Abstract
Disruption of the function of the A-type Aurora kinase of Drosophila by mutation or RNAi leads to a reduction in the length of astral microtubules in syncytial embryos, larval neuroblasts, and cultured S2 cells. In neuroblasts, it can also lead to loss of an organized centrosome and its associated aster from one of the spindle poles, whereas the centrosome at the other pole has multiple centrioles. When centrosomes are present at the poles of aurA mutants or aurA RNAi spindles, they retain many antigens but are missing the Drosophila counterpart of mammalian transforming acidic coiled coil (TACC) proteins, D-TACC. We show that a subpopulation of the total Aurora A is present in a complex with D-TACC, which is a substrate for the kinase. We propose that one of the functions of Aurora A kinase is to direct centrosomal organization such that D-TACC complexed to the MSPS/XMAP215 microtubule-associated protein may be recruited, and thus modulate the behavior of astral microtubules.
Keywords: Aurora A; D-TACC; mitosis; centrosomes; microtubule
Introduction
Correct regulation of the organization and dynamics of microtubules is an essential aspect of entry into M-phase. Microtubules are nucleated by the γ-tubulin ring complex (Pereira and Schiebel, 1997), the amount of which increases markedly at the centrosome upon entry into mitosis. Microtubule nucleation at the centrosome requires the cooperation of other microtubule-associated proteins (MAPs),* notably the abnormal spindle protein (Asp) in Drosophila (do Carmo Avides and Glover, 1999). MAPs also play a central role in regulating microtubule dynamics (Hyman and Karsenti, 1996; Shirasu et al., 1999). For example, Xenopus XMAP215 promotes the elongation rate of microtubules at their plus ends (less so at the minus ends), and appears to counteract the catastrophe-promoting activity (the transition from polymerization to a depolymerization) promoted by XKCM1 (Vasquez et al., 1994; Tournebize et al., 2000). The counterpart of XMAP215 in Drosophila is encoded by the gene minispindles (MSPS), mutations that appear to destabilize spindle microtubules which become small and associated with single chromosomes (Cullen et al., 1999). The MSPS protein was recently shown to form a complex with the Drosophila counterpart of mammalian transforming acidic coiled coil (TACC) proteins, the centrosomally associated protein D-TACC (Gergely et al., 2000b; Cullen and Ohkura, 2001; Lee et al., 2001). Injections of antibodies against D-TACC or mutations in the d-tacc gene result in centrosomal microtubules that are abnormally short, as well as in the accumulation of mitotic defects. The D-TACC protein is found at the spindle poles and its recruitment of MSPS protein has been postulated to stabilize centrosomal microtubules (Lee et al., 2001). In the acentriolar spindles of female meiosis, both the motor protein Ncd and D-TACC are required for the proper localization of MSPS (Cullen and Ohkura, 2001).
A number of protein kinases are known to regulate the behavior of microtubules upon mitotic entry by phosphorylating MAPs. For example, MAP4, which induces the formation of long and stable microtubules by increasing the rescue frequency (the transition from depolymerization to polymerization) has to be inactivated in mitosis. This is achieved by cdk1–cyclin B, thus promoting the increase in the microtubule dynamics when the cell enters mitosis (Ookata et al., 1995). Cdk1 also activates the microtubule binding properties of the kinesin-related protein Eg5 and dynactin to promote their interaction with spindle microtubules (Blangy et al., 1995; Sawin and Mitchison, 1995). The mitotic Polo-like kinases are also required to recruit γ-tubulin to the centrosome and thus increase its nucleating activity (Lane and Nigg, 1996; Donaldson et al., 2001). Polo kinase also phosphorylates the Asp protein and so activates its role to increase the nucleating activity of centrosomes (do Carmo Avides et al., 2001). Recent studies suggest that a Polo-like kinase also seems to phosphorylate and inactivate stathmin/Op18, a microtubule-destabilizing protein, around chromatin to promote local microtubule stabilization (Belmont et al., 1996; Andersen et al., 1997; Budde et al., 2001)
Another major family of mitotic kinases is the Aurora-related enzymes (Giet and Prigent, 1999). Most is known about the B-type subfamily, which first localizes to condensing chromosomes and centromeres, and subsequently to the central spindle and the midbody in anaphase and telophase, respectively. The Aurora B protein kinase is the functional subunit of a complex containing INCENP (Adams et al., 2000; Kaitna et al., 2000), and the BIR-1/survivin protein is required for its localization (Speliotes et al., 2000); it is required in chromosome segregation and it phosphorylates histone H3, which correlates with recruitment of the condensin complex (Adams et al., 2001; Giet and Glover, 2001). In contrast, less is known of the exact function of the Aurora A–type kinases, although ectopic expression of the human enzyme leads to aneuploidy, centrosome amplification, and transformation (Bischoff et al., 1998; Zhou et al., 1998). Mutations in the aurora A gene of Drosophila melanogaster lead to formation of spindles with abnormally organized poles, including characteristic monopolar structures (Glover et al., 1995). Bipolar spindles having abnormally organized centrosomes, and microtubules were observed after double-stranded RNA mediated interference directed against the air1 gene of Caenorhabditis elegans (Schumacher et al., 1998). A catalytically inactive or truncated version of the pEg2 Aurora A–like kinase promotes the collapse of spindles assembled in Xenopus egg extracts (Roghi et al., 1998). This would be consistent with the known ability of Aurora A kinase to phosphorylate the kinesin-like protein XlEg5 that is also required for spindle assembly and stability (Sawin et al., 1992; Giet et al., 1999). To gain a better understanding of the role of Aurora A kinase, we have examined the requirement for the enzyme in organizing spindle poles in different mutant alleles. We now show that in mutant cells or after RNA interference to eliminate the enzyme, the spindle poles have abnormal organization and abnormally short arrays of astral microtubules. The latter defect correlates with the loss of D-TACC from centrosomes. We show that D-TACC interacts with a subpopulation of Aurora A in vivo, and that D-TACC is a substrate of the kinase. We propose a model in which one of the centrosomal functions of Aurora A kinase is to control microtubule dynamics at the spindle poles by regulating the recruitment of D-TACC and its associated MAP, the minispindles/XMAP215 protein.
Results
Aurora A kinase localizes to the centrosome in mitosis
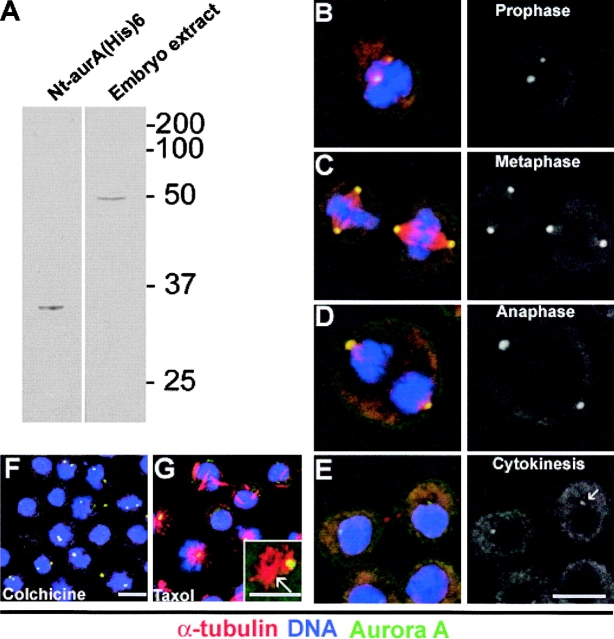
To examine the subcellular localization of Aurora A kinase in Drosophila cells, we first raised a polyclonal antibody that was specifically directed against the NH2-terminal domain of the enzyme expressed in E. coli (Fig. 1 A, left lane), and which recognized a single 47-kD protein in an extract of 2-h--old Drosophila embryos (Fig. 1 A, right lane). We found the antibody strongly decorated the centrosomes of S2 cells in prophase (Fig. 1 B), metaphase (Fig. 1 C), and anaphase (Fig. 1 D). At cytokinesis (Fig. 1 E), we noticed a decrease in the intensity of staining at the centrosome that may be a consequence of anaphase-promoting complex–mediated degradation of the enzyme as reported previously (Walter et al., 2000). To determine if the localization of Aurora A to centrosomes required microtubules, we treated S2 cells with the microtubule-depolymerizing drug colchicine (Fig. 1 F) or the microtubule-stabilizing drug taxol (Fig. 1 G). Neither treatment disrupted the centrosomal association of the enzyme, and although taxol treatment induced the formation of ectopic asters, Aurora A only remained associated with asters nucleated by centrosomes. Thus, the Aurora A kinase appears to associate with centrosomes independently of microtubules, as previously described for Aurora A orthologues (Gopalan et al., 1997; Roghi et al., 1998).
Figure 1.
Aurora A localizes to the centrosomes and the spindle poles independently of microtubules. (A) An anti–Aurora A anti-serum recognizes the NH2-terminal recombinant histidine-tagged protein domain used for immunization (left) and the 47-kD endogenous Aurora A protein kinase in Drosophila embryo extracts (right) by Western blotting. Similar results were obtained using affinity purified antibodies. (B–E) The Aurora A antibody (green) decorates centrosomes and spindle poles in S2 cultured cells in prophase (B), metaphase (C), anaphase (D), and cytokinesis (E). Microtubules are in red, DNA in blue. Right hand panels show Aurora A staining alone. Note the decrease in the Aurora A staining during cytokinesis (E, right, arrow). Bar is 10 μm. Colchicine (F) and taxol (G) treatments have no effects on the Aurora A centrosome localization. Note that aurora remains on the asters containing the centrosome (G, inset). Microtubules are red, aurora is green and DNA is blue and the scale bar represents 10 μm. Inset, α-tubulin (red); bar, 5 μm.
