Abstract
Yeast Ypt1p-interacting protein (Yip1p) belongs to a conserved family of transmembrane proteins that interact with Rab GTPases. We encountered Yip1p as a constituent of ER-derived transport vesicles, leading us to hypothesize a direct role for this protein in transport through the early secretory pathway. Using a cell-free assay that recapitulates protein transport from the ER to the Golgi complex, we find that affinity-purified antibodies directed against the hydrophilic amino terminus of Yip1p potently inhibit transport. Surprisingly, inhibition is specific to the COPII-dependent budding stage. In support of this in vitro observation, strains bearing the temperature-sensitive yip1-4 allele accumulate ER membranes at a nonpermissive temperature, with no apparent accumulation of vesicle intermediates. Genetic interaction analyses of the yip1-4 mutation corroborate a function in ER budding. Finally, ordering experiments show that preincubation of ER membranes with COPII proteins decreases sensitivity to anti-Yip1p antibodies, indicating an early requirement for Yip1p in vesicle formation. We propose that Yip1p has a previously unappreciated role in COPII vesicle biogenesis.
Keywords: ER; Golgi; vesicles; coat proteins; trafficking
Introduction
The eukaryotic secretory pathway responsively delivers proteins and lipids to their correct destinations. Transport though the secretory pathway is mediated by membrane vesicles and/or tubules that form from a donor compartment and fuse selectively with an acceptor. Specific cytosolic coat protein complexes and conserved membrane fusion factors such as Rab GTPases and SNARE proteins are known to catalyze many of these intracellular transport reactions (Mellman and Warren, 2000). Although several of these transport components are characterized, it is less clear how compartment-specific factors interface with the conserved machinery and how budding and fusion stages are coordinated.
Transport between the ER and Golgi complex in the early secretory pathway also relies on GTPases and SNARE proteins. More specifically, the Rab GTPase Ypt1p is required for transport to the Golgi complex in yeast, and is localized primarily to Golgi membranes (Segev et al., 1988). To identify other cellular factors that bind to Ypt1p, yeast two-hybrid approaches uncovered a Ypt1p-interacting protein (Yip1p) that is essential for transport through the early secretory pathway (Yang et al., 1998). Yip1p is a 27-kD integral membrane protein predicted to span the membrane multiple times. The amino-terminal hydrophilic domain of Yip1p faces the cytosol and is sufficient for Ypt1p interaction. Yip1p also displays direct interactions with several other Rab GTPases in yeast including Ypt31p and Sec4p. Yip1p binding to Rabs depends on an intact carboxy-terminal CAAX motif and prenylation of these GTPases (Yang et al., 1998; Calero and Collins, 2002). The nucleotide status of the GTPase seems less critical for these associations.
Yip1p not only associates with Rab GTPases, but forms a heteromeric complex with a related integral membrane protein termed Yip1p-interacting factor (Yif1p). Yif1p shares a common domain topology with Yip1p, binds to Rab GTPases through a cytoplasmically exposed amino-terminal domain, and is required for transport through the early secretory pathway (Matern et al., 2000). Additional reports have shown that yeast cells contain an extended family of Yip1p related proteins that appear to form mixed heteromeric complexes with one another. This family displays some functional overlap and may act more broadly in intracellular transport (Calero and Collins, 2002; Calero et al., 2002). The subcellular distribution and molecular function of the Yip family of proteins remains to be determined.
In this report, we investigate the role of Yip1p in protein transport between the ER and Golgi complex. Using cell-free assays that recapitulate subreactions in this transport process, we observed that antibody inhibition of Yip1p activity and mutations within the amino-terminal cytosolic domain of Yip1p inhibit COPII-dependent vesicle budding from the ER. Moreover, these Yip1p-specific inhibitors do not directly interfere with the vesicle tethering or fusion stages of ER-derived vesicles with Golgi membranes. Our in vivo analyses also support a role for Yip1p in budding from the ER. These results are surprising in light of the fact that Ypt1p does not appear to be required for COPII-dependent budding.
Results
Anti-Yip1p antibodies inhibit transport to the Golgi complex in vitro
Genetic and biochemical experiments have indicated a requirement for Yip1p in transport between the ER and the Golgi complex. Conditionally lethal yip1 mutants display defects in protein secretion, and morphological analyses demonstrated that cells depleted of Yip1p accumulate membranes of the ER (Yang et al., 1998). Biochemical experiments have shown that Yip1p can physically associate with Ypt1p (Yang et al., 1998), a small GTPase required for ER/Golgi transport (Segev et al., 1988). Given these findings, we sought to define the function of Yip1p more specifically using a reconstituted cell-free assay that measures protein transport to the Golgi complex. For this assay, washed semi-intact cell membranes containing [35S]glycopro-α-factor (gpαf) in the ER are incubated with purified factors (COPII, Uso1p, and LMA1) to drive transport of [35S]gpαf to the Golgi complex (Barlowe, 1997). Upon delivery to the Golgi complex, gpαf receives outer-chain α1,6-mannose residues that can be immunoprecipitated with α1,6-mannose–specific serum to quantify [35S]gpαf transport (Baker et al., 1988). To investigate Yip1p function in this assay, we first prepared affinity-purified antibodies against the hydrophilic amino terminus of Yip1p (aa residues 1–99). These anti-Yip1p antibodies were then added to cell-free transport assays in an attempt to neutralize Yip1p function. As seen in Fig. 1 A, reconstituted transport was sensitive to anti-Yip1p antibodies, whereas preimmune IgGs at comparable concentrations did not inhibit transport. The inhibition of anti-Yip1p antibodies was alleviated if purified MBP-Yip1p was included in the reaction. This observation indicates MBP-Yip1p can compete with endogenous Yip1p for antibody binding, and demonstrates that the antibodies act in a specific manner.
Figure 1.
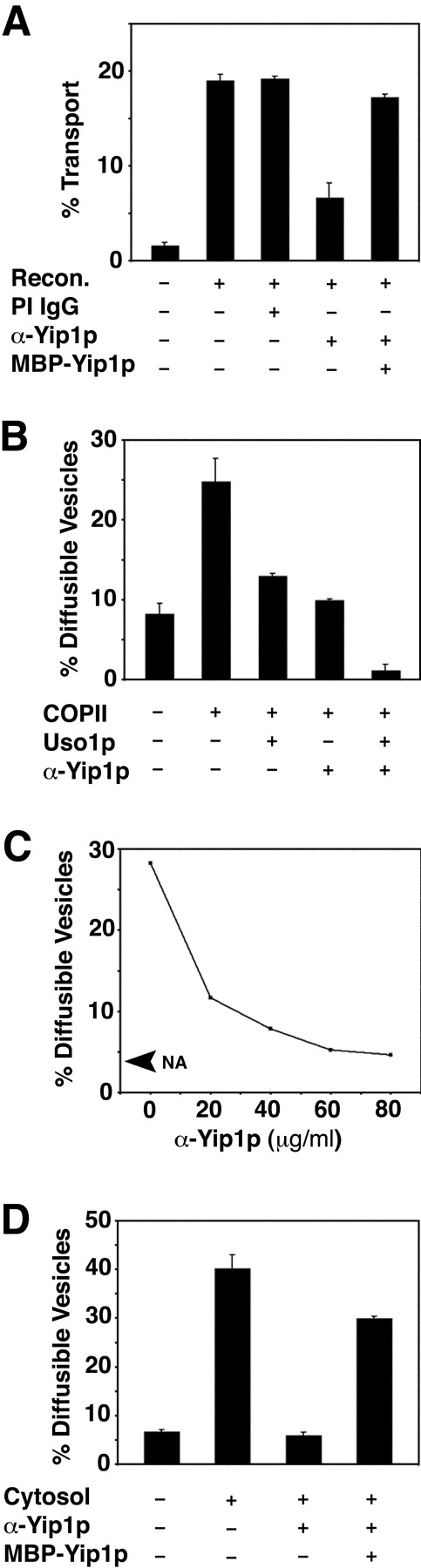
Anti-Yip1p antibodies inhibit in vitro transport between the ER and the Golgi complex at the budding stage. (A) Washed wild-type (FY834) semi-intact cells containing [35S]gpαf were incubated with Recon proteins (COPII, Uso1p, and LMA1) and an ATP regeneration system. After 75 min at 23°C, the amount of Golgi-modified [35S]gpαf was measured to determine transport efficiency. Where indicated, anti-Yip1p antibodies (40 μg/ml), preimmune IgGs (40 μg/ml), or MBP-Yip1p (144 μg/ml) were added to reactions. (B) Semi-intact cells prepared as in A were incubated with COPII or COPII plus Uso1p to measure budding and tethering in the presence or absence of anti-Yip1p antibodies (20 μg/ml). After 30 min at 23°C, freely diffusible vesicles containing [35S]gpαf were separated from semi-intact cell membranes by centrifugation at 18,000 g and [35S]gpαf quantified by Con A precipitation. (C) Vesicle budding as in B with increasing amounts of anti-Yip1p antibodies (20–80 μg/ml). No addition (NA) shows level of budding minus COPII. (D) Vesicle budding as in B, except cytosol was used to drive reactions. Where indicated, anti-Yip1p antibodies (40 μg/ml) and MBP-Yip1p (144 μg/ml) were added.
Subreactions in cell-free transport can be monitored by following the sedimentation properties of membranes containing [35S]gpαf (Barlowe, 1997). Incubation of washed semi-intact cell membranes with purified COPII proteins catalyzes the formation of diffusible vesicles that can be separated from larger membranes by centrifugation. When purified Uso1p is included in this reaction, a significant fraction of the diffusible vesicles pellet with heavier membranes, providing a measurement of vesicle tethering. We found that the inhibitory anti-Yip1p antibodies did not affect vesicle tethering to the Golgi complex, but instead inhibited the budding of COPII vesicles (Fig. 1 B). Titrating the inhibitory effect of anti-Yip1p antibodies on budding showed that increasing amounts of antibodies inhibited COPII-dependent vesicle budding in a dose-dependent manner (Fig. 1 C). We also examined the influence of anti-Yip1p antibodies on vesicle budding when reactions were supplied with a crude cytosolic fraction. As shown in Fig. 1 D, budding remained sensitive to the anti-Yip1p antibodies under this condition. This observation indicates that other factors present in a cytosolic extract cannot circumvent the inhibitory effect of the anti-Yip1p antibodies on vesicle budding.
