Abstract
Cell migration and invasion are fundamental components of tumor cell metastasis. Increased focal adhesion kinase (FAK) expression and tyrosine phosphorylation are connected with elevated tumorigenesis. Null mutation of FAK results in embryonic lethality, and FAK−/− fibroblasts exhibit cell migration defects in culture. Here we show that viral Src (v-Src) transformation of FAK−/− cells promotes integrin-stimulated motility equal to stable FAK reexpression. However, FAK−/− v-Src cells were not invasive, and FAK reexpression, Tyr-397 phosphorylation, and FAK kinase activity were required for the generation of an invasive cell phenotype. Cell invasion was linked to transient FAK accumulation at lamellipodia, formation of a FAK–Src-p130Cas–Dock180 signaling complex, elevated Rac and c-Jun NH2-terminal kinase activation, and increased matrix metalloproteinase expression and activity. Our studies support a dual role for FAK in promoting cell motility and invasion through the activation of distinct signaling pathways.
Keywords: motility; invasion; FAK; Src; JNK
Introduction
Focal adhesion kinase (FAK)* is a protein tyrosine kinase involved in the regulation of cell cycle progression, cell survival, and cell migration (for review see Schaller, 2001). Through FAK COOH-terminal domain interactions with integrin-associated proteins, FAK is activated upon cell binding to extracellular matrix proteins and forms a transient signaling complex with Src family protein tyrosine kinases (for review see Schlaepfer et al., 1999). FAK-null (FAK−/−) fibroblasts form an abundance of actin stress fibers and focal contact sites in cell culture that result in refractory motility responses (Ilic et al., 1995). FAK−/− reconstitution studies have shown that cellular Src (c-Src) recruitment to FAK is an initial event promoting focal contact turnover and enhanced cell motility (Owen et al., 1999; Sieg et al., 1999). FAK also associates with activated growth factor receptors through its NH2-terminal FERM domain and the FAK–Src complex is important in the regulation of growth factor–stimulated cell migration (Sieg et al., 2000). Whereas activation of the FAK–Src complex facilitates the association with and/or phosphorylation of multiple signaling proteins (for review see Schlaepfer et al., 1999), both FAK (Ren et al., 2000) and Src act to transiently inhibit p21 Rho–GTPase activity in part through targets such as p190RhoGAP (Arthur et al., 2000). Notably, inhibition of Rho signaling can partially reverse the morphological and motility defects of FAK−/− cells (Chen et al., 2002).
Many malignant human tumor samples exhibit increased FAK expression and tyrosine phosphorylation (Owens et al., 1995). These changes are correlated with the acquisition of an invasive cell phenotype and increased metastasis (Kornberg, 1998; Cance et al., 2000). Since specific signaling pathways associated with FAK and cell invasion have not been elucidated, it has been hypothesized that FAK-mediated cell invasion may represent FAK effects on tumor cell motility (Hauck et al., 2001b; Slack et al., 2001). Notably, the viral Src (v-Src) oncogene can directly transform a variety of cell types and can promote an invasive cell phenotype in vitro and experimental metastases in vivo (Hamaguchi et al., 1995; Aguirre-Ghiso et al., 1999). FAK is a v-Src substrate, and the formation of a v-Src–FAK signaling complex is associated with increased cell invasion and the generation of invadopodia (Hauck et al., 2002a). Stable expression of a dominant-negative (DN) inhibitor of FAK termed FAK-related nonkinase (FRNK) in v-Src–transformed NIH3T3 fibroblasts resulted in the inhibition of Matrigel cell invasion in vitro and experimental metastasis in vivo without effects on cell motility or v-Src–enhanced cell growth (Hauck et al., 2002b). These studies with v-Src and FRNK suggest that the role of FAK in promoting cell motility and/or invasion might be distinguishable events.
Cell invasion is a complex process that can be initiated by alterations in integrin surface expression (Hood and Cheresh, 2002), by the release or activation of proteases that degrade the extracellular matrix (McCawley and Matrisian, 2000), and by changes in gene expression during cell transformation (Ozanne et al., 2000). Although FAK may not be a critical determinant regulating v-Src–altered cell morphology or cell growth (Roy et al., 2002), we show that v-Src transformation reverses the integrin-stimulated motility defects of FAK−/− fibroblasts as does FAK reexpression. However, FAK−/− v-Src cells fail to exhibit an invasive phenotype as do v-Src–transformed FAK-reconstituted cells. For the first time, we show that serum stimulation promotes FAK accumulation at lamellipodia and that this is associated with increased Rac and c-Jun NH2-terminal kinase (JNK) activation. Our studies support the novel hypothesis that FAK recruitment to lamellipodia/invadopodia promotes cell invasion and FAK localization to focal contacts promotes integrin-stimulated cell motility, each in part through the activation of distinct signaling pathways.
Results
v-Src transformation promotes fibronectin-stimulated FAK− /− cell motility
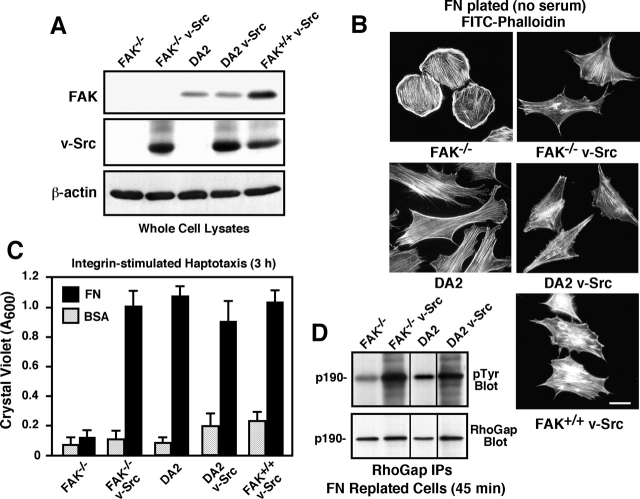
To establish a model system where the role of FAK in cell transformation could be defined, primary FAK−/−, FAK-reconstituted (DA2), or FAK+/+ fibroblasts were transformed by stable v-Src expression (Fig. 1) . Importantly, v-Src protein levels (Fig. 1 A) and in vitro kinase (IVK) activity (unpublished data) were equal in pooled populations of FAK−/− and DA2 cells. FAK−/− cells possess a rounded cell shape when plated onto fibronectin (FN), and FAK reexpression rescues this defect by promoting DA2 cell spreading (Fig. 1 B). v-Src promoted a transformed fusiform morphology with reduced levels of actin stress fibers in both FAK−/− and DA2 cells (Fig. 1 B). Pooled populations of FAK+/+ v-Src cells exhibited an intermediate morphological conversion (Fig. 1 B) that was associated with lower levels of v-Src expression (Fig. 1 A). These results support the conclusion that FAK is not a critical determinant of v-Src–stimulated morphological changes (Roy et al., 2002).
Figure 1.
v-Src transformation rescues spreading and haptotaxis defects of FAK −/− cells. (A) Whole cell lysates from the indicated cells were blotted with antibodies to FAK, v-Src, or β-actin. (B) The indicated cells were plated on FN-coated (10 μg/ml) coverslips in the absence of serum for 6 h. Phalloidin staining of actin shows that v-Src transformation promotes the conversion of FAK−/− and DA2 cells to a fusiform morphology. Bar, 20 μm. (C) FN-stimulated haptotaxis assays show that expression of FAK or v-Src can rescue FAK−/− motility defects. Random motility was assessed in the presence of BSA. Values are means ± SD of triplicates from three independent experiments. (D) p190 RhoGAP IPs were made from the indicated cells plated onto FN, analyzed by anti-pTyr blotting, and show enhanced pTyr levels stimulated by FAK or v-Src expression. Blots were reprobed by anti-p190RhoGAP blotting.
Refractory FAK−/− cell motility is due in part to the enhanced stability of focal contact structures (Ilic et al., 1995) and to defects in Rho regulation (Ren et al., 2000; Chen et al., 2002). Surprisingly, FAK−/− v-Src cells exhibited an approximately sixfold increase in FN-stimulated cell migration compared with FAK−/− cells. This level of FAK−/− v-Src cell motility was equivalent to DA2, DA2 v-Src, and FAK+/+ v-Src cell migration (Fig. 1 C). As integrins suppress Rho activity through Src-dependent p190RhoGAP tyrosine phosphorylation (Arthur et al., 2000), p190RhoGAP tyrosine phosphorylation was analyzed after FN stimulation of cells (Fig. 1 D). Elevated p190RhoGAP tyrosine phosphorylation was observed in lysates from FN-stimulated FAK−/− v-Src, DA2, and DA2 v-Src compared with FAK−/− cells. Our results show that both v-Src and FAK can promote cell motility, enhanced p190RhoGAP tyrosine phosphorylation, and morphological alterations of FAK−/− cells.
v-Src localizes to lamellipodia in FAK-containing cells
High levels of v-Src expression result in the turnover of peripheral focal contacts and the formation of ventral adhesion structures termed podosomes (Tarone et al., 1985). In the absence of serum, the fusiform-shaped FAK−/− v-Src cells (Fig. 1 B) make podosomes (unpublished data). In the presence of serum, FAK−/− v-Src cells assume a more spread morphology and make peripheral focal contacts as visualized by integrin-linked kinase (ILK) immunofluorescence (IF) staining (Fig. 2 A). Interestingly, v-Src was not strongly localized to peripheral focal contact sites in FAK−/− cells but instead exhibited a punctate perinuclear distribution (Fig. 2 A). Nevertheless, v-Src expression increased FAK−/− cell motility in scratch assays (Fig. 2 B). However, FAK−/− v-Src cell repopulation of the wounded area was less than DA2 cells (Fig. 2 B).
Figure 2.