Hypomorphic mutations in the ATP binding domain of Aurora A kinase reduce the density of astral microtubules
We previously described an allelic series of mutations at the aurora locus (that we now term aurora A) of Drosophila that show disruption to mitotic progression at differing developmental stages (Glover et al., 1995). All of these mutations were fully rescued by a transgene carrying an aurora A gene (Glover et al., 1995). The allele aurA 287 exemplifies a weak hypomorph that displays no apparent mitotic defects in larval brains (Table I). Females homozygous for aurA 287 survive to adulthood, but show a maternal effect and produce embryos that cannot complete the 13 rapid cycles of syncytial mitosis as a consequence of accumulating defects that include asynchronous mitotic cycles, loss of centrosomes, and fusion of mitotic spindles (Glover et al., 1995). The aurA e209 allele is a strong hypomorph; the aurA e209 larval central nervous system showed a 5–6-fold elevated mitotic index with the majority of cells delayed in a metaphase-like state (Fig. 3; Table I). In most of these cells, metaphase chromosomes were arranged in a configuration consistent with their association with bipolar spindles (Fig. 3, C and D), but a substantial proportion were in circular arrays (Fig. 3, E and F) previously shown to correspond to monopolar spindles (Glover et al., 1995).
Table I. Quantitation of the mitotic figures in Aurora A mutants.
| Genotype | Number of brains |
Number of mitoses |
Mitotic index % |
Metaphase | Aneuploid + polyploid % |
Circular figures % |
|---|---|---|---|---|---|---|
| Ore R (wild-type) | 5 | 50 | 1 | 4:1 | 0 | 0 |
| aur287/aur287 | 2 | 12 | 0.6 | 5:1 | 0 | 0 |
| aure209/aure209 | 12 | 752 | 6.3 | no anaphases recorded | 2.5 | 23.9 |
| aure209/Df(3R)T47 | 5 | 270 | 5.4 | 53:1 | 4 | 13.7 |
| aure209/Df(3R)T61 | 19 | 913 | 9.1 | 50:1 | 1.1 | 16 |
| aure209/Df(3R)P79 | 3 | 238 | 7.9 | 79:1 | 6 | 12.2 |
Squashed preparations of larval brains were prepared as previously described (Glover et al., 1995). The mitotic index was scored as the percentage of cells in mitosis for each of the indicated genotypes. The proportions of aneuploid and polyploid cells and of those with circular mitotic figures are expressed as a percentage of the total number of mitotic cells. The deficiency chromosomes (Df) have deletions that remove the Aurora A locus and differing extents of the flanking genome (Flybase).
Figure 3.
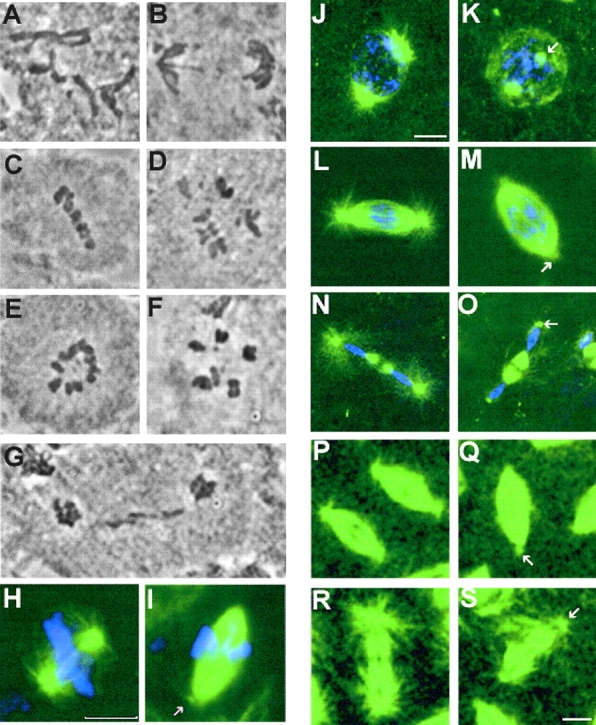
Mutation in aurora A leads to metaphase arrest in neuroblasts and decreased length of astral microtubules in both neuroblasts and embryos. Squashed preparations of larval brains from wild-type (A and B) or aurA e209/Df(3R)T61 (C–G) were stained with aceto-orcein and the mitotic figures were scored. A and B wild-type metaphase and anaphase figures, respectively. (C and D) aurA e209Df(3R)T61 metaphase-like figures spindles and E and F circular mitotic figures. Note the high chromosome condensation in panels C–F. (G) An abnormal anaphase figure with lagging chromosomes. (H–S) aurA mutants have short astral microtubules in mitosis. (H and I) Spindles from wild- type and aurA e209/Df(3R)T61 mutant neuroblasts, respectively. Arrow point to a small aster at the mutant spindle poles. Bar, 10 μm. Wild-type embryos (J, prophase; L, early anaphase; N, telophase) and aurA 287-derived embryos (K, prophase; M, early anaphase; O, telophase) that have been fixed and stained for tubulin (green) or DNA (blue). Note the decreased length of the astral microtubules in the mutant embryos (arrows). (P–S) Microtubule behavior monitored in live wild type (P, early anaphase; R, telophase) and aurA 287-derived embryos (Q, early anaphase; S, telophase) expressing a tau–GFP transgene under the control of the polyubiquitin promoter. Mutant embryos show a reduced number of apparently shorter astral microtubules (arrows). Bar, 5 μm.
We have now determined that the weak hypomorphic allele, aurA 287, encodes a protein with the amino acid substitutions H3Y, D47A, T90P, and R162C. The amino acid at position 47 represents polymorphism of the wild-type sequence. This modified enzyme is likely to have a reduced kinase activity because R162 is a conserved arginine in the ATP binding pocket in subdomain I of the catalytic domain of all protein kinases. Consistent with the sequencing data, we found that the full-length protein is expressed in embryos derived from homozygous aurA e287 mothers (Fig. 2 B, lane 3), but that an Aurora A immunoprecipitate has kinase activity (Fig. 2 B, lane 3) <35% of a wild-type immunoprecipitate (Fig. 2 B, lane 2). This reduced activity is consistent with the very weak hypomorphic nature of the allele. We previously showed that aurA e209 encodes a full-length protein with two amino acid substitutions at other sites, D47A and E202K (Glover et al., 1995). The latter mutation changes a conserved acidic amino acid to a basic one in subdomain III of the catalytic domain, known to be required for the binding of the Mg–ATP complex (Hunter and Sefton, 1981). Consequently, we expect the aurA e209 gene to encode a kinase that is likely to be catalytically inactive, thus accounting for the strongly hypomorphic nature of the mutation. It is noteworthy that brains from hemizygous aurA e209 larvae show no significant increase in mitotic index over homozygotes, but do show a reduction of circular figures and a concomitant increase in bipolar spindles (Table I). Thus, although by the criterion of mitotic index alone aurA e209 appears to exhibit extreme loss of function, it remains possible that the increase in circular mitotic figures seen in homozygous rather than hemizygous aurA e209 larvae is favored by the increase in dose of such a catalytically inactive protein that can behave as a neomorphic mutant with respect to the formation of circular figures.
Figure 2.
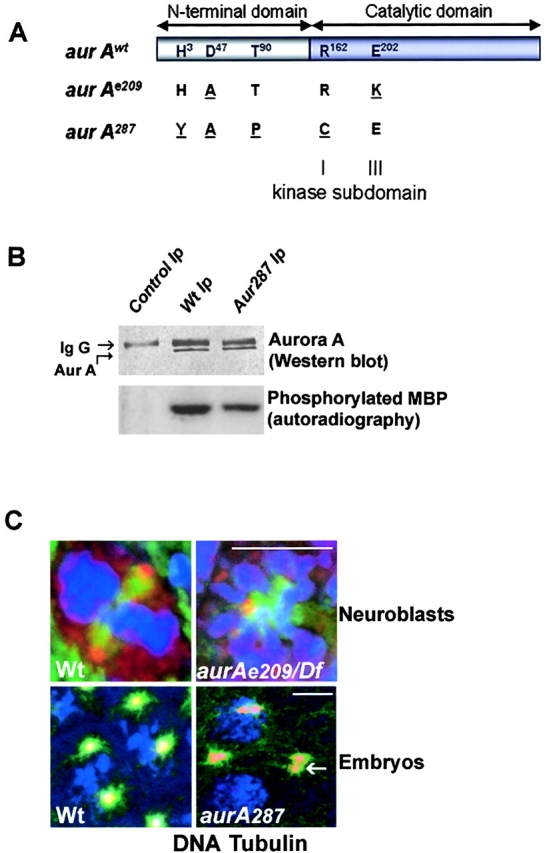
aurA287 encodes an enzyme of reduced activity that localizes to the centrosome. aurA e209 and aurA 287 encode full-length Aurora A kinases with the indicated amino acid changes (underlined). The amino acid changes in aurA e209 were previously reported (Glover et al., 1995). Such a mutation was described to be essential for kinase activity (Hunter and Sefton, 1981). (B) The aurA 287 mutant shows reduced protein kinase activity. Extracts of wild-type or aurA 287-derived embryos (lanes 2 and 3, respectively) were submitted to immunoprecipitation using anti–Aurora A antibodies. One aliquot of immunoprecipitate was subjected to Western blotting (top panels) and a second (bottom panels) assayed for its ability to phosphorylate myelin basic protein (Materials and methods). Quantification using a phosphoimager identified the mutant Aurora A287 kinase had 35% of the activity of wild-type. (C) Mutations in aurA do not prevent its centrosome localization. Wild-type (top left) or auroraA e209 (top right) neuroblasts showing localization of Aurora A (red) to centrosomes of a bipolar and monopolar spindle respectively. Embryos derived from wild-type (bottom left) or homozygous aurora A 287 mothers (bottom right) showing Aurora A (red) at centrosomes. The arrow shows a centrosome pair that has dissociated from the nucleus. DNA is stained blue and tubulin, green. Bars, 5 μM.
To determine whether centrosomal localization was affected in either mutant form, we performed immunostaining with anti–Aurora A antibodies on the brains of wild-type and hemizygous aurA e209 larvae (heterozygous for the recessive aurA e209 allele and a deletion of the locus and surrounding genes) (Fig. 2 C, top). We found that the mutant kinase did localize to centrosomes of both bipolar and monopolar (Fig. 2 C) spindles found in the mutant brains. Similarly, the mutant kinase in aurA 287-derived embryos was also found on centrosomes (Fig. 2 C, bottom right panel). Thus, the mutations present in neither of these two mutant enzymes affect their ability to localize to centrosomes.
