Abstract
Pathophysiological activity and various kinds of traumatic insults are known to have deleterious long-term effects on neuronal Cl− regulation, which can lead to a suppression of fast postsynaptic GABAergic responses. Brain-derived neurotrophic factor (BDNF) increases neuronal excitability through a conjunction of mechanisms that include regulation of the efficacy of GABAergic transmission. Here, we show that exposure of rat hippocampal slice cultures and acute slices to exogenous BDNF or neurotrophin-4 produces a TrkB-mediated fall in the neuron-specific K+–Cl− cotransporter KCC2 mRNA and protein, as well as a consequent impairment in neuronal Cl− extrusion capacity. After kindling-induced seizures in vivo, the expression of KCC2 is down-regulated in the mouse hippocampus with a spatiotemporal profile complementary to the up-regulation of TrkB and BDNF. The present data demonstrate a novel mechanism whereby BDNF/TrkB signaling suppresses chloride-dependent fast GABAergic inhibition, which most likely contributes to the well-known role of TrkB-activated signaling cascades in the induction and establishment of epileptic activity.
Keywords: chloride homeostasis; neurotrophic factors; GABAA depolarization; inhibition; hippocampus
Introduction
Brain-derived neurotrophic factor (BDNF)* has well-documented long-term effects on neuronal survival and differentiation, as well as on synapse formation and functional maturation (Marty et al., 1997; Huang and Reichardt, 2001). In the adult brain, an enhancement in the expression of BDNF and its receptor TrkB is thought to predispose cortical areas to seizure (Binder et al., 2001). BDNF-induced hyperexcitability has been proposed to be partly due to effects on fast GABAergic inhibition. In rat hippocampal slices, BDNF acting via TrkB receptors inhibits GABAA synaptic responses of CA1 pyramidal neurons (Kim et al., 1994; Tanaka et al., 1997; Frerking et al., 1998; Brunig et al., 2001).
GABAA receptor–mediated inhibition can be modulated by a variety of mechanisms, including changes in the firing rate of GABAergic interneurons, the kinetics of quantal release, or by postsynaptic changes at the GABAA receptor level (Ben Ari and Cossart, 2000; Dalby and Mody, 2001). In addition, GABAA-mediated responses are sensitive to changes in the electrochemical gradients for the permeant anions (Kaila, 1994), a factor that is often ignored. In most central neurons, fast inhibition is based on a postsynaptic intracellular [Cl−] that is lower than expected from passive distribution (Thompson et al., 1988; Thompson and Gähwiler, 1989). The generation and maintenance of the chloride gradient required for hyperpolarizing ionotropic responses is attributable to the neuron-specific K+–Cl− cotransporter, KCC2 (Rivera et al., 1999; DeFazio et al., 2000; Kakazu et al., 2000; Hubner et al., 2001). Here, we present several lines of evidence showing that BDNF, acting via TrkB, down-regulates KCC2, leading to impairment of Cl− extrusion from mature hippocampal neurons.
Results and discussion
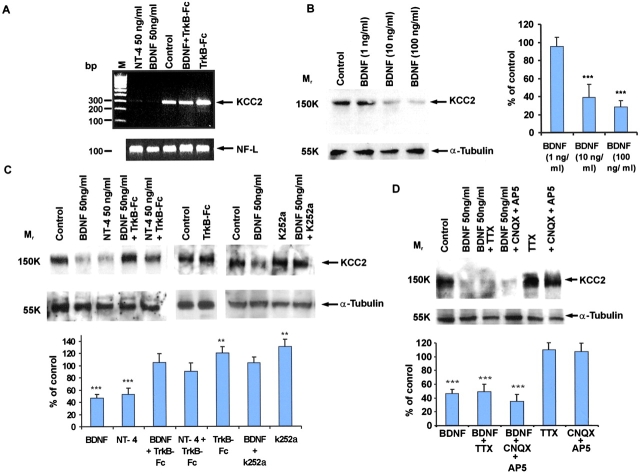
We examined the effects of the TrkB receptor ligands BDNF and neurotrophin-4 (NT-4) on the expression of KCC2 in rat organotypic hippocampal cultures. RT-PCR analysis (Rivera et al., 1999) showed a clear down-regulation of KCC2 mRNA expression by BDNF or NT-4, indicating that this effect is mediated by TrkB (Fig. 1 A) and acts at the transcriptional level. Treatment of the organotypic cultures with increasing concentrations of BDNF in the culture medium (1–100 ng/ml) for 17–19 h (Fig. 1 B) resulted in a dose-dependent decrease in the intensity of KCC2 protein expression. At 10 ng/ml of BDNF, there was a 61 ± 14% decrease, and at 100 ng/ml, an 82 ± 7% decrease in the expression of KCC2 as compared with control levels. A similar down-regulation of KCC2 protein was observed when NT-4 was applied (Fig. 1 C). The effects of both BDNF and NT-4 on KCC2 expression were blocked by either inhibiting tyrosine kinase activity with 1 μM K252a or by specifically scavenging these growth factors with 200 ng/ml of the TrkB-soluble receptor body (TrkB-Fc; Fig. 1 C). An intriguing finding was that application of TrkB-Fc or K252a as such produced a significant increase in KCC2 mRNA as well as protein levels (Fig. 1, A and C), indicating that KCC2 levels were regulated by an endogenous BDNF/TrkB-mediated action. TTX (1 μM) or the glutamate antagonists AP5 (20 μM) and CNQX (50 μM) did not inhibit the BDNF-induced decrease in KCC2 protein (Fig. 1 D), which shows that the TrkB-mediated effects on KCC2 were not caused by a general increase in neuronal excitability and network activity (Scharfman, 1997; Kafitz et al., 1999; Scharfman et al., 1999). Application of NGF, which acts via TrkA, or heat-inactivated BDNF and NT-4 had no effect on KCC2 expression levels (unpublished data).
