Abstract
Phosphatidylinositol 3,5-bisphosphate (PtdIns[3,5]P2) was first identified as a nonabundant phospholipid whose levels increase in response to osmotic stress. In yeast, Fab1p catalyzes formation of PtdIns(3,5)P2 via phosphorylation of PtdIns(3)P. We have identified Vac14p, a novel vacuolar protein that regulates PtdIns(3,5)P2 synthesis by modulating Fab1p activity in both the absence and presence of osmotic stress. We find that PtdIns(3)P levels are also elevated in response to osmotic stress, yet, only the elevation of PtdIns(3,5)P2 levels are regulated by Vac14p. Under basal conditions the levels of PtdIns(3,5)P2 are 18–28-fold lower than the levels of PtdIns(3)P, PtdIns(4)P, and PtdIns(4,5)P2. After a 10 min exposure to hyperosmotic stress the levels of PtdIns(3,5)P2 rise 20-fold, bringing it to a cellular concentration that is similar to the other phosphoinositides. This suggests that PtdIns(3,5)P2 plays a major role in osmotic stress, perhaps via regulation of vacuolar volume. In fact, during hyperosmotic stress the vacuole morphology of wild-type cells changes dramatically, to smaller, more highly fragmented vacuoles, whereas mutants unable to synthesize PtdIns(3,5)P2 continue to maintain a single large vacuole. These findings demonstrate that Vac14p regulates the levels of PtdIns(3,5)P2 and provide insight into why PtdIns(3,5)P2 levels rise in response to osmotic stress.
Keywords: VAC14; FAB1; PtdIns(3,5)P2; vacuole; phosphatidylinositol
Introduction
Phosphatidylinositol polyphosphates function in diverse cellular events. This diversity is facilitated by the variety of sterically distinct compounds generated via phosphorylation of inositol at any or all of its six hydroxyls. These compounds are rapidly synthesized and degraded via kinases, phosphatases, and lipases, and thus are ideal regulatory molecules (De Camilli et al., 1996; Michell, 1997). Phosphatidylinositol polyphosphates regulate cell growth and differentiation (Joly et al., 1995; Toker and Cantley, 1997), cytoskeletal rearrangements (Lanier and Gertler, 2000), Ca2+ mobilization (Berridge, 1993), and membrane traffic (De Camilli et al., 1996; Roth, 1999; Odorizzi et al., 2000).
The involvement of phosphatidylinositol polyphosphates in membrane traffic has been demonstrated by the requirement of enzymes that synthesize specific phosphatidylinositol polyphosphates. For example, Ca2+-dependent secretory granule exocytosis requires both PEP1 and PEP3 activity (Hay et al., 1995), enzymes that together increase the local concentrations of phosphatidylinositol 4,5-bisphosphate (PtdIns[4,5]P2)* (Liscovitch and Cantley, 1995; Kearns et al., 1998). Likewise, synthesis of PtdIns(3)P is required for intracellular membrane transport. Mutations in the yeast phosphatidylinositol 3 kinase, VPS34, result in secretion of vacuolar proteins, such as carboxypeptidase Y (Herman et al., 1992; Schu et al., 1993; Stack et al., 1995). Moreover, the lysosomal protein, cathepsin D, requires mammalian Vps34p for proper sorting (Brown et al., 1995; Davidson, 1995). Thus, the ability to increase the local concentration of these phosphatidylinositol polyphosphates is intrinsic to some intracellular membrane traffic events.
One of the primary roles for phosphatidylinositol polyphosphates in membrane traffic is recruitment of specific proteins to designated regions of membranes. Many of the proteins required for formation of clathrin-coated vesicles, dynamin, the α subunit of AP-2, and synaptojanin, contain PtdIns(4,5)P2 binding sites (Jost et al., 1998) and have been shown to bind PtdIns(4,5)P2 in vitro (Beck and Keen, 1991; Barylko et al., 1998; Bottomley et al., 1999). More recently, it has been shown that the epsin homology NH2-terminal domains of CALM (Ford et al., 2001) and epsin (Itoh et al., 2001) bind PtdIns(4,5)P2 as well. Likewise, PtdIns(3)P binds specifically to several proteins required for membrane traffic, including Vac1p, EEA1, and Vps27p. Each of these proteins contains a zinc-binding FYVE finger motif, which specifically binds PtdIns(3)P but not other phosphatidylinositols (Burd and Emr, 1998; Patki et al., 1998; Stenmark and Aasland, 1999). PtdIns(3)P is required for recruitment of these proteins to membranes and may also regulate protein function (Peterson et al., 1999; Tall et al., 1999; Gaullier et al., 2000).