The inhibition of budding by anti-Yip1p antibodies was surprising, as there is no apparent requirement for Ypt1p in the COPII-dependent vesicle-budding assay (Cao et al., 1998). Therefore, we considered the possibility that these anti-Yip1p antibodies interfered with budding in a nonspecific manner, perhaps by coating ER membranes and preventing COPII association. Alternatively, the antibodies could cross-link vesicles to ER membranes or to one another, producing an aggregate that would pellet under the conditions of our budding assay. These effects would then mask any later stage requirements for Yip1p in vesicle tethering or fusion. To address these possibilities, we performed an additional series of experiments. First, it should be noted that antibodies against other abundant vesicle proteins such as Sec22p, Sed5p, and Erv29p do not block budding nonspecifically (Cao et al., 1998; Belden and Barlowe, 2001b; Liu and Barlowe, 2002). Second, we prepared Fab fragments from the affinity-purified anti-Yip1p antibodies and observed specific inhibition of the vesicle-budding stage of cell-free transport (unpublished data). Finally, we generated COPII vesicles containing [35S]gpαf for use in second-stage transport reactions to determine if anti-Yip1p antibodies influenced any of the post-budding assays. As seen in Fig. 2 A, addition of Uso1p to reactions containing isolated vesicles and wild-type acceptor membranes produced an ∼3.7-fold reduction in diffusible vesicles, indicating efficient tethering of vesicles to Golgi membranes (Fig. 2 A, compare column 1 with column 2). Vesicle tethering was unaffected by the addition of anti-Yip1p antibodies in amounts that effectively block vesicle budding. In columns 4 and 5 of Fig. 2 A, vesicles and acceptor membranes or vesicles alone were combined with anti-Yip1p antibodies in the absence of Uso1p. No reduction in diffusible vesicles was observed for either condition, indicating that an antibody cross-linking event was not responsible for the observed decrease in vesicle budding.
Figure 2.
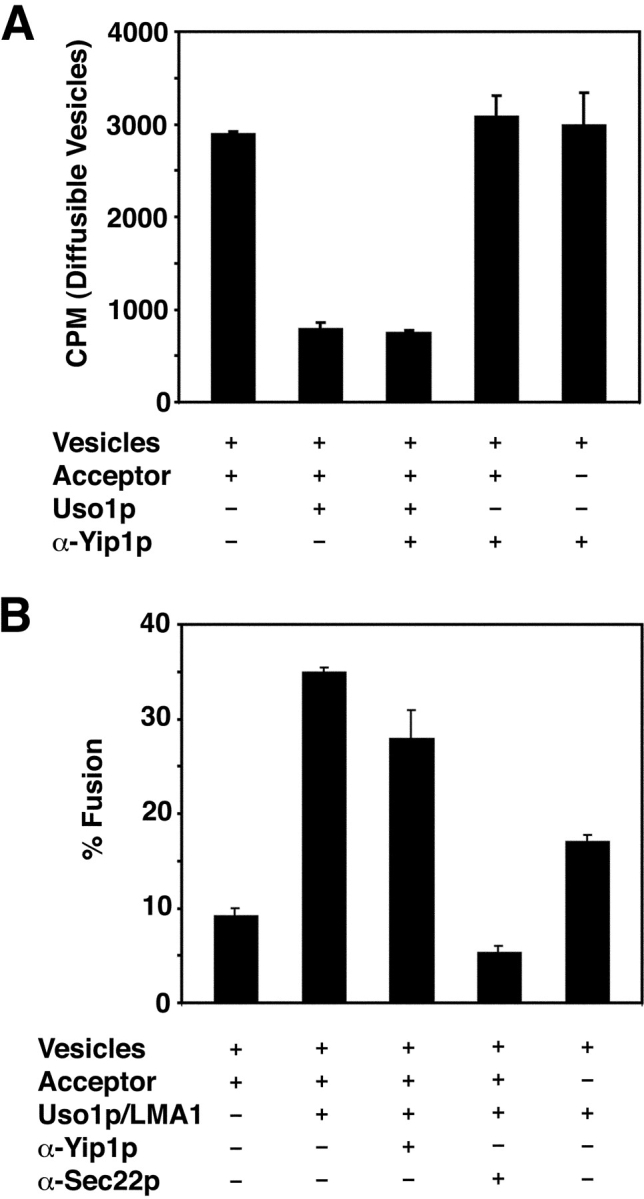
Anti-Yip1p antibodies do not inhibit vesicle tethering or vesicle fusion in a two-stage reaction. (A) COPII vesicles containing [35S]gpαf were synthesized from wild-type (FY834) membranes and mixed with wild-type semi-intact cell acceptor membranes. Reactions were incubated with Uso1p in the presence or absence of anti-Yip1p antibodies (120 μg/ml) where indicated. Freely diffusible vesicles containing [35S]gpαf were quantified by Con A precipitation. (B) COPII vesicles prepared as in A were mixed with wild-type semi-intact cell acceptor membranes. Reactions contained Uso1p and LMA1 in the presence or absence of anti-Yip1p antibodies (120 μg/ml) or anti-Sec22p antibodies (60 μg/ml) where indicated. After 75 min at 23°C, the amount of Golgi-modified [35S]gpαf was measured to determine fusion efficiency.
We also examined the ability of the anti-Yip1p antibodies to inhibit vesicle fusion in a two-stage reaction. Vesicles containing [35S]gpαf were added to wild-type acceptor membranes with Uso1p and the fusion factor LMA1, in the presence or absence anti-Yip1p antibodies. As shown in Fig. 2 B, the addition of Uso1p and LMA1 to the reaction stimulated fusion ∼3.8-fold (Fig. 2 B, compare column 1 with column 2). The addition of anti-Yip1p antibodies to this reaction did not inhibit fusion (Fig. 2 B, column 3). In contrast, affinity-purified antibodies against the SNARE protein Sec22p effectively block vesicle fusion under these conditions as previously reported (Liu and Barlowe, 2002). Together, the results in Fig. 1 and Fig. 2 indicate that Yip1p is required for in vitro transport of [35S]gpαf to the Golgi complex. These data show that the anti-Yip1p antibodies potently and specifically inhibit the budding of COPII vesicles, suggesting a role for Yip1p in vesicle biogenesis.
Anti-Yip1p antibodies block the COPII-dependent budding of vesicle proteins
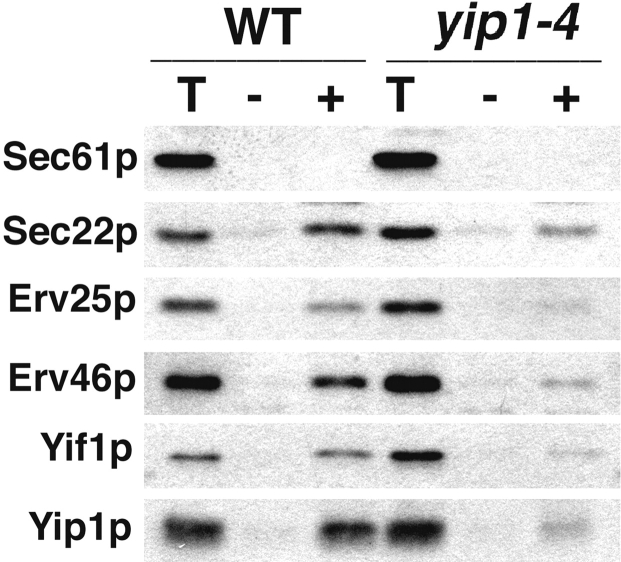
Next, we examined whether the inhibition of vesicle budding by the anti-Yip1p antibodies was restricted to [35S]gpαf monitored in the budding assay, or reflected a more complete block of vesicle biogenesis from ER membranes. Previous reports have shown that ER/Golgi SNARE proteins are efficiently packaged into COPII vesicles (Barlowe et al., 1994; Rexach et al., 1994). In addition, a group of conserved transmembrane proteins, termed ER vesicle (Erv) proteins, are efficiently incorporated into COPII vesicles (Rexach et al., 1994; Otte et al., 2001). We investigated whether anti-Yip1p antibodies affected the incorporation of these proteins into COPII vesicles. Washed semi-intact cells were incubated with COPII proteins and an energy regeneration system in the presence or absence of anti-Yip1p antibodies. The vesicles synthesized in each condition were then isolated and analyzed by immunoblotting. As seen in Fig. 3, COPII proteins catalyzed the efficient and specific incorporation of certain proteins into vesicles. The SNARE protein Sec22p, as well as Erv25p, Erv41p, Yif1p, and Yip1p, were efficiently packaged under these conditions (Fig. 3, lane 2). The ER resident proteins Sec12p and Sec61p were not efficiently packaged into COPII vesicles, demonstrating selective sorting in this budding assay. The presence of anti-Yip1p antibodies in the reaction effectively inhibited budding of all vesicle proteins examined, whereas preimmune IgGs at a comparable concentration had no effect (Fig. 3, lane 3 and lane 4). These results demonstrate the inhibition of vesicle budding by the anti-Yip1p antibodies occurs in a general manner, and is not restricted to [35S]gpαf.
Figure 3.