Figure 1.
Exogenous BDNF and NT-4 down-regulate KCC2 mRNA and protein expression in organotypic hippocampal slices. (A) A representative RT-PCR experiment (out of four similar ones) showing the down-regulatory effect of BDNF and NT-4 on KCC2 mRNA expression. The effects of BDNF and NT-4 were inhibited by 200 ng/ml TrkB-Fc. The amplified light neurofilament (NF-L) fragment indicates an equal amount of mRNA in the reactions. Note the increase in KCC2 mRNA brought about by exposure to TrkB-Fc only. (B) Western blot analysis showing the dose-dependent effect of BDNF. A representative blot (left) and the average normalized optical densities displayed as a percentage of control (right, mean ± SD, n = 5). (C) Representative Western blot (top) showing that KCC2 protein levels are down-regulated by BDNF or NT-4. The Trk inhibitor K252a and TrkB-Fc receptor body inhibited this effect. Note the significant increase in KCC2 protein level after treatment with TrkB-FC or K252a only. The bottom panel shows the average normalized optical densities displayed as percentage of control (mean ± SD, n = 24). (D) Inhibition of network activity with TTX or with glutamate blockers (CNQX and AP5) does not inhibit the down-regulation of KCC2 by BDNF in organotypic slices. The top panel shows a representative Western blot, and the bottom one the normalized optical densities (mean ± SD, n = 7; ***, P < 0.001; **, P < 0.05 as compared with control using the t test).
To examine the consequences of a BDNF-induced reduction in KCC2 expression on neuronal Cl− extrusion, we exposed acute hippocampal slices to 100–200 ng/ml BDNF for different periods of time. Free-floating in situ hybridization showed that KCC2 mRNA levels were down-regulated in all hippocampal regions (Fig. 2 A), and a fast reduction of KCC2 protein was seen in Western blots (Fig. 2 B). The decrease in KCC2 protein expression was already observed after 2 h of incubation, and became even more pronounced after 4 h (Fig. 2 B). Here, one should note that this surprisingly brief time delay reflects a number of steps, including the diffusion of BDNF into the slice and the activation of TrkB-mediated signaling cascades, as well as the consequent changes in KCC2 expression. Again, the effect of exogenous BDNF was not attributable to a network-mediated action because a prompt BDNF-dependent down-regulation of KCC2 mRNA was also seen in the continuous presence of the glutamate antagonists CNQX and AP5 (Fig. 2 B).
Figure 2.
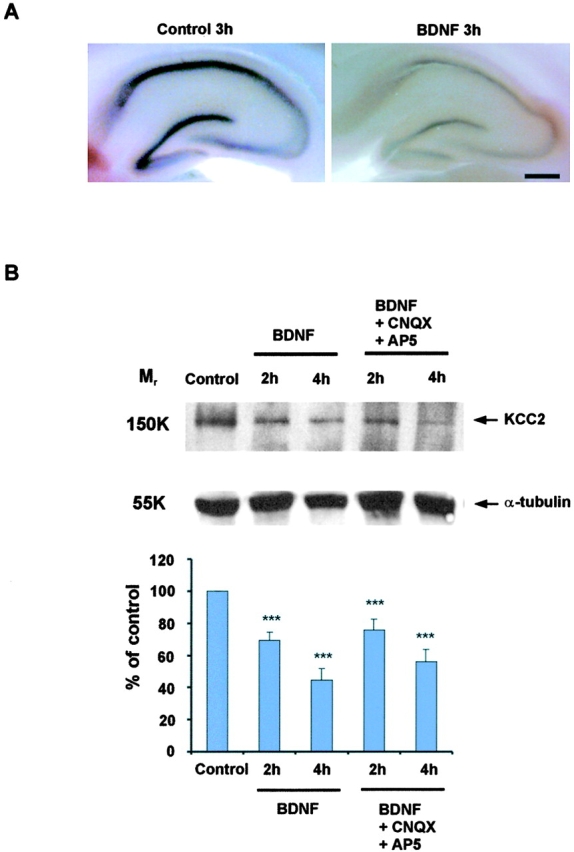
Rapid BDNF-induced down-regulation of KCC2 in acute hippocampal slices. (A) Free-floating in situ hybridization of acute hippocampal slices exposed to 100 ng/ml BDNF showing decreased KCC2 mRNA expression in all hippocampal regions as compared with control. (B) The top panel shows a representative Western blot of KCC2 expression at different time points after adding 100 ng/ml BDNF to the extracellular solution in the absence or presence of glutamate antagonists (CNQX and AP5). Normalized optical densities are shown in the bottom panel (n = 5; ***, P < 0.001 as compared with control using the t test). Note that the KCC2 protein is already down-regulated after 2 h. Bar, 1 mm.