The discovery of phosphatidylinositol 3,5-bisphosphate (PtdIns[3,5]P2) has expanded the list of biologically relevant phosphatidylinositol polyphosphates (Dove et al., 1997; Rameh et al., 1997; Whiteford et al., 1997). In Saccharomyces cerevisiae, PtdIns(3,5)P2 is a low abundance phosphatidylinositol whose synthesis increases in cells exposed to osmotic stress (Dove et al., 1997). Synthesis of PtdIns(3,5)P2 in yeast requires Vps34p, demonstrating that PtdIns(3,5)P2 is produced by phosphorylation of PtdIns(3)P (Dove et al., 1997). The subsequent phosphorylation of PtdIns(3)P is catalyzed by Fab1p, the sole phosphatidylinositol (3)P 5-kinase in yeast (Cooke et al., 1998; Gary et al., 1998). Unlike vps34 mutants which are defective in membrane transport from the Golgi region to the vacuole, fab1 mutants have only a partial defect, demonstrating that PtdIns(3,5)P2 and PtdIns(3)P have distinct functions (Yamamoto et al., 1995; Gary et al., 1998).
Phenotypic analysis of two yeast mutants that cannot synthesize PtdIns(3,5)P2, fab1 and vac7, suggested that this phospholipid is required for selected vacuole membrane functions (Bonangelino et al., 1997; Gary et al., 1998). The most prominent phenotypes include an enlarged, unlobed vacuole and defects in both vacuole inheritance and acidification. A third mutant, vac14 shares all of these phenotypes.
Here we present the corresponding wild-type gene, VAC14, which encodes a newly discovered protein that, like Fab1p and Vac7p, resides on the vacuole membrane and is required for normal cellular levels of PtdIns(3,5)P2. We find that Vac14p is both a general activator and a specific osmotic response regulator of the PtdIns(3)P 5-kinase, Fab1p. Moreover, we show that PtdIns(3,5)P2 levels are tightly correlated with vacuole morphology and that this ability to regulate the size and number of vacuole lobes, through increased PtdIns(3,5)P2, is a physiological response to a sudden increase in extracellular osmolarity. Furthermore, we find that after exposure to osmotic stress, the cellular levels of PtdIns(3,5)P2 increase 16–20-fold. These levels are much higher than previously reported and are similar to the basal levels observed for PtdIns(3)P, PtdIns(4)P, and PtdIns(4,5)P2. Together, these findings suggest that PtdIns(3,5)P2 may function similarly to PtdIns(3)P and PtdIns(4,5)P2 (i.e., by recruiting membrane coat proteins) to regulate a specific vacuole-related membrane-trafficking pathway(s) that is required for the observed changes in vacuole morphology.
Results
Identification of the VAC14 gene
The Class III vac mutant, vac14-1, was isolated via fluorescence-activated cell sorting (for description of approach see Wang et al. [1996]). Like vac7 and fab1 mutants, vac14-1 cells are defective in vacuole inheritance, acidification, and morphology. These mutants have a single, unlobed, enlarged vacuole. Frequently, the vacuole spans both the mother and daughter cell resulting in an “open figure eight” vacuole morphology (Bonangelino et al., 1997).
We determined (see Materials and methods) that the VAC14 open reading frame is YLR386W (sequence deposited by the Yeast Genome Sequencing Project). The VAC14 2.64-kb open reading frame encodes a novel polypeptide of 880 amino acids. There are no notable motifs except for a putative transmembrane domain (see below). However, VAC14 displays a high degree of identity with open reading frames present in other eukaryotic organisms. The two regions of highest identity are near the NH2 terminus (residues 1–171) (Fig. 1, left) and COOH terminus (residues 578–746) (Fig. 1, right). Both mouse and human sequences matching either end were identified in the corresponding EST databases. Moreover, a human hypothetical protein, FLJ10305, found on chromosome 16, shows a high degree of identity with the COOH-terminal region of Vac14p and its sequence matches human EST sequences. Both the NH2- and COOH-terminal ESTs map to human chromosome 16, suggesting that they correspond to the same gene. No obvious Vac14p homologues were found in any of the published bacterial genomes.
