Abstract
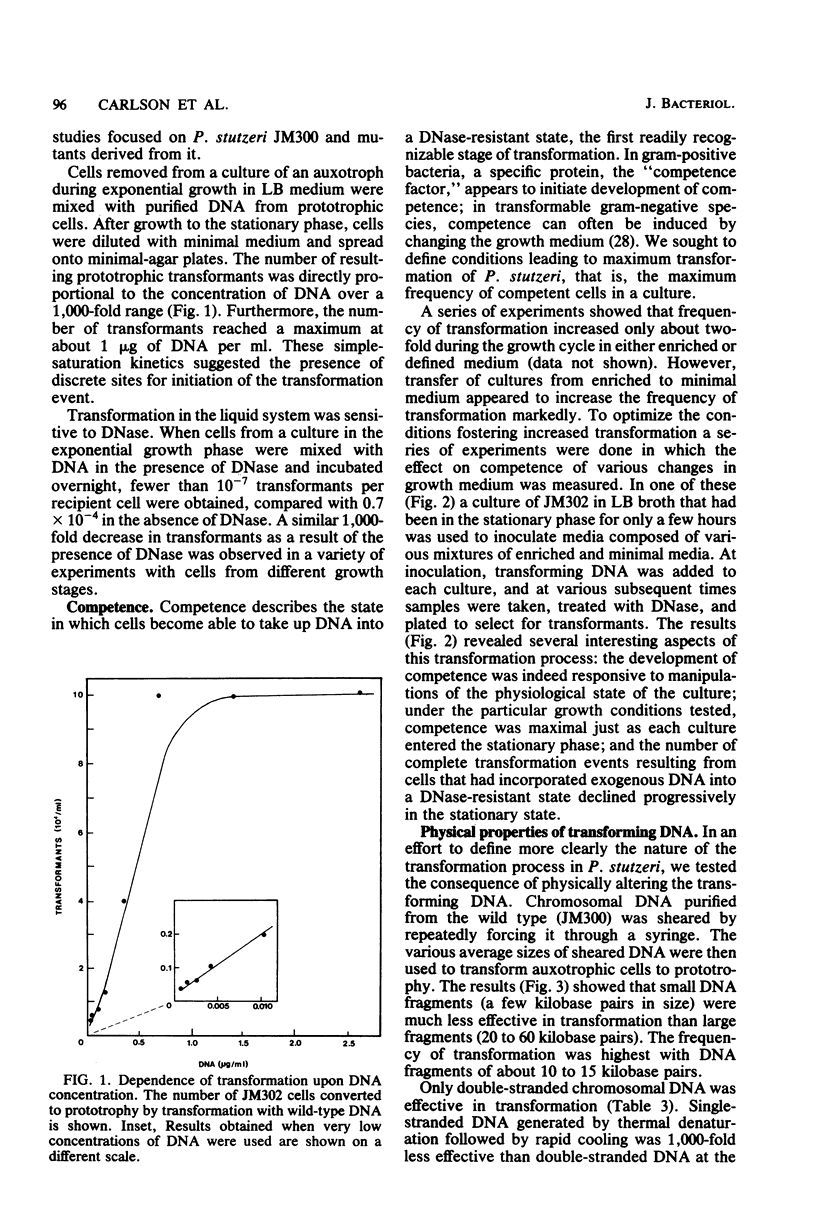
Cells of Pseudomonas stutzeri are naturally transformed by homologous chromosomal DNA; they do not require chemical treatment to become competent. This capacity to undergo natural transformation was found to be shared by the closely related species P. mendocina, P. alcaligenes, and P. pseudoalcaligenes, but was not detectable in strains of P. aeruginosa, P. perfectomarinus, P. putida, P. fluorescens, or P. syringae. P. stutzeri could be transformed either on plates or in liquid medium. Only double-stranded chromosomal DNA was effective; single-stranded DNA and plasmid DNA were not. DNA fragments larger than 10 kilobase pairs were more effective than smaller fragments. The transformation frequency was proportional to DNA concentration from 1 ng/ml to 1 microgram/ml; higher concentrations were saturating. The maximum frequency, about 10(-4) transformants per recipient cell, was obtained with cells from a culture in the early stationary growth phase. A variety of chromosomal mutations have been transformed, including mutations to auxotrophy and to antibiotic resistance. Other systems for genetic exchange in P. stutzeri have not yet been found; transformation offers a means for the genetic analysis of this metabolically versatile organism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Bukhari A. I. Analysis of bacteriophage mu and lambda-mu hybrid DNAs by specific endonucleases. J Mol Biol. 1975 Mar 15;92(4):529–540. doi: 10.1016/0022-2836(75)90307-1. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker R. J., Loutit J. S. The order of replication of chromosomal markers in Pseudomonas aeruginosa strain 1. I. Marker frequency analysis by transduction. Genet Res. 1974 Apr;23(2):145–153. doi: 10.1017/s0016672300014762. [DOI] [PubMed] [Google Scholar]
- Boucher C. A., Sequeira L. Evidence for the cotransfer of genetic markers in Pseudomonas solanacearum strain K60. Can J Microbiol. 1978 Jan;24(1):69–72. doi: 10.1139/m78-013. [DOI] [PubMed] [Google Scholar]
- Cohn W., Crawford I. P. Regulation of enzyme synthesis in the tryptophan pathway of Acinetobacter calcoaceticus. J Bacteriol. 1976 Jul;127(1):367–379. doi: 10.1128/jb.127.1.367-379.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. B., Smith H. O., Narang S. A. Construction of DNA recognition sites active in Haemophilus transformation. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2393–2397. doi: 10.1073/pnas.79.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet. 1976 Mar 30;144(3):243–251. doi: 10.1007/BF00341722. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Cresswell J. M., Hedges R. W., Coetzee J. N., Beringer J. E. Properties of plasmids constructed by the in vitro insertion of DNA from Rhizobium leguminosarum or Proteus mirabilis into RP4. Mol Gen Genet. 1976 Sep 23;147(3):315–323. doi: 10.1007/BF00582883. [DOI] [PubMed] [Google Scholar]