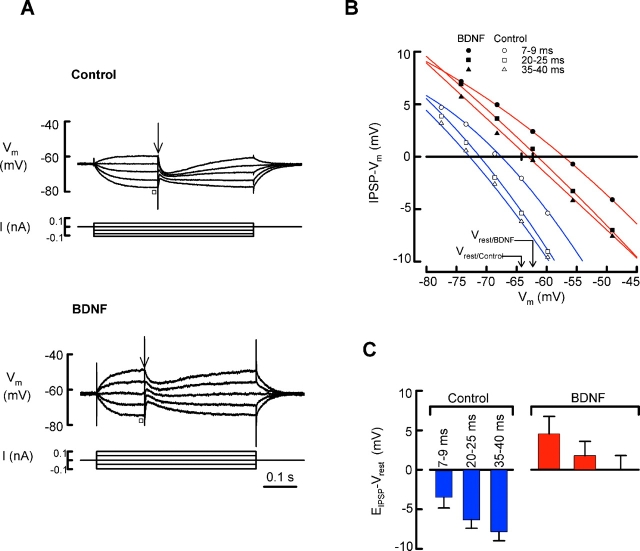
Next, we asked whether the down-regulation of KCC2 seen in acute slices is paralleled by a decrease in the capacity of neuronal Cl− extrusion in CA1 pyramidal neurons. The synaptic and ionic mechanisms underlying hyperpolarizing inhibitory postsynaptic potentials (IPSPs) in these neurons have been extensively examined (Thompson et al., 1988; Thompson and Gähwiler, 1989; Kaila, 1994), and a wealth of data points to the K+–Cl− cotransporter KCC2 as the main Cl− extrusion mechanism (Rivera et al., 1999; DeFazio et al., 2000; Kakazu et al., 2000). Furthermore, exogenous BDNF is known to activate TrkB in these neurons (Huang and Reichardt, 2001). With a fixed exogenous chloride load imposed by leakage from sharp microelectrodes filled with a solution containing 0.5 M Cl−, a fall in the level of functional KCC2 should lead to a higher steady-state level of [Cl−]i, and to a consequent positive shift in the reversal potential of pharmacologically isolated IPSPs (EIPSP). Therefore, we injected constant current pulses into CA1 pyramidal neurons in order to bring the membrane potential (Vm) to different steady levels. Subsequently, IPSPs were evoked by stimulating the GABAergic inputs to the pyramidal neurons (Fig. 3 A, arrow). With the exogenous Cl− load, the control cells were able to maintain hyperpolarizing IPSPs at the resting membrane potential (Vrest) level, whereas those in slices exposed to BDNF were depolarizing (Fig. 3 A), indicating, indeed, a significant reduction in functional Cl− extrusion capacity. The synaptic responses were fully blocked by 100 μM picrotoxin or 50 μM bicuculline (unpublished data). We plotted the amplitudes of IPSPs (IPSP − Vm) against the Vm to obtain reversal potentials (Fig. 3 B). IPSPs such as those evoked presently are caused by mass stimulation of both somatic and dendritic inputs (Buhl et al., 1995), which explains the observation that they had distinct reversal potentials at various points of time (Fig. 3, A and B). The fastest postsynaptic response, which had the most positive reversal potential with respect to Vrest in both control cells and those preexposed to BDNF (Fig. 3 B), peaked between 7–9 ms, most likely reflecting the input from basket cells (Buhl et al., 1995) that target the somata of pyramidal neurons. Using three time windows when measuring the values of EIPSP under steady-state conditions (Fig. 3, B and C), the IPSP driving force (defined here as EIPSP−Vrest) at 7–9, 20–25, and 35–40 ms after the stimulus pulse was −3.52 ± 1.33, −6.33 ± 1.07, and −7.85 ± 1.13 mV, respectively, in control slices (n = 9), and 4.55 ± 2.21, 1.79 ± 1.81, and 0.03 ± 1.79 mV, respectively, in slices exposed to BDNF (n = 10). These results are consistent with an intracellular somatodendritic Cl− gradient that is caused by the diffusional steady state between the somatic Cl− load and KCC2-mediated dendritic Cl− extrusion (Gulyas et al., 2001). The results clearly show that the efficacy of net Cl− extrusion in the BDNF-treated neurons was significantly reduced, which resulted in an estimated mean increase of roughly 50% in intracellular Cl− under the exogenous load used in this work (Kaila, 1994).
Figure 3.