Figure 1.

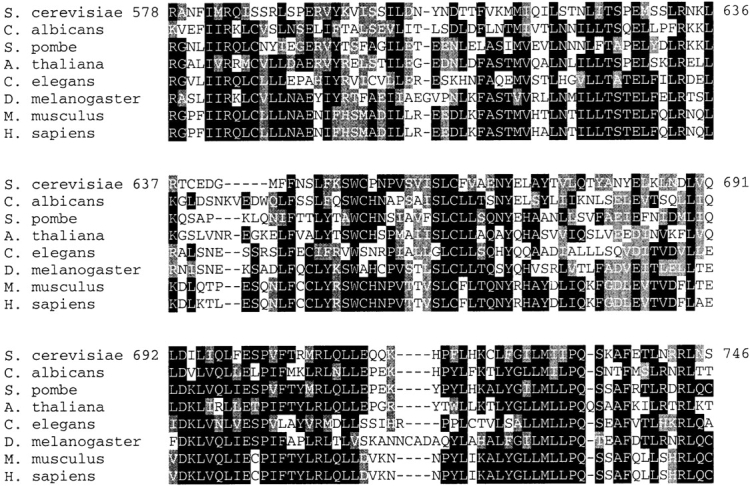
Proteins with identity to Vac14p exist in higher eukaryotes. Identical amino acids (black) and similar amino acids (gray) are highlighted. (Left) The NH2-terminal sequence of S. cerevisiae VAC14 and related ORFs were aligned using ClustalW (http://searchlauncher.bcm.tmc.edu:9331/multi-align/multi-align.html). Sequences were identified by searching the indicated databases via the BLAST algorithm (Altschul et al., 1990). The C. albicans sequence was found in the C. albicans database (http://sequence-www.stanford.edu/group/candida/search.html). ORFs from S. pombe (EMBL/GenBank/DDBJ accession no. CAB08779.1), A. thaliana (EMBL/GenBank/DDBJ accession no. AAD12702.1), C. elegans (EMBL/GenBank/DDBJ accession no. CAB00043.1) and D. melanogaster (EMBL/GenBank/DDBJ accession no. AAF54829.1) were in the GenBank database (http://www.ncbi.nlm.nih.gov/BLAST). The M. musculus sequence was identified in the mouse EST database (http://www.ncbi.nlm.nih.gov/BLAST). The sequence shown is a consensus of two similar ESTs (EMBL/GenBank/DDBJ accession nos. BE573148 and BF162275). The H. sapiens sequence was in the human EST database (http://www.ncbi.nlm.nih.gov/BLAST). The consensus sequence of 14 similar ESTs from chromosome 16 (EMBL/GenBank/DDBJ accession nos. AL527155, AL535971, AL555680, AL556062, BE409891, BE696780, BE728471, BE893810, BE901196, BE937614, BF081182, BF091052, BF325708, and BG107035) is shown. The sequences contain at least 25% global identity and 42% global similarity to S. cerevisiae VAC14. (Right) The COOH-terminal sequence of yeast Vac14p and similar ORFs were identified and aligned as in the left sequence. The M. musculus sequence is a consensus of 12 similar ESTs (EMBL/GenBank/DDBJ accession nos. AA036005, AA050423, AA058300, AA276168, AA497446, AA670618, BE862623, BF023070, BF237130, BF720417, BG079707, W09660). The H. sapiens sequence is hypothetical protein, FLJ10305 found on chromosome 16, deposited in the human genome database (http://www.ncbi.nlm.nih.gov/genome/seq). One of the mouse ESTs (clone ID 468926) had been mapped to chromosome VIII (106 cM offset) with an inferred position on human chromosome 16 (16q22.1-qter).