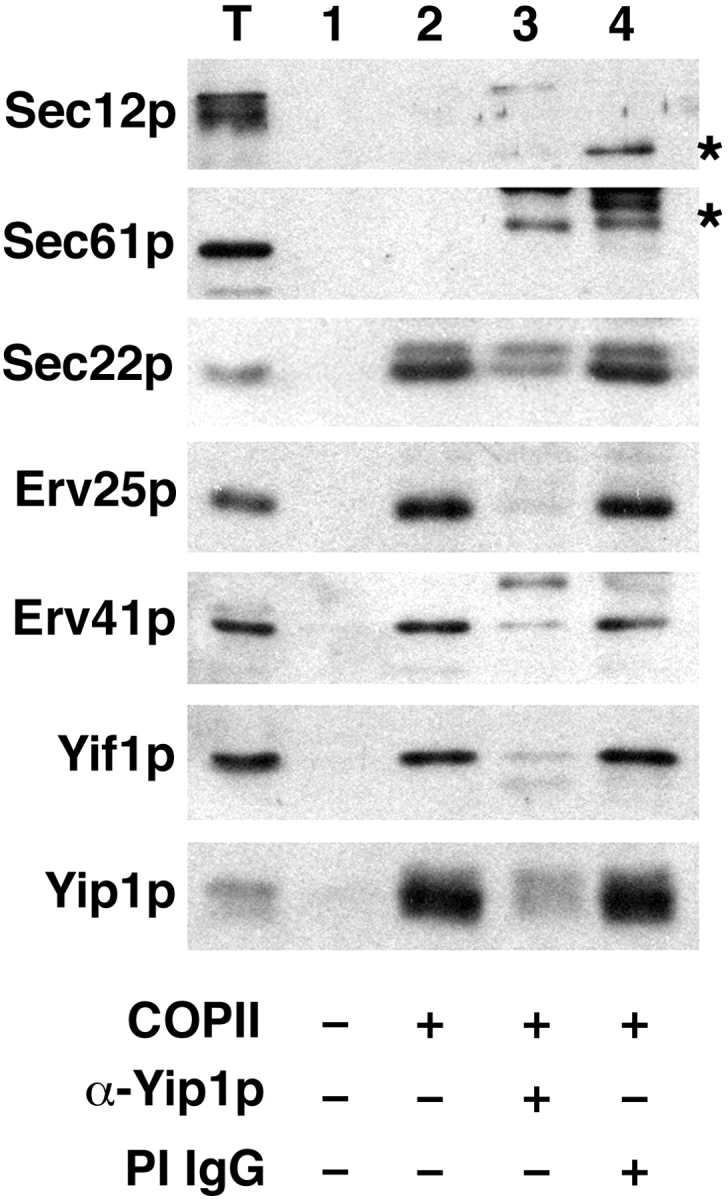
Anti-Yip1p antibodies block COPII-dependent budding of vesicle proteins. COPII-budding reactions were performed from wild-type semi-intact cells (FY834) in the presence or absence of anti-Yip1p antibodies (40 μg/ml) or preimmune IgGs (40 μg/ml) as indicated. One tenth of a total reaction (T), or budding reactions without COPII proteins (lane 1), with COPII proteins (lane 2), with COPII plus anti-Yip1p antibodies (lane 3), and with COPII plus preimmune antibodies (lane 4) were separated on a 12.5% polyacrylamide gel. ER resident proteins (Sec61p and Sec12p) and vesicle proteins (Sec22p, Erv25p, Erv41p, Yif1p, and Yip1p) were detected using immunoblot. Asterisks indicate antibody heavy chain cross-reactivity with secondary antibodies.
Mutant yip1-4 strains accumulate ER membranes in vivo
We sought additional approaches to study the role of Yip1p in transport between the ER and Golgi complex. The thermosensitive yip1-4 allele, which contains a single point mutation (E70K) in the cytoplasmic domain of Yip1p, blocks secretion and growth when shifted to a restrictive temperature (Calero et al., 2003). To characterize the morphology of transport intermediates that may accumulate in yip1-4 strains, thin-section EM was used under conditions that highlight membrane and vesicle structures (Kaiser and Schekman, 1990). Upon shift to 37°C for 40 min, yip1-4 strains show a massive proliferation of ER membranes (Fig. 4), which is commonly observed in the secretory mutants that block vesicle production from the ER (Novick et al., 1980). This type of ER accumulation was also reminiscent of the karmellae-like ER exaggerations formed by overexpression of HMG1 in that the accumulating ER membrane frequently formed multi-layered aggregates that were asymmetrically localized in the cell (Wright et al., 1988). However, unlike the HMG1 overexpression strains, the ER in yip1-4 strains did not accumulate around the nucleus in an ordered array, but appeared to extend into the cytoplasm, sometimes lacking any obvious nuclear association, and was often positioned underneath the plasma membrane. A very similar terminal phenotype has been reported under conditions of Yip1p depletion after placing YIP1 expression under GAL10 control (Yang et al., 1998).
Figure 4.
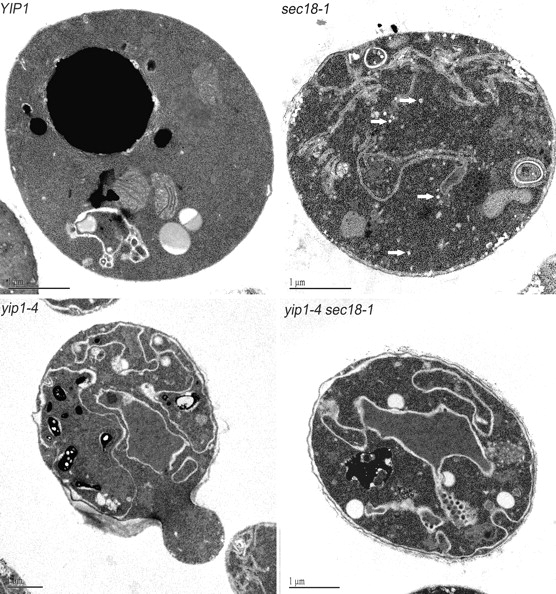
Themosensitive yip1-4 mutants display morphological phenotypes characteristic of an early block in the secretory pathway. Wild-type and mutant cells were shifted to 37°C for 40 min and then fixed and prepared for EM as described under the Materials and methods section. Representative thin sections are shown for each condition. The white arrows in the sec18-1 panel point to transport vesicles that have accumulated in this strain. Note that the accumulation of vesicles was absent in the yip1-4 and yip1-4 sec18-1 strains. Bars, 1 μm.
Small 50–60-nm vesicles did not accumulate in the yip1-4 strain, but were clearly observed in a sec18 mutant (Fig. 4). The sec18-1 mutation produces a strong vesicle accumulation phenotype because the corresponding Sec18p protein is required for vesicle fusion (Wilson et al., 1989; Kaiser and Schekman, 1990). When the yip1-4 and sec18-1 alleles were combined in a single strain, we observed an ER accumulation phenotype without an accumulation of transport vesicles. This epistatic relationship between yip1-4 and sec18-1 indicates yip1-4 inhibits vesicle formation from the ER, and therefore prevents the accumulation of vesicles that are characteristic of fusion mutants. A similar reversal of the sec18-1 vesicle accumulation phenotype has been reported when sec mutants that specifically block COPII budding were combined with sec18-1 (Kaiser and Schekman, 1990). Thus, our morphological experiments place YIP1 function in the vesicle-budding stage of transport between the ER and Golgi compartments and before the action of Sec18p.
We also examined the distribution of an ER-localized protein in yip1-4 cells by fluorescence microscopy of GFP-KDEL. When yip1-4 mutants were shifted to a restrictive temperature for 60 min, striking elaborations of the ER were apparent (Fig. 5 A) while nuclear structures remained intact. Very similar elaborations of the ER were observed when GFP-KDEL was expressed in a sec12 strain under restrictive conditions (unpublished data). These observations are in accord with the images obtained by EM showing accumulation of ER membranes in yip1-4 mutants.
Figure 5.
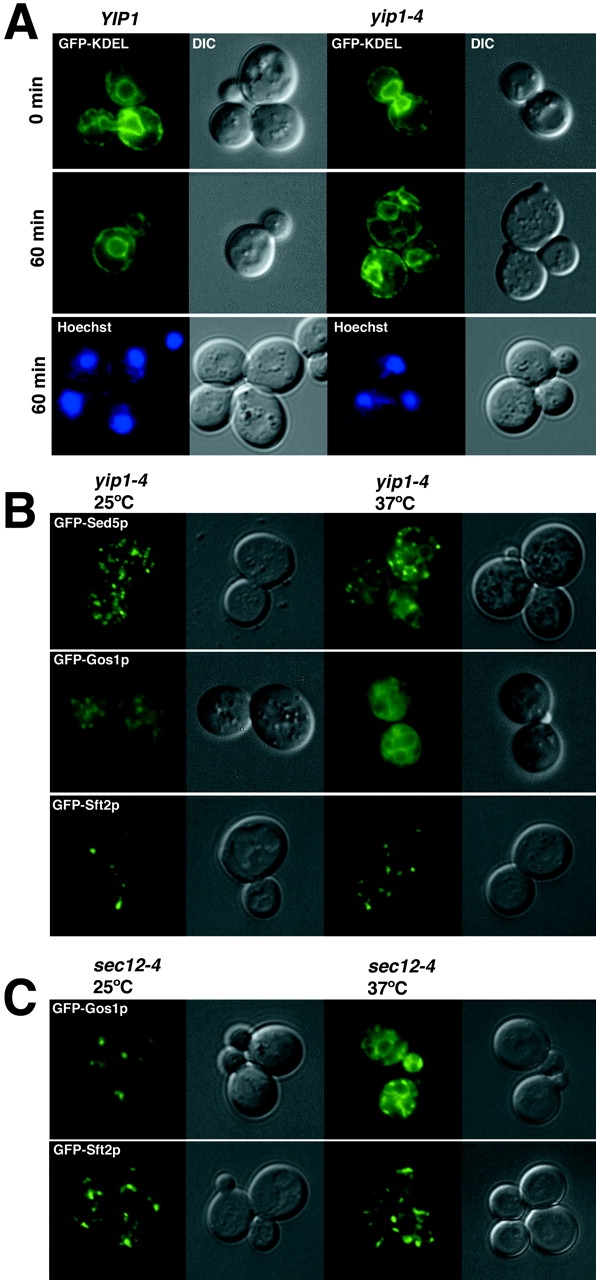
Distribution of GFP-tagged proteins in wild-type and yip1-4 strains. (A) Fluorescence images of GFP-KDEL showing the outline of ER membranes in wild-type (RCY1768) and yip1-4 (RCY1764) strains after 60 min at 37°C. Hoechst stain for DNA indicates nuclei remain intact in yip1-4 cells after a 60-min shift to the restrictive temperature. (B) GFP-tagged versions of Sed5p, Gos1p, and Sft2p were expressed in the yip1-4 strain and monitored by fluorescence microscopy at 25°C or after shift to 37°C for 30 min. Note the partial localization to perinuclear structures for GFP-Sed5p and GFP-Gos1p upon shift to the restrictive temperature. (C) GFP-Gos1p shifts to a perinuclear distribution when ER export is blocked in a sec12-4 strain, whereas GFP-Sft2p remains in a punctate pattern as in the yip1-4 strain.
Redistribution of Golgi-localized proteins to the ER in yip1-4 strains
Proteins that localize to early Golgi compartments continually cycle through the ER at varying rates. If export from the ER is blocked, cycling proteins accumulate in the ER (Schroder et al., 1995; Ward et al., 2001). For example, Emp47p and Sed5p display Golgi localization patterns in wild-type cells; however, in a sec12 strain shifted to the restrictive temperature, these proteins redistribute to the ER (Schroder et al., 1995; Wooding and Pelham, 1998). We used this approach to determine the influence of the yip1-4 mutation on the localization of Golgi-localized proteins. If YIP1 is required for budding from the ER, we hypothesized that early Golgi proteins would become ER-localized in the absence of YIP1 function.