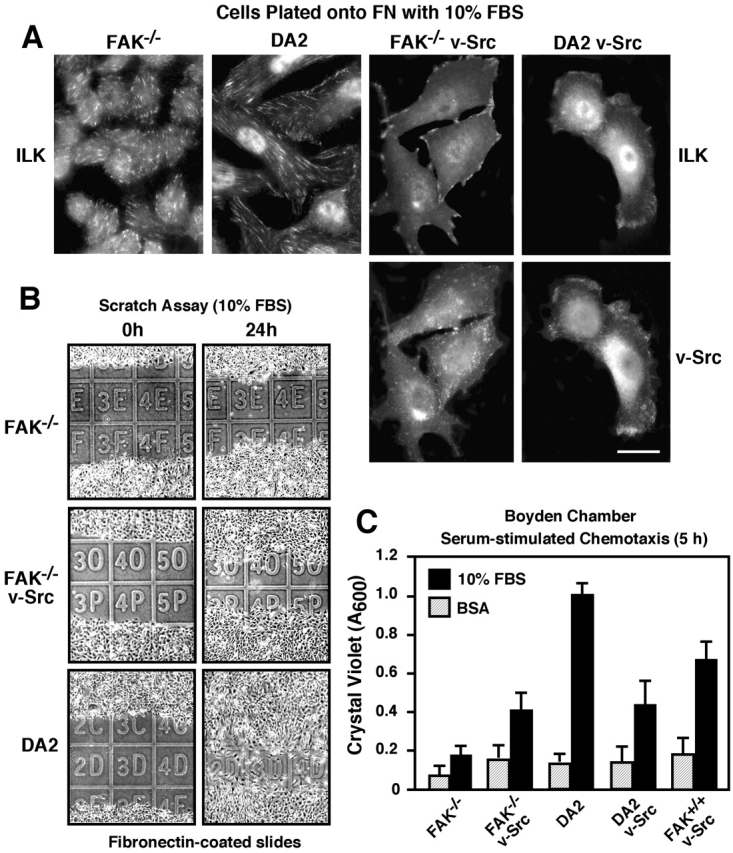
Chemotaxis motility and serum-stimulated redistribution of v-Src to lamellipodia in FAK-containing cells. (A) ILK indirect IF of cells plated on FN in the presence of serum shows reduced numbers of focal contacts in FAK−/− v-Src and DA2 v-Src cells. Indirect v-Src IF shows that FAK promotes v-Src association with focal contacts and membrane ruffles. Bar, 20 μm. (B) v-Src enhances the wound closure activity of FAK−/− cells. Cell migration was assessed by 1-mm grid comparisons of four image sets; representative images are shown. (C) Serum-stimulated chemotaxis assays show that v-Src–transformed FAK−/−, DA2, and FAK+/+ cells exhibit similar properties of reduced migration compared with DA2 cells. Random motility was assessed in the presence of BSA. Values are means ± SD of triplicates from two independent experiments.
In contrast to FAK−/− v-Src cells, DA2 v-Src cells plated on FN in the presence of serum exhibited extensive spreading and lamellipodia formation (Fig. 2 A). Both v-Src and ILK were similarly distributed within lamellipodial regions of DA2 v-Src cells (Fig. 2 A). However, DA2 v-Src cells also exhibited lower levels of wound closure activity compared with DA2 cells (unpublished data). As analyzed by Boyden Chamber chemotaxis assays, DA2 v-Src cell motility was ∼2.5-fold less than DA2 cells (Fig. 2 C). Since cell motility responses are dependent on a balance of focal contact formation and turnover (Lauffenburger and Horwitz, 1996), the lower levels of DA2 v-Src cell motility are consistent with the combined actions of FAK and v-Src in promoting focal contact turnover and reduced cell adhesion (Fincham and Frame, 1998; Datta et al., 2001). Interestingly, although FAK−/− v-Src and DA2 v-Src cells exhibited distinctly different cell morphologies in the presence of serum (Fig. 2 A), they exhibited equivalent levels of serum-stimulated motility (Fig. 2 C).
FAK expression promotes Matrigel invasion
In addition to altered growth and motility responses, v-Src can promote an invasive phenotype (Hamaguchi et al., 1995). This can be evaluated in three-dimensional (3-D) invasion assays wherein the cells degrade and move through a reconstituted (Matrigel) extracellular matrix barrier. Surprisingly, FAK−/− v-Src cells did not significantly invade through Matrigel (Fig. 3, A and B) . Normal DA2 cells exhibited greater cell invasion than FAK−/− v-Src cells. Both DA2 v-Src and FAK+/+ v-Src cells exhibited two- to threefold increased invasion compared with DA2 cells and eight to tenfold increased invasion compared with FAK−/− v-Src cells (Fig. 3, A and B). The differences in DA2 v-Src compared with FAK−/− v-Src cell invasion were not due to altered motility responses, since these cells exhibited equal wound closure activity on Matrigel-coated glass slides in the presence of serum (Fig. 3 C). These results show that v-Src promotes an invasive phenotype in FAK-reconstituted cells but not in FAK-deficient cells.
Figure 3.
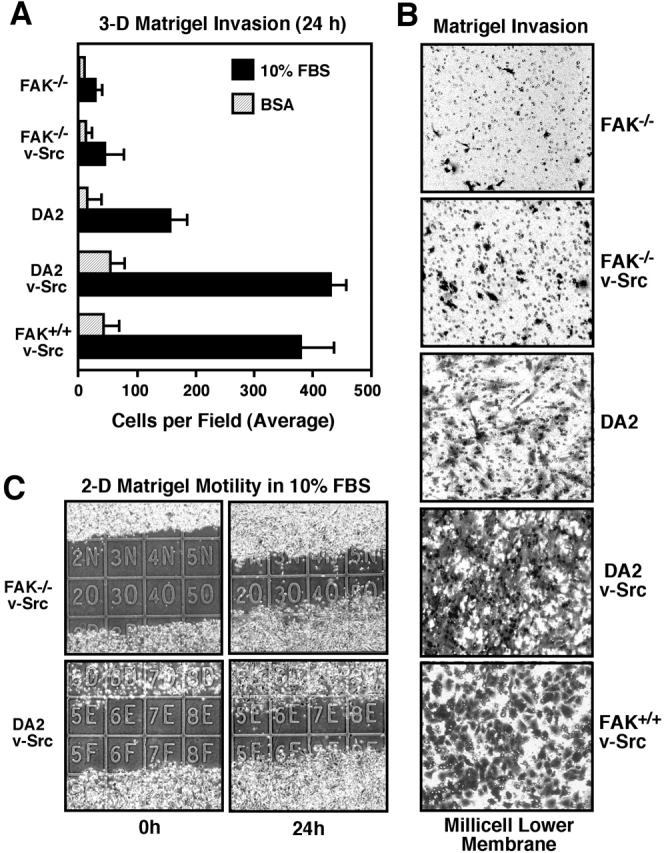
FAK is required for 3-D cell invasion through Matrigel. (A) FAK−/− v-Src cells do not invade through Matrigel as do FAK-expressing cells. Random cell invasion was assessed in the presence of BSA. Values are means of triplicates ± SD from three independent experiments. (B) Crystal violet–stained cells that invaded through the Matrigel can be distinguished from the 8-μm membrane pores. (C) Equivalent wound closure motility of FAK−/− v-Src and DA2 v-Src cells on Matrigel-coated slides in the presence of serum. Cell migration was assessed as in the legend to Fig. 2.
v-Src–FAK signaling enhances basal and serum-stimulated JNK activation
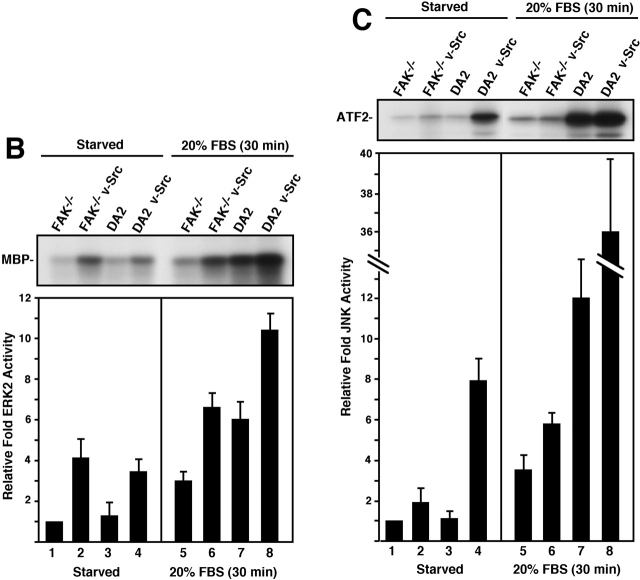
v-Src transformation results in the activation of multiple downstream targets such as the Ras–extracellular signal–regulated kinase (ERK)2/MAP kinase cascade, the Rac-JNK/SAP kinase cascade, phosphatidylinositol (PI) 3-kinase–Akt, and the phosphorylation of STATs (for review see Irby and Yeatman, 2000). To determine the contribution of FAK- to v-Src–stimulated signaling, whole cell lysates from serum-starved cells were analyzed with a series of phospho-specific antibodies (Fig. 4 A). Anti-phosphotyrosine (pTyr) blotting and phospho-specific antibodies to STAT3 (pSTAT3) revealed that v-Src promoted similar increases in tyrosine phosphorylation in both FAK−/− and DA2 cells (Fig. 4 A, lanes 2 and 4). Phospho-specific antibodies directed to Akt (pAkt) and to ERK (pERK) showed that these targets were equally activated in v-Src–transformed FAK−/− and DA2 cells (Fig. 4 A, lanes 6 and 8). However, blotting with phospho-specific antibodies to JNK (pJNK) revealed that this pathway was constitutively activated only in DA2 v-Src cells (Fig. 4 A, lane 8).
Figure 4.
FAK expression promotes elevated v-Src and serum-stimulated JNK activity. (A) Whole cell lysates from the indicated serum-starved cells were blotted with phospho-specific antibodies to tyrosine (pTyr), Tyr-705 of STAT3 (pSTAT3), Ser-473 of Akt (pAkt), Thr-202 and Tyr-204 of ERK2 (pERK), and Thr-183 and Tyr-185 of JNK (pJNK). Equal protein expression was verified by reprobing blots with antibodies to STAT3, Akt, ERK2, or JNK1. Numbers represent M r in kD. (B) ERK2 IP/IVK assays were performed with lysates from the indicated starved or 20% FBS-stimulated cells. Values are means ± SD from two independent experiments that were normalized for protein levels in IPs and represent fold differences in ERK2 activity from starved FAK−/− cells. (Inset) Representative image of MBP phosphorylation. (C) JNK IP/IVK assays were performed as described for ERK2 above. (Inset) Representative image of GST-ATF2 phosphorylation.
Since phospho-specific blotting provides only a qualitative measure of target protein activation, IVK assays were performed with lysates from starved or serum-stimulated cells (Fig. 4, B and C). Although no significant differences in basal ERK2 activity were found between v-Src–transformed FAK−/− and DA2 cells (Fig. 4 B, lanes 2 and 4), normal and v-Src–transformed DA2 cells exhibited slightly increased serum-stimulated ERK2 IVK activity compared with FAK−/− and FAK−/− v-Src cells, respectively (Fig. 4 B, lanes 5–8). This result is consistent with previous findings that FAK enhances the integrin signaling requirement for serum-stimulated ERK2 activation (Renshaw et al., 1999). Analyses of basal and serum-stimulated Akt IVK activity yielded similar results as shown for ERK2 (unpublished data). However, JNK IVK activity was elevated four- to fivefold in DA2 v-Src lysates compared with FAK−/− v-Src cells under both starved and serum-stimulated conditions (Fig. 4 C). Additionally, the serum-stimulated JNK IVK activity in DA2 lysates was approximately twofold higher than FAK−/− v-Src cells (Fig. 4 C, lanes 6 and 7). Since the level of serum-stimulated JNK activity correlated with increased DA2 and DA2 v-Src cell invasion (Fig. 3 A and Fig. 4 C), our results support the hypothesis that serum-stimulated and FAK-enhanced signaling to JNK may promote cell invasion.