Chromosomal deletion of VAC14
A heterozygous diploid containing a chromosomal deletion of VAC14 was created by replacing the VAC14 open reading frame with TRP1. The resulting diploid was sporulated. In all 20 tetrads examined the TRP1 gene cosegregated with the vac14 phenotypes. vac14-Δ1 cells are viable and, like vac14-1, they display abnormal vacuole morphology (Fig. 2 a), are defective in vacuole inheritance, and have a vacuole acidification defect (Fig. 2 e). vac14-1 and vac14-Δ1 cells have nearly identical growth rates, with a doubling time that is similar to that of wild-type cells (unpublished data). Because vac14-1 and vac14-Δ1 behave identically in all tests performed, it is likely that the original vac14-1 allele is a loss of function mutation.
Figure 2.
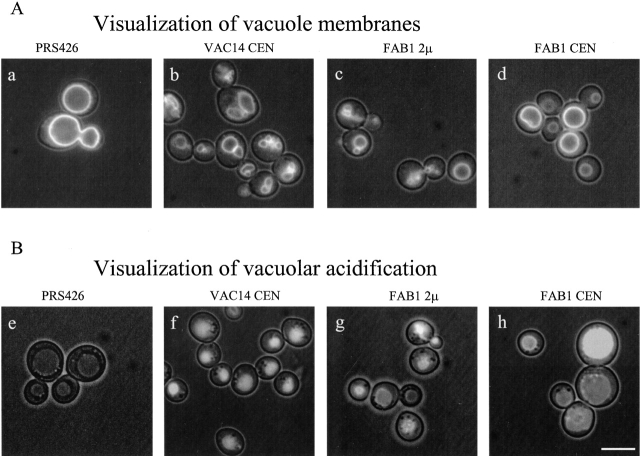
FAB1 supresses the vacuole inheritance, morphology, and acidification defects of vac14-Δ1. vac14-Δ1 mutants expressing pRS426 (a and e), VAC14 on a low copy plasmid (b and f), FAB1 on a multicopy plasmid (c and g), or FAB1 from a low copy plasmid (d and h) were labeled with either FM4-64 to visualize vacuole membranes (A) or with 200 μM quinacrine to assess vacuole acidification (B). The photographs were taken with both fluorescence and a low level of transmitted light. Bar, 5 μm.
The growth defects of the double mutants, vac7-Δ1, vac14Δ and fab1-Δ1, vac14Δ were similar to vac7-Δ1, or fab1-Δ1 mutants alone, consistent with VAC14, VAC7, and FAB1 functioning in the same pathway. We were unable to determine whether VAC7 functions upstream or downstream of FAB1 or VAC14 because VAC7 expressed from either a low-copy or multi-copy plasmid did not suppress the vac14-1, vac14-Δ1 or fab1-Δ1 mutant phenotypes (Bonangelino et al., 1997), and neither FAB1 nor VAC14 suppressed vac7-Δ1. However, a similar analysis suggested that VAC14 functions upstream of FAB1. Overexpression of FAB1 suppressed the vacuolar morphology, inheritance (Fig. 2, c and d) and acidification defects of the vac14-Δ1 mutant (Fig. 2, g and h), and the vac14-1 mutant (Bonangelino et al., 1997). This suppression required the kinase activity of Fab1p since overexpression of a kinase-dead fab1-D2134R mutant (Gary et al., 1998) did not suppress the vac14-Δ1 mutant phenotypes (unpublished data). These data suggest that Vac14p may be an activator of Fab1p kinase activity.
Vac14p associates with vacuolar membranes
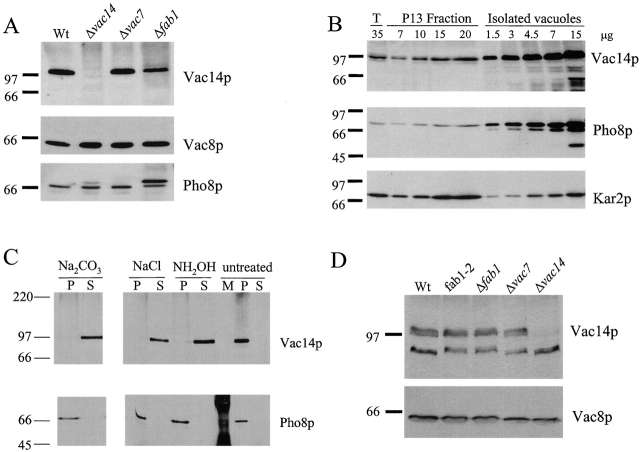
Antibodies raised to a glutathione S-transferase (GST)–Vac14p fusion protein recognized a polypeptide with a molecular weight of ∼99 kD, the predicted molecular weight of Vac14p (Fig. 3 A). (A second, 70-kD polypeptide is nonspecific as it appears in both wild-type and vac14-Δ1 extracts.) The majority of Vac14p was found in a membrane-associated fraction that pelleted at 13,000 g (P13) (Fig. 3). The P13 fraction contains large organelles such as vacuoles, ER, and nuclei. A similar fractionation pattern was observed for other vacuolar proteins, such as Vac8p (Wang et al., 1998) (Fig. 3 C).