In this set of experiments, GFP-tagged versions of Sed5p, Gos1p, and Sft2p were expressed in the yip1-4 mutant and monitored by fluorescence at permissive and restrictive temperatures (Fig. 5 B). GFP-Sed5p displayed a typical punctate pattern at a permissive temperature, but upon temperature shift, a distinct perinuclear localization emerged. Some GFP-Sed5p appeared to remain in spots and may represent Sed5p that functions in later Golgi compartments (Pelham, 2001). GFP-Gos1p also redistributed to the ER when ER export was blocked, consistent with its proposed role in transport through early Golgi compartments (McNew et al., 1998; Tsui et al., 2001). In contrast, GFP-Sft2p remained in a punctate pattern after temperature shift as expected for a protein that functions in later Golgi compartments (Wooding and Pelham, 1998). A very similar effect on the distribution of GFP-Gos1p and GFP-Sft2p was observed in sec12 strains (Fig. 5 C). These results provide further support for a Yip1p requirement in export from the ER.
Yip1p cycles between the ER and Golgi compartments
Although Yip1p was detected in ER-derived vesicles, previous reports indicated that the protein was largely Golgi localized (Yang et al., 1998). We generated a GFP-Yip1p fusion protein under its native promoter to monitor the location of Yip1p in live cells. Strains expressing this GFP-Yip1p fusion as the sole source of Yip1p activity displayed growth rates that were comparable to wild-type strains (Fig. 6 A). Fluorescence imaging of GFP-Yip1p cells at various cell cycle stages (Fig. 6 B) revealed both a punctate pattern typical of Golgi-localized proteins and ring-like perinuclear structures indicative of ER localization. We also examined the fate of GFP-Yip1p in a sec12 mutant shifted to 37°C (Fig. 6 C). If GFP-Yip1p traffics through the ER, we would expect it to accumulate in the ER when exit from this compartment is blocked. Indeed, GFP-Yip1p redistributed to ER structures under this condition. Finally, we compared the distribution of endogenous Yip1p under wild-type conditions or under a sec12 block using subcellular fractionation schemes. Larger ER membranes pellet at a lower g-force (∼13,000 g) than Golgi membranes (100,000 g) when cell lysates are prepared under specified conditions (Wooding and Pelham, 1998). As seen in Fig. 6 D, Yip1p was found in both ER and Golgi fractions in wild-type strains, but shifts to the ER fraction under a sec12 block. Together with our budding experiments showing Yip1p is efficiently packaged into ER-derived vesicles, these results indicate Yip1p cycles between the ER and Golgi compartments and is dynamically localized to the early secretory pathway.
Figure 6.
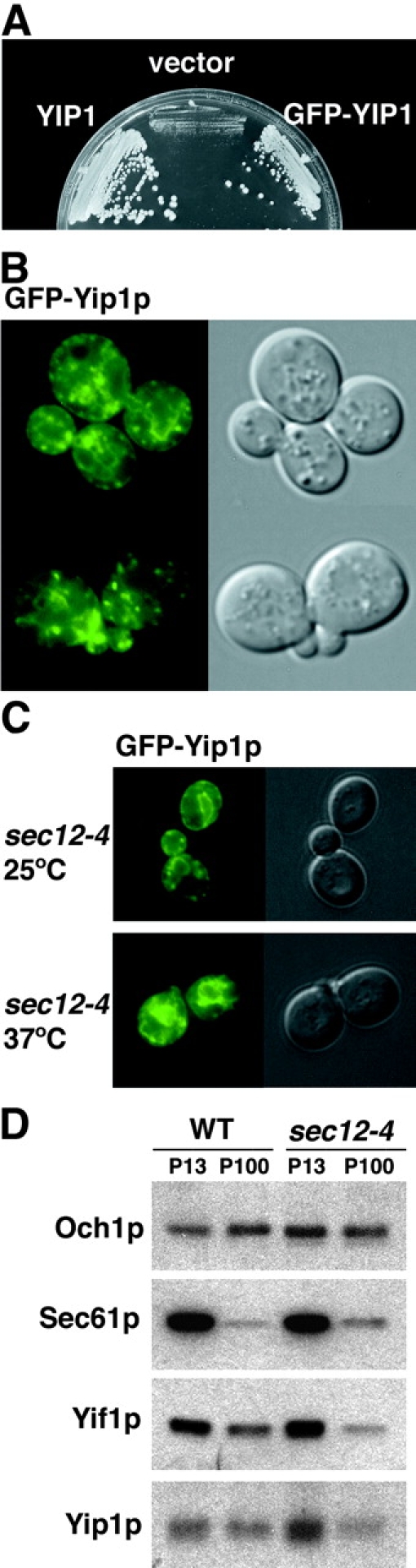
Yip1p cycles between the ER and Golgi compartments. (A) GFP fused to the amino terminus of Yip1p (GFP-Yip1p) complements a yip1Δ strain. (B) Cells expressing GFP-Yip1p were analyzed by fluorescence imaging. A punctate fluorescence pattern typical of Golgi-localized proteins in addition to ring-like perinuclear structures of the ER were observed. (C) GFP-Yip1p redistributes to perinuclear structures when transport from the ER is blocked in a sec12 strain shifted to 37°C for 30 min (D) ER and Golgi membrane fractions from WT and sec12-4. Cells were shifted to 37°C for 45 min before lysis and collection of membrane fractions. The contents of ER (P13) and Golgi (P100) fractions were monitored by immunoblot. Sec61p serves as an ER marker, whereas Och1p is a Golgi-localized protein.
Mutant yip1-4 cells display a defect in COPII vesicle budding in vitro
Our in vivo analyses of yip1-4 mutants indicated a block in budding from the ER. Next, we examined the yip1-4 strain in cell-free transport assays to further characterize transport defects. As shown in Fig. 7 A, yip1-4 cells exhibited a significant defect in transport of [35S]gpαf to the Golgi complex in comparison to an isogenic wild-type strain at 23°C. Specifically, the yip1-4 membranes displayed a 41% reduction in [35S]gpαf transport compared with wild-type. A similar result was obtained when these strains were compared at 29°C, thus, we could not replicate thermosensitivity of the yip1-4 allele in vitro (unpublished data). However, we examined the ability of the yip1-4 strain to bud and tether COPII vesicles to identify the stage at which transport was compromised (Fig. 7 B). We observed that upon the addition of COPII proteins, the wild-type membranes budded vesicles at an efficiency of ∼33%, whereas the yip1-4 membranes budded vesicles at an efficiency of only ∼9%. Importantly, translocation of [35S]gpαf into the yip1-4 membranes was near the wild-type level, indicating that the ER membranes were not generally compromised. When the tethering factor Uso1p was included in these reactions, we found that wild-type membranes tethered ∼45% of the diffusible vesicles and yip1-4 membranes tethered ∼47% of the diffusible vesicles. These results indicate that the yip1-4 membranes can effectively tether COPII vesicles to the Golgi, but are defective in their ability to bud COPII vesicles from ER membranes.
Figure 7.
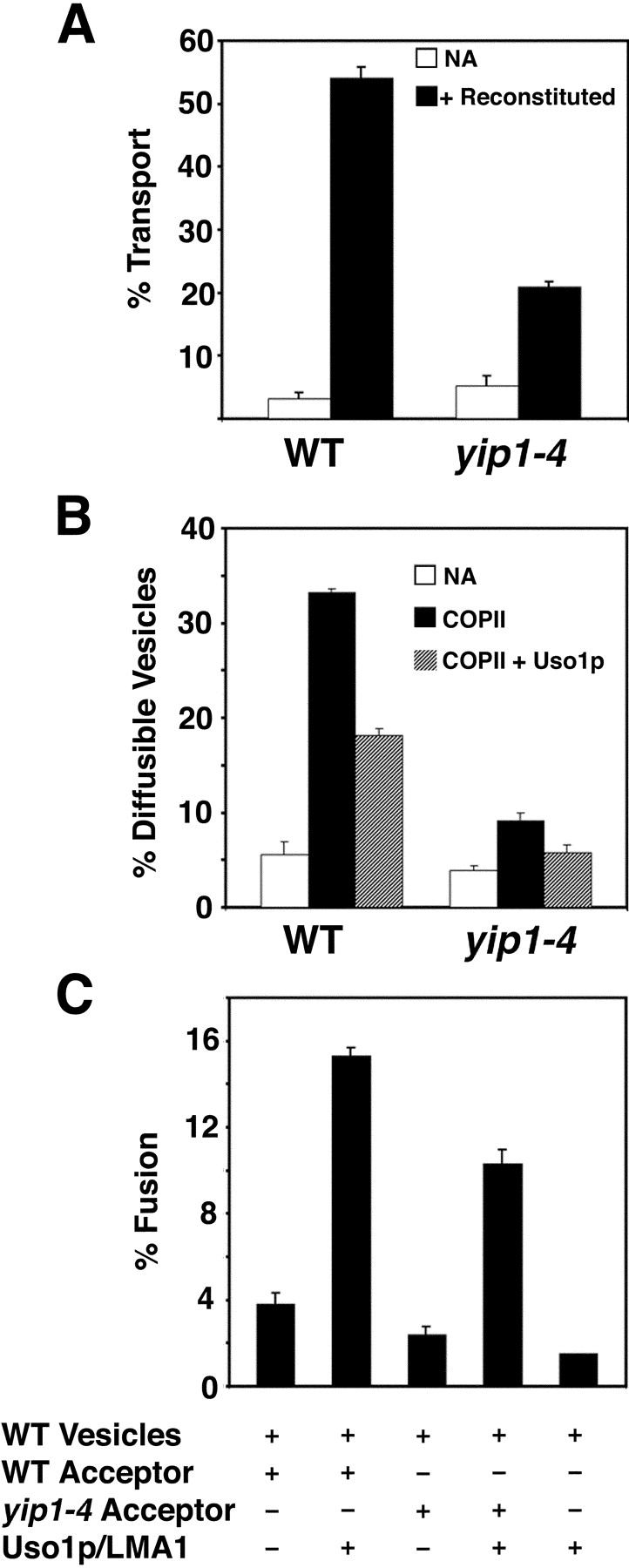
yip1-4 membranes display a defect in COPII vesicle budding in vitro. (A) Washed semi-intact cells containing [35S]gpαf were prepared from wild-type (RCY1768) and yip1-4 (RCY1764) strains. Semi-intact cells were incubated with COPII proteins, Uso1p, LMA1, and an ATP regeneration system. After 75 min at 23°C, the amount of Golgi-modified [35S]gpαf was measured to determine transport efficiency. (B) Semi-intact cells from wild-type and mutant strains were prepared as in A and incubated with COPII or COPII plus Uso1p to measure vesicle budding and tethering. (C) COPII vesicles containing [35S]gpαf were synthesized from wild-type microsomes and purified on density gradients. Purified vesicles were mixed with wild-type or yip1-4 semi-intact cell acceptor membranes in second-stage assays in the presence or absence of Uso1p and LMA1. After 75 min at 23°C, the amount of Golgi-modified [35S]gpαf was measured to determine transport efficiency.