Although the ultimate consequences of the two mutations differ at the two developmental stages they affect, their phenotypes suggested that aurora A was required for aspects of centrosome function. Therefore, we examined the bipolar spindles produced either in syncytial embryos derived from aurA 287 mothers, or in the central nervous systems of aurA e209 larvae to determine whether there were any common defects affecting the spindle poles. In addition to the previously described characteristics of aurA 287-derived embryos (Glover et al., 1995), we found that in contrast to wild-type embryos, the astral microtubules at the spindle poles appeared as though very short and/or reduced in density (Fig. 3, compare panels J and K, L and M, and N and O). We confirmed this observation by introducing a gene encoding a Tau–GFP fusion protein into the aurA 287 line to enable us to observe centrosome-associated microtubules in live embryos. This also revealed that astral microtubules seemed short and apparently reduced in number throughout mitosis compared with wild-type embryos carrying the same Tau-GFP transgene (Fig. 3, compare P and Q, early anaphase, with R and S, telophase). Examination of the poles of bipolar spindles in neuroblasts from aurA e209 larvae also revealed a similar apparent reduction in length and/or number of the astral microtubules relative to wild-type (Fig. 3, compare H and I). Taken together, this suggested that one role of the Aurora A protein kinase is to regulate microtubule dynamics at the spindle poles.
Major centrosomal proteins associate with abnormal aurora spindle poles
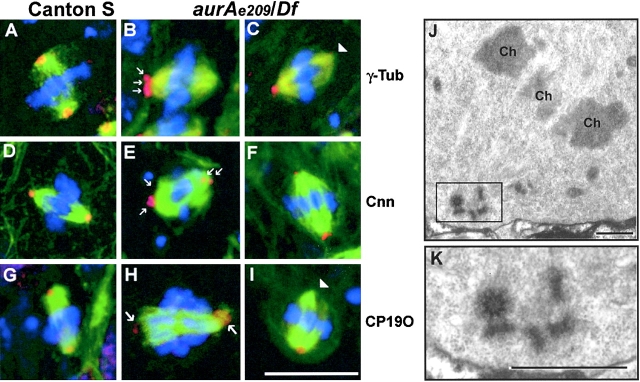
The reduction in length and/or density of microtubules at the spindle poles after mutation of aurA led us to ask whether this might correlate with changes in the association of any known centrosomal antigen with the poles. We chose to assess this by immunostaining neuroblasts from hemizygous mutant larvae of genotype aurA e209/Df(3R)T61. As expected from our previous examination of squashed preparations of mutant larval brains, we found an elevated frequency of mitotic cells in whole-mount preparations, the majority of which contained bipolar metaphase spindles. We found that many of these spindles appeared to be bipolar but monoastral. The asters were diminutive and were nucleated around centrosomal antigens frequently only present at one pole (Fig. 4, B, C, and I, arrows). However, when centrosomal bodies were present, the intensity of staining given by antibodies to detect either γ-tubulin, centrosomin, CP190 (Fig. 4), or Asp (unpublished data) was comparable to that observed at the poles of wild-type spindles. These centrosomal antigens were often not present as a single body at the poles, but as multiple punctate bodies (Fig. 4, B, E, and H, arrows). Such punctate bodies could arise either by fragmentation of centrosomes or by the accumulation of multiple centrosomes that have failed to segregate during mitosis. To attempt to distinguish between these possibilities, we sectioned preparations of aurA e209/Df(3R)T61 brains for electron microscopy (Fig. 4 J). Poles of bipolar spindles in such mutant brains either showed focused microtubules with no evidence of an organized centrosome, or spindle microtubules nucleated from bodies containing multiple centrioles. For example, the spindle pole shown in the electron micrograph in Fig. 4 K shows five centrioles at this plane, one in transverse, and four in the longitudinal section. The latter were of comparable length to the centrioles of wild-type centrosomes. Thus mutation in aurA appears to result, at least in part, in a block to centrosome separation resulting in the accumulation of multiple centrioles. These are surrounded by electron-dense pericentriolar material that is likely to correspond to the multiple regions of punctate staining of centrosomal antigens that we observe at these spindle poles by light microscopy. Similar defects were observed in ten cells that were examined in this way.
Figure 4.
aurAe209/Df(3R)T61 neuroblasts show abnormal mitotic spindle poles. Localization of γ-tubulin (A–C), centrosomin (D–F) and CP190 (G–I) in wild-type Canton S (A, D, and G) or aurA e209/Df(3R)T61 metaphase spindles (B, C, E, F, H, and I). Note the absence of centrosomal antigens at the poles indicated by arrowheads (C and I) and the presence of additional bodies of staining (B, E, H, arrows). Cnn, γ-tubulin and CP190 are shown in red, microtubules in green and DNA in blue. Bar is 10 μm. (J and K) ultrastructure of an aurA e209/Df(3R)T61 spindle pole by electron microscopy (one section). Chromosomes are indicated Ch. Note the presence of 5 centrioles on the spindle pole, boxed and shown enlarged in K. Bars: (J) 5 μm; (K) 1 μm.
Centrosomal association of D-TACC and MSPS is prevented after aurA mutation
A reduction in the length of astral microtubules comparable to that seen after reduction in Aurora A kinase function has also been described in embryos derived from mothers carrying mutations in the d-tacc gene (Gergely et al., 2000a) and in brains derived from flies having mutations in the MSPS gene (Cullen et al., 1999). Therefore, we decided to examine whether the distribution of D-TACC might be affected in Aurora A–deficient cells. We first examined the distribution of D-TACC in wild-type and mutant embryos derived from homozygous aurA 287 mothers. We found that the anti–D-TACC antibodies strongly decorated the centrosomes and weakly stained the spindle microtubules of wild-type embryos (Fig. 5, A, prophase, C, telophase), in agreement with the original findings of Gergely et al. (2000a). In contrast, we found that in aurA 287-derived embryos, D-TACC protein was poorly localized to centrosomes (Fig. 5 B, prophase, D, telophase). However, D-TACC protein was clearly present and could be seen through increased cytoplasmic staining and in association with the spindle microtubules, indicating that this latter aspect of its localization is not dependent on Aurora A kinase. We then examined the localization of D-TACC in larval neuroblasts hemizygous for the aurA e209 mutation. In wild-type cells from this larval tissue, D-TACC was once again found to have a centrosomal association (Fig. 5 E). However, in the aurA mutant cells, we found D-TACC was absent from the poles of the mitotic spindle, but was distributed in the region occupied by the spindle microtubules and throughout the cytoplasm in a punctate array (Fig. 5 F). Thus, the altered distribution of D-TACC after perturbation of Aurora A function with different mutations suggests that Aurora A kinase function is necessary for localization of D-TACC to the centrosomes, but not the spindle microtubules in two cell types.
Figure 5.
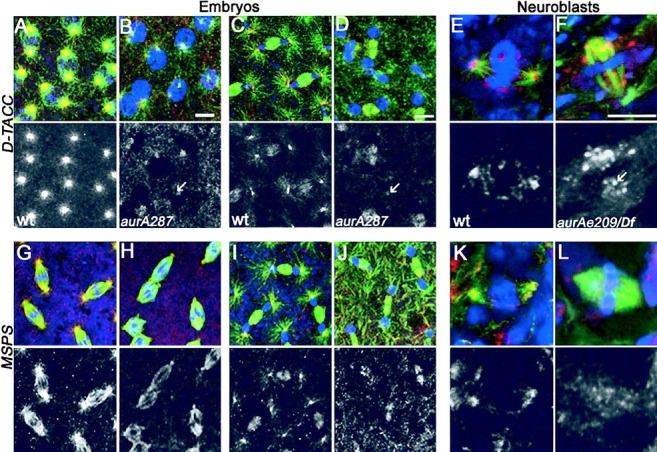
D-TACC and MSPS proteins are mislocalized in aurA mutant cells. D-TACC (A–F) and MSPS (G–L) localization in wild-type (A, prophase; C and I, telophase; G, metaphase) or AurA 287 mutant embryos (B, prophase; H, metaphase; D and J, telophase). Staining was also performed in wild-type (E and K) or aurA e209/Df neuroblasts (F and L). In all color panels, D-TACC and MSPS are red, microtubules are green and DNA is blue. Lower panels show D-TACC or MSPS staining alone in monochrome. Note that D-TACC and MSPS disappears from the poles of aurA mutant cells (arrows) but remains associated with spindle microtubules. In aurA e209 neuroblasts, both D-TACC and MSPS tends to generate aggregates in the cytoplasm or that stick on the mitotic spindles. Bars, 5 μm.
The product of the MSPS gene encodes a MAP that stabilizes microtubules and was recently found to be in a complex with the D-TACC protein (Cullen et al., 1999; Lee et al., 2001). To determine whether MSPS localization was also disrupted when Aurora A kinase function was compromised, we examined embryos derived from wild-type (Fig. 5 G, metaphase, I telophase) and homozygous aurA 287 mothers (Fig. 5 H, metaphase, J telophase). We found that the MSPS staining was highly reduced from spindle poles, but that the protein remains on spindle microtubules. In aurA e209 neuroblasts, we noted that MSPS was also absent from the centrosome, but that it remained associated with the region of the spindle (Fig. 5 L) compared with wild-type cells where it was associated with both (Fig. 5 K). Thus, in aurora A mutant cells, neither MSPS nor D-TACC properly localize to the centrosome.
Reduced levels of Aurora A kinase after RNAi also lead to similar centrosomal defects as seen in mutants
As the mutant Aurora A kinases we have studied still localized to the centrosome, and because the stronger mutant allele seemed to display some neomorphic function in the absence of zygotically expressed wild-type protein, we wished to determine whether there were similar spindle pole defects upon depletion of Aurora A protein. In the absence of protein-null mutants, we turned to RNAi as a means of reducing levels of the enzyme. We found that three days after transfection of S2 cells with ds aurA RNA, the protein kinase had disappeared from the centrosome (Fig. 6, B and C) and was reduced in levels by >95% as judged by Western blotting (Fig. 6 J). Examination of Aurora A–depleted cells indicated a high frequency of metaphase abnormalities in which chromosomes were frequently misaligned on the metaphase plate (Table II). However, cells appeared to undertake anaphase with such defects and in contrast to the aurA mutant neuroblasts did not arrest at metaphase. At present, we cannot be certain of the reason for this difference, but suggest it is likely to be a consequence of different checkpoint responses in these two cell types. Such differences are known in Drosophila, where for example meiocytes only show a transient checkpoint response evident by a lengthening of prometaphase in response to spindle damage (Rebollo and Gonzalez, 2000; Savoian et al., 2000). We noted that there was a significant increase in the number of cells with fragmented DNA after aurA RNAi, indicating that defective mitotic cells may be eliminated by an apoptotic pathway.