- Juni E., Heym G. A. Transformation assay for identification of psychrotrophic achromobacters. Appl Environ Microbiol. 1980 Dec;40(6):1106–1114. doi: 10.1128/aem.40.6.1106-1114.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E. Simple genetic transformation assay for rapid diagnosis of Moraxella osloensis. Appl Microbiol. 1974 Jan;27(1):16–24. doi: 10.1128/am.27.1.16-24.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY M. S., PRITCHARD R. H. UNSTABLE LINKAGE BETWEEN GENETIC MARKERS IN TRANSFORMATION. J Bacteriol. 1965 May;89:1314–1321. doi: 10.1128/jb.89.5.1314-1321.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. C., Sen S. P. Further observations on genetic transformation in pseudomonas. J Gen Microbiol. 1974 Aug;83(2):251–259. doi: 10.1099/00221287-83-2-251. [DOI] [PubMed] [Google Scholar]
- Khan N. C., Sen S. P. Genetic transformation in Pseudomonas. J Gen Microbiol. 1967 Nov;49(2):201–209. doi: 10.1099/00221287-49-2-201. [DOI] [PubMed] [Google Scholar]
- LAMBINA V. A., MIKHAILOVA T. N. KOLICHESTVENNYE ZAKONOMERNOSTI TRANSFORMATSII STREPTOMITSINOUSTO ICHIVOSTI U PSEUDOMONAS FLUORESCENS. Mikrobiologiia. 1964 Sep-Oct;33:800–806. [PubMed] [Google Scholar]
- Mandel M. Deoxyribonucleic acid base composition in the genus Pseudomonas. J Gen Microbiol. 1966 May;43(2):273–292. doi: 10.1099/00221287-43-2-273. [DOI] [PubMed] [Google Scholar]
- Palleroni N. J., Doudoroff M., Stanier R. Y., Solánes R. E., Mandel M. Taxonomy of the aerobic pseudomonads: the properties of the Pseudomonas stutzeri group. J Gen Microbiol. 1970 Feb;60(2):215–231. doi: 10.1099/00221287-60-2-215. [DOI] [PubMed] [Google Scholar]
- Sawula R. V., Crawford I. P. Mapping of the tryptophan genes of Acinetobacter calcoaceticus by transformation. J Bacteriol. 1972 Nov;112(2):797–805. doi: 10.1128/jb.112.2.797-805.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Danner D. B., Deich R. A. Genetic transformation. Annu Rev Biochem. 1981;50:41–68. doi: 10.1146/annurev.bi.50.070181.000353. [DOI] [PubMed] [Google Scholar]
- VAN NIEL C. B., ALLEN M. B. A note on Pseudomonas stutzeri. J Bacteriol. 1952 Sep;64(3):413–422. doi: 10.1128/jb.64.3.413-422.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. M., Holloway B. W. Suppressor mutations in Pseudomonas aeruginosa. J Bacteriol. 1976 Mar;125(3):780–786. doi: 10.1128/jb.125.3.780-786.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]