Given that the yip1-4 strain exhibited a budding defect even at the permissive temperature of 23°C, we were concerned that this defect could be an indirect consequence of this point mutation on both ER and Golgi membranes. To further monitor the integrity of Golgi membranes, we also determined the acceptor activity of yip1-4 membranes when supplied with wild-type vesicles. Vesicles containing [35S]gpαf were generated from wild-type microsomes and purified on density gradients (Barlowe, 1997). The vesicles were then added to wild-type or yip1-4 acceptor membranes in the presence or absence of purified fusion factors. As shown in Fig. 7 C, the combination of wild-type vesicles, wild-type acceptor membranes, and fusion factors produced a fusion efficiency of ∼15% (column 2). As shown in column 4, the combination of wild-type vesicles, yip1-4 acceptor membranes, and fusion factors produced a comparable amount of fusion (∼10%), indicating that the mutant cells retained functional acceptor activity. These results suggest the Golgi complex in the yip1-4 strain is functionally competent for tethering and fusion in vitro. Based on these findings, we conclude that the transport defect in the yip1-4 strain is most specific to the budding stage, and that the membranes of the early secretory pathway are not generally compromised.
The yip1-4 cells were also examined for their ability to package proteins other than [35S]gpαf into COPII vesicles. COPII vesicles were generated from equivalent amounts of wild-type or yip1-4 semi-intact cells and analyzed by immunoblot. As shown in Fig. 8, wild-type membranes efficiently packaged Sec22p, Erv25p, Erv46p, Yif1p, and Yip1p into vesicles in a COPII-dependent fashion. In contrast, yip1-4 membranes packaged these vesicle proteins at significantly lower efficiencies upon reconstitution of COPII budding. This result mirrors the decrease observed in packaging of [35S]gpαf, indicating an overall defect in COPII vesicle biogenesis. In summary, the in vitro budding defects caused by the yip1-4 mutation are in accord with the phenotypes observed by microscopic inspection of the yip1-4 strain and with our observation that anti-Yip1p antibodies block the production of COPII vesicles in vitro.
Figure 8.
COPII-dependent budding of vesicle proteins is reduced in the yip1-4 strain. COPII-budding reactions in wild-type (RCY1768) or yip1-4 (RCY1764) semi-intact cells. One tenth of a total reaction (T), budded vesicles isolated after incubation with COPII proteins (+), or a mock reaction without COPII proteins (−) were separated on a 12.5% polyacrylamide gel. Sec61p (ER resident protein) and Sec22p, Erv25p, Erv46p, Yif1p, and Yip1p (vesicle proteins) were detected by immunoblot.
Genetic interaction analysis of yip1-4
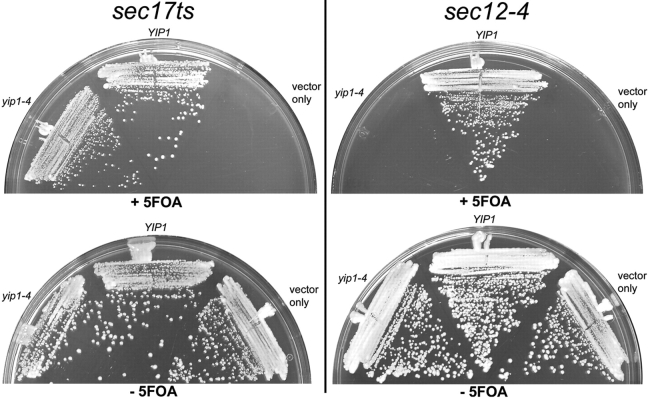
The growth phenotype of strains that combine two distinct mutations can indicate a functional connection between two genes. To test if the yip1-4 allele influences the growth properties of other ER/Golgi transport mutants, specific mutants were combined with yip1-4. The double mutants were constructed in haploid strains in which viability was ensured by expression of YIP1 from an extrachromosomal URA3-linked plasmid. In these strains, loss of the YIP1-URA3 plasmid can be scored by growth on media containing 5-fluorouracil (5-FOA) and demonstrates viability of the double mutant. As examples of this approach, no growth of the yip1-4 sec12-4 strain was observed on 5-FOA plates, whereas viable yip1-4 sec17ts colonies were observed (Fig. 9). This result indicates that yip1-4 displays a synthetic lethal relationship with the budding mutant sec12-4. Several other trafficking mutants that act in specific stages of transport between the ER and Golgi complex have been characterized. We constructed pair-wise combinations of these mutants with yip1-4 and tested for growth on 5-FOA media (Table I). Interestingly, we observed synthetic lethal relationships between yip1-4 and mutants involved in COPII budding from the ER, including sec12-4, sec13-1, and sec23-1. The only other lethal combinations observed were with the sec21-1 and uso1-1 mutations, which act in COPI- and COPII-dependent transport pathways, respectively (Hosobuchi et al., 1992; Cao et al., 1998). A variety of other ER/Golgi mutants did not display synthetic lethality when combined with yip1-4, indicating specificity in the interactions detected. These genetic tests provide further evidence for Yip1p function in vesicle budding from the ER and are consistent with our biochemical and morphological experiments.
Figure 9.
Genetic interaction analysis of yip1-4. Strains carrying a thermosensitive mutation in genes involved in ER/Golgi transport were combined with a yip1Δ allele together with a wild-type YIP1 on a URA3 plasmid. Strains also contained vector only, wild-type YIP1 LEU2 vector, or a yip1-4 LEU vector. All transformants were assessed by plasmid shuffling on 5-fluorouracil at 25°C. Plates containing yip1-4 sec17ts double mutants are shown as an example where no genetic interaction was observed. In contrast, yip1-4 sec12-4 double mutants are shown as an example of complete lethality between two mutants. A summary of genetic interaction results is shown in Table I.
Table I. Summary of genetic interactions.
| Allele | Viability at 25°C in combination with yip1-4 |
|---|---|
| sec12-4 | − |
| sec13-1 | − |
| sec23-1 | − |
| ypt1-3 | +/− |
| sec16-2 | + |
| ret1-1 | + |
| sec20-1 | + |
| sec21-1 | − |
| sec17ts | + |
| sec18-1 | + |
| sec22ts | + |
| bos1-1 | + |
| sed5ts | + |
| bet3-1 | + |
| uso1-1 | − |
| sec34-2 | + |
| sec35-1 | + |
| bet1-1 | + |
Preincubation of membranes with COPII results in a decrease in sensitivity to anti-Yip1p antibodies
Our experiments establish a role for Yip1p in vesicle budding from the ER. To further refine the stage at which Yip1p functions during the process of COPII vesicle biogenesis, we attempted to order the temporal requirements for the COPII proteins and Yip1p activity. Wild-type ER membranes containing [35S]gpαf were preincubated with either COPII or anti-Yip1p antibodies for 5 min at 15°C. In a second incubation, factors were then added to each condition, and the reaction proceeded for an additional 25 min before vesicle budding was quantified. As shown in Fig. 10 A, preincubation of membranes with COPII proteins followed by buffer produced a vesicle budding efficiency of ∼23%. Simultaneous addition of COPII proteins and anti-Yip1p antibodies reduced the budding efficiency to ∼5%. Similarly, preincubation of membranes with anti-Yip1p antibodies for 5 min followed by the addition of COPII proteins inhibited vesicle budding. Notably, preincubation of membranes with COPII followed by the addition of anti-Yip1p antibodies produced a budding efficiency of ∼22%, very similar to the control COPII reaction. One explanation for this result may be that a majority of vesicle budding occurred during the 5-min COPII preincubation. In this way, second-stage addition of the anti-Yip1p antibodies would have little or no effect. To address this issue, we measured COPII budding after a 5-min incubation at 15°C and found that the level of budding only reached ∼8% during the COPII preincubation stage (Fig. 10 A). It should also be noted that preincubation of membranes with COPII proteins on ice did not produce resistance to anti-Yip1p antibodies. Therefore, we conclude that membranes preincubated with COPII proteins at elevated temperatures become insensitive to anti-Yip1p antibodies. These observations suggest that Yip1p is required early in the budding reaction.
Figure 10.
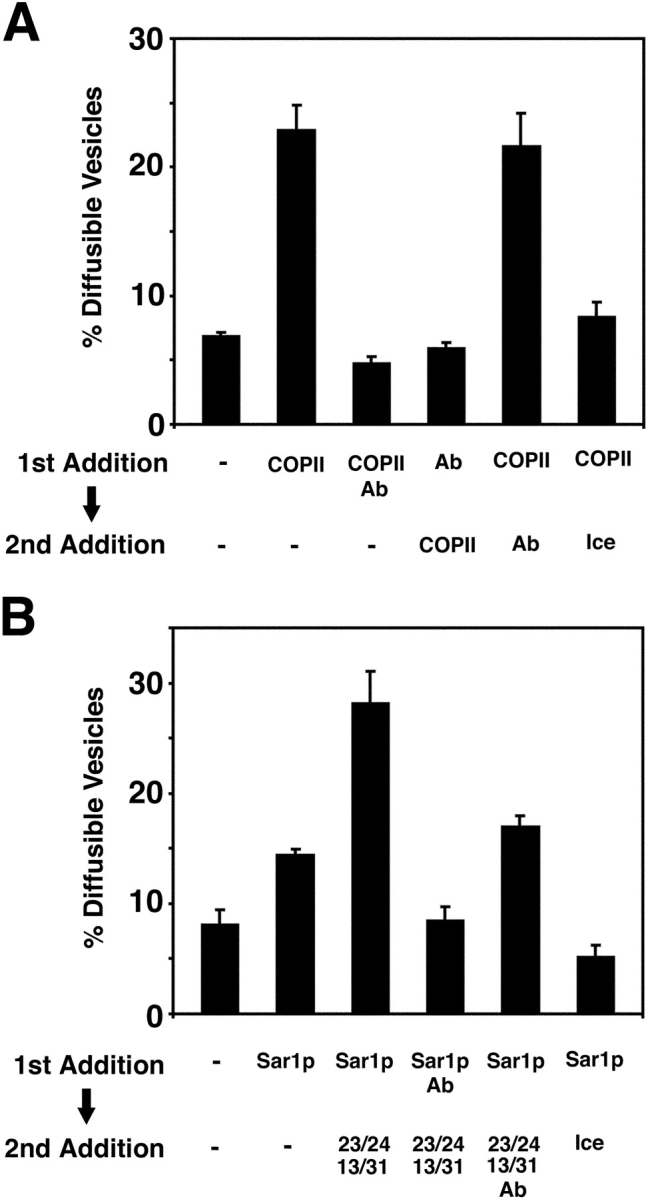
Preincubation of donor membranes with COPII produces a decrease in sensitivity to anti-Yip1p antibodies. (A) Washed semi-intact cells containing [35S]gpαf were prepared from a wild-type strain (FY834) and treated with either COPII or anti-Yip1p antibodies (40 μg/ml) for 5 min at 15°C. Secondary factors were then added as indicated, and the reactions proceeded for an additional 25 min. Freely diffusible vesicles containing [35S]gpαf were then separated from semi-intact cell membranes by centrifugation and quantified by Con A precipitation. (B) Semi-intact cells prepared as in A were treated with Sar1p or anti-Yip1p antibodies alone (40 μg/ml) for 5 min at 15°C. Secondary factors were then added and diffusible vesicles were quantified as in A.