Adenoviral-mediated FAK expression rescues FAK− /− v-Src invasion defects
To establish the importance of FAK in promoting cell invasion, a transient adenovirus (Ad)-mediated expression strategy was employed to express various hemagglutinin (HA)-tagged FAK constructs in FAK−/− v-Src cells (Fig. 5) . HA-FAK expression is controlled by the coinfection of cells with a recombinant Ad construct producing the tetracycline transactivator (TA) protein, and this serves as a control for the effects of Ad uptake into cells. Previous studies showed that FAK−/− fibroblasts can be readily infected with Ad constructs at low multiplicity of infection (MOI) (Li et al., 2000). Ad infection of FAK−/− v-Src cells yielded equal expression of HA-tagged FAK, Tyr-397–mutated FAK (Y397F), kinase-inactive FAK (K454R), or FAK mutated within both proline-rich SH3-binding sites in the COOH-terminal domain (Pro-null) (Fig. 5 A). Surprisingly, all FAK constructs were tyrosine phosphorylated in FAK−/− v-Src cells, and phospho-specific antibody blotting revealed that wild-type (WT), K454R, and Pro-null FAK were phosphorylated at Tyr-397 (Fig. 5 A). However, only WT FAK expression promoted an approximately fourfold increase in FAK−/− v-Src Matrigel cell invasion compared with Ad-TA–infected cells (Fig. 5 B). This corresponded to an ∼80% rescue of FAK−/− v-Src cell invasion compared with DA2 v-Src cells.
Figure 5.

FAK Tyr-397 phosphorylation, kinase activity, and SH3-binding sites in the FAK COOH-terminal domain are required to promote JNK activation and cell invasion. (A) HA tag IPs from FAK−/− v-Src cells infected with Ad-TA or Ad-TA in combination with the indicated Ad-FAK constructs were used to evaluate Ad-FAK activation by blotting with antibodies to HA, pTyr, and phosphorylated FAK at Tyr-397 (pY397). (B) Matrigel invasion assays were performed with FAK−/− v-Src cells infected with Ad-TA or Ad-TA in combination with the indicated Ad-FAK constructs and with noninfected DA2 v-Src cells. Values are means ± SD of triplicates from two separate experiments. (C) Ad-FAK expressed in FAK−/− v-Src cells is localized to the tips of invadopodia emerging from Matrigel and is phosphorylated at Tyr-397 (arrowheads) as detected by indirect IF. Only the 8 μm (scale bar) Matrigel-filled membrane pores are seen in assays with FAK Pro-null–infected cells. (D) JNK IP/IVK assays were performed in Ad-TA– or Ad-FAK–infected FAK−/− v-Src cells. Values are means ± SD of duplicates from independent experiments. (E) Matrigel invasion assays were performed with DA2 v-Src cells in the presence of DMSO or the JNK inhibitor (SP600125) at the indicated concentration in the top chamber. Values are means ± SD from two independent experiments.
Since the v-Src–FAK signaling complex is localized within invadopodia that emerge from the Matrigel barrier (Hauck et al., 2002a), IF staining with HA tag or FAK phospho-specific antibodies was used to analyze the activation state of Ad-FAK within FAK−/− v-Src cells during Matrigel invasion (Fig. 5 C). WT FAK expression was localized to the tips of invadopodia and was highly phosphorylated at Tyr-397 (Fig. 5 C). However, the FAK mutants that did not function to promote cell invasion also did not show invadopodia emergence through the Matrigel-filled 8-μm pores. Together, the IF results support the importance of FAK Tyr-397 phosphorylation during the processes of cell invasion.
To determine whether FAK-enhanced cell invasion was associated with increased signaling to JNK, endogenous JNK IVK activity was measured after Ad-FAK infection of FAK−/− v-Src cells (Fig. 5 D). WT FAK expression promoted a greater than fourfold increase in JNK activity compared with Ad-TA–infected FAK−/− v-Src cells, whereas the FAK mutants did not activate JNK. The approximately fourfold increase in FAK-stimulated JNK activity (Fig. 5 D) was correlated with similar changes in FAK−/− v-Src cell invasion (Fig. 5 B) and was consistent with the differences in JNK activity between FAK−/− v-Src and DA2 v-Src cells (Fig. 4 C). The importance of JNK activation promoting cell invasion of FAK-expressing cells was confirmed by the dose-dependent inhibition of DA2 v-Src Matrigel invasion by SP600125, a pharmacological inhibitor of JNK kinase activity (Fig. 5 E).
FAK localizes to lamellipodia and promotes Rac activation
In many cells, JNK activation is regulated in part through small GTPases such as Rac1 (Davis, 2000). To determine whether there were differences in Rac activity between FAK−/− and DA2 cells, the Rac-binding region (residues 67–150) of p65PAK kinase (GST-PAK) was used as an affinity probe for activated Rac in pull-down assays (Fig. 6 A). Under serum-starved conditions, activated Rac was only detected in lysates from DA2 v-Src cells, whereas total Rac expression levels was equal in all cells. After serum stimulation, Rac activation was greater in DA2 and DA2 v-Src cells compared with FAK−/− and FAK−/− v-Src cells, respectively (Fig. 6 A). To evaluate the contribution of FAK expression in promoting Rac activation, FAK−/− v-Src cells were infected with Ad-FAK constructs. WT FAK promoted endogenous Rac activation in FAK−/− v-Src cells as determined by GST-PAK binding (Fig. 6 B). No Rac activation was detected in cells infected with Y397F, K454R, or Pro-null FAK. These results support the conclusion that FAK-mediated Rac activation is associated with increased cell invasion.
Figure 6.
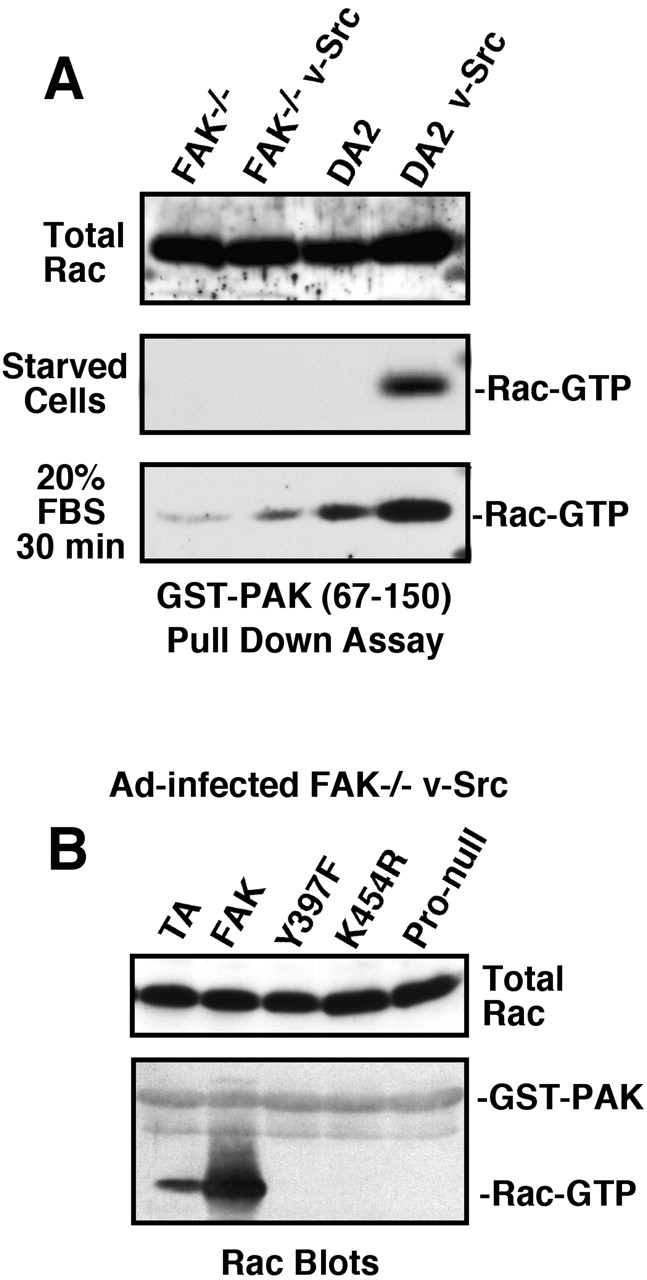
FAK activates Rac and serum stimulation promotes GFP-FAK localization to lamellipodia. (A) Rac activation was visualized in the indicated cells by pull-down assays using GST-PAK (67–150) followed by Rac blotting. Total Rac expression is shown in whole cell lysates. (B) FAK−/− v-Src cells were infected with Ad-TA or the indicated Ad-FAK constructs, and Rac activation was evaluated by GST-PAK binding. (C) FAK−/− v-Src cells were transfected with GFP-FAK, starved, and then stimulated with 10% FBS. Time-lapse confocal video-microscopy at 1-min intervals was obtained over 3 h (Video 1 available at http://www.jcb.org/cgi/content/full/jcb.200212114/DC1). Panels show a 9-min time series where GFP-FAK is stably localized to focal contacts and transiently recruited to lamellipodia extensions (arrows).
Since Rac activation occurs in membrane-associated regions in close proximity to lamellipodia, whereas integrin-stimulated FAK signaling is initiated from focal contact sites, FAK−/− v-Src cells were transfected with GFP-FAK to visualize the real-time localization of FAK (Fig. 6 C). Not surprisingly, GFP-FAK was localized to ventral and perimeter focal contact sites in FAK−/− v-Src cells. However, upon serum stimulation a transient accumulation of GFP-FAK was also visualized at distinct plasma membrane regions that were associated with the initiation of lamellipodial projections (Fig. 6 C and see Video 1 available at http://www.jcb.org/cgi/content/full/jcb.200212114/DC1). This GFP-FAK lamellipodial association lasted from 1 to 3 min, and it was not detectably accompanied by the loss of GFP-FAK from focal contact sites. Instead, the lamellipodial-associated FAK became nucleated at numerous point contact sites that formed behind lamellipodial projections. The GFP-FAK–containing point contacts were transient structures with a small percentage becoming mature focal contact sites that moved toward the center of the cell (Fig. 6 C). These analyses show that serum stimulation promotes the transient localization of FAK to lamellipodia. This places FAK at an appropriate intracellular site to facilitate signaling leading to Rac and JNK activation.
p130Cas as a mediator of FAK-initiated signals to JNK
Several different cellular proteins associate with FAK through SH3-mediated binding interactions at two proline-rich sites within the FAK COOH-terminal domain (Schaller, 2001). Since a trimeric complex between Src, FAK, and p130Cas is stabilized by Src SH2 binding to FAK Tyr-397, Src SH3 binding to p130Cas, and p130Cas SH3 binding to FAK (Schlaepfer et al., 1997), recombinant Ad constructs were used to overexpress WT p130Cas or SH3 domain-mutated p130Cas (Cas ΔSH3) in FAK−/− v-Src cells (Fig. 7 A). Although Ad-FAK expression increased endogenous p130Cas pTyr levels, both WT Cas and Cas ΔSH3 were highly tyrosine phosphorylated in FAK−/− v-Src cells (Fig. 7 A). However, only WT Cas functioned to rescue FAK−/− v-Src cell invasion defects approximately equal to Ad-FAK expression (Fig. 7 B). Importantly, Cas-stimulated cell invasion was associated with elevated JNK IVK activity in FAK−/− v-Src cells (Fig. 7 C). Cas ΔSH3 was highly tyrosine phosphorylated but did not stimulate JNK activity (Fig. 7 C).