Figure 6.
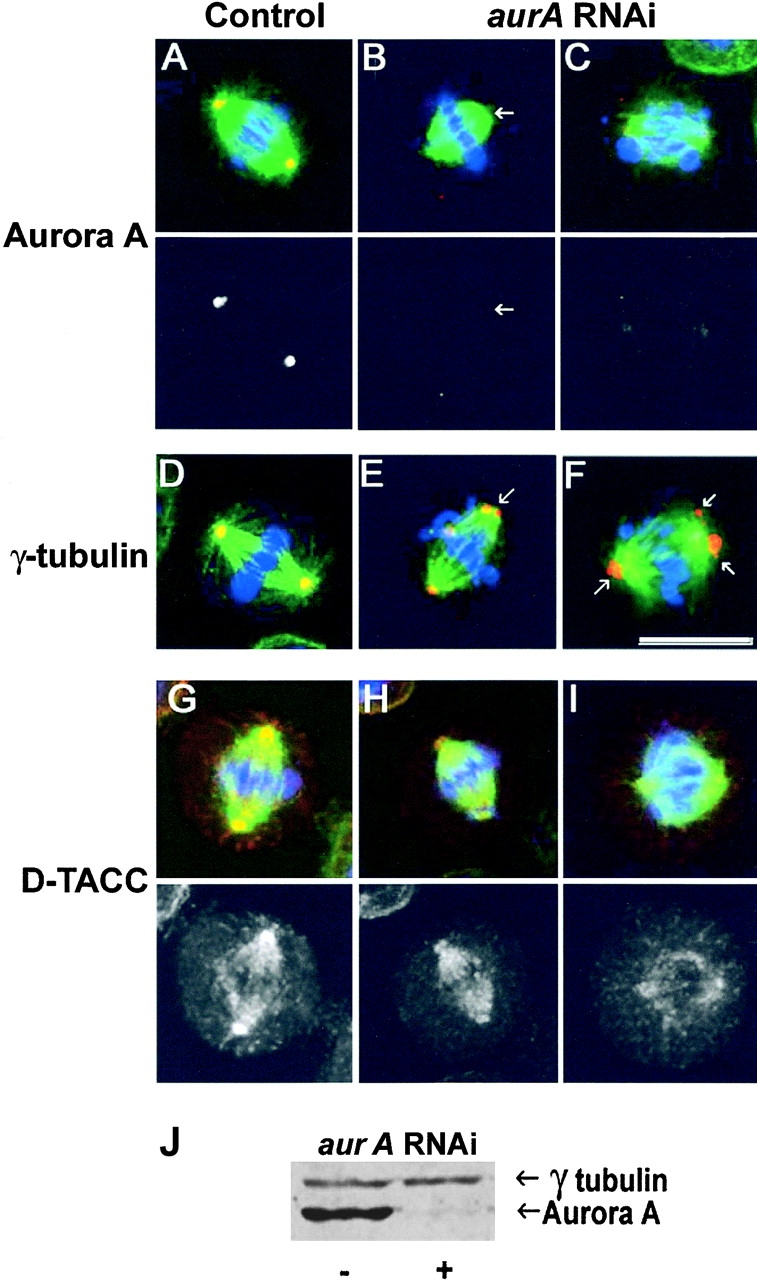
S2 cells display spindle pole abnormalities following aurA RNAi. Control (A, D, and G) and aurA RNAi treated (B, C, E, F, H, and I) S2 cells immunostained to reveal either Aurora A (A-C), gamma-tubulin (D–F), or D-TACC (G–I) in red. DNA is stained blue and microtubules, green. Monochrome images show the red channel alone and the scale bar represents 10 μm. Arrows in panel B indicate reduced levels of Aurora A staining and reduced length and density of astral microtubules following aurA RNAi. Arrows in panels E and F indicate multiple gamma-tubulin containing bodies at the poles and reduced length and density of astral microtubules in aurA RNAi cells. J, aurora A and γ-tubulin Western blot of S2 cells 3 d after aurA RNAi treatment. Aurora A levels were reduced by >95%, whereas Aurora B, or D-TACC protein levels were unchanged (unpublished data).
Table II. Quantification of the mitotic figures after Aurora A RNAi experiments in S2 cells.
| Prophase % |
Metaphase % |
Anaphase % |
Cytokinesis % |
Abnormal metaphase % |
Abnormal anaphase % |
Mitotic index % | DNA fragmentation % | |
|---|---|---|---|---|---|---|---|---|
| Control | 9 ± 0.5 | 40.5 ± 4.5 | 2.7 ± 1.0 | 19.3 ± 3.4 | 2.65 ± 2.4 | 1.6 ± 1.4 | 7.8 ± 0.9 | 6.9 ± 0.4 |
| RNAi | 5.7 ± 0.7 | 13.6 ± 2.6 | 4.1 ± 1.0 | 26.5 ± 1.0 | 32.5 ± 5.0 | 6.1 ± 1.4 | 7.2 ± 1 | 13.8 ± 0.5 |
The abnormal metaphases correspond to cells with unaligned chromosomes and/or punctuate gamma-tubulin staining at the poles. The abnormal anaphases represent cells with chromosome separation defects and/or punctuate gamma-tubulin staining at the poles (Fig. 6). Note that the proportion of abnormal mitoses in control S2 cells can be ∼10%. The proportions of each mitotic stage are expressed as a percentage of the total number of mitotic cells, with the exception of the DNA fragmented cells, that is expressed as a percentage of the total number of cells.
In spite of these differences, we observed striking similarities defects at the spindle poles of aurA RNAi–treated cells that compare with those seen in the mutants. First of all, both the length and number of astral microtubules were reduced in Aurora A–depleted cells (Fig. 6). Second, most of the aurA RNAi mitotic spindles had abnormal accumulations of gamma-tubulin, frequently as multiple bodies at the spindle poles (Fig. 6, E and F) similar to those seen in aurA e209 neuroblasts (compare Figs. 4 and 6). Finally, although D-TACC was associated with centrosomes and the mitotic spindle in S2 cells (Fig. 6 G), its levels on centrosomes were greatly reduced (Fig. 6, H and I). This also correlated with a reduction of MSPS protein at the spindle poles of aurA RNAi cells (unpublished data).
D-TACC is not required for Aurora A localization onto the centrosome
Together, the above experiments showed that when Aurora A kinase activity is reduced, either as a consequence of mutation or depletion of protein, there are similar effects upon centrosome behavior in three different division cycles: in syncytial embryos, neuroblasts, and cultured embryonic cells. In each case the astral microtubules appear short and reduced in number and centrosomal levels of D-TACC are reduced. We found that D-TACC remains strongly associated with the centrosome in the absence of microtubules after nocodazole treatment (unpublished data). Thus, the decrease microtubule length and density with reduced Aurora A activity is likely to be due to reduced D-TACC–MSPS complex on the centrosome. To determine whether the association of Aurora A kinase with the centrosome was itself dependent on D-TACC, we monitored Aurora A localization in embryos derived from wild-type (Fig. 7 A) or homozygous d-tacc mothers (Fig. 7, B and C). We found that Aurora A remained associated with the centrosome in mutant embryos containing either ∼10% (d-tacc1–derived, Fig. 7 B) or null levels (d-tacc stella–derived, Fig. 7 C) of D-TACC protein. This indicates that Aurora A localization to the centrosome is independent of the D-TACC protein. It strengthens our previous result that even when D-TACC protein was mislocalized in Aurora A mutants, the mutant Aurora A protein was always found on the centrosome and never in association with D-TACC (Fig. 5). Our experiments suggest that Aurora A kinase might phosphorylate centrosomal proteins, D-TACC itself, or both in such a way as to target D-TACC to the centrosome and thereby stabilize microtubules.
Figure 7.
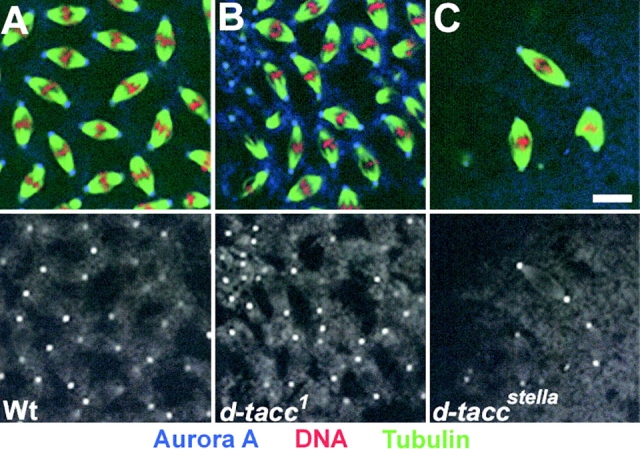
Aurora A is found on centrosomes in d-tacc mutants. Embryos derived from wild-type (A) d-tacc 1, (B) or d-tacc stella, (C) mothers stained to reveal microtubules (green), DNA (red) and aurora A (blue). Bar, 10 μm.