BDNF reduces the capacity of neuronal Cl − extrusion in rat hippocampal slices. (A) The reversal potential of IPSPs (EIPSP) was measured using 0.5 M Cl−-containing microelectrodes from acute slices incubated with 100–200 ng/ml BDNF for 2–4 h (bottom) and from control slices (top). The traces are superimposed responses to constant current pulses injected to bring the membrane potential (Vm) to different levels before evoking an IPSP by stimulation (arrow). The amplitudes of the IPSPs (i.e., IPSP − Vm) from the recordings in A have been plotted against the Vm measured 5–15 ms before (square) the time of stimulation in B. (C) Summary of the mean (± SEM) driving forces of IPSPs (EIPSP − Vrest) obtained from control cells (n = 9), and from cells exposed to BDNF (n = 11), all measured under steady-state conditions in the presence of the electrode-induced Cl− load. Note that the time-dependent negative shift in reversal cannot be accounted for by inadequate space clamp because a time-dependent change in IPSP polarity was often seen in BDNF-incubated slices in the absence of injected current (A, bottom, and C).
In vivo kindling as well as several other experimental paradigms involving strong stimulation are known to lead to a massive up-regulation and release of BDNF and to an activation of TrkB receptors (Binder et al., 2001; Huang and Reichardt, 2001). The results presented here predict that KCC2 is down-regulated under such conditions.
Strikingly, the KCC2 mRNA decreased in all hippocampal regions after in vivo hippocampal kindling–induced seizures (Fig. 4 A). In the dentate gyrus (DG) granule layer, where TrkB expression as well as activation is most pronounced on kindling (Bengzon et al., 1993; Binder et al., 1999), a large decrease in KCC2 expression was already observed 2 h after kindling (55 ± 2%), and the lowest level was reached after 6 h (30 ± 3%). After 24 h, KCC2 mRNA levels had partially recovered (77 ± 3%). The KCC2 mRNA in the CA1 and CA3 regions was significantly lower than in control animals, and they did not recover within 24 h.
Figure 4.
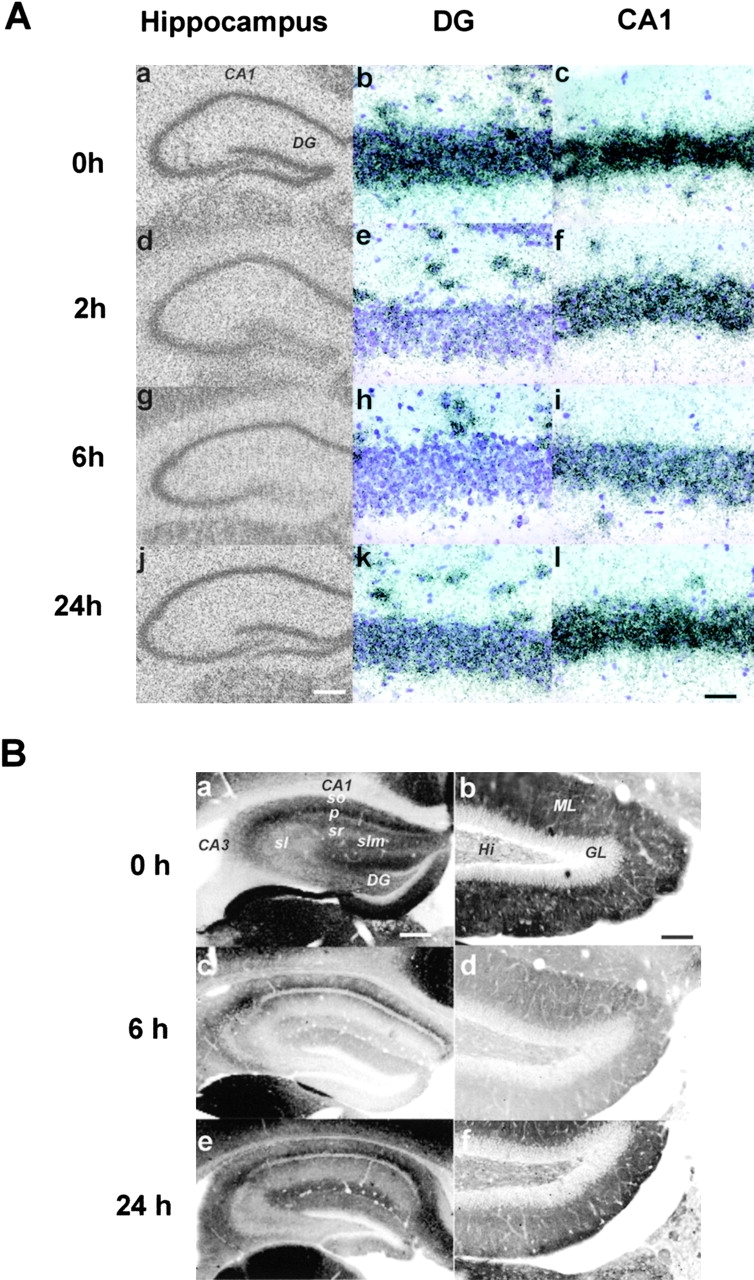
Down-regulation of KCC2 in vivo after hippocampal kindling. (A) The photomicrographs show changes in the distribution of KCC2 mRNA in transverse sections from mice 2, 6, and 24 h after the last stimulus-evoked seizure (a, d, g, and j). The mice experienced 4.6 ± 0.5 generalized (grade 4–5) seizures during the series of 40 hippocampal kindling stimulations where the mean duration of focal hippocampal epileptiform activity after each stimulus was 46 ± 2 s (n = 16). Control animals (0 h) were operated, but did not receive stimulation. Note the conspicuous down-regulation of KCC2 mRNA in the dentate gyrus (DG) 2 and 6 h after stimulation (b vs. h). KCC2 levels were low in the CA1–CA3 region even after 24 h (j and l). The contralateral side showed identical changes and is therefore not shown. Bars: left panel, 1 mm; right panel, 100 μm. (B) Representative pictures of transverse brain sections from kindled mice showing changes in the distribution of KCC2 immunostaining in the hippocampus (dark areas indicate high expression, n = 3). (a) Control section shows intense staining in CA1–CA3 stratum oriens (so) proximal to the pyramidal layer (p) and moderate staining in str. lacunosum-moleculare (slm). A lower level of KCC2 immunoreactivity is observed in str. radiatum (sr) and str. lucidum (sl). (b) Higher magnification of the DG. Here, the molecular layer (ML) shows strong KCC2 immunoreactivity, whereas the granular layer (GL) and the hilus (Hi) show weak staining. (c) Note a significant reduction of KCC2 immunoreactivity in all hippocampal regions 6 h after the last seizure. The CA1 region, especially the proximal dendrites in the str. oriens, although displaying lower levels than control, shows higher KCC2 immunoreactivity than the CA3 region and DG. (d) The ML in the DG is the region with the most conspicuous fall in KCC2 expression at 6 h. (e and f) A partial recovery of the KCC2 immunostaining intensity is observed 24 h after the last stimulus-evoked seizure. The DG still shows lower levels of KCC2 expression. Bars: left panel, 350 μm; right panel, 100 μm.