Figure 7.

Ad-p130Cas rescues FAK −/− v-Src invasion defects; multiple connections to Dock180. (A) FAK−/− v-Src cells were infected with Ad-TA, Ad-FAK, Ad-p130Cas, or Ad-Cas ΔSH3. Total p130Cas was isolated by IP and sequentially analyzed by Cas and pTyr blotting. (B) Matrigel invasion assays were performed with FAK−/− v-Src cells infected with Ad-TA, Ad-FAK, or the indicated Ad-Cas constructs. Values are means ± SD of triplicates from two independent experiments. (C) JNK IP/IVK assays were performed with lysates of FAK−/− v-Src cells infected with Ad-TA, Ad-FAK, or the indicated Ad-Cas constructs and with lysates from DA2 v-Src cells. Shown is GST–ATF-2 phosphorylation and values reflect fold changes in JNK activity from two independent experiments normalized to Ad-TA. (D) Lysates (1 mg) were prepared from FAK−/− v-Src cells, incubated with GST or the indicated GST-SH3 domain fusion proteins, and collected by binding to glutathione-agarose beads. The GST-associated Dock180 was visualized by blotting, and bead-associated GST fusion proteins were visualized by Coomassie blue staining. (E) Lysates from serum-stimulated FAK−/− v-Src or DA2 v-Src cells were prepared, and IPs were performed with antibodies to Crk, p130Cas, Dock180, and FAK. The IP- associated proteins were resolved by SDS-PAGE and visualized by anti-pTyr blotting. The membrane was cut and the indicated regions reprobed with antibodies to Dock180, p130Cas, v-Src, or Crk. The p130Cas membrane region was sequentially reprobed by HA tag blotting to visualize HA-FAK expression in lysates from DA2 v-Src cells.
Our results show that Cas SH3 domain function is needed for v-Src–stimulated cell invasion. Although Crk SH2 binding to Cas within the substrate domain can promote signaling to JNK, overexpression of a Cas substrate domain-deleted mutant blocked FN- but not v-Src–stimulated JNK activation (Dolfi et al., 1998). Therefore, to elucidate a Cas SH3 domain–mediated and potential Crk-independent linkage to Rac and JNK, GST pull-down assays were performed with the Cas SH3 domain and compared with the binding activities of SH3 domains from other proteins (Fig. 7 D). Whereas the Cas SH3 domain can bind to targets such as guanine nucleotide exchange factor C3G in addition to FAK (Kirsch et al., 1998), we identified the guanine nucleotide exchange factor Dock180 as a target of both the Crk and Cas SH3 domains within lysates of FAK−/− v-Src cells (Fig. 7 D).
FAK expression facilitates the formation of a Dock180 signaling complex
To determine the potential participation of Dock180 in FAK-mediated signaling, serum-stimulated FAK−/− v-Src and DA2 v-Src cells were analyzed by co-immunoprecipitation (IP) analyses with antibodies to Crk, p130Cas, Dock180, or FAK (Fig. 7 E). Strikingly, highly tyrosine phosphorylated multiprotein complexes containing Crk, v-Src, p130Cas, Dock180, and HA-tagged FAK were isolated from DA2 v-Src but not FAK−/− v-Src lysates (Fig. 7 E). Importantly, equal amounts of Crk, p130Cas, or Dock180 were immunoprecipitated using the same primary antibodies, whereas multiprotein complexes were isolated only from DA2 v-Src lysates. Additionally, antibodies to FAK did not co-IP protein complexes in lysates from FAK−/− v-Src cells.
For Crk IPs, there was a strong association with v-Src and a weak association with highly tyrosine-phosphorylated p130Cas in FAK−/− v-Src lysates. In DA2 v-Src lysates, there was an increased association of FAK, Cas, and Dock180 with Crk without a change in either Crk tyrosine phosphorylation or v-Src association. For p130Cas, the Cas immunoreactive band was super shifted in DA2 v-Src lysates, and this was correlated with increased Cas tyrosine phosphorylation and the strong association with HA-FAK and v-Src (Fig. 7 E). Both Dock180 and Crk were associated with Cas only in DA2 v-Src lysates. For Dock180 IPs, strong associations with Cas and Crk and weaker associations with v-Src and HA-FAK were detected only in DA2 v-Src cell lysates. For FAK IPs, there was a strong association with v-Src and weaker associations with Cas and Crk. No association of Dock180 was visualized in FAK IPs, indicating that FAK expression in DA2 v-Src cells facilitates the formation of a Dock180-containing signaling complex likely through direct and indirect multiprotein interactions.
FAK and Rac regulate cell invasion and matrix metalloproteinase-9 secretion
Since the FAK−/− v-Src cells exhibit equivalent motility responses as DA2 v-Src cells, experiments were performed to determine whether enhanced matrix metalloproteinase (MMP) secretion and/or activity were associated with FAK-mediated cell invasion (Fig. 8) . Cells were analyzed by Matrigel invasion assays (Fig. 8 B) and by gelatin zymography of cell conditioned media (Fig. 8 C). FAK−/− and FAK−/− v-Src–conditioned media contained secreted but not activated 72 kD MMP-2 (Fig. 8 C, lanes 1 and 2). In zymography assays, pro–MMP-2 exhibits high levels of apparent activity due to the dissociation of TIMP inhibitor–MMP complexes during gel electrophoresis. Activation of pro–MMP-2 occurs in part through proteolytic cleavage (Nagase and Woessner, 1999). DA2-conditioned media exhibited both pro- and activated MMP-2 (Fig. 8 C, lane 3).
Figure 8.
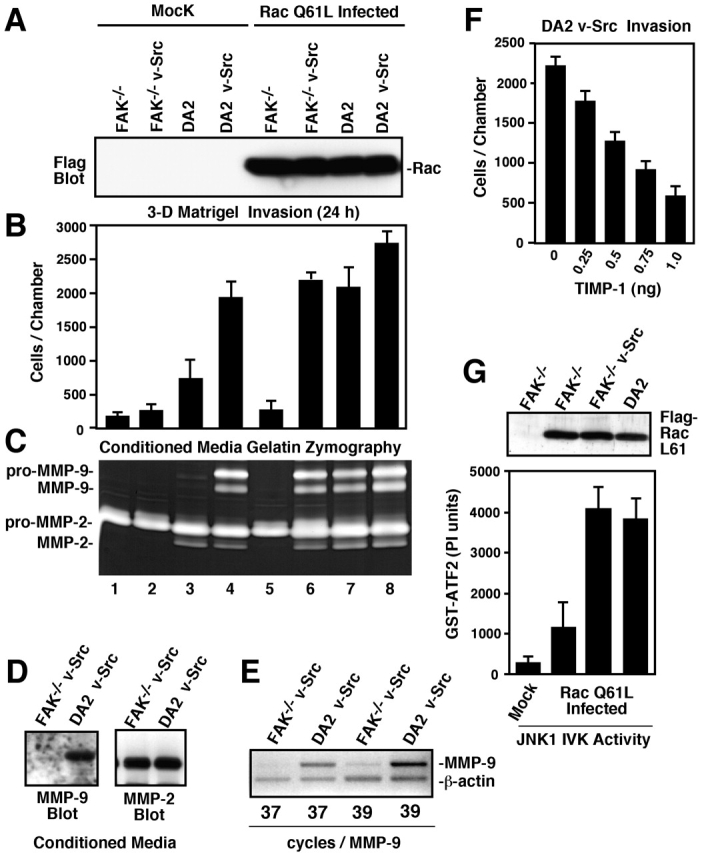
FAK and activated Q61L Rac synergize to promote JNK activation, increased MMP-9 expression, and an invasive cell phenotype. (A) Flag tag blotting was used to visualize Q61L Rac expression in the indicated Ad- infected cells. (B) Matrigel invasion assays were performed with the indicated Mock or Ad-Q61L Rac-infected cells. Values are means ± SD of triplicates from two independent experiments. (C) Gelatin zymography was performed with conditioned media from the indicated Mock or Ad-Q61L Rac-infected cells. Migration of pro and active forms of MMP-9 and MMP-2 are shown. (D) Conditioned media from FAK−/− v-Src or DA2 v-Src cells was concentrated, and MMP-9 or MMP-2 secretion was evaluated by blotting. (E) Semiquantitative RT-PCR was performed using primers to MMP-9 and to β-actin and showed that MMP-9 mRNA levels were 6.5-fold higher in DA2 v-Src compared with FAK−/− v-Src cells. (F) Matrigel invasion assays were performed with DA2 v-Src cells with the addition of recombinant TIMP-1 at the indicated concentration in the top chamber. Values are means ± SD of triplicates from two independent experiments. (G) JNK IP/IVK assays performed with lysates from FAK−/− cells or Ad-Q61L Rac-infected FAK−/−, FAK−/− v-Src, or DA2 v-Src cells. Values are means ± SD of duplicates from two independent experiments. Flag tag blotting of whole cell lysates was used to verify equivalent Q61L Rac expression.
Additional bands of gelatinase activity at ∼92 kD were also weakly visualized in DA2 cell conditioned media, prominently visualized in DA2 v-Src cell media, but not detected in FAK−/− v-Src cell media (Fig. 8 C, lanes 2–4). This activity corresponds to the predicted size of MMP-9. By protein blotting of conditioned media samples, MMP-9 was secreted only from DA2 v-Src cells, whereas equal levels of MMP-2 were secreted by DA2 v-Src and FAK−/− v-Src cells (Fig. 8 D). RT-PCR analyses revealed that MMP-9 mRNA levels were 6.5-fold higher in DA2 v-Src compared with FAK−/− v-Src cells (Fig. 8 E). The importance of increased MMP-9 secretion as a contributing factor promoting DA2 v-Src invasion was verified by the dose-dependent inhibition of DA2 v-Src cell invasion by recombinant TIMP-1 addition (Fig. 8 F).