Figure 3.
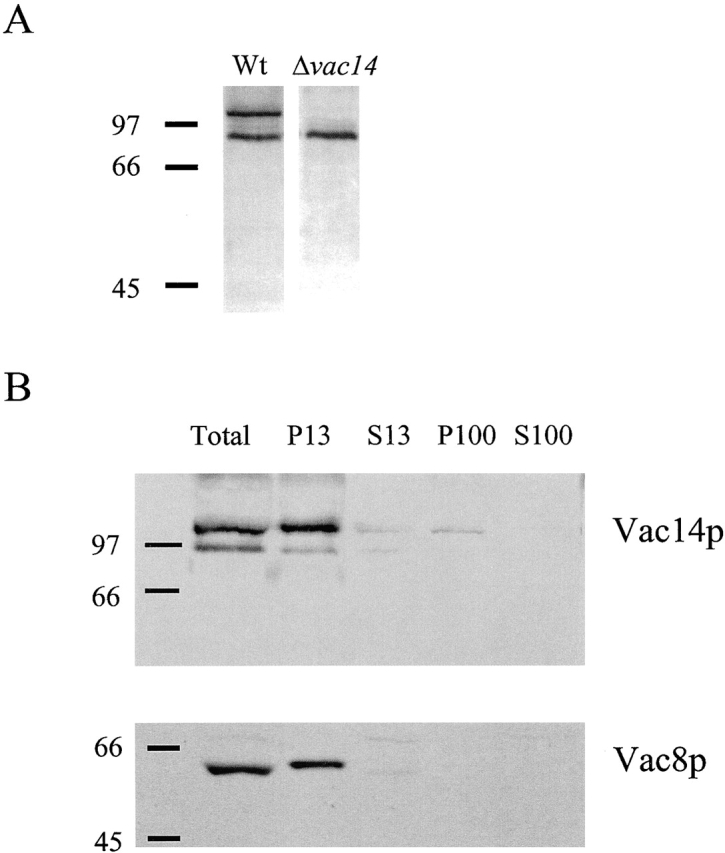
Vac14p associates with a membrane fraction. (A) Wild-type and vac14-Δ1 yeast were lysed with glass beads. Equivalent amounts of the cell extracts were separated by SDS-PAGE and transferred to nitrocellulose. Western blot analysis was performed with a 1:5,000 dilution of anti-Vac14p antibody. (B) Whole cell extracts of wild-type were subjected to differential centrifugation. Equivalent amounts were loaded in each lane. Lane 2, total crude extract; lane 3, P13, 13,000 g spin pellet; lane 4, S13, supernatant from the 13,000 g spin; lane 5, P100, 100,000 g spin pellet; and lane 6, S100, the supernatant from the 100,000 g spin. Western blot analysis with anti-Vac14 antibodies was performed. The nitrocellulose membrane was reprobed with anti-Vac8 polyclonal rabbit antibodies (Wang et al., 1998) at a 1:5,000 dilution.
Vac14p was present on vacuoles isolated on a Ficoll flotation gradient (Fig. 4 A) and was enriched on purified vacuoles to a similar extent as the vacuolar membrane protein, Pho8p (Fig. 4 B). Sequence analysis of Vac14p revealed a putative transmembrane domain between residues 430 and 451 (determined by PSORT and hydropathy plot analysis). Despite this prediction, Vac14p was peripherally associated with vacuole membranes. Treatments with either 0.1 M Na2CO3, pH 11.5, 1 M NaCl, or 0.8 M NH2OH-solubilized Vac14p, whereas Pho8p was not extracted by any of these treatments (Fig. 4 C).