Next, we investigated the possibility that an individual COPII component was responsible for producing the decrease in sensitivity to anti-Yip1p antibodies during the preincubation step. Given the role of Sar1p in initiating coat assembly, we tested Sar1p as a candidate for this activity. Wild-type ER membranes containing [35S]gpαf were preincubated with Sar1p or anti-Yip1p antibodies for 5 min at 15°C. In a second incubation, factors were added to each condition, and then budding reactions continued for 25 min. As shown in Fig. 10 B, preincubation of membranes with Sar1p alone followed by addition of buffer produced a budding efficiency of ∼14%. Preincubation of membranes with Sar1p followed by the secondary addition of Sec23/24p and Sec13/31p stimulated budding to an efficiency of ∼28%. In contrast, preincubation of membranes with Sar1p and anti-Yip1p antibodies followed by secondary addition of Sec23/24p and Sec13/31p produced a budding efficiency of ∼8%. Interestingly, preincubation of membranes with Sar1p followed by addition of Sec23/24p, Sec13/31p, and anti-Yip1p antibodies yielded a budding efficiency of ∼17%, which is intermediate when compared with the secondary addition of Sec23/24p and Sec13/31p alone. We also measured the amount of budding during the 5-min Sar1p preincubation stage and observed an ∼5% budding efficiency. Therefore, the amount of vesicle budding that occurred during the Sar1p preincubation step cannot account for the fraction of vesicle budding that becomes insensitive to anti-Yip1p antibodies during the second stage. These results indicate that preincubation of membranes with Sar1p alone decreases sensitivity to anti-Yip1p antibodies; however, preincubation with all of the COPII components provides greater resistance.
Discussion
The Yip1 family of proteins is widespread in nature and has been implicated in Rab/Ypt function (Yang et al., 1998; Matern et al., 2000; Calero and Collins, 2002; Calero et al., 2002). Although these reports indicate that Yip1p and Yif1p are essential for transport through the early secretory pathway, their mechanisms of action remain to be elucidated. We had encountered the Yip1p and Yif1p proteins as constituents of ER-derived transport vesicles in experiments to identify proteins involved in ER/Golgi transport (Otte et al., 2001). Given that these proteins appear to cycle between the ER and Golgi compartments as well as interact with Ypt1p, we hypothesized that Yip1p would participate in a vesicle tethering and/or membrane fusion stage. Surprisingly, our experiments indicate that Yip1p function is required for biogenesis of COPII-derived vesicles. Antibodies directed against the amino-terminal cytosolic domain of Yip1p potently inhibited budding of COPII vesicles in assays that followed packaging of the soluble secretory cargo gpαf and other integral membrane vesicle proteins. Vesicle tethering and fusion were not significantly affected by the presence of anti-Yip1p antibodies.
Genetic analyses provided an independent line of evidence for Yip1p function in vesicle budding. The thermosensitive yip1-4 allele caused an accumulation of ER membranes with no apparent accumulation of 50–60-nm transport vesicle intermediates. When the yip1-4 mutation was combined with the vesicle accumulating sec18-1 mutation, the double mutant accumulated ER structures when shifted to restrictive temperatures. This result demonstrates a requirement for YIP1 in the production of vesicles that accumulate in sec18-1 cells. Furthermore, pair-wise combinations of yip1-4 with other ER/Golgi transport mutants revealed specific interactions with genes (SEC12, SEC13, and SEC23) involved in formation of COPII vesicles. Mutations that influence COPI vesicle biogenesis (SEC21) and Golgi tethering (USO1) also displayed synthetic lethal relationships with yip1-4, revealing potential roles for Yip1p in Golgi structure or function. Finally, in vitro analysis of the yip1-4 allele revealed a significant defect in COPII-dependent vesicle budding, a result that is in good accord with the antibody inhibition analysis. Based on these observations, we propose that Yip1p acts in vesicle biogenesis and may be required for the assembly of coat structures, or possibly in the scission of coated vesicles from the ER.
Our findings are unexpected, given that much of the current data regarding Yip1p suggest a role for this protein in regulating aspects of Rab GTPase function. Indeed, Yip1p was originally identified as a Ypt1p- and Ypt31p-binding protein using yeast two-hybrid screens (Yang et al., 1998). Subsequent reports identified Yif1p as a Yip1p-binding partner, and it has been proposed that a Golgi-localized Yif1p–Yip1p complex acts to bind Ypt1p and Ypt31p to facilitate vesicle docking and fusion (Matern et al., 2000). More recent data demonstrate the presence of an extended family of Yip1-related proteins that form mixed heteromeric complexes with one another (Calero et al., 2002). Yip1p and the Yip1-related proteins interact with multiple Rabs and in a manner that depends on carboxy-terminal prenylation of the Rab protein. Based on these observations, the Yip1 family of proteins has been proposed to act in the pathway by which Rab proteins are recruited to membrane compartments (Calero et al., 2002). Analyses on the mammalian homologue of yeast Yip1p have been undertaken as well. The mammalian protein Yip1A shares 31% identity with yeast Yip1p and functionally complements the loss of yeast YIP1 (Calero et al., 2003), indicating a functional conservation of mechanism. Interestingly, Yip1A localizes to vesicular structures composing ER export sites, and interacts with the Sec23/24 subunit of the mammalian COPII complex (Tang et al., 2001). The authors of this report suggest that Yip1A is involved in the regulation of ER/Golgi traffic at the level of ER exit sites. This conclusion is consistent with our findings that inhibitors of yeast Yip1p function block COPII-dependent budding from the ER and provides support for the hypothesis that Yip1p acts in COPII vesicle biogenesis.
If Yip1p functions in the process of vesicle budding, how are Yip1p interactions with Rab GTPases integrated into this model? Rab proteins have been traditionally thought to act after vesicle formation to regulate the subsequent targeting and fusion of vesicles to their acceptor membranes (Segev, 2001). However, recent evidence has also suggested a role for Rab proteins in the process of vesicle formation. For example, experiments in mammalian cells indicate that Rab1 acts during budding to program COPII vesicles for docking and fusion competency. Here, Rab1 activity was proposed to recruit the vesicle tethering factor p115 into COPII vesicles to promote targeting to the Golgi apparatus (Allan et al., 2000). Other reports implicate Rab9 in coordinating cargo selection with vesicle formation. Specifically, the authors of this paper suggest that activated Rab9 binds directly to the protein TIP47, which in turn facilitates the recruitment of the mannose-6-phosphate receptor into a forming vesicle (Carroll et al., 2001). Taking these observations into account when considering Yip1p function, a model can be envisioned in which Yip1p acts to recruit Rabs/Ypts into forming transport vesicles. In this way, vesicle biogenesis would be coupled to the incorporation of cargos necessary for the subsequent docking and fusion of the vesicle. If the ability of Yip1p to recruit Rabs into forming vesicles is inhibited, this might then cause overall vesicle formation to become blocked as well. However, it should be noted that other vesicle proteins required for subsequent fusion, such as the SNARE proteins Sec22p, Bos1p, Bet1p, rbet1, and membrin, can be efficiently depleted from forming COPII vesicles, yet vesicle formation is not compromised (Allan et al., 2000; Liu and Barlowe, 2002; Miller et al., 2002).
Although some of the data support a role for Rab proteins in vesicle formation, additional reports suggest that certain Rabs are not required for budding, but are required for the docking and fusion of transport vesicles. For example, sec4 and ypt1 thermosensitive mutant strains accumulate transport vesicles at nonpermissive temperatures (Novick et al., 1980; Becker et al., 1991). These observations indicate that Sec4p and Ypt1p are not required for the formation of vesicles that accumulate, but instead act to fuse vesicles to the correct target membrane. In vitro experiments also demonstrate that inhibition of Ypt1p activity does not block formation of COPII vesicles, but inhibits post-budding stages of transport to the Golgi (Rexach and Schekman, 1991; Segev, 1991; Cao and Barlowe, 2000). Together, these results argue against a requirement for Ypt1p in COPII vesicle formation and suggest Yip1p does not rely on Ypt1p in this stage of transport.
During the course of our investigation, a similar analysis of Yip1p and Yif1p function in ER/Golgi transport was reported (Barrowman et al., 2003). This report showed that antibodies directed against Yip1p or Yif1p blocked transport to the Golgi complex, but only reduced budding efficiencies by one half. Interestingly, the vesicles formed in the presence of their inhibitory antibodies failed to fuse with Golgi membranes. In agreement with our findings, their anti-Yip1p antibodies did not inhibit vesicle tethering or fusion when added after vesicle production. The authors concluded that the Yip1p–Yif1p complex is required during vesicle formation to produce fusion competent vesicles (Barrowman et al., 2003). Although we cannot easily explain the differential effects of anti-Yip1p antibodies on the level of vesicle budding, both reports indicate a role for Yip1p function during COPII vesicle biogenesis. Additional experiments will be needed to clarify the role of Yip1p in post-budding transport stages.
Barrowman and colleagues also reported that depletion of cellular Yip1p did not affect membrane binding or localization of Ypt1p (Barrowman et al., 2003). Similarly, we have observed that thermosensitive mutations in Yip1p and Yif1p as well as antibodies directed against Yip1p did not inhibit Ypt1p membrane association in vitro (unpublished data). Although it is possible that other Yip family members may drive membrane association of Ypt1p in the absence of Yip1p and Yif1p, it may be useful to consider a model for Yip1p function in budding that is independent of Ypt1p or Rab protein activity. It is also possible that Yip1p serves as a regulatory checkpoint in vesicle budding to ensure that ER-derived vesicles can ultimately interact with Rab GTPases. In such a model, the Rab protein, per se, may not be required for vesicle budding, but a Rab-binding activity would be required.