Since only low levels of Rac activation were detected in lysates of FAK−/− v-Src cells (Fig. 6 A), Ad infection was used to deliver activated (Gln-61 mutated to Leu, Q61L) Flag-tagged Rac to cells to test whether activated Rac could promote cell invasion (Fig. 8 A). However, Rac Q61L expression in FAK−/− cells did not promote Matrigel invasion or detectable changes in MMP secretion (Fig. 8, B and C, lane 5). Additionally, Q61L Rac expression led to only modest increases in JNK activity within FAK−/− cells (Fig. 8 G). Since Rho activity is elevated in FAK−/− cells (Ren et al., 2000), it is possible that Rac-mediated signaling may be inhibited through the constitutive activation of Rho targets such as p160Rock (Chen et al., 2002; Tsuji et al., 2002).
In contrast to FAK−/− cells, equivalent Rac Q61L expression in DA2 cells increased cell invasion approximately threefold compared with mock-infected DA2 cells (Fig. 8 B, lanes 3 and 7). The level of Rac-induced DA2 cell invasion was equal to DA2 v-Src cells and was associated with elevated MMP-9 secretion as detected by zymography (Fig. 8 C, lane 7). Rac Q61L also functioned to promote high levels of JNK activity in DA2 compared with FAK−/− cells (Fig. 8 G). Since the JNK SP600125 inhibitor blocked DA2 v-Src cell invasion (Fig. 5 E) and reduced MMP-9 secretion from DA2 v-Src cells (unpublished data), the Rac Q61L results with DA2 cells support the hypothesis that FAK expression coupled with Rac activation can promote an invasive cell phenotype in part by enhancing MMP-9 expression and secretion.
Rac Q61L expression in FAK−/− v-Src cells also resulted in the rescue of FAK−/− v-Src invasion defects (Fig. 8 B, lane 6). High levels of JNK IVK activity were stimulated by Rac Q61L expression in FAK−/− v-Src cells (Fig. 8 G) compared with equivalent low levels of JNK activity in lysates of FAK−/− and FAK−/− v-Src cells (Fig. 4 C and unpublished data). These results are consistent with the hypothesis that either v-Src or FAK expression within FAK−/− cells creates a permissive environment for maximal Rac activation. To this end, zymography analyses showed that Rac Q61L expression promoted both MMP-2 activation and increased MMP-9 secretion in FAK−/− v-Src cells compared with mock-infected cells (Fig. 8 C, lanes 2 and 6). Since zymography evaluates only relative MMP activity in the absence of associated TIMP inhibitors, solution gelatinase activity measurements were also performed and showed that total gelatinase activity in conditioned media samples paralleled the level of cell invasion (unpublished data). Although Rac Q61L expression slightly increased DA2 v-Src cell invasion further, the number of invasive cells enumerated on the lower cell membrane (Fig. 3 B) was close to saturation (Fig. 8 B). In contrast, Ad-mediated expression of DN Rac (Thr-17 mutated to Asn) in DA2 v-Src cells blocked cell invasion and reduced both MMP-2 and MMP-9 expression (unpublished data). These results show that FAK and Rac signaling can promote changes in MMP-2 activity or MMP-9 expression associated with cell invasion.
The importance of JNK activity in enhancing cell invasion
Since DA2 v-Src cell invasion (Fig. 5 E) and MMP-9 gene expression are sensitive to SP600125 JNK inhibition (Shin et al., 2002), Ad-mediated expression of WT or DN JNK1 (Thr-180 and Tyr-182 mutated to Ala and Phe) was used to test the role of JNK activation as a critical Rac target promoting cell invasion (Fig. 9) . WT JNK overexpression in FAK−/− v-Src cells led to its activation as evaluated by phospho-JNK blotting (Fig. 9 A). Serum stimulation in combination with WT JNK overexpression was sufficient to promote increased signaling to downstream targets such as the c-Jun transcription factor as measured by phospho-specific blotting (Fig. 9 B). These signaling changes were sufficient to promote a four- to fivefold increase in FAK−/− v-Src cell Matrigel invasion equivalent to DA2 v-Src cell invasion (Fig. 9 C).
Figure 9.
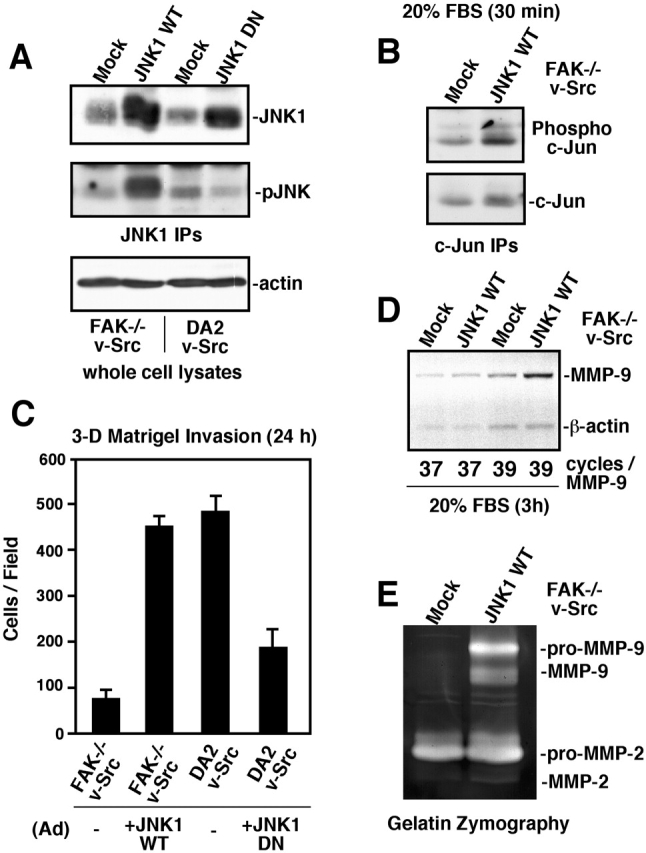
Ad-JNK rescues FAK −/− v-Src invasion defects and promotes increased MMP-9 expression. (A) Polyclonal IPs were used to isolate total JNK1 from mock- and Ad-JNK1 WT-infected FAK−/− v-Src cells or from mock- and JNK1 DN- (Thr-180 and Tyr-182 mutated to Ala and Phe) infected DA2 v-Src cells. JNK1 expression was evaluated by blotting (top), and JNK activity was determined by blotting phospho-specific antibodies to Thr-183 and Tyr-185 of JNK (pJNK, middle). Equivalent total lysate protein was verified by β-actin blotting. (B) c-Jun IPs were prepared from either mock or Ad-JNK WT-infected FAK−/− v-Src cells after serum stimulation (20% FBS for 30 min). Activating c-Jun phosphorylation was determined by blotting with phospho-specific antibodies to Ser-63 of c-Jun. (C) Matrigel invasion assays were preformed with the indicated mock- or Ad-infected cells. Values are means ± SD of triplicates. (D) Semiquantitative RT-PCR was performed with RNA isolated from mock or Ad-JNK WT-infected FAK−/− v-Src cells after serum stimulation (20% FBS for 3 h) using primers to MMP-9 and to β-actin. JNK overexpression promoted a twofold increase in MMP-9 mRNA. (E) Gelatin zymography of conditioned media from mock- or Ad-JNK WT-infected FAK−/− v-Src cells.
In contrast, DN JNK overexpression resulted in the inhibition of JNK in DA2 v-Src cells as detected by phospho-JNK blotting (Fig. 9 A) and the 2.5-fold inhibition of DA2 v-Src cell invasion (Fig. 9 C). By RT-PCR analyses, WT JNK overexpression promoted a twofold increase in MMP-9 mRNA levels in FAK−/− v-Src cells (Fig. 9 D). These JNK-induced changes were dependent on a serum stimulus as only minor changes in MMP-9 mRNA levels were detected in unstimulated FAK−/− v-Src cells overexpressing JNK (unpublished data). Notably, gelatin zymography analyses of Ad-JNK–infected FAK−/− v-Src cells showed elevated secretion of MMP-9 and activation of MMP-2 compared with mock-infected FAK−/− v-Src cells (Fig. 9 E). These results show the importance of JNK activity in promoting increased cell invasion.
Discussion
FAK plays important roles in promoting growth factor– and integrin-stimulated cell motility in both normal and transformed cells. FAK expression and tyrosine phosphorylation are elevated as a function of human tumor cell malignancy, and these changes are associated with increased tumor metastasis (Cance et al., 2000). Whereas FAK-increased cell motility is a fundamental component of cell invasion, the significance of our findings is that defects in FAK−/− cell motility are distinct from the role of FAK in promoting cell invasion. This connection was determined by the fact that both FAK and v-Src can rescue FAK−/− motility defects, yet FAK expression was required for the generation of an invasive cell phenotype.
Localization influences signaling connections: FAK at focal contacts
FAK associates with a variety of cell surface receptors, cytoskeletal-associated proteins, and SH2/SH3-containing adaptor proteins. Multiple connections link FAK to the activation of ERK2 kinase, PI 3-kinase, or JNK kinase signaling cascades (Schlaepfer et al., 1999; Schaller, 2001). However, it is not known whether FAK activation leads to either generalized or specific downstream signaling events. Our results support the hypothesis that FAK localization to focal contacts promotes integrin-stimulated cell motility and that FAK accumulation at lamellipodia/invadopodia promotes cell invasion, each in part through the activation of distinct signaling pathways.
FAK−/− fibroblasts form an abundance of actin stress fibers and focal contacts in cell culture, and these abnormalities limit cell motility responses (Ilic et al., 1995). As summarized in Fig. 10 A, FAK reconstitution reverses FAK−/− cell defects, FAK activity is dependent on its localization to focal contacts, and FAK–Src signaling enhances the extent and duration of FN-stimulated ERK2 kinase activation (Sieg et al., 1999; Klingbeil et al., 2001). v-Src transformation of FAK−/− cells resulted in the constitutive activation of ERK2 kinase activity, and FAK−/− v-Src cells exhibited equivalent haptotaxis motility as FAK-reconstituted cells. MEK-ERK2 signaling is required for haptotaxis motility (Klingbeil et al., 2001), and ERK2 can promote cell contractility through increased myosin light chain phosphorylation (Klemke et al., 1997). However, FAK−/− cell motility defects are associated with excessive contractility and are partially reversed through the inhibition of myosin light chain kinase or Rho kinase signaling (Chen et al., 2002).
Figure 10.