Interestingly, in several epilepsy models, the time course of up-regulation of BDNF protein levels has been shown to follow its mRNA levels with an ∼4-h delay starting in the DG, followed by the hilus and CA3 stratum lucidum 12–24 h later (Binder et al., 2001). Here, we found that KCC2 mRNA reached its minimum value after 6 h in the DG, whereas in the CA3, this occurred after 24 h. In agreement with previous data, the expression levels of TrkB and BDNF mRNAs showed a time-dependent increase upon kindling-induced seizures (Bengzon et al., 1993). TrkB mRNA levels increased bilaterally in all hippocampal regions with a prominent peak after 2 h in the granule cells of the DG (160 ± 5% of control), returning to control levels after 6 h. The increase of TrkB mRNA levels in CA1 and CA3 was not as steep as in the DG, but remained high 6 h after kindling (138 ± 2% in CA1, 139 ± 3% in CA3) and returned to control values after 24 h. BDNF mRNA showed a strong increase in all hippocampal regions with a 3–5-fold increase over control levels 2 h after kindling. The BDNF mRNA levels gradually returned back to control values after 24 h, except for the DG that still showed elevated levels (198 ± 5% of control).
A general decrease in KCC2 immunostaining was found 6 h after kindling (Fig. 4 B). A regional comparison of these data with the in situ hybridization results (Fig. 4 A) showed that the kindling-induced changes in KCC2 mRNA are directly translated into corresponding alterations in protein expression, indicating that this effect appears to take place at the level of transcription of KCC2, although we cannot rule out changes at the level of regulation of KCC2 mRNA stability and/or of translation or trafficking of the KCC2 protein (Kelsch et al., 2001). Activity-dependent down-regulation of KCC2 is also observed in vitro in the classical 0-Mg slice model. Here, the effects of continuous neuronal activity on KCC2 can be blocked by K252a or by scavenging endogenous BDNF with TrkB receptor bodies (unpublished data).
In conclusion, our results disclose a novel BDNF/TrkB-mediated signaling mechanism that is likely to have a profound action on neuronal Cl− homeostasis. Here, it is worth pointing out that changes in intraneuronal [Cl−] do not only affect the amplitude and polarity of GABAergic responses (Kaila, 1994). They are also intimately involved in the control of both neuronal and interstitial volume, which play a critical role in the modulation of neuronal excitability, especially under conditions that promote epileptiform activity (Jefferys, 1995, 1998; Azouz et al., 1997).
Materials and methods
All experimental procedures were performed according to ethical guidelines approved by local authorities.
Kindling
Male adult C57BL/6 mice (n = 16) were housed under 12 h of light/12 h of dark with food and water ad libitum. Stimulating/recording electrodes were implanted in the left ventral hippocampus and 40 threshold stimulations with 5-min intervals (1-ms pulses, 10-Hz frequency, and 10-s duration) were delivered 7–10 d after electrode implantation as described previously (Kokaia et al., 1999). Animals were killed at 2, 6, and 24 h after the last stimulus-evoked seizure (four animals in each group). Four electrode-implanted nonstimulated mice were used as controls. Brains were immediately frozen on powdered dry ice. For in situ hybridization, brains were sectioned on a cryostat at 14 μm in the frontal plane at the level of the dorsal hippocampus, and thaw-mounted onto ProbeOn slides (Fisher Scientific) and stored at −70°C. For free-floating immunohistochemistry, 40-μm sections were prepared and stored at −20°C in a cryoprotecting solution.