Aurora A interacts with and phosphorylates D-TACC
The Aurora A–dependent localization of D-TACC to centrosomes prompted us to ask whether the two proteins showed any physical interactions. To this end we used anti–Aurora A antibodies to immunoprecipitate Aurora A protein from Drosophila embryo extracts and analyzed the precipitate for the presence of both Aurora A and D-TACC proteins by Western blotting. The presence of D-TACC in the Aurora A immunoprecipitate indeed suggests that the proteins interact either directly or via intermediate proteins (Fig. 8 A, lane 2). When we performed the reciprocal experiment and immunoprecipitated D-TACC from the extract, we were unable to detect Aurora A in the precipitate. One possible interpretation of this result is that the anti–D-TACC antibody might interfere with the D-TACC–Aurora A interaction. Therefore, we decided to repeat the experiment using a Drosophila line expressing a GFP–D-TACC fusion protein under the control of the polyubiquitin promoter and to perform immunoprecipitation using anti-GFP antibodies in an attempt to avoid possible antibody interference. We found that GFP-tagged D-TACC protein could be immunoprecipitated from the extracts of such embryos, and that Aurora A kinase was present in this immunoprecipitate (Fig. 8 A, lane 4). However, Aurora A was not found in the immunoprecipitate obtained using the same anti-GFP antibody on the extract from embryos expressing a Tau–GFP fusion protein (Fig. 8 A, lane 3). Together, these experiments demonstrated an interaction between the Aurora A and D-TACC proteins in extracts of wild-type embryos.
Figure 8.
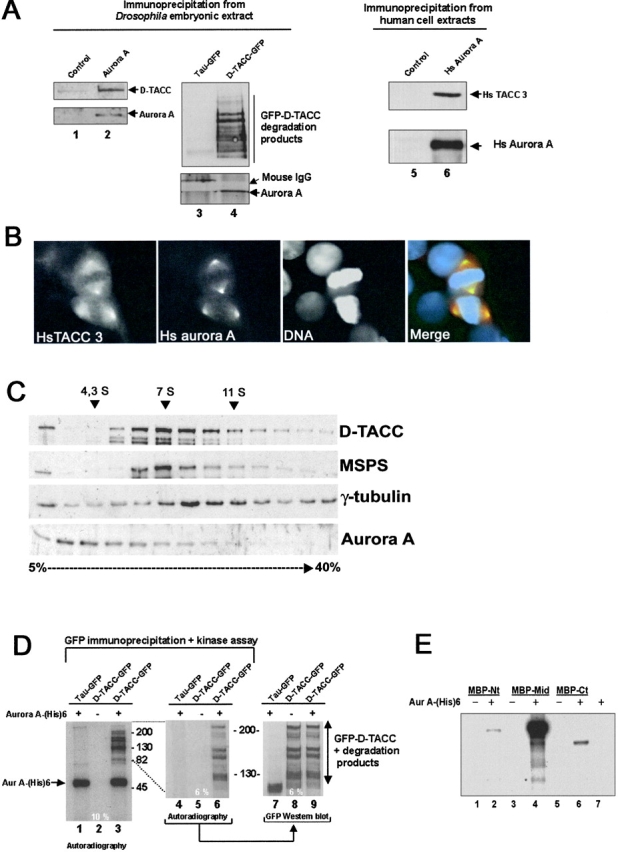
Aurora A interacts with and phosphorylates D-TACC. (A) Drosophila Aurora A (lane 2) and GFP–D-TACC coimmunoprecipitate (lane 4) and human (Hs) Aurora A precipitates with Hs TACC3 (lane 6). Lanes 1 and 2 show control preimmune and anti–Aurora A immunoprecipitates blotted to reveal D-TACC (top) or Aurora A (bottom). Lanes 3 and 4 show anti-GFP immunoprecipitates from transgenic lines expressing Tau–GFP and D-TACC–GFP fusion proteins, respectively and blotted with anti–D-TACC (top) or anti–Aurora A (bottom). Lanes 5 and 6 show control preimmune and anti-human Aurora A immunoprecipitates blotted to reveal human TACC3 (top) or human Aurora A (bottom). (B) Hs aurora (green) colocalizes with Hs TACC3 (red) to the centrosomes and the spindle poles. DNA is blue. (C) Extracts of a 2-h collection of Drosophila embryos were fractionated on a 5–40% sucrose gradient. Aliquots from each fraction were subjected to Western blotting with anti-TACC antibodies (top), anti-minispindle (second panel from the top), anti–γ-tubulin (second panel from the bottom) and anti–Aurora A (bottom). Molecular mass marker (4.3, 7.4, and 11 S are indicated). (D) GFP–D-TACC (lanes 2, 3, 5, 6, 8, and 9) or GFP-Tau (lanes 1, 4, and 7) were immunoprecipitated from Drosophila embryos, heat treated to inactivate potential endogenous kinases, and incubated with recombinant aurA-(His)6 kinase (lanes labeled +) and [γ-32P] ATP. Enzyme was not added to the reaction mixtures analyzed on lanes marked −. Left and middle panels show the radioactive products of the same kinase assay analyzed by 10 and 6% SDS-PAGE, respectively. The right panel shows an equivalent aliquot of material from a 6% gel analyzed by Western blotting with an anti-GFP antibody. Note the correspondence between the phosphorylated bands in the middle pannel and the presence of the GFP-D-TACC protein and its degradation products in the right panel. (E) Bacterially expressed chimeras of Maltose Binding Protein fused to the NH2-terminal (MBP–Nt amino acids 2–433), middle (MBP-Mid, amino acids 433–889), or COOH-terminal domain (MBP–Ct, amino acids 853–1189) of D-TACC were incubated in the presence (+, lanes 2, 4, and 6) or absence (−, lanes 1, 3, and 5) of purified aurora A-(His) 6 kinase and [γ-32P] ATP. Note the strong phosphorylation of the middle fragment of D-TACC (lane 4) and the very weak phosphorylation of the MBP–Nt and MBP–Ct fusion proteins (lanes 2 and 6).
If this interaction has functional significance, it might be expected to have been conserved through evolution. Therefore, we chose to examine whether the human counterpart of Aurora A showed association with HsTACC3, the closest human counterpart to D-TACC (Gergely et al., 2000b). We found that just as in Drosophila cells an immunoprecipitate of Hs Aurora A contained HsTACC3 (Fig. 8 A, lane 6). Moreover, immunostaining revealed both proteins to colocalize around centrosomes in cultured human cells (Fig. 8 B).
The syncytial Drosophila embryo is provided with a rich dowry of maternal proteins that enable it to undertake the 13 rapid cycles of nuclear division before cellularization. Therefore, any collection of embryos will have not only representation of different cell cycle stages, but also of differing extents of development to which the maternally provided mitotic proteins make an increasing contribution. Thus, we wished to determine whether the interaction between Aurora A and D-TACC was mirrored by a major multisubunit complex that could represent either a maternal store or some other functional unit. Therefore, we fractionated an extract from a 2-h collection of predominantly syncytial embryos by sedimentation on a 5–40% sucrose gradient (Fig. 8 C). Western blotting of the fractions revealed that D-TACC (Fig. 8 C, top) sedimented at 6–8 s, suggesting it is present in a complex with MSPS (Fig. 8 C, second panel from the top) as shown by Lee and colleagues (2001). The small, previously described complex of γ-tubulin thought to be a heterotetramer of two molecules of γ-tubulin, and one molecule each of Dgrip84 and Dgrip91, by comparison sediments slightly ahead at 7 s. Aurora A, on the other hand, sediments predominantly at 3–4 s (Fig. 8 C, bottom) showing only a small proportion cosedimenting with the D-TACC complex. Thus, it seems that there are substantial maternal pools of both proteins, but that they are not stockpiled in the same multiprotein complex, suggesting that only a small fraction of Aurora A interacts with the D-TACC complex.
Nevertheless, as immunoprecipitation experiments suggested that the two proteins could associate, we wondered whether Aurora A was also able to phosphorylate D-TACC. We chose to test this in two ways. In the first, we affinity purified GFP-tagged D-TACC protein from Drosophila embryos (Materials and methods) and then subjected the fusion protein to a heat treatment to inactivate any endogenous protein kinases before incubating it with a preparation of active recombinant Aurora A-(His)6 protein kinase and radiolabeled ATP. We analyzed the products of the kinase reaction by SDS-PAGE on either a 10 (Fig. 8 D, lanes 1–3) or a 6% gel, followed by autoradiography (Fig. 8 D, lanes 4–6). As control, we analyzed the ability of the enzyme to phosphorylate proteins that had been affinity purified using the same anti-GFP antibody from embryos expressing a Tau–GFP fusion protein (Fig. 8 D, lanes 1 and 4). We also performed Western blotting (Fig. 8 D, lanes 7–9) on the same membrane where the kinase reaction products were analyzed by autoradiography (Fig. 8 D, lanes 4–6). The results showed first that Aurora A cannot phosphorylate purified Tau–GFP protein that migrated at ∼100 kD (Fig. 8 D, lane 1). Second, there were no protein kinases in the immunoprecipitate capable of phosphorylating GFP-tagged D-TACC protein in the absence of exogenous Aurora A kinase (Fig. 8 D, lanes 2 and 5). When Aurora A kinase is added, we observed the appearance of several phosphorylated bands (Fig. 8 D, lanes 3 and 6). These bands comigrated exactly with the GFP–D-TACC protein and its degradation products when observed by autoradiography (Fig. 8 D, lane 6) or by Western blotting (Fig. 8 D, lanes 8 and 9). In a second test of whether D-TACC was an Aurora A substrate, we used bacterially expressed and purified fusion proteins between Maltose Binding protein and different domains of the D-TACC protein (Fig. 8 E). We found that Aurora A kinase would only phosphorylate the NH2- or the COOH-terminal domains of D-TACC very poorly (Fig. 8 E, lanes 2 and 6). In contrast, the middle fragment of the protein was a highly favored substrate (Fig. 6 E, lane 4). Thus, it appears that not only does D-TACC requires active Aurora A kinase for its localization to the centrosome, but it is also a good in vitro substrate of the enzyme.