Models of differential FAK signaling connections at focal contacts or lamellipodia/invadopodia. (A) Integrin-associated FAK and Src activation promotes increased p190RhoGAP tyrosine phosphorylation or ERK2/MAP kinase activation. FAK–Src modulation of p21 Rho activity in FAK−/− cells is associated with increased cell motility, focal contact turnover, and actin cytoskeletal rearrangements. (B) In either EGF-stimulated human adenocarcinoma cells (Hauck et al., 2001b) or serum-stimulated v-Src–transformed fibroblasts, FAK functions to coordinate a Src-p130Cas signaling complex localized to lamellipodia in two-dimensional cell culture or to invadopodia in 3-D invasion assays. Through direct Cas SH3-mediated and indirect Crk-mediated interactions with the Dock180 guanine-nucleotide exchange factor, the FAK–Src signaling complex promotes Rac and JNK activation with effects upon gene expression. Although PI 3-kinase is a direct target of both v-Src and Rac (Liu et al., 1993; Bishop and Hall, 2000), FAK−/− v-Src cells exhibit defects in JNK but not Akt activation. Overexpression of JNK in FAK−/− v-Src cells coupled with serum stimulation promoted c-Jun Ser-63 phosphorylation, MMP-9 expression, MMP-2 activation, and rescued the FAK−/− v-Src cell invasion defects.
v-Src–mediated ERK2 activation also has been associated with the disruption of actin stress fibers in part through the down-regulation of Rho kinase expression (Pawlak and Helfman, 2002). However, transfection of FAK−/− cells with activated MEK1 did not rescue FAK−/− haptotaxis defects (Klingbeil et al., 2001). Since FAK reexpression in FAK−/− cells transiently inhibits Rho activity upon FN stimulation cells (Ren et al., 2000), p190RhoGAP was investigated as an upstream regulator of Rho. Both v-Src and FAK expression enhanced FN-stimulated p190RhoGAP tyrosine phosphorylation compared with FAK−/− cells, and v-Src–mediated p190RhoGAP phosphorylation is associated with actin and focal contact reorganization (Fincham et al., 1999). Although integrins can suppress Rho activity through c-Src–dependent p190RhoGAP tyrosine phosphorylation (Arthur et al., 2000), it is possible that other FAK-associated proteins, such as Graf, may additionally contribute to Rho regulation (Taylor et al., 1999). The Graf SH3 domain binds to FAK COOH-terminal proline-rich sites that are required for full FAK function in promoting FAK−/− haptotaxis motility (Sieg et al., 1999). Our results showing that v-Src does not strongly localize to focal contacts in FAK−/− cells yet rescues haptotaxis motility is also consistent with recent studies, showing that Src catalytic but not scaffolding function is needed for integrin-regulated cell migration (Cary et al., 2002).
FAK signaling at lamellipodia or invadopodia
Although transient suppression of Rho activity may alleviate contractile forces that would otherwise impede protrusion at the leading edge of migrating cells (Arthur et al., 2000), FAK−/− v-Src cells did not exhibit serum-stimulated membrane ruffling activity or possess an invasive phenotype. Notably, both Rac and JNK activation were attenuated in cells lacking FAK. In contrast, transient or stable FAK expression in FAK−/− v-Src cells promoted membrane ruffling, and serum stimulation resulted in the localization of both FAK and v-Src either to lamellipodia or invadopodia cell projections. Importantly, the accumulation of FAK at lamellipodia was not accompanied by the loss of FAK at focal contacts, indicating that distinct intracellular pools of FAK may differentially respond to particular extracellular signals. Although the signals and mechanism(s) promoting FAK recruitment to lamellipodia are not known, the localization of FAK to membrane ruffles places FAK at an appropriate intracellular site to facilitate Rac activation.
As summarized in Fig. 10 B, we showed that FAK expression within FAK−/− cells facilitated the formation of a multiprotein signaling complex comprised of v-Src, p130Cas, Crk, and Dock180. The role of FAK in linking serum stimulation to Rac and JNK activation is not limited to reconstituted FAK−/− cells, since FAK antisense treatment of human A549 carcinoma cells disrupted the EGF-stimulated formation of a c-Src–p130Cas signaling complex (Hauck et al., 2001b). Loss of FAK expression in A549 cells also inhibited EGF-stimulated JNK activation and invasive cell motility. Our Ad-mediated rescue assays performed with FAK−/− v-Src cells showed that FAK phosphorylation at Tyr-397, FAK kinase activity, and the SH3-binding sites in the FAK COOH-terminal domain were individually required to facilitate Rac and JNK activation and cell invasion. Similar results linking FAK to JNK with respect to cell cycle and cell survival signaling have been obtained through the expression of DN FAK-related constructs (Oktay et al., 1999; Almeida et al., 2000). However, our results with Ad-p130Cas expression in FAK−/− v-Src cells revealed the unexpected importance of p130Cas SH3 domain function in cell invasion that is distinct from the role of p130Cas in promoting Src-stimulated cell growth (Huang et al., 2002).
We found that SH3 domain–mutated p130Cas was highly tyrosine phosphorylated in FAK−/− v-Src cells but that it did not function to promote either JNK activation or cell invasion as did WT p130Cas. Although p130Cas–Crk interactions form a scaffolding complex promoting Rac-induced JNK activation (Girardin and Yaniv, 2001) and have been proposed as a molecular “switch” promoting cell motility (Klemke et al., 1998), overexpression of a Crk-binding site mutant of p130Cas does not block v-Src–induced JNK activation (Dolfi et al., 1998). We found that FAK expression facilitated the formation of a v-Src–p130Cas–Dock180 signaling complex and that the Cas SH3 domain bound to Dock180 in pull-down assays. As a consensus (PXXPXR), Cas SH3-binding site (Schlaepfer et al., 1999) lies within the Dock180 COOH-terminal domain and Crk SH3 binding to Dock180 facilitates both Rac and JNK activation (Kiyokawa et al., 1998), we speculate that Dock180 may function as both a Crk-dependent and -independent guanine nucleotide exchange factor promoting v-Src–stimulated Rac activation.
Notably, FAK expression was required for maximal Rac activation in both reconstituted normal and v-Src–transformed cells. Moreover, we found that activated Rac expression in normal FAK-reconstituted cells yielded a highly invasive phenotype equivalent to v-Src transformation. The findings suggest that FAK, v-Src, and Rac may coordinately regulate common targets involved in promoting cell invasion. Interestingly, activated Rac expression in normal FAK-reconstituted cells yielded a similar profile of MMP-2 activation and increased MMP-9 secretion as did v-Src transformation. The importance of these MMPs was supported by our findings that exogenous TIMP-1 addition blocked Matrigel cell invasion in a dose-dependent manner. Although previous studies have shown that both FAK and Rac can modulate both MMP activity and expression (Shibata et al., 1998; Hauck et al., 2001b, 2002b; Zhuge and Xu, 2001), gene array analyses reveal that these targets are part of a larger set of FAK-regulated and metastases-associated gene products (unpublished data).
FAK to JNK signaling promoting an invasive cell phenotype
Our results using the SP600125 JNK kinase inhibitor and DN JNK expression support the importance of JNK activation downstream of Rac as being necessary for cell invasion. This is consistent with studies using Rac effector domain mutants where cell invasion was disrupted by the Rac Y40H mutation that uncouples Rac-mediated JNK activation (Banyard et al., 2000). Although FAK has been shown to recruit JNK to focal contacts (Almeida et al., 2000), we hypothesize that FAK–Src signaling through JNK also acts to alter the transcriptional regulation of cell invasion-associated gene targets such as MMP-9 (Shibata et al., 1998; Ozanne et al., 2000; Shin et al., 2002). Since Src activation contributes to the metastatic spread of carcinoma cells (Boyer et al., 2002) and FAK is a key regulator of cytotrophoblast invasion of the uterus during placental formation (Ilic et al., 2001), we propose that FAK–Src signaling may synchronize MMP-mediated extracellular matrix proteolysis and cell motility, thereby facilitating an invasive phenotype in both normal developmental and neoplastic cell settings.
Materials and methods
Cells and constructs
FAK−/−p53−/− and FAK+/+p53−/− fibroblasts were generated as described (Ilic et al., 1995). DA2 cells express HA-tagged FAK and were generated from FAK−/− cells as described (Sieg et al., 1999). Prague C v-Src retrovirus was used as described (Hauck et al., 2002a). Cells were infected for 24 h in medium containing 5 μg/ml polybrene, selected for growth in 3 μg/ml puromycin, and drug-resistant colonies were pooled and expanded. Pro-null FAK (Hauck et al., 2001a), GFP-FAK (Ilic et al., 1998), and GST-SH3 domains for c-Src, Grb2, and PLCγ (Schlaepfer et al., 1994) were generated as described.
Antibodies and reagents
Anti-pTyr (4G10) mAb, avian-specific mAb to v-Src (EC10), and Rac mAb (23A8) were from UBI. HA epitope tag mAb (16B12) was from Covance Research. p130Cas, p190RhoGAP, and Crk mAbs were from BD Biosciences. Flag tag mAb (M2) and β-actin mAb (AC-74) were from Sigma-Aldrich. Polyclonal antibodies to Dock180 (C19), p130Cas (C20), MMP-2 (C19), MMP-9 (C20), JNK1 (C17), ERK2 (C14), and Akt1 (C20) were from Santa Cruz Biotechnology, Inc. Phospho-specific mAbs to activated ERK2 (E10 to pT202/pY204), activated JNK1 (G9 to pT183/pY185), activated Akt (pS473), phosphorylated STAT-3 (pY705), t-phosphorylated c-Jun (pS63), and polyclonal antibodies to STAT-3 and c-Jun were from Cell Signaling Technology. Phospho-specific antibodies to FAK pTyr-397 motif were from Biosource International. Polyclonal antibodies to FAK were used as described (Sieg et al., 1999). Recombinant human TIMP-1 was from Chemicon. The pharmacological inhibitor to JNK (SP600125) was from Calbiochem.
Blotting and pull-down assays
Cells were solubilized in RIPA lysis buffer containing 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS. Antibodies (2.5 μg) were incubated with lysates for 3 h at 4°C and collected by binding to protein G plus (Oncogene Research Products) or protein A (Repligen) agarose beads. Blotting and sequential membrane reprobing was performed as described (Hauck et al., 2002b). GST-PAK (residues 67–150) or GST-SH3 domains were purified from bacterial lysates by glutathione-agarose affinity binding, and 15 μg of agarose bead-associated GST fusion protein was incubated with cell lysates for 3 h at 4°C. The beads were washed three times in lysis buffer containing 1% Triton X-100, bound proteins were resolved by SDS-PAGE, and target protein association was determined by blotting.
IVK assays
ERK2 IVK activity was measured using myelin basic protein, JNK IVK activity was measured using GST-ATF2, and Akt IVK activity was measured using GST–GSK-3 as described (Hauck et al., 2002b). After SDS-PAGE and electrophoretic transfer to PVDF membranes (Millipore), labeled proteins were quantified by a PhosphorImager (Molecular Dynamics), and the equal recovery of the immuno-isolated kinase was verified by blotting.