In situ hybridization
The following cDNA constructs were used as templates for the synthesis of labeled cRNA probes: a 1,039-bp mouse KCC2 EST clone (EMBL/GenBank/DDBJ AA982489), corresponding to nucleotides 4,605–5,566 of the full-length rat KCC2 cDNA (Payne et al., 1996), and a 366-bp rat BDNF cDNA construct (Hiltunen et al., 1996). This fragment (nucleotides 517–882) of the rat BDNF cDNA sequence (EMBL/GenBank/DDBJ M61178) is 99% identical to the corresponding mouse BDNF sequence (EMBL/GenBank/DDBJ X55573); a 483-bp insert of mouse TrkB cDNA extracellular domain (Klein et al., 1989; Hiltunen et al., 1996). Radioactive in situ hybridization was performed on frozen sections as described previously (Kokaia et al., 1999).
Free-floating in situ hybridization was performed on 100-μm sections obtained from thick acute hippocampal slices (350 μm) using digoxigenin-labeled riboprobes as described previously (Nieto et al., 1996).
Quantification of mRNA levels
Quantification of hybridization signals on the X-ray films was performed by computerized image analysis using Image 1.57 software (provided by Wayne Rasband, National Institutes of Health, Bethesda, MD). Gray levels from 14C radioactive standards were used in a third degree polynominal calibration to obtain equivalent values of tissue radioactivity (nCi/g) for different brain regions. In each section, measurements for every structure were performed in both left and right hemispheres. Because there were no significant differences in tissue radioactivity between the two sides, values were pooled to obtain the mean value for each animal and brain region. For each probe, averaged data from two sets of adjacent sections from two frontal levels per animal (n = 4 animals in each group) were used for statistical analysis. Evaluation of statistical differences in kindling characteristics and mRNA levels between groups was performed using one-way analysis of variance (ANOVA) followed by the Bonferroni/Dunn post hoc test. All values are given as means ± SEM.
Organotypic cultures
Organotypic hippocampal cultures (Stoppini et al., 1991) were prepared and maintained as described previously (Lahtinen et al., 2001), using transverse slices (350-μm thickness) from the dorsal two-thirds (septal end) of hippocampi from postnatal day 9–13 Wistar rats. All cultures used in this work had a total age (age at the start of experiment + days in vitro) of 19–27 d, and retained their morphological organization over the time used for culturing. The quality of every slice was controlled and slices showing signs of degeneration were discarded.
Recombinant BDNF, NT-4, and NGF were acquired from PeproTech. TrkB-Fc was acquired from R&D Systems, and K252a was acquired from Calbiochem.
Western blotting
Membrane fractions prepared from hippocampal slices were analyzed by Western blotting as described previously (Payne et al., 1996; Rivera et al., 1999), using the following source of pAbs: affinity-purified rabbit anti–rat KCC2 (a gift from Dr. John Payne, University of California, Davis, CA) and monoclonal anti–α-tubulin clone DM1A (Sigma-Aldrich). The corresponding bands were developed using ECL-plus (Amersham Biosciences) on X-ray films as well as on a phosphorimager (model BAS-1500; Fuji) and were analyzed with the TINA program (Tamro). The linearity of the system was assayed with increasing protein concentrations, and a protein concentration of 15–20 μg/ml was calculated to be optimal.
Immunohistochemistry
For free-floating immunostaining, 50-μm brain sections and organotypic slices were washed several times with PBS, dehydrated through methanol series, and incubated in Dent's fixative (20% DMSO in methanol) for 1 h. Sections were washed with TBST (0.1% Triton X-100 in TBS), incubated overnight with rabbit anti-KCC2 antibody (1:600), and diluted in TBSTD (5% DMSO in TBST) containing 5% BSA and 0.4% sheep serum. After washing, the sections were incubated with corresponding Cy3-conjugated secondary antibody (1:200; Jackson ImmunoResearch Laboratories) overnight and mounted on Superfrost plus (Menzel-Glaser) in gelvatol (Biomedia Corp.).
RT-PCR
Total RNA from rat hippocampal slices was reverse-transcribed and PCR-amplified as described previously (Rivera et al., 1999). Primers were synthesized over specific regions for full-length TrkB, forward, 5′-TCAAGTTGGCGAGACATT-3′; reverse, 5′-ATGTACTCAAAGACCATGATGAG-3′, targeting nucleotides 1,869–2,367; for KCC2, forward, 5′-CTCAACAACC-TGACGGACTG-3′; reverse, 5′-GCAGAAGGACTCCATGATGCCTGCG-3′, targeting nucleotides 119–518; for neuron-specific neurofilament light chain, forward, 5′-GCACATCTCCAGCGTGCGCAG-3′; reverse, 5′- GGATCTGAGCCTGCAGCTCGG-3.