Discussion
Our present work casts new light onto some of the functions of the A-type Aurora protein kinases. A role for the Drosophila member of this family in regulating centrosome behavior was suggested by our earlier analysis of the mutant phenotype, a characteristic of which was the generation of circular arrays of mitotic chromosomes around a single body of apparently duplicated centrosomes (Glover et al., 1995). It is possible that such a phenotype could arise through two different routes. One possibility, that Aurora A function is required to maintain spindle pole separation, receives support from the observation that bipolar mitotic spindles formed in frog egg extracts collapse following the addition of dominant negative mutant forms of Eg2, the Xenopus A-type Aurora kinase (Giet and Prigent, 2000). However, our present observations of multiple centrioles at the spindle poles in aurora A mutant cells suggests that at least in these cells there has been a failure of centriole segregation at the onset of mitosis. An allelic series of mutations offers the possibility of studying multiple functions of a protein and its role at different developmental stages where the cell cycle may be under differing modes of regulation. In this study we have concentrated on two mutants, one of which displays a mitotic phenotype in syncytial embryos that undertake rapidly alternating S and M phases, and the other in larval neuroblasts, cells that undergo conventional cell cycles with active checkpoints. In both situations we have focused on events at the spindle poles that provide a common aspect of mutant phenotype in these differing cell cycles. In the first case, we reexamined the centrosomes and astral microtubules in syncytial embryos derived from mothers homozygous for aurA 287, a weak hypomorphic aurA mutant that produces poorly functional protein that allows repeated, but increasingly abnormal mitoses in the syncytium. In the second case we studied the spindle poles in aurA e209, a strongly hypomorphic mutant that shows an equally elevated mitotic index whether the mutation is homozygous or hemizygous. In each mutant we observed a diminution of the length and number of astral microtubules even though the ultimate consequences for mitotic progression differs markedly in these two circumstances; the embryonic mitotic cycles can continue whereas the larval cycles are blocked at metaphase. The origins of the monopolar spindles seen in larval neuroblasts are still unclear. The increase in proportion of such structures seen in the presence of two copies rather than a single copy of the aurA e209 allele (in both cases in the absence of wild-type protein) points toward a neomorphic function for the mutant protein. Consistent with this is the finding that no monopolar spindles were seen following air-1 RNAi in C. elegans (Schumacher et al., 1998). The interphase-like arrays of microtubules seen in the air-1–depleted embryos resemble those seen in the cytoplasm of aurA 287-derived embryos. However, depletion of Aurora A by RNAi did not block mitotic progression in the embryonic cell line S2, but nevertheless it did lead to similar defects at the spindle poles to those in the aurA mutants.
Examination of the ultrastructure of the spindle poles in aurA e209 mutant larval neuroblasts revealed that when centrosomes were present they were comprised of multiple centrioles surrounded by electron-dense material having a distribution similar to centrosomal antigens revealed by light microscopy. These abnormal centrosomes contained core centrosomal antigens such as centrosomin (Heuer et al., 1995), proteins required for the nucleation of microtubules such as γ-tubulin (Moritz et al., 1995) and Asp (do Carmo Avides and Glover, 1999) and which can be removed by salt washes from the core centrosome, and proteins such as CP-190 whose association with the centrosome may be mediated through an interaction with γ-tubulin (Whitfield et al., 1995). However, a characteristic of the aurA e209 mutant centrosomes, also seen in the aurA 287-derived embryos and after aurA RNAi in S2 cells, was the reduced amounts of the D-TACC protein and the MSPS proteins, known to be required to maintain the length and/or number of astral microtubules (Cullen et al., 1999; Gergely et al., 2000b; Lee et al., 2001). Thus, the failure of D-TACC–MSPS complex to be recruited to the centrosome when Aurora A kinase function is compromised provides an explanation for the correlation of the apparent diminution of length and/or number of astral microtubules in such circumstances.
D-TACC is not only associated with centrosomes, but also with spindle microtubules. This latter association, which appears not to require Aurora A kinase, has been shown to depend on binding to the MSPS MAP, a member of the ch-TOG/XMAP 215 family of proteins (Cullen and Ohkura, 2001; Lee et al., 2001). We have shown that Aurora A kinase can bind to the D-TACC protein in Drosophila, as can the orthologues of these proteins in human cells. However, in extracts of Drosophila embryos, only a minor proportion of each protein appears to be associated in the same complex probably reflecting the fact that both Aurora A and D-TACC proteins are supplied as an abundant maternal dowry that is only used for mitosis as development proceeds. We also show that Aurora A is capable of phosphorylating D-TACC, leading us to propose that this is required to recruit D-TACC to the centrosome early in mitosis. However, it is equally possible that Aurora A phosphorylates other centrosomal proteins in such a way as to facilitate the recruitment of D-TACC. The association of D-TACC with the centrosome could occur either independently or when it is already complexed with MSPS. In either case, the docking of the D-TACC–MSPS complex to the centrosome could then allow the complex access to microtubules nucleated by the γ-tubulin ring complex in concert with the Asp protein. Association of MSPS may then promote the growth of the microtubules at both minus and plus ends as is normally seen in mitotic asters. This is suggested by the known properties of the Xenopus counterpart of MSPS, XMAP215, that promote microtubule elongation rates strongly at the plus end but also at the minus end (Vasquez et al., 1994; Popov et al., 2001). Thus, the complex may also be carried on the extending microtubule giving it the appearance of accumulating near the plus ends as well as on the centrosome. We have observed such localization of D-TACC near to the putative plus ends of microtubules on the spindles of S2 cells (unpublished data) as have also Lee and colleagues (2001) using real time imaging of GFP fusion proteins in Drosophila embryos. It is also possible that the D-TACC–MSPS complex acts to prevent ejection of microtubules from the centrosome.
Although both hypomorphic and null alleles of d-tacc lead to female sterility, the gene is not essential for the larval division cycles. Thus, homozygotes mutants can transit the earliest stages of development using wild-type protein from their heterozygous mothers and develop to adulthood. One possible explanation is that a need for D-TACC to target MSPS to centrosomes and thereby provide an efficient means of stabilizing astral microtubules is of particular importance in the rapid division cycles of the syncytial embryo. The longer cell cycle of larval cells coupled with their strong metaphase checkpoint could permit time to correctly assemble spindle poles in the absence of D-TACC protein. However, strong hypomorphic aurora A mutants do arrest at metaphase, pointing toward additional functions of the Aurora A enzyme beyond D-TACC recruitment, possibly in aspects of the metaphase–anaphase transition itself.
Our ultrastructural studies outlined above showed that bipolar spindles in aurA e209 neuroblasts were missing centrioles from one of their poles and had multiple centrioles and pericentriolar material at the other pole. Serial sectioning for electron microscopy was a time-consuming process in which we only examined sections from ten cells. However, this limited number of observations correlates well with our observations made by immunostaining with the light microscope. Such studies revealed many mitotic figures in which multiple centrosomal antigens were missing from one of the poles and multiple bodies containing centrosomal antigens were present at the other. The ability to make a stabilized and focused spindle pole in the absence of centrosomes is well known. Such focused spindle poles have been shown to form in the absence of centrosomes both in Xenopus extracts (Heald et al., 1996) and in Drosophila (Megraw et al., 2001) through the concerted action of microtubule motors and MAPS to organize and stabilize focused microtubule minus ends. Such acentriolar spindle poles are also seen in female meiosis in Drosophila where the minus end directed motor Ncd is essential to organize the poles (Endow and Komma, 1997). Cullen and Ohkura (2001) have proposed that complexes of D-TACC and MSPS at the acentriolar poles of the spindles of female meiosis could stabilize the bipolar structure, thus accounting for its loss of bipolarity in MSPS or d-tacc mutants. Such a function is unlikely to be essential to maintain the mitotic spindle in larval neuroblasts, which can adopt a stable bipolar structure in the absence of Aurora A function and hence D-TACC accumulation, and indeed in the absence of centrosomes (Megraw et al., 2001).
Could the failure to recruit the D-TACC protein to the centrosome also explain the accumulation of replicated centrioles at the spindle poles and their failure to segregate? The dispersed distribution of centrosomal antigens at the spindle pole is explained at the ultrastructural level by the finding of multiple centrioles surrounded by electron-dense pericentriolar material. It is possible that the presence of D-TACC is required to maintain aspects of the structural integrity of the centrosome, as the molecule is endowed with a coiled coil region. It has also been demonstrated to form polymers that could be of structural importance (Gergely et al., 2000a). Overexpression of the TACC domain of D-TACC alone results in the formation of TACC aggregates that bind MSPS and nucleate asters of microtubules (Lee et al., 2001). Overexpression of the human counterpart of D-TACC, HsTACC3, in mammalian cells also leads to the formation of aggregates to bind ch-TOG and appears to increase the numbers of microtubules (Gergely et al., 2000a). This apparent tendency of the TACC proteins to form large polymers could perhaps account for the cytoplasmic accumulation of D-TACC aggregates in aurA mutant neuroblasts. These show some tendency to cluster over the spindle microtubules. However, their failure to affect spindle structure might reflect the lower levels of MSPS that we find in this tissue compared with the syncytial embryo. It may be particularly important during mitosis that this tendency of D-TACC to aggregate is controlled. Aurora A kinase could fulfil such a role by permitting recruitment of D-TACC once mitosis is underway and when the centriole pairs have separated before prophase. Regulation of the behavior of astral microtubules may be important at the time that centrosomes are migrating around the nuclear envelope before the nuclear lamins depolymerize. The length, density, and dynamics of these microtubules may be essential for the migration of the centrosome not only around the nuclear envelope, but in other developmental processes. Indeed, one developmental failure seen in d-tacc mutant embryos is the failure of centrosomes to migrate to the cortex of the syncytial embryo in cycles 9 and 10 (Gergely et al., 2000b), a process known to be microtubule dependent (Raff and Glover, 1989). Failure of centrosomes to migrate to opposite sides of the nucleus could explain the origins of monoastral biploar spindles. We propose that accumulation of microtubule nucleating centers at one pole could in its extreme disrupt the inherent tendency to form a bipolar structure leading to formation of monopolar spindles. The failure of duplicated centrioles to segregate to both ends of the mitotic spindle as it forms raises the possibility that D-TACC/MSPS recruitment may also be required to stably associate the replicated centriole pair. D-TACC is only the second substrate of the A-type Aurora kinases to be identified, the first being the Eg5 kinesin-like protein (Giet et al., 1999). Undoubtedly there exist many others, and indeed it is possible that D-TACC is only one of several centrosomal substrates of the Aurora A kinase that may play a role in facilitating the equitable segregation of centrioles to the spindle poles. Indeed as d-tacc does not appear to be essential for viability it may be functionally redundant and so the larval lethality shown by strongly hypomorphic alleles of aurA may reflect a role for the kinase in modifying other mitotic targets. Identifying other substrates of the Aurora A kinases and evaluating their roles in mitotic progression remains a future challenge.