Migration and invasion assays
Millicell (12 mm diameter with 8-μm pores; Millipore) haptotaxis assays with 10 μg/ml FN (Sigma-Aldrich) were performed as described (Sieg et al., 1999). Chemotaxis assays with using 10% FBS added to the lower chamber were performed as described (Sieg et al., 2000). Wound-healing scratch assays and growth factor–reduced Matrigel (BD Biosciences) invasion assays were performed as described (Hauck et al., 2002b). One-way analysis of variance (ANOVA) was used to determine significance.
Gelatinase activity
Conditioned media samples from 5 × 106 cells were separated in nonreducing gels containing 0.1% (wt/vol) gelatin and processed for zones of clearing activity by zymography as described (Hauck et al., 2002b). For blotting analyses, proteins in the conditioned media were precipitated by cold acetone and separated by SDS-PAGE. Analysis of soluble MMP-2 + MMP-9 activity was performed using the Chemicon Gelatinase Activity kit (ECM700) as per the manufacturer's instructions.
Ad production and infection
Murine HA-tagged FAK cDNA constructs were subcloned into pADtet7 containing Tet-responsive enhancer sequences within a minimal cytomegalovirus (CMV) promoter. pADtet7 also contains SV40 late poly(A) cassette, Ad E1A, and a single loxP site. Recombinant Ads were produced by pADtet7-FAK cotransfection of 293-Cre cells with Ad DNA (Ad5-ψ5) that contains an E1A/E3-deleted Ad genome. Recombinant Ads were expanded on 293-Cre cells, and stocks were titered by limiting dilution. FAK protein expression was driven by coinfection with Ad-TA expressing the Tet transactivator as described (Streblow et al., 1999). Cells were infected at an MOI at 10 or 30 plaque forming units (pfu)/cell for Ad-TA or Ad-TA with Ad-FAK, respectively. Cells were analyzed 48 h postinfection.
The CMV promoter and bovine growth hormone poly(A) cassette from pCI-Neo (Promega) were subcloned into pE1sp1B (Microbix) to generate pAd/CMV. Flag-tagged Rac1 mutants (Q61L and T17N) were cloned into pAd/CMV, and recombinant Ads were generated by cotransfection of 293 cells with pJM17 (Microbix) containing the E1A-deleted Ad genome. Recombinant Ads were isolated by plaque formation, and Rac-producing virus were identified by flag tag blotting. MOI at 50 pfu/cell was used.
A mutation was introduced into the ATG start site of the rat cDNA encoding the alternate spliced short form of p130Cas (Sakai et al., 1994). Translation of this construct begins at Met-30 within the p130Cas SH3 domain (CasΔSH3). Both WT and CasΔSH3 were cloned into pShuttle-CMV, and recombinant Ads were produced using the Ad-Easy System (Stratagene) as described (Hauck et al., 2002a). Cells were infected at an MOI of 50 pfu/cell. Ads for WT JNK or DN JNK (APF) were produced as described (Huang et al., 2000). An MOI of 80 pfu/cell was used.
RT-PCR
Total RNA was isolated using Trizol (Invitrogen). Reverse transcription was performed using 2 μg RNA and the SuperScript first strand cDNA synthesis kit (GIBCO-BRL). 1.5 μl of the RT product was used for coamplification of MMP-9 with β-actin primer pairs (R&D Systems) as an internal standard. Semiquantitative MMP-9 PCR was performed for 37 and 39 cycles, and β-actin primers were added after PCR cycle 11. Values were quantified using ImageQuant and normalized to β-actin (Molecular Dynamics).
IF staining
2 × 104 cells were plated on FN-coated (5 μg/ml) glass coverslips for 6 h. Cells were fixed with 4% PFA in PBS for 15 min, permeabilized for 10 min with 0.2% saponin in PBS containing normal goat serum (blocking solution), and incubated for 1 h in blocking solution containing TRITC-phalloidin (Molecular Probes). Anti–v-Src mAb or ILK rabbit polyclonal antibody (Zymed Laboratories) costaining was performed using biotinylated donkey anti–mouse (Jackson ImmunoResearch Laboratories) followed by streptavidin-FITC (Vector) and TRITC-conjugated donkey anti–rabbit as described (Hauck et al., 2002a). Staining of invadopodia emerging through Matrigel was performed at hour 16 of 24 during the invasion assay as described (Hauck et al., 2002a).
Live cell microscopy
FAK−/− v-Src cells were transfected with GFP-FAK using Lipofectamine Plus (GIBCO-BRL), FACS® sorted for GFP expression after 24 h, and plated onto FN-coated (10 μg/ml) 25-mm coverslips in DME with 0.5% BSA. After 18 h, 20 mM Hepes, pH 7.4, and 10% FBS were added, cells were placed into an Attofluor chamber (Molecular Probes), covered with light mineral oil (Sigma-Aldrich) to prevent evaporation, and placed into a 20/20 Technologies Bionomic microscope stage chamber maintained at 37°C. GFP-FAK was monitored using a 40× Plan-Apochromat objective on a ZEISS Axiovert 100TV microscope, coupled to a Bio-Rad Laboratories 1024 laser-scanning confocal focused at the ventral surface of cells. Images were taken at 1-min intervals, adjusted using Adobe Photoshop®, and exported to QuickTime®.
Online supplemental material
Time-lapse images are included as an online video available at http://www.jcb.org/cgi/content/full/jcb.200212114/DC1. Video 1 shows the distribution of GFP-FAK transiently transfected into FAK−/− v-Src cells. The first image was taken ∼15 min after serum stimulation. The time interval for Video 1 is 15 s at 12 frames/s.
Supplemental Material
Acknowledgments
We thank Gary Bokoch (The Scripps Research Institute) for GST-PAK (67-150), Jean-Francois Cote (Burnham Institute, La Jolla, CA) for GST-CAS SH3 and GST-Dock180 SH3 constructs, Kristiina Vuori for helpful discussions, Stephan Feller (Oxford University, Oxford, UK) for the GST-Crk SH3 construct, and Amanda Moore for administrative assistance.
C. Hauck was supported in part by a fellowship from the Deutsche Forschungsgemeinschaft (HA-2856/1-1). This work was supported by grants from the National Institutes of Health to D. Cheresh (CA50286, CA45726, and CA78045), J. Nelson (HL65754), G. Nemerow (HL54352), and D. Schlaepfer (CA75240 and CA87038). This work was initiated with support from the American Cancer Society (RPG-98-109-TBE) to D. Schlaepfer. This is manuscript number 15085-IMM from The Scripps Research Institute.
The online version of this article contains supplemental material.
C.R. Hauck's present address is Zentrum für Infektionsforschung, Universität Würzburg, Röntgenring 11, D-97070 Würzburg, Germany.
J. Leng's present address is Chemicon International Inc., Temecula, CA 92590.
Footnotes
Abbreviations used in this paper: Ad, adenovirus; CMV, cytomegalovirus; c-Src, cellular Src; 3-D, three dimensional; DN, dominant-negative; ERK; extracellular signal–regulated kinase; FAK, focal adhesion kinase; FRNK, FAK-related nonkinase; FN, fibronectin; HA, hemagglutinin; ILK, integrin-linked kinase; IF, immunofluorescence; IP, immunoprecipitation; IVK, in vitro kinase; JNK, c-Jun NH2-terminal kinase, MMP, matrix metalloproteinase; MOI, multiplicity of infection; pfu, plaque-forming units; PI, phosphatidylinositol; pTyr, phosphotyrosine; TA, transactivator; v-Src, viral Src; WT, wild-type.