Electrophysiology
350-μm transverse hippocampal slices from 100–150-g Wistar rats were cut using a Vibratome (Technical Products International, Inc.), and the slices were allowed to recover in standard physiological solution containing 124 mM NaCl, 3 mM KCl, 2 mM CaCl2, 25 mM NaHCO3, 1.1 mM NaH2PO4, 2 mM MgSO4, and 10 mM d-glucose, and were equilibrated with 95% O2 and 5% CO2. Slices were placed into Falcon® tissue culture filters (treated with 2% BSA overnight) in a 6-well culture tray containing 8–10 ml of physiological solution and continuously gassed with hunified 95% O2 and 5% CO2 at 32°C. Slices were exposed to 100–200 ng/ml BDNF for 2–4 h before transferring to either a submerged-type (double-sided perfusion) recording chamber (32–34°C) or processing for free-floating in situ hybridization. The procedure with control slices was identical except that the BDNF application was omitted.
Intracellular recordings were obtained blind in CA1 stratum pyramidale using microelectrodes filled with 0.5 M potassium acetate plus 0.5 M KCl (pH 6.6–6.8; resistance 135–220 MΩ), and 50 mM QX-314 (Tocris Cookson Ltd.) to block spiking. Data accepted for analysis were taken from cells that had a stable Vrest of at least −50 mV (control = −64.74 ± 1.91 mV; BDNF = −67.60 ± 1.09 mV) and an input resistance of 68–163 MΩ (control = 93 ± 8 MΩ; BDNF = 129 ± 12 MΩ). Pharmacologically isolated IPSPs were evoked in the presence of the ionotropic glutamate antagonists NBQX and DL-AP5 (10 and 40 μM, respectively; Tocris Cookson Ltd.) by stimuli (5–25 V, 60–100 μsec, and frequency 1/10 or 1/15 Hz) delivered via a bipolar tungsten electrode positioned close (≤500 μm) to the recording electrode (Davies et al., 1990). Picrotoxin and bicuculline were acquired from Sigma-Aldrich. Measurements were performed with an Axoclamp 2B amplifier (Axon Instruments, Inc.) in bridge mode. Data were digitized and analyzed offline using WinWCP v3.2.0 software (Strathclyde University, Glasgow, UK).
Acknowledgments
We would like to thank Eila Kujamäki, Miika Palviainen, and Marjo Heikura for excellent technical assistance, and Dr. J. Payne for providing anti-KCC2 antibodies.
This work has been supported by the Academy of Finland, Biocentrum Helsinki, Sigrid Jusélius Foundation, and The Swedish Research Council.
Footnotes
Abbreviations used in this paper: BDNF, brain-derived neurotrophic factor; DG, dentate gyrus; EIPSP, reversal potential of IPSP; IPSP, inhibitory postsynaptic potential; NT-4, neurotrophin-4; TrkB-Fc, TrkB-soluble receptor body; Vm, membrane potential; Vrest, resting membrane potential.
References
- Azouz, R., G. Alroy, and Y. Yaari. 1997. Modulation of endogenous firing patterns by osmolarity in rat hippocampal neurones. J. Physiol. 502:175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ari, Y., and R. Cossart. 2000. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 23:580–587. [DOI] [PubMed] [Google Scholar]
- Bengzon, J., Z. Kokaia, P. Ernfors, M. Kokaia, G. Leanza, O.G. Nilsson, H. Persson, and O. Lindvall. 1993. Regulation of neurotrophin and trkA, trkB and trkC tyrosine kinase receptor messenger RNA expression in kindling. Neuroscience. 53:433–446. [DOI] [PubMed] [Google Scholar]
- Binder, D.K., M.J. Routbort, and J.O. McNamara. 1999. Immunohistochemical evidence of seizure-induced activation of trk receptors in the mossy fiber pathway of adult rat hippocampus. J. Neurosci. 19:4616–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, D.K., S.D. Croll, C.M. Gall, and H.E. Scharfman. 2001. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 24:47–53. [DOI] [PubMed] [Google Scholar]
- Brunig, I., S. Penschuck, B. Berninger, J. Benson, and J.M. Fritschy. 2001. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur. J. Neurosci. 13:1320–1328. [DOI] [PubMed] [Google Scholar]
- Buhl, E.H., S.R. Cobb, K. Halasy, and P. Somogyi. 1995. Properties of unitary IPSPs evoked by anatomically identified basket cells in the rat hippocampus. Eur. J. Neurosci. 7:1989–2004. [DOI] [PubMed] [Google Scholar]
- Dalby, N.O., and I. Mody. 2001. The process of epileptogenesis: a pathophysiological approach. Curr. Opin. Neurol. 14:187–192. [DOI] [PubMed] [Google Scholar]
- Davies, C.H., S.N. Davies, and G.L. Collingridge. 1990. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J. Physiol. 424:513–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio, R.A., S. Keros, M.W. Quick, and J.J. Hablitz. 2000. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J. Neurosci. 20:8069–8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking, M., R.C. Malenka, and R.A. Nicoll. 1998. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J. Neurophysiol. 80:3383–3386. [DOI] [PubMed] [Google Scholar]