Materials and methods
Fly stocks
The aurora A e209 allele (Glover et al., 1995) originates from Gausz et al. (1981). The aurora A 287 allele is described in Tearle and Nusslein-Volhard (1987).
Production of recombinant proteins
To produce antibodies against Aurora A, the NH2-terminal domain of Aurora A was first amplified by PCR using the following primers: (a) 5′- GTTGTCGAGTGCGAATTCCGTCATGTCCCATCCG-3′ containing a EcoRI restriction site; and (b) 5′-GGGGCTGGGTTTCAAGCTTGGTTTTCTCTTTTTC-3′containing a HindIII restriction site. To make full-length active protein kinase, the primer 5′-GTGTGCGCAAGCTTCCAAGGATGCACC-3′ also containing the HindIII restriction site was used in combination with primer (a). Both PCR products were cleaved with HindIII and EcoRI and cloned into pET23b. Recombinant proteins were purified following the manufacturer's instructions (QIAGEN) and stored in 50% glycerol at –20°C.
Antibodies and Western blotting
The NH2-terminal domain of Aurora A was purified on an Ni-NTA agarose column (Amersham Pharmacia Biotech) and used for rabbit immunization. This Aurora A antibody was diluted 100 and 5,000 times for immunostaining and Western blots, respectively. Alternatively, antibody was affinity purified on nitrocellulose membrane and was used at the concentration of 5 ng/ml. This showed identical staining in immunolocalization and Western blotting to the complete serum.
Antibodies used in immunostaining were as follows: rat anti-tubulin antibody clone YL1/2 (SIGMA); Anti-CP190 (Whitfield et al., 1995); anti-CNN, a gift of Tom Kaufman (Indiana University, Bloomington, IN) (Heuer et al., 1995); anti–gamma-tubulin clone GTU-88 (Sigma-Aldrich); anti–human Hs TACC3 and TACC (Gergely et al., 2000b); and anti-MSPS (Cullen et al., 1999; Lee et al., 2001). Peroxidase-, Cy-2–, or Texas red–conjugated goat anti–rat, –mouse, and –rabbit secondary antibodies used in immunofluorescence were obtained from Jackson ImmunoResearch Laboratories.
Immunofluorescence analysis
S2 cells, grown on glass coverslips were fixed in PHEM buffer (60 mM Pipes, 25 mM Hepes, pH 6.8, 10 mM EGTA, 4 mM MgCl2) containing 3.7% formaldehyde. The cells were permeabilized using PBST (PBS containing 0.1% Triton X-100). Alternatively, the cells were fixed in –20°C methanol. Sequential incubations of primary and secondary antibodies were performed in PBST containing 1% BSA. DNA was stained by 1 μM TOTO3-iodide (Molecular Probes). Coverslips were mounted onto slides using Vectashield mounting medium H-1000 (Vector Laboratories).
Embryos were collected for 2–4 h for immunostaining, bleach dechorionated, and fixed in heptane/methanol for 10 min. The embryos were then rinsed three times in methanol, and incubated 30 min in 0.3% hydrogen peroxide in methanol. The subsequent antibody incubations were performed in PBST containing 1% BSA.
Brains from wild-type or mutant larvae were dissected in PBS, fixed 20 min in PBS containing 10% formaldehyde, permeabilized in PBST for 2 min, and preincubated in PBST containing 1% BSA. Incubations with the primary antibody were overnight followed by 2 ×20–min washes before incubation for 2 h with secondary antibodies. DNA was stained using 1 μM TOTO-3 iodode (Molecular Probes). For MSPS immunostaining in neuroblasts, we found that the best fixation was 20 min in methanol. The tissue was then processed like for the formaldehyde fixation. Images were acquired using a Biorad 1024 Confocal Scaning head mounted on a Nikon microscope and prepared for publication using Adobe Photoshop.
RNAi in S2 cultured cells
S2 cells were grown in Schneider's Drosophila media (GIBCO BRL) supplemented with 10% Fetal Calf Serum (GIBCO BRL) and 50 μg/ml streptomycin and penicillin. A 1.2-Kb Aurora A fragment corresponding to the open reading frame was amplified by PCR using the oligonucleotides: 5′-GTTGTCGAGTGCGAATTCCGTCATGTCCCATCCG-3′ and 5′-GAAAATTAAACAAGAATGTGAAAGCTTCGTGTGCCGC-3′.
The PCR amplification product was cloned into pGEMt easy vector (Promega). Clones with inserts in the opposite orientations were selected, mixed in equal proportions and digested by SpeI. The digested plasmids were used to produce Aurora A dsRNA using MEGASCRIPT T7 transcription kit (Ambion). RNA was purified following the manufacturer's instructions, denaturated for 20 min at 94°C, and annealed by a cooling overnight at room temperature. DsRNA was analyzed by 1% electrophoresis in agarose gel to ensure that the dsRNA migrated as a single band of ∼1.2 Kb.
Cells were inoculated into 6-well plates the day before transfection into 6-well plates. Before transfection of dsRNA, the cells were washed three times with media without serum and antibiotics and transfected with Transfast lipid reagent (Promega) following the manufacturer's instructions. 5 μg was used to transfect each well. Cells were incubated for 3 or 4 d to permit the cyclical degradation of the Aurora A protein kinase. Effects on spindle morphology could be first seen 2 d posttransfection. Samples were taken at various time post transfection and analyzed by FACS, Western blot, or indirect immunofluorescence.
Immunoprecipitations and kinase assays
The embryonic extract was made from a 0–8-h collection of embryos that had been bleach dechorionated as described before (Moritz et al., 1998). For immunoprecipitation, 0.3 ml of extract was diluted five times in EB buffer (50 mM Hepes, 100 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.25% NP-40, 2 mM β-glycerophosphate, 1 mM β-mercaptoethanol) supplemented with 10 μg/ml of leupeptin, pepstatin, chymostatin. The diluted extract was incubated with 10 μg of purified Aurora or mouse GFP antibodies linked with sheep anti–rabbit Dynabeads (Dynal) or protein G Sepharose, respectively. After 4 h at 4°C on a rotating wheel, the beads were washed three times in EB buffer, and the associated proteins were analyzed by SDS-PAGE and immunoblotting.
The potential substrates were immunoprecipitated from Drosophila extracts as described before, except that β-glycerophosphate was omitted from the EB buffer. After washing, the beads were heat inactivated to inactivate potential kinases in the IP and submitted to kinase assay using 100 ng of the recombinant Aurora A-(His) 6 kinase. The reaction assay was made in kinase buffer (KB containing 10 mM Tris, 25 mM NaCl, 10 mM MgCl2, 100 μM, ATP, 1 mM DTT, and 5 μCi of γ32PATP, 30,00 Ci/mmole, NEN). The reaction was stopped after 20 min by addition of Laemmli buffer. The boiled samples were submitted to SDS-PAGE analysis, autoradiography, and Western blotting. Maltose binding protein D-TACC proteins were used directly in the kinase assay (without heat inactivation) and were provided by Jordan Raff (Gergely et al., 2000b).
To monitor the kinase activity of wild-type and mutant Aurora kinases, Canton-S embryos or derived from aurA 287 mothers were lysed in 500 μl of lysis buffer (LB containing 80 mM K/Hepes, 1 mM EGTA, 1 mM MgCl2, 150 mM KCl, 100 mM β-glycerophosphate, 1 mM DTT, 0,5% Triton X-100) supplemented with 10 μg/ml of leupeptin, pepstatin, and chymostatin). The extract was filtered and clarified by centrifugation (10,000 g at 4°C). The extract was then incubated 1 h at 4°C with sheep anti–rabbit Dynabeads (DYNAL) prebounded with 5 μg of control or purified Aurora A antibodies. The beads were washed three times with LB and one time with KB without ATP. The Aurora kinase activity was then monitored by incubating the beads in KB containing 4 μg of Myelin Basic Protein for 15 min at room temperature. Activities were quantified using a Phosphorimager (Packard).
Extracts fractionation and centrosome preparation
Extracts fractionation was performed as previously described (Moritz et al., 1998). BSA (4 and 3 s) aldolase (7 and 4 s), and catalase (11 s) were run in parallel as controls.
Acknowledgments
We would especially like to thank Adelaide Carpenter for her skilled tuition in carrying out electron microscopy on Drosophila larval brains, and Endre Mathe for helpful advice and stimulating discussions.
We would like to thank the Cancer Research Campaign for a Program Grant to D.M. Glover. R. Giet was supported by Fellowships from the Marie Curie Program of the EEEC and the Human Frontiers Science Program. C. Prigent is supported by the Centre National de la Recherche Scientifique, L'association pour la Recherche sur le Cancer, and the Ligue Nationale Contre le Cancer. S. Deschamps is a fellow of the Ligue Nationale Contre le Cancer. J.W. Raff thanks the Wellcome Trust for a Senior Research Fellowship in Basic Biomedical Sciences, and M.J. Lee thanks the Medical Research Council for a research studentship.
Footnotes
Abbreviations used in this paper: Asp, abnormal spindle protein; MAP, microtubule-associated protein; MSPS, minispindles; TACC, transforming acidic coiled coil.