References
- Aguirre-Ghiso, J.A., P. Frankel, E.F. Farias, Z. Lu, H. Jiang, A. Olsen, L.A. Feig, E.B. de Kier Joffe, and D.A. Foster. 1999. RalA requirement for v-Src- and v-Ras-induced tumorigenicity and overproduction of urokinase-type plasminogen activator: involvement of metalloproteases. Oncogene. 18:4718–4725. [DOI] [PubMed] [Google Scholar]
- Almeida, E.A.C., D. Ilic, Q. Han, C.R. Hauck, F. Jin, H. Kawakatsu, D.D. Schlaepfer, and C.H. Damsky. 2000. Matrix survival signaling from fibronectin via FAK to JNK. J. Cell Biol. 149:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur, W.T., L.A. Petch, and K. Burridge. 2000. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10:719–722. [DOI] [PubMed] [Google Scholar]
- Banyard, J., B. Anand-Apte, M. Symons, and B.R. Zetter. 2000. Motility and invasion are differentially modulated by Rho family GTPases. Oncogene. 19:580–591. [DOI] [PubMed] [Google Scholar]
- Bishop, A.L., and A. Hall. 2000. Rho GTPases and their effector proteins. Biochem. J. 2:241–255. [PMC free article] [PubMed] [Google Scholar]
- Boyer, B., Y. Bourgeois, and M.F. Poupon. 2002. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene. 21:2347–2356. [DOI] [PubMed] [Google Scholar]
- Cance, W.G., J.E. Harris, M.V. Iacocca, E. Roche, X. Yang, J. Chang, S. Simkins, and L. Xu. 2000. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin. Cancer Res. 6:2417–2423. [PubMed] [Google Scholar]
- Cary, L.A., R.A. Klinghoffer, C. Sachsenmaier, and J.A. Cooper. 2002. SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading. Mol. Cell. Biol. 22:2427–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B.H., J.T. Tzen, A.R. Bresnick, and H.C. Chen. 2002. Roles of Rho-associated kinase and myosin light chain kinase in morphological and migratory defects of focal adhesion kinase-null cells. J. Biol. Chem. 277:33857–33863. [DOI] [PubMed] [Google Scholar]
- Datta, A., Q. Shi, and D.E. Boettiger. 2001. Transformation of chicken embryo fibroblasts by v-src uncouples beta1 integrin-mediated outside-in but not inside-out signaling. Mol. Cell. Biol. 21:7295–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, R.J. 2000. Signal transduction by the JNK group of MAP kinases. Cell. 103:239–252. [DOI] [PubMed] [Google Scholar]
- Dolfi, F., M. Garcia-Guzman, M. Ojaniemi, H. Nakamura, M. Matsuda, and K. Vuori. 1998. The adaptor protein Crk connects multiple cellular stimuli to the JNK signaling pathway. Proc. Natl. Acad. Sci. USA. 95:15394–15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham, V.J., and M.C. Frame. 1998. The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J. 17:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham, V.J., A. Chudleigh, and M.C. Frame. 1999. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J. Cell Sci. 112:947–956. [DOI] [PubMed] [Google Scholar]
- Girardin, S.E., and M. Yaniv. 2001. A direct interaction between JNK1 and CrkII is critical for Rac1-induced JNK activation. EMBO J. 20:3437–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi, M., S. Yamagata, A.A. Thant, H. Xiao, H. Iwata, T. Mazaki, and H. Hanafusa. 1995. Augmentation of metalloproteinase (gelatinase) activity secreted from Rous sarcoma virus-infected cells correlates with transforming activity of src. Oncogene. 10:1037–1043. [PubMed] [Google Scholar]
- Hauck, C.R., T. Hunter, and D.D. Schlaepfer. 2001. a. The v-Src SH3 domain facilitates a cell adhesion-independent association with focal adhesion kinase. J. Biol. Chem. 276:17653–17662. [DOI] [PubMed] [Google Scholar]
- Hauck, C.R., D.J. Sieg, D.A. Hsia, J.C. Loftus, W.A. Gaarde, B.P. Monia, and D.D. Schlaepfer. 2001. b. Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor-stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res. 61:7079–7090. [PubMed] [Google Scholar]
- Hauck, C.R., D.A. Hsia, D. Ilic, and D.D. Schlaepfer. 2002. a. v-Src SH3-enhanced interaction with focal adhesion kinase at β1 integrin-containing invadopodia promotes cell invasion. J. Biol. Chem. 277:12487–12490. [DOI] [PubMed] [Google Scholar]
- Hauck, C.R., D.A. Hsia, X.S. Puente, D.A. Cheresh, and D.D. Schlaepfer. 2002. b. FRNK blocks v-Src-stimulated invasion and experimental metastasis without effects on cell motility or growth. EMBO J. 21:6289–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood, J.D., and D.A. Cheresh. 2002. Role of integrins in cell invasion and migration. Nat. Rev. 2:91–100. [DOI] [PubMed] [Google Scholar]
- Huang, J., H. Hamasaki, T. Nakamoto, H. Honda, H. Hirai, M. Saito, T. Takato, and R. Sakai. 2002. Differential regulation of cell migration, actin stress fiber organization and cell transformation by functional domains of Cas. J. Biol. Chem. 277:27265–27272. [DOI] [PubMed] [Google Scholar]
- Huang, S., L. New, Z. Pan, J. Han, and G.R. Nemerow. 2000. Urokinase plasminogen activator/urokinase-specific surface receptor expression and matrix invasion by breast cancer cells requires constitutive p38alpha mitogen-activated protein kinase activity. J. Biol. Chem. 275:12266–12272. [DOI] [PubMed] [Google Scholar]
- Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, T. Yamamoto, and S. Aizawa. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 377:539–544. [DOI] [PubMed] [Google Scholar]
- Ilic, D., E.A. Almeida, D.D. Schlaepfer, P. Dazin, S. Aizawa, and C.H. Damsky. 1998. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J. Cell Biol. 143:547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic, D., O. Genbacev, F. Jin, E. Caceres, E.A.C. Almeida, V. Bellingard-Dubouchaud, E.M. Schaefer, C.H. Damsky, and S.J. Fisher. 2001. Plasma membrane-associated pY397-FAK is a marker of cytotrophoblast invasion in vivo and in vitro. Am. J. Pathol. 159:93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irby, R.B., and T.J. Yeatman. 2000. Role of Src expression and activation in human cancer. Oncogene. 19:5636–5642. [DOI] [PubMed] [Google Scholar]
- Kirsch, K.H., M.M. Georgescu, and H. Hanafusa. 1998. Direct binding of p130(Cas) to the guanine nucleotide exchange factor C3G. J. Biol. Chem. 273:25673–25679. [DOI] [PubMed] [Google Scholar]
- Kiyokawa, E., Y. Hashimoto, S. Kobayashi, H. Sugimura, T. Kurata, and M. Matsuda. 1998. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 12:3331–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke, R.L., S. Cai, A.L. Giannini, P.J. Gallagher, P. de Lanerolle, and D.A. Cheresh. 1997. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 137:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke, R.L., J. Leng, R. Molander, P.C. Brooks, K. Vuori, and D.A. Cheresh. 1998. Cas/Crk coupling serves as a “molecular switch” for induction of cell migration. J. Cell Biol. 140:961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingbeil, C.K., C.R. Hauck, D.A. Hsia, K.C. Jones, S.R. Reider, and D.D. Schlaepfer. 2001. Targeting Pyk2 to β1-integrin–containing focal contacts rescues fibronectin-stimulated signaling and haptotactic motility defects of focal adhesion kinase-null cells. J. Cell Biol. 152:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg, L.J. 1998. Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck. 20:745–752. [DOI] [PubMed] [Google Scholar]
- Lauffenburger, D.A., and A.F. Horwitz. 1996. Cell migration: a physically integrated molecular process. Cell. 84:359–369. [DOI] [PubMed] [Google Scholar]
- Li, E., D.G. Stupack, S.L. Brown, R. Klemke, D.D. Schlaepfer, and G.R. Nemerow. 2000. Association of p130CAS with phosphatidylinositol-3-OH kinase mediates adenovirus cell entry. J. Biol. Chem. 275:14729–14735. [DOI] [PubMed] [Google Scholar]
- Liu, X., L.E. Marengere, C.A. Koch, and T. Pawson. 1993. The v-Src SH3 domain binds phosphatidylinositol 3′-kinase. Mol. Cell. Biol. 13:5225–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCawley, L.J., and L.M. Matrisian. 2000. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol. Med. Today. 6:149–156. [DOI] [PubMed] [Google Scholar]
- Nagase, H., and J.F. Woessner, Jr. 1999. Matrix metalloproteinases. J. Biol. Chem. 274:21491–21494. [DOI] [PubMed] [Google Scholar]
- Oktay, M., K.K. Wary, M. Dans, R.B. Birge, and F.G. Giancotti. 1999. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J. Cell Biol. 145:1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, J.D., P.J. Ruest, D.W. Fry, and S.K. Hanks. 1999. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 19:4806–4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens, L.V., L.H. Xu, R.J. Craven, G.A. Dent, T.M. Weiner, L. Kornberg, E.T. Liu, and W.G. Cance. 1995. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 55:2752–2755. [PubMed] [Google Scholar]
- Ozanne, B.W., L. McGarry, H.J. Spence, I. Johnston, J. Winnie, L. Meagher, and G. Stapleton. 2000. Transcriptional regulation of cell invasion: AP-1 regulation of a multigenic invasion programme. Eur. J. Cancer. 36:1640–1648. [DOI] [PubMed] [Google Scholar]
- Pawlak, G., and D.M. Helfman. 2002. MEK mediates v-Src-induced disruption of the actin cytoskeleton via inactivation of the Rho-ROCK-LIM-kinase pathway. J. Biol. Chem. 277:26927–26933. [DOI] [PubMed] [Google Scholar]
- Ren, X., W.B. Kiosses, D.J. Sieg, C.A. Otey, D.D. Schlaepfer, and M.A. Schwartz. 2000. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 113:3673–3678. [DOI] [PubMed] [Google Scholar]
- Renshaw, M.W., L.S. Price, and M.A. Schwartz. 1999. Focal adhesion kinase mediates the integrin signaling requirement for growth factor activation of MAP kinase. J. Cell Biol. 147:611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, S., P.J. Ruest, and S.K. Hanks. 2002. FAK regulates tyrosine phosphorylation of CAS, paxillin, and PYK2 in cells expressing v-Src, but is not a critical determinant of v-Src transformation. J. Cell. Biochem. 84:377–388. [DOI] [PubMed] [Google Scholar]
- Sakai, R., A. Iwamatsu, N. Hirano, S. Ogawa, T. Tanaka, H. Mano, Y. Yazaki, and H. Hirai. 1994. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 13:3748–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, M.D. 2001. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta. 1540:1–21. [DOI] [PubMed] [Google Scholar]
- Schlaepfer, D.D., S.K. Hanks, T. Hunter, and P. van der Geer. 1994. Integrin-mediated signal transduction linked to Ras pathway by Grb2 binding to focal adhesion kinase. Nature. 372:786–791. [DOI] [PubMed] [Google Scholar]
- Schlaepfer, D.D., M.A. Broome, and T. Hunter. 1997. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130Cas, and Nck adaptor proteins. Mol. Cell. Biol. 17:1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer, D.D., C.R. Hauck, and D.J. Sieg. 1999. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71:435–478. [DOI] [PubMed] [Google Scholar]
- Shibata, K., F. Kikkawa, A. Nawa, A.A. Thant, K. Naruse, S. Mizutani, and M. Hamaguchi. 1998. Both focal adhesion kinase and c-Ras are required for the enhanced matrix metalloproteinase 9 secretion by fibronectin in ovarian cancer cells. Cancer Res. 58:900–903. [PubMed] [Google Scholar]
- Shin, M., C. Yan, and D. Boyd. 2002. An inhibitor of c-jun amino terminal kinase (SP600125) represses c-Jun activation, DNA-binding and PMA-inducible 92-kDa type IV collagenase expression. Biochim. Biophys. Acta. 8:311–316. [DOI] [PubMed] [Google Scholar]
- Sieg, D.J., C.R. Hauck, and D.D. Schlaepfer. 1999. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 112:2677–2691. [DOI] [PubMed] [Google Scholar]
- Sieg, D.J., C.R. Hauck, D. Ilic, C.K. Klingbeil, E. Schaefer, C.H. Damsky, and D.D. Schlaepfer. 2000. FAK integrates growth factor and integrin signals to promote cell migration. Nat. Cell Biol. 2:249–256. [DOI] [PubMed] [Google Scholar]
- Slack, J.K., R.B. Adams, J.D. Rovin, E.A. Bissonette, C.E. Stoker, and J.T. Parsons. 2001. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene. 20:1152–1163. [DOI] [PubMed] [Google Scholar]
- Streblow, D.N., C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J.A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell. 99:511–520. [DOI] [PubMed] [Google Scholar]
- Tarone, G., D. Cirillo, F.G. Giancotti, P.M. Comoglio, and P.C. Marchisio. 1985. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp. Cell Res. 159:141–157. [DOI] [PubMed] [Google Scholar]
- Taylor, J.M., M.M. Macklem, and J.T. Parsons. 1999. Cytoskeletal changes induced by GRAF, the GTPase regulator associated with focal adhesion kinase, are mediated by Rho. J. Cell Sci. 112:231–242. [DOI] [PubMed] [Google Scholar]
- Tsuji, T., T. Ishizaki, M. Okamoto, C. Higashida, K. Kimura, T. Furuyashiki, Y. Arakawa, R.B. Birge, T. Nakamoto, H. Hirai, and S. Narumiya. 2002. ROCK and mDia1 antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts. J. Cell Biol. 157:819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuge, Y., and J. Xu. 2001. Rac1 mediates type I collagen-dependent MMP-2 activation. role in cell invasion across collagen barrier. J. Biol. Chem. 276:16248–16256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.