biochem Biophys Res Commun. 1963 Apr 2;11:34–38. doi: 10.1016/0006-291x(63)90023-8. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. Specificity and biological distribution of coenzyme M (2-mercaptoethanesulfonic acid). J Bacteriol. 1979 Jan;137(1):256–263. doi: 10.1128/jb.137.1.256-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaylock B. A. Cobamide-dependent methanol-cyanocob(I)alamin methyltransferase of Methanosarcina barkeri. Arch Biochem Biophys. 1968 Mar 20;124(1):314–324. doi: 10.1016/0003-9861(68)90333-0. [DOI] [PubMed] [Google Scholar]
- Blaylock B. A., Stadtman T. C. Methane biosynthesis by Methanosarcina barkeri. Properties of the soluble enzyme system. Arch Biochem Biophys. 1966 Sep 26;116(1):138–152. doi: 10.1016/0003-9861(66)90022-1. [DOI] [PubMed] [Google Scholar]
- Daniels L., Zeikus J. G. One-carbon metabolism in methanogenic bacteria: analysis of short-term fixation products of 14CO2 and 14CH3OH incorporated into whole cells. J Bacteriol. 1978 Oct;136(1):75–84. doi: 10.1128/jb.136.1.75-84.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Role of component C in the methylreductase system of Methanobacterium. J Biol Chem. 1980 Sep 25;255(18):8388–8389. [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. ATP activation and properties of the methyl coenzyme M reductase system in Methanobacterium thermoautotrophicum. J Bacteriol. 1978 Sep;135(3):851–857. doi: 10.1128/jb.135.3.851-857.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Stimulation of CO2 reduction to methane by methylcoenzyme M in extracts Methanobacterium. Biochem Biophys Res Commun. 1977 Jun 6;76(3):790–795. doi: 10.1016/0006-291x(77)91570-4. [DOI] [PubMed] [Google Scholar]
- Hermans J. M., Hutten T. J., van der Drift C., Vogels G. D. Analysis of coenzyme m (2-mercaptoethanesulfonic acid) derivatives by isotachophoresis. Anal Biochem. 1980 Aug;106(2):363–366. doi: 10.1016/0003-2697(80)90533-3. [DOI] [PubMed] [Google Scholar]
- Hippe H., Caspari D., Fiebig K., Gottschalk G. Utilization of trimethylamine and other N-methyl compounds for growth and methane formation by Methanosarcina barkeri. Proc Natl Acad Sci U S A. 1979 Jan;76(1):494–498. doi: 10.1073/pnas.76.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten T. J., Bongaerts H. C., van der Drift C., Vogels G. D. Acetate, methanol and carbon dioxide as substrates for growth of Methanosarcina barkeri. Antonie Van Leeuwenhoek. 1980;46(6):601–610. doi: 10.1007/BF00394016. [DOI] [PubMed] [Google Scholar]
- Hutten T. J., De Jong M. H., Peeters B. P., van der Drift C., Vogels G. D. Coenzyme M derivatives and their effects on methane formation from carbon dioxide and methanol by cell extracts of Methanosarcina barkeri. J Bacteriol. 1981 Jan;145(1):27–34. doi: 10.1128/jb.145.1.27-34.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno S., Toraya T., Fukui S. Chemical modification of coenzyme B12-dependent diol dehydrase with pyridoxal 5'-phosphate: lysyl residue essential for interaction between two components of the enzyme. Arch Biochem Biophys. 1981 Oct 15;211(2):722–730. doi: 10.1016/0003-9861(81)90508-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mah R. A., Smith M. R., Baresi L. Studies on an acetate-fermenting strain of Methanosarcina. Appl Environ Microbiol. 1978 Jun;35(6):1174–1184. doi: 10.1128/aem.35.6.1174-1184.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Wolfe R. S. A new coenzyme of methyl transfer, coenzyme M. Biochemistry. 1971 Jun 8;10(12):2317–2324. doi: 10.1021/bi00788a022. [DOI] [PubMed] [Google Scholar]
- STADTMAN T. C., BARKER H. A. Studies on the methane fermentation. IX. The origin of methane in the acetate and methanol fermentations by methanosarcina. J Bacteriol. 1951 Jan;61(1):81–86. doi: 10.1128/jb.61.1.81-86.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Shapiro S., Wolfe R. S. Methyl-coenzyme M, an intermediate in methanogenic dissimilation of C1 compounds by Methanosarcina barkeri. J Bacteriol. 1980 Feb;141(2):728–734. doi: 10.1128/jb.141.2.728-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Mah R. A. Growth and methanogenesis by Methanosarcina strain 227 on acetate and methanol. Appl Environ Microbiol. 1978 Dec;36(6):870–879. doi: 10.1128/aem.36.6.870-879.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. A simplified assay for coenzyme M (HSCH2CH2SO3). Resolution of methylcobalamin-coenzyme M methyltransferase and use of sodium borohydride. J Biol Chem. 1974 Aug 10;249(15):4886–4890. [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate metabolism in Methanosarcina barkeri. Arch Microbiol. 1978 Nov 13;119(2):175–182. doi: 10.1007/BF00964270. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Moura I., Moura J. J., Santos M. H., Xavier A. V., LeGall J., Scandellari M. Role of vitamin B12 in methyl transfer for methane biosynthesis by Methanosarcina barkeri. Science. 1982 Apr 16;216(4543):303–305. doi: 10.1126/science.7063887. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