The production of COPII-coated vesicles has been extensively studied in reconstituted budding reactions that use purified COPII proteins and ER microsomes or defined synthetic liposomes as the source of donor membrane (Matsuoka et al., 1998). In contrast to microsomes, budding from synthetic liposomes requires addition of nonhydrolyzable analogs of GTP to prolong the Sar1p-GTP state until COPII coats assemble. In addition, synthetic liposomes with an acidic phospholipid content that approximates the composition of ER membranes must be used in the minimal budding reaction. Given these distinct requirements, Yip1p could serve a role in stabilizing Sar1p-GTP during vesicle formation from microsomal membranes, or it could influence local lipid concentrations at transitional ER sites. Further investigations are required to fully define the mechanism of Yip1p in COPII-dependent budding from the ER. One approach may be to accumulate arrested budding intermediates in the presence of anti-Yip1p antibodies. Such intermediates could then be characterized biochemically and morphologically to provide further insights into Yip1p function.
Materials and methods
Yeast strains and media
Yeast strains used in this report are listed in Table II. Unless noted otherwise, cultures were grown at 25°C (for mutant strains) or 30°C (for wild-type strains) in rich yeast extract, peptone, dextrose medium (1% Bacto-yeast extract, 2% Bacto-peptone, and 2% dextrose). Standard yeast (Sherman, 1991) and cloning protocols (Ausubel et al., 1987) were used.
Table II. Yeast strains used in this paper.
| Strain | Genotype | Source |
|---|---|---|
| FY834 | MATα his3Δ200 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63 | Winston et al. (1995) |
| RCY927 | MATa sec21-1 ura3-52 leu2-3,112 | This paper |
| RCY1610 | MATα YIP1ΔKANR ura3-52 leu2-3,112 [URA3 CEN YIP1] | This paper |
| RCY1612 | MATa YIP1ΔKANR ura3-52 leu2-3,112 [URA3 CEN YIP1] | This paper |
| RCY1633 | MATa YIP1ΔKANR ura3-52 leu2-3,112 hisΔ200 [URA3 CEN YIP1 GDI1] | This paper |
| RCY1634 | MATα YIP1ΔKANR ura3-52 leu2-3,112 hisΔ200 [URA3 CEN YIP1 GDI1] | This paper |
| RCY1764 | MATa ura3-52 leu2-3,112 YIP1ΔKANR [pRS315 yip1-4] | Calero et al. (2003) |
| RCY1768 | MATa ura3-52 leu2-3,112 YIP1ΔKANR [pRS315 YIP1] | Calero et al. (2003) |
| RCY2057 | MATa YIP1ΔKANR ura3-52 leu2-3,112 hisΔ200 lys2-801 [URA3 CEN YIP1] | This paper |
| RCY2141 | MATα sec12-4 YIP1ΔKANR ura3-52 leu2-3,112 [URA3 CEN YIP1] | This paper |
| RCY2143 | MATα sec18-1 YIP1ΔKANR ura3-52 leu2-3,112 [URA3 CEN YIP1] | This paper |
| RCY2146 | MATα sec21-1 YIP1ΔKANR ura3-52 leu2-3,112 hisΔ200 [URA3 CEN YIP1 GDI1] | This paper |
| RCY2153 | MATa sec13-1 YIP1ΔKANR ura3-52 leu2-3,112 [URA3 CEN YIP1] | This paper |
| RCY2226 | MATa sec17ts YIP1ΔKANR ura3-52 leu2-3,112 lys2 [URA3 CEN YIP1 GDI1] | This paper |
| RCY2232 | MATa sec22ts YIP1ΔKANR ura3-52 leu2-3,112 [URA3 CEN YIP1 GDI1] | This paper |
| RCY2234 | MATa sec16-2 YIP1ΔKANR ura3-52 leu2-3,112 lys2-801 his [URA3 CEN YIP1 GDI1] | This paper |
| RCY2237 | MATa sec23-1 YIP1ΔKANR ura3-52 leu2-3,112 his [URA3 CEN YIP1 GDI1] | This paper |
| RCY2239 | MATα ypt1-3 YIP1ΔKANR ura3-52 leu2-3,112 hisΔ200 [URA3 CEN YIP1 GDI1] | This paper |
| RCY2258 | MATα sec18-1 YIP1ΔKANR ura3-52 leu2-3,112 [LEU2 CEN YIP1] | This paper |
| RCY2259 | MATα sec18-1 YIP1ΔKANR ura3-52 leu2-3,112 [LEU2 CEN yip1-4] | This paper |
| RCY2303 | MATα sec20-1 YIP1ΔKANR ura3-52 leu2-3,112 [URA3 CEN YIP1 GDI1] | This paper |
| RCY2318 | MATa bos1-1 YIP1ΔKANR ura3-52 leu2-3,112 hisΔ200 [URA3 CEN YIP1 GDI1] | This paper |
| RCY2321 | MATα bet3-1 YIP1ΔKANR ura3-52 leu2-3,112 his [URA3 CEN YIP1 GDI1] | This paper |
| RCY2325 | MATa sec17ts YIP1ΔKANR ura3-52 leu2-3,112 lys2 [LEU2 CEN YIP1] | This paper |
| RCY2326 | MATa sec17ts YIP1ΔKANR ura3-52 leu2-3,112 lys2 [LEU2 CEN yip1-4] | This paper |
| RCY2384 | MATα sed5ts YIP1ΔKANR ura3-52 leu2-3,112 his [URA3 CEN YIP1 GDI1] | This paper |
| RCY2386 | MATa ret1-1 YIP1ΔKANR ura3-52 leu2-3,112 trp1 [URA3 CEN YIP1 GDI1] | This paper |
| RCY2389 | MATa uso1-1 YIP1ΔKANR ura3 leu2 hisΔ200 lys2 [URA3 CEN YIP1 GDI1] | This paper |
| RCY2474 | MATα bet1-1 YIP1ΔKANR ura3 leu2 hisΔ200 [URA3 CEN YIP1 GDI1] | This paper |
| RCY2390 | MATα sec35-1 YIP1ΔKANR ura3 leu2 hisΔ200 lys2 [URA3 CEN YIP1 GDI1] | This paper |
| RCY2400 | MATa sec34-2 YIP1ΔKANR ura3 leu2 hisΔ200 lys2 [URA3 CEN YIP1 GDI1] | This paper |
GDI1, GDP dissociation inhibitor 1. Note that the presence of GDI1 is not relevant to these experiments.
Antibodies and immunoblotting
Antibodies against α1,6-mannose linkages (Cao et al., 1998), Sec12p, Sec61p, Erv25p, Erv41p, Erv46p, Och1p (Otte et al., 2001), Yif1p (Matern et al., 2000), and Sec22p (Liu and Barlowe, 2002) have been described previously. pAbs were raised against a GST-Yip1p (amino-terminal 1–99 aa) fusion protein expressed from plasmid pGEX-2T-YIP1 in Escherichia coli. The fusion protein was purified according to the manufacturer's specifications (Amersham Biosciences) and was used to immunize rabbits by standard procedures (Covance). Polyclonal anti-Yip1p antibodies were affinity purified on an Affi-Gel 15 column with maltose-binding protein–Yip1p fusion (MBP-Yip1p) coupled as described by the manufacturer (Bio-Rad Laboratories). MBP-Yip1p (amino-terminal 1–99 aa) was generated using the expression vector pMAL-c2x (New England Biolabs, Inc.). The fusion protein was purified according to the manufacturer's specifications. Affinity-purified anti-Yip1p Fab fragments and preimmune IgGs were prepared as described previously (Harlow and Lane, 1988). In membrane fractionation experiments, ER (P13) and Golgi (P100) fractions were prepared as described previously (Belden and Barlowe, 2001a), except that pellets were resuspended in 0.15 ml SDS-PAGE sample buffer.
In vitro vesicle budding, tethering, and transport assays
Yeast semi-intact cells from wild-type and mutant strains were prepared as described previously (Baker et al., 1988). Vesicle budding, tethering, and fusion assays following [35S]gpαf were published previously (Barlowe, 1997; Cao et al., 1998). For two-stage fusion assays with wild-type and yip1-4 acceptor membranes, wild-type vesicles containing [35S]gpαf were first isolated from density gradients (Barlowe, 1997). Vesicles were then added to acceptor membranes to measure fusion (Cao and Barlowe, 2000). Experiments to assay packaging of proteins into vesicles by Western blot were performed as described previously (Liu and Barlowe, 2002) using semi-intact cell membranes. For ordering experiments, wild-type semi-intact cells containing translocated [35S]gpαf were first incubated with either COPII proteins or anti-Yip1p antibodies (40 μg/ml) for 5 min at 15°C. Secondary factors were then added, and cells were incubated at 23°C for an additional 25 min. Reactions were then processed to measure the level of freely diffusible vesicles (Cao et al., 1998). For in vitro assays, data points are the average of duplicate determinations and the error bars represent the range.
Microscopy
For EM experiments, cells were grown overnight to a final cell density of 0.4–0.7 A600. After shift to the restrictive temperature, cells were washed once with buffer (0.1 M Pipes, 0.1 M sorbitol, and 50 mM KPi, pH 7.3) and then fixed with fixative (2% glutaraldehyde, 2% PFA, 0.1 M Pipes, pH 6.8, 0.1 M sorbitol, 1 mM MgCl2, 1 mM CaCl2, and 10 μM CuCl2) for 1 h at RT and then overnight at 4°C. The cell walls were removed by treatment with 0.2 mg/ml zymolyase 100T in KPi buffer, pH 7.3 (1 ml/5 OD unit cells). An aliquot of the cells was collected in a microfuge tube and the pellet was incubated with 2% OsO4 for 1 h followed by incubation with 1% uranyl acetate (aqueous) at 4°C for 30 min. The pellets were dehydrated with sequential ethanol washes and incubated with 50% ethanol/50% SPURR resin (Electron Microscopy Sciences), then changed to 100% SPURR, and the sample was transferred to beem capsules (Electron Microscopy Sciences) and baked at 70°C for at least 24 h. Thin sections were cut onto 3-mm-diam 75/300-type mesh copper specimen grids (Veco), contrasted with lead citrate and uranyl acetate, and examined in an electron microscope (model 201; Philips) at 80 kV.