References
- Adams, R.A., H. Maiato, W.C. Earnshaw, and M. Carmena. 2001. Essential roles of Drosophila inner centromere protein (INCENP) and Aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction and chromosome segregation. J. Cell Biol. 153:865–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, R.R., S.P. Wheatleya, A.M. Gouldsworthy, S.E. Kandels-Lewis, M. Carmena, C. Smythe, D.L. Gerloff, and W.C. Earnshaw. 2000. INCENP binds the aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol. 10:1075–1078. [DOI] [PubMed] [Google Scholar]
- Andersen, S.S., A.J. Ashford, R. Tournebize, O. Gavet, A. Sobel, A.A. Hyman, and E. Karsenti. 1997. Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature. 389:640–643. [DOI] [PubMed] [Google Scholar]
- Belmont, L., T. Mitchison, and H.W. Deacon. 1996. Catastrophic revelations about Op18/stathmin. Trends Biochem. Sci. 21:197–198. [PubMed] [Google Scholar]
- Bischoff, J.R., L. Anderson, Y. Zhu, K. Mossie, L. Ng, B. Souza, B. Schryver, P. Flanagan, F. Clairvoyant, C. Ginther, et al. 1998. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17:3052–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy, A., H.A. Lane, P. d'Herin, M. Harper, M. Kress, and E.A. Nigg. 1995. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 83:1159–1169. [DOI] [PubMed] [Google Scholar]
- Budde, P.P., A. Kumagai, W.G. Dunphy, and R. Heald. 2001. Regulation of Op18 during spindle assembly in Xenopus egg extracts. J. Cell Biol. 153:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, C.F., and H. Ohkura. 2001. MSPS protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat. Cell Biol. 3:637–642. [DOI] [PubMed] [Google Scholar]
- Cullen, C.F., P. Deak, D.M. Glover, and H. Ohkura. 1999. Mini spindles: a gene encoding a conserved microtubule-associated protein required for the integrity of the mitotic spindle in Drosophila. J. Cell Biol. 146:1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo Avides, M., and D.M. Glover. 1999. Abnormal spindle protein, Asp, and the integrity of mitotic centrosomal microtubule organizing centers. Science. 283:1733–1735. [DOI] [PubMed] [Google Scholar]
- do Carmo Avides, M., A. Tavares, and D.M. Glover. 2001. Polo kinase and Asp are needed to promote the mitotic organizing activity of centrosomes. Nat. Cell Biol. 3:421–424. [DOI] [PubMed] [Google Scholar]
- Donaldson, M.M., A.A. Tavares, H. Ohkura, P. Deak, and D.M. Glover. 2001. Metaphase arrest with centromere separation in polo mutants of Drosophila. J. Cell Biol. 153:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow, S.A., and D.J. Komma. 1997. Spindle dynamics during meiosis in Drosophila oocytes. J. Cell Biol. 137:1321–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gausz, J., H. Gyurkovics, G. Bencze, A. Awad, J.J. Holden, and D. Ish-Horowicz. 1981. Genetic-characterization of the region between 86F1,2 and 87B15 on chromosome 3 of Drosophila-melanogaster. Genetics. 98:775–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely, F., C. Karlsson, I. Still, J. Cowell, J. Kilmartin, and J.W. Raff. 2000. a. The TACC domain identifies a family of centrosomal proteins that can interact with microtubules. Proc. Natl. Acad. Sci. USA. 97:14352–14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely, F., D. Kidd, K. Jeffers, J.G. Wakefield, and J.W. Raff. 2000. b. D-TACC: a novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J. 19:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet, R., and D.M. Glover. 2001. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152:669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet, R., and C. Prigent. 1999. Aurora/Ipl1p-related kinases: a new oncogenic familly of mitotic serine/threonine kinases. J. Cell Sci. 112:3591–3601. [DOI] [PubMed] [Google Scholar]
- Giet, R., and C. Prigent. 2000. The Xenopus laevis aurora/Ip11p-related kinase pEg2 participates in the stability of the bipolar mitotic spindle. Exp. Cell Res. 258:145–151. [DOI] [PubMed] [Google Scholar]
- Giet, R., R. Uzbekov, F. Cubizolles, K. Le Guellec, and C. Prigent. 1999. The Xenopus laevis aurora-related protein kinase pEg2 associates with and phosphorylates the kinesin-related protein XlEg5. J. Biol. Chem. 274:15005–15013. [DOI] [PubMed] [Google Scholar]
- Glover, D.M., M.H. Leibowitz, D.A. McLean, and H. Parry. 1995. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 81:95–105. [DOI] [PubMed] [Google Scholar]
- Gopalan, G., C.S.M. Chan, and P.J. Donovan. 1997. A novel mammalian, mitotic spindle-associated kinase is related to yeast and fly chromosome segregation regulators. J. Cell Biol. 138:643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald, R., R. Tournebize, T. Blank, R. Sandaltzopoulos, P. Becker, A. Hyman, and E. Karsenti. 1996. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 382:420–425. [DOI] [PubMed] [Google Scholar]
- Heuer, J.G., K. Li, and T.C. Kaufman. 1995. The Drosophila homeotic target gene centrosomin (cnn) encodes a novel centrosomal protein with leucine zippers and maps to a genomic region required for midgut morphogenesis. Development. 121:3861–3876. [DOI] [PubMed] [Google Scholar]
- Hunter, T., and B.M. Sefton. 1981. Protein phosphorylation. Part A. Protein kinases: assays, purification, antibodies, functional analysis, cloning, and expression. Methods Enzymol. 200:1–763. [PubMed] [Google Scholar]
- Hyman, A.A., and E. Karsenti. 1996. Morphogenetic properties of microtubules and mitotic spindle assembly. Cell. 84:401–410. [DOI] [PubMed] [Google Scholar]
- Kaitna, S., M. Mendoza, V. Jantsch-Plunger, and M. Glotzer. 2000. INCENP and an Aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr. Biol. 10:1172–1181. [DOI] [PubMed] [Google Scholar]
- Lane, H.A., and E.A. Nigg. 1996. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol. 135:1701–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.J., F. Gergely, S.Y. Peak-Chew, and J.W. Raff. 2001. MSPS/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat. Cell Biol. 3:643–648. [DOI] [PubMed] [Google Scholar]
- Megraw, T.L., L.R. Kao, and T.C. Kaufman. 2001. Zygotic development without functional mitotic centrosomes. Curr. Biol. 11:116–120. [DOI] [PubMed] [Google Scholar]
- Moritz, M., M.B. Braunfeld, J.W. Sedat, B. Alberts, and D.A. Agard. 1995. Microtubule nucleation by gamma-tubulin-containing rings in the centrosome. Nature. 378:638–640. [DOI] [PubMed] [Google Scholar]
- Moritz, M., Y. Zheng, B.M. Alberts, and K. Oegema. 1998. Recruitment of the gamma-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J. Cell Biol. 142:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookata, K., S. Hisanaga, J.C. Bulinski, H. Murofushi, H. Aizawa, T.J. Itoh, H. Hotani, E. Okumura, K. Tachibana, and T. Kishimoto. 1995. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J. Cell Biol. 128:849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, G., and E. Schiebel. 1997. Centrosome-microtubule nucleation. J. Cell Sci. 110:295–300. [DOI] [PubMed] [Google Scholar]
- Popov, A.V., A. Pozniakovsky, I. Arnal, C. Antony, A.J. Ashford, K. Kinoshita, R. Tournebize, A.A. Hyman, and E. Karsenti. 2001. XMAP215 regulates microtubule dynamics through two distinct domains. EMBO J. 20:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff, J.W., and D.M. Glover. 1989. Centrosomes, and not nuclei, initiate pole cell formation in Drosophila embryos. Cell. 57:611–619. [DOI] [PubMed] [Google Scholar]
- Rebollo, E., and C. Gonzalez. 2000. Visualizing the spindle checkpoint in Drosophila spermatocytes. Embo Reports. 1:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghi, C., R. Giet, R. Uzbekov, N. Morin, I. Chartrain, R. Le Guellec, A. Couturier, M. Doree, M. Philippe, and C. Prigent. 1998. The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J. Cell Sci. 111:557–572. [DOI] [PubMed] [Google Scholar]
- Savoian, M.S., M.L. Goldberg, and C.L. Rieder. 2000. The rate of poleward chromosome motion is attenuated in Drosophila zw10 and rod mutants. Nat. Cell Biol. 2:948–952. [DOI] [PubMed] [Google Scholar]
- Sawin, K.E., and T.J. Mitchison. 1995. Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc. Natl. Acad. Sci. USA. 92:4289–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin, K.E., K. LeGuellec, M. Philippe, and T.J. Mitchison. 1992. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 359:540–543. [DOI] [PubMed] [Google Scholar]
- Schumacher, J.M., N. Ashcroft, P.J. Donovan, and A. Golden. 1998. A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development. 125:4391–4402. [DOI] [PubMed] [Google Scholar]
- Shirasu, M., A. Yonetani, and C.E. Walczak. 1999. Microtubule dynamics in Xenopus egg extracts. Microsc. Res. Tech. 44:435–445. [DOI] [PubMed] [Google Scholar]
- Speliotes, E.K., A. Uren, D. Vaux, and H.R. Horvitz. 2000. The survivin-like C. elegans BIR-1 protein acts with the Aurora-like kinase AIR-2 to affect chromosomes and the spindle midzone. Mol. Cell. 6:211–223. [DOI] [PubMed] [Google Scholar]
- Tearle, R., and C. Nusslein-Volhard. 1987. Tubingen mutants and stocklist. Dros. Inf. Ser. 66:209–226. [Google Scholar]
- Tournebize, R., A. Popov, K. Kinoshita, A.J. Ashford, S. Rybina, A. Pozniakovsky, T.U. Mayer, C.E. Walczak, E. Karsenti, and A.A. Hyman. 2000. Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat. Cell Biol. 2:13–19. [DOI] [PubMed] [Google Scholar]
- Vasquez, R.J., D.L. Gard, and L. Cassimeris. 1994. XMAP from Xenopus eggs promotes rapid plus end assembly of microtubules and rapid microtubule polymer turnover. J. Cell Biol. 127:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, A.O., W. Seghezzi, W. Korver, J. Sheung, and E. Lees. 2000. The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene. 19:4906–4916. [DOI] [PubMed] [Google Scholar]
- Whitfield, W.G., M.A. Chaplin, K. Oegema, H. Parry, and D.M. Glover. 1995. The 190 kDa centrosome-associated protein of Drosophila melanogaster contains four zinc finger motifs and binds to specific sites on polytene chromosomes. J. Cell Sci. 108:3377–3387. [DOI] [PubMed] [Google Scholar]
- Zhou, H., J. Kuang, L. Zhong, W.L. Kuo, J.W. Gray, A. Sahin, B.R. Brinkley, and S. Sen. 1998. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat. Genet. 20:189–193. [DOI] [PubMed] [Google Scholar]