Figure 4.
Vac14p is peripherally associated with isolated vacuoles. (A) Vacuoles from wild-type, vac14-Δ1, vac7-Δ1, and fab1-Δ1 were isolated on Ficoll flotation gradients. Equivalent protein amounts were loaded in each lane. Western blot analysis was performed with anti-Vac14 and anti-Vac8p antibodies. The nitrocellulose blot was then reprobed with anti-Pho8p antibodies at a 1:1,000 dilution. (B) Vacuoles were isolated from LWY7235. 35 μg of the total cell extract (lane 2) and increasing amounts of the P13 fraction (lanes 3–7), as well as increasing amounts of vacuoles (lanes 8–13), were loaded onto a 7.5% SDS-polyacrylamide gel. Western blot analyses were performed using polyclonal anti-Vac14p, anti-Pho8p, and anti-Kar2p antibodies. (C) Vacuoles were prepared, divided into six equal aliquots, and treated on ice with one of the following conditions: 0.1 M Na2CO3, pH 11.5, 1 M NaCl, 0.8 M NH2OH, or left untreated. Samples were centrifuged at 100,000 g for 1 h at 4°C. The resultant supernatant fractions (S100) were separated and the pellets (P100) were resuspended in 100 μl cytosol cocktail. Equal amounts of each were separated on a 7.5% SDS-PAGE, transferred to nitrocellulose, and probed with anti-Vac14 antibody. The nitrocellulose blots were then reprobed with monoclonal anti-Pho8p antibodies. (D) Equal amounts of protein from total cell extracts were loaded into each lane of a 7.5% SDS-polyacrylamide gel. The proteins were then transferred to nitrocellulose. Western blot analyses were performed using anti-Vac14 antibodies and anti-Vac8p antibodies.
Vac14p was also present in vacuoles isolated from vac7-Δ1 and fab1-Δ1 strains (Fig. 4 A). Thus, defects observed in vac7-Δ1 cells are not due to a gross mislocalization of Vac14p. Interestingly, although fab1-Δ1 cell extracts had normal levels of Vac14p (Fig. 4 D), the vacuoles contained ∼50% less Vac14p than wild-type vacuoles (Fig. 4 A). The decrease of Vac14p on fab1-Δ1 vacuoles was observed in four independent experiments and suggests that Vac14p may physically associate with Fab1p. Since some Vac14p remained associated with vacuoles in the absence of Fab1p, there are likely to be additional proteins that bind Vac14p and help mediate its association with vacuolar membranes. Vac7p is not a candidate because vac7-Δ1 vacuoles contain normal levels of Vac14p. We were unable to detect a direct physical interaction between Vac14p and Fab1p either by yeast two-hybrid, coimmunoprecipitation, or cross-linking experiments. Thus, any interaction is likely to be transient or indirect, and therefore difficult to detect by standard biochemical techniques. Cellular fractionation alone was used to determine Vac14p localization because we were unable to use the goat serum for indirect immunofluorescence.
Proper GFP-Fab1p localization does not depend on Vac7p or Vac14p
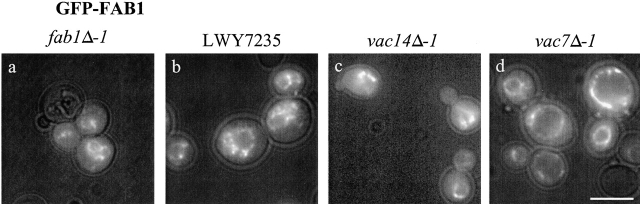
Fab1p, like Vac14p, is peripherally associated with vacuolar membranes (Gary et al., 1998). A GFP–Fab1p fusion protein was constructed to visualize Fab1p in various mutant strains. The GFP–Fab1p localized to endosomal and vacuolar membranes in wild-type yeast, fab1-Δ1, vac7-Δ1, and vac14-Δ1 mutants (Fig. 5). This demonstrates that defects in PtdIns(3,5)P2 levels in vac7-Δ1 and vac14-Δ1 mutants are not due to Fab1p mislocalization. Moreover, both Vac14p and Fab1p are localized on the vacuole, consistent with Vac14p serving as an activator of Fab1p.