- Gulyas, A.I., A. Sik, J.A. Payne, K. Kaila, and T.F. Freund. 2001. The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur. J. Neurosci. 13:2205–2217. [DOI] [PubMed] [Google Scholar]
- Hiltunen, J.O., U. Arumäe, M. Moshnyakov, and M. Saarma. 1996. Expression of mRNAs for neurotrophins and their receptors in developing rat heart. Circ. Res. 79:930–939. [DOI] [PubMed] [Google Scholar]
- Huang, E.J., and L.F. Reichardt. 2001. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24:677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner, C.A., V. Stein, I. Hermans-Borgmeyer, T. Meyer, K. Ballanyi, and T.J. Jentsch. 2001. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 30:515–524. [DOI] [PubMed] [Google Scholar]
- Jefferys, J.G. 1995. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol. Rev. 75:689–723. [DOI] [PubMed] [Google Scholar]
- Jefferys, J.G. 1998. Mechanisms and experimental models of seizure generation. Curr. Opin. Neurol. 11:123–127. [DOI] [PubMed] [Google Scholar]
- Kafitz, K.W., C.R. Rose, H. Thoenen, and A. Konnerth. 1999. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 401:918–921. [DOI] [PubMed] [Google Scholar]
- Kaila, K. 1994. Ionic basis of GABAA receptor channel function in the nervous system. Prog. Neurobiol. 42:489–537. [DOI] [PubMed] [Google Scholar]
- Kakazu, Y., S. Uchida, T. Nakagawa, N. Akaike, and J. Nabekura. 2000. Reversibility and cation selectivity of the K+-Cl− cotransport in rat central neurons. J. Neurophysiol. 84:281–288. [DOI] [PubMed] [Google Scholar]
- Kelsch, W., S. Hormuzdi, E. Straube, A. Lewen, H. Monyer, and U. Misgeld. 2001. Insulin-like growth factor 1 and a cytosolic tyrosine kinase activate chloride outward transport during maturation of hippocampal neurons. J. Neurosci. 21:8339–8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.G., T. Wang, P. Olafsson, and B. Lu. 1994. Neurotrophin 3 potentiates neuronal activity and inhibits gamma-aminobutyratergic synaptic transmission in cortical neurons. Proc. Natl. Acad. Sci. USA. 91:12341–12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, R., L.F. Parada, F. Coulier, and M. Barbacid. 1989. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 8:3701–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia, Z., M.S. Airaksinen, A. Nanobashvili, E. Larsson, E. Kujamäki, O. Lindvall, and M. Saarma. 1999. GDNF family ligands and receptors are differentially regulated after brain insults in the rat. Eur. J. Neurosci. 11:1202–1216. [DOI] [PubMed] [Google Scholar]
- Lahtinen, H., A.M. Autere, P. Paalasmaa, S.E. Lauri, and K. Kaila. 2001. Post-insult activity is a major cause of delayed neuronal death in organotypic hippocampal slices exposed to glutamate. Neuroscience. 105:131–137. [DOI] [PubMed] [Google Scholar]
- Marty, S., M. Berzaghi, and B. Berninger. 1997. Neurotrophins and activity-dependent plasticity of cortical interneurons. Trends Neurosci. 20:198–202. [DOI] [PubMed] [Google Scholar]
- Nieto, M.A., K. Patel, and D.G. Wilkinson. 1996. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 51:219–235. [DOI] [PubMed] [Google Scholar]
- Payne, J.A., T.J. Stevenson, and L.F. Donaldson. 1996. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J. Biol. Chem. 271:16245–16252. [DOI] [PubMed] [Google Scholar]
- Rivera, C., J. Voipio, J.A. Payne, E. Ruusuvuori, H. Lahtinen, K. Lamsa, U. Pirvola, M. Saarma, and K. Kaila. 1999. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 397:251–255. [DOI] [PubMed] [Google Scholar]
- Scharfman, H.E. 1997. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J. Neurophysiol. 78:1082–1095. [DOI] [PubMed] [Google Scholar]
- Scharfman, H.E., J.H. Goodman, and A.L. Sollas. 1999. Actions of brain-derived neurotrophic factor in slices from rats with spontaneous seizures and mossy fiber sprouting in the dentate gyrus. J. Neurosci. 19:5619–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini, L., P.A. Buchs, and D. Muller. 1991. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 37:173–182. [DOI] [PubMed] [Google Scholar]
- Tanaka, T., H. Saito, and N. Matsuki. 1997. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J. Neurosci. 17:2959–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, S.M., and B.H. Gähwiler. 1989. Activity-dependent disinhibition. II. Effects of extracellular potassium, furosemide, and membrane potential on ECl− in hippocampal CA3 neurons. J. Neurophysiol. 61:512–523. [DOI] [PubMed] [Google Scholar]
- Thompson, S.M., R.A. Deisz, and D.A. Prince. 1988. Outward chloride/cation co-transport in mammalian cortical neurons. Neurosci. Lett. 89:49–54. [DOI] [PubMed] [Google Scholar]