For fluorescence microscopy, the GFP-KDEL construct was generated in plasmid pRS316 (Sikorski and Hieter, 1989) using standard molecular biology techniques. In brief, the construct comprises 363 bp of the KAR2 promoter with 45 amino acids of the Kar2p presequence fused to a linker (GGPGG) followed by yeast-enhanced GFP (yEGFP; Cormack et al., 1997), which in turn is followed by second linker (GGPGG) and the sequence HDEL. The ADH1 3′ region (572 bp) was added after the stop codon to provide transcription termination. The GFP amino-terminal fusions of GOS1, SED5, and SFT2 were also constructed in plasmid pRS316. Each construct contains 238 amino acids of yEGFP fused to the start methionine of the tagged protein preceded by a linker (GGPGG). The fusions are driven by 452 bp of the YOP1 promoter and contain the endogenous gene terminator (441 bp, 378 bp, and 553 bp of the noncoding 3′ region for SED5, GOS1, and SFT2, respectively). GFP-YIP1 was constructed by inserting yEGFP after the initiator methionine in pRS315-YIP1, leaving the endogenous promoter intact. This construct (pRC693) was expressed at wild-type levels in haploid cells as the only source of YIP1. Cells containing GFP fusion plasmids were examined with a microscope (Eclipse E600; Nikon) equipped with a 60× objective and 2× optovar. A Spot-RT monochrome CCD camera (Diagnostic Instruments)with version 3.5 software was used for image capture. All images shown are representative images from cells during logarithmic phase growth in minimal media supplemented as necessary.
Acknowledgments
We thank Dieter Gallwitz (Max Planck Institute, Göttingen, Germany) for providing strains and antibodies used in the early stages of this work. We also thank A. Damon Ferguson for valuable assistance with EM and Susan Henry (Cornell University, Ithaca, NY) for providing strains.
This work was supported by grants from the American Heart Association (0030316T) and the National Science Foundation (MCB0079045) to R.S. Collins, and from the National Institutes of Health (GM52549) to C. Barlowe.
Abbreviations used in this paper: Erv, ER vesicle; gpαf, glycopro-α-factor; Yif1p, Yip1p-interacting factor; Yip1p, Ypt1p-interacting protein.
References
- Allan, B.A., B.D. Moyer, and W.E. Balch. 2000. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding of COPII vesicles for fusion. Science. 289:444–448. [DOI] [PubMed] [Google Scholar]
- Ausubel, R.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1987. Current Protocols in Molecular Biology. Greene Publishing Associates and Wiley-InterScience, New York. 3.0.1–3.14.3.
- Baker, D., L. Hicke, M. Rexach, M. Schleyer, and R. Schekman. 1988. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 54:335–344. [DOI] [PubMed] [Google Scholar]
- Barlowe, C. 1997. Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J. Cell Biol. 139:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe, C., L. Orci, T. Yeung, M. Hosobuchi, S. Hamamoto, N. Salama, M. Rexach, M. Ravazzola, M. Amherdt, and R. Schekman. 1994. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the ER. Cell. 77:895–907. [DOI] [PubMed] [Google Scholar]
- Barrowman, J., W. Wang, Y. Zhang, and S. Ferro-Novick. 2003. The Yip1p/Yif1p complex is required for the fusion competence of endoplasmic reticulum-derived vesicles. J. Biol. Chem. 278:19878–19884. [DOI] [PubMed] [Google Scholar]
- Becker, J., T.J. Tan, H.-H. Trepte, and D. Gallwitz. 1991. Mutational analysis of the putative effector domain of the GTP-binding Ypt1 protein in yeast suggests specific regulation by a novel GAP activity. EMBO J. 10:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden, W.J., and C. Barlowe. 2001. a. Deletion of yeast p24 genes activates the unfolded protein response. Mol. Biol. Cell. 12:957–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden, W.J., and C. Barlowe. 2001. b. Role of Erv29p in collecting soluble secretory proteins into ER-derived transport vesicles. Science. 294:1528–1531. [DOI] [PubMed] [Google Scholar]
- Calero, M., and R.N. Collins. 2002. Saccharomyces cerevisiae Pra1p/Yip3p interacts with Yip1p and Rab proteins. Biochem. Biophys. Res. Commun. 290:676–681. [DOI] [PubMed] [Google Scholar]
- Calero, M., N.J. Winand, and R.N. Collins. 2002. Identification of the novel proteins Yip4p and Yip5p as Rab GTPase interacting factors. FEBS Lett. 515:89–98. [DOI] [PubMed] [Google Scholar]
- Calero, M., C.Z. Chen, W. Zhu, N. Winand, K.A. Havas, P.M. Gilbert, C.G. Burd, and R.N. Collins. 2003. Dual prenylation is required for Rab protein localization and function. Mol. Biol. Cell. 14:1852–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X., and C. Barlowe. 2000. Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J. Cell Biol. 149:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X., N. Ballew, and C. Barlowe. 1998. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 17:2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, K.S., J. Hanna, I. Simon, J. Krise, P. Barbero, and S.R. Pfeffer. 2001. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science. 292:1373–1376. [DOI] [PubMed] [Google Scholar]
- Cormack, B.P., G. Bertram, M. Egerton, N.A. Gow, S. Falkow, and A.J. Brown. 1997. Yeast-enhanced green fluorescent protein (yEGFP) a reporter of gene expression in Candida albicans Microbiol. 143:303–311. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 312–319.
- Hosobuchi, M., T. Kreis, and R. Schekman. 1992. SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of the yeast coatomer. Nature. 360:603–605. [DOI] [PubMed] [Google Scholar]
- Kaiser, C., and R. Schekman. 1990. Distinct sets of SEC genes govern transport vesicle formation and fusion in the early secretory pathway. Cell. 61:723–733. [DOI] [PubMed] [Google Scholar]
- Liu, Y., and C. Barlowe. 2002. Analysis of Sec22p in endoplasmic reticulum/Golgi transport reveals cellular redundancy in SNARE protein function. Mol. Biol. Cell. 13:3314–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern, H., X. Yang, E. Andrulis, R. Sternglanz, H.-H. Trepte, and D. Gallwitz. 2000. A novel Golgi membrane protein is part of a GTPase-binding protein complex involved in vesicle targeting. EMBO J. 19:4485–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka, K., L. Orci, M. Amherdt, S.Y. Bednarek, S. Hamamoto, R. Schekman, and T. Yeung. 1998. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 93:263–275. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., J.G. Coe, M. Sogaard, B.V. Zemelman, C. Wimmer, W. Hong, and T.H. Sollner. 1998. Gos1p, a Saccharomyces SNARE protein involved in Golgi transport. FEBS Lett. 435:89–95. [DOI] [PubMed] [Google Scholar]
- Mellman, I., and G. Warren. 2000. The road taken: past and future foundations of membrane traffic. Cell. 100:99–112. [DOI] [PubMed] [Google Scholar]
- Miller, E., B. Antonny, S. Hamamoto, and R. Schekman. 2002. Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO J. 21:6105–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick, P., C. Field, and R. Schekman. 1980. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 21:205–215. [DOI] [PubMed] [Google Scholar]
- Otte, S., W.J. Belden, M. Heidtman, J. Liu, O.N. Jensen, and C. Barlowe. 2001. Erv41p and Erv46p: new components of COPII vesicles involved in transport between the ER and the Golgi complex. J. Cell Biol. 152:503–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, H.R.B. 2001. SNAREs and the specificity of membrane fusion. Trends Cell Biol. 11:99–101. [DOI] [PubMed] [Google Scholar]
- Rexach, M.F., and R. Schekman. 1991. Distinct biochemical requirements for the budding, targeting, and fusion of ER-derived transport vesicles. J. Cell Biol. 114:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach, M.F., M. Latterich, and R.W. Schekman. 1994. Characteristics of endoplasmic reticulum-derived transport vesicles. J. Cell Biol. 126:1133–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder, S., F. Schimmoler, B. Singer-Kruger, and H. Riezman. 1995. The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1-1 mutation in α-COP. J. Cell Biol. 131:895–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev, N. 1991. Mediation of the attachment or fusion step in vesicular transport by the GTP-binding Ypt1 protein. Science. 252:1553–1556. [DOI] [PubMed] [Google Scholar]
- Segev, N. 2001. Ypt and Rab GTPases: insight into functions through novel interactions. Curr. Opin. Cell Biol. 13:500–511. [DOI] [PubMed] [Google Scholar]
- Segev, N., J. Mulholland, and D. Botstein. 1988. The yeast GTP-binding Ypt1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 52:915–924. [DOI] [PubMed] [Google Scholar]
- Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3–20. [DOI] [PubMed] [Google Scholar]
- Sikorski, R.S., and P.A. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 122:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, B.L., Y.S. Ong, B. Huang, S. Wei, E.S. Wong, R. Qi, H. Horstmann, and W. Hong. 2001. A membrane protein enriched in endoplasmic reticulum exit sites interacts with COPII. J. Biol. Chem. 276:40008–40017. [DOI] [PubMed] [Google Scholar]
- Tsui, M.M.K., W.C.S. Tai, and D.K. Banfield. 2001. Selective formation of Sed5p-containing SNARE complexes is mediated by combinatorial binding interactions. Mol. Biol. Cell. 12:521–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, T.H., R.S. Polishchuk, S. Caplan, K. Hirschberg, and J. Lippincott-Schwartz. 2001. Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 155:557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, D.W., C.A. Wilcox, G.C. Flynn, E. Chen, W. Kuang, W.J. Henzel, M.R. Block, A. Ullrich, and J.E. Rothman. 1989. A fusion protein is required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 339:355–359. [DOI] [PubMed] [Google Scholar]
- Winston, F., C. Dollard, and L.L. Ricupero-Hovasse. 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast. 11:53–55. [DOI] [PubMed] [Google Scholar]
- Wooding, S., and H.R.B. Pelham. 1998. The dynamics of Golgi protein traffic visualized in living yeast cells. Mol. Biol. Cell. 9:2667–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, R., M. Basson, L. D'Ari, and J. Rine. 1988. Increased amounts of HMG-CoA reductase induce “karmellae”: a proliferation of stacked membrane pairs surrounding the yeast nucleus. J. Cell Biol. 107:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., H.T. Matern, and D. Gallwitz. 1998. Specific binding to a novel and essential Golgi membrane protein (Yip1p) functionally links the transport GTPases Ypt1p and Ypt31p. EMBO J. 17:4954–4963. [DOI] [PMC free article] [PubMed] [Google Scholar]