Figure 5.
GFP-Fab1p is localized to the vacuole membranes in vac14-Δ1 and vac7-Δ1. Wild-type (A), fab1-Δ1 (B), vac14-Δ1 (C), and vac7-Δ1 (D) strains expressing GFP-Fab1p from a 2μ plasmid (pRS426) were visualized using fluorescence light combined with low levels of transmitted light. Bar, 8 μm.
An improved method to quantify phosphatidylinositol polyphosphate levels in yeast
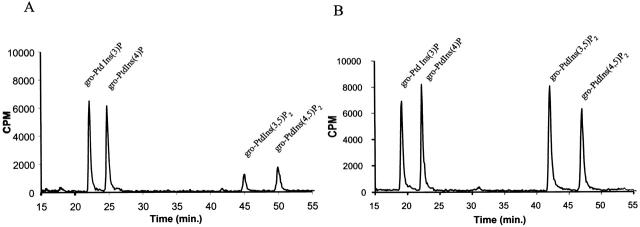
Standard methods for analyzing phosphatidylinositol levels in yeast have consisted of lysing cells in acidified chloroform/methanol/HCl with the concomitant extraction of total cellular lipids. We tested this method on wild-type yeast in the absence and presence of osmotic shock and obtained phosphatidylinositol polyphosphate levels similar to those reported previously (Dove et al., 1997; Gary et al., 1998) (Fig. 6 and Table I). However, we also noted that there were substantial fluctuations in the levels of PtdIns(3,5)P2 and PtdIns(4,5)P2 even in identically prepared samples. Therefore, we adapted a method described for analyzing PtdIns(3,5)P2 levels in tissue culture cells, where cells are lysed in 4.5% perchloric acid (Whiteford et al., 1997). This treatment precipitates most macromolecules, including proteins and lipids. The lipids are then deacylated with methylamine and the released glycero-phospho-inositols are extracted with water. Notably, we obtained significantly higher quantities of the corresponding inositol monophosphates and biphosphates (Fig. 6 and Table I) with far less variability among identically prepared samples for the inositol bisphosphates. This allowed us to reproducibly detect basal levels of PtdIns(3,5)P2, which are ∼15-fold higher than reported previously.
Figure 6.
Use of 4.5% perchloric acid results in a significantly higher extraction of phosphatidylinositol monophosphates and bisphosphates. Cells were labeled with myo-[2-3H]inositol for 12 h at 24°C and exposed to 0.9 M NaCl for 10 min. (A) Cells were lysed with glass beads in 1.5 ml chloroform/methanol/1 N HCl (1:1:1) at room temperature. Phospholipids were extracted in the organic phase and dried under a N2 stream. (B) Cells were lysed with glass beads in 0.8 ml of 4.5% perchloric acid. Cell extracts were centrifuged at 14,000 rpm at 4°C to obtain a phospholipid-containing pellet. The phospholipids from both protocols were then deacylated and analyzed by HPLC. An online scintillation counter was used to detect the tritiated compounds. Approximately 3.2 × 106 cpm (A, chloroform/methanol/HC1) and 3.6 × 106 cpm (B, 4.5% perchloric acid) were injected, respectively.
Table I. Cellular lipid extractions utilizing 4.5% perchloric acid provide a more representative measure of phosphatidylinositol levels.
| PtdIns(3)P | PtdIns(4)P | PtdIns(3,5)P2 | PtdIns(4,5)P2 | |||||
|---|---|---|---|---|---|---|---|---|
| 0.9 M NaCl | + | − | + | − | + | − | + | − |
| 4.5% HClO4 | 70 ± 15 | 100 ± 15 | 65 ± 10 | 90 ± 15 | 65 ± 10 | 4 ± 1 | 85 ± 20 | 70 ± 15 |
| Chloroform: MeOH | 38 ± 5 | 60 ± 5 | 43 ± 5 | 68 ± 3 | 8 ± 3 | ≤1.5 | 12 ± 5 | 10 ± 5 |
Cells were grown in inositol-free media in the presence of 0.01 mCi/ml 3H inositol. 0.9 M NaCl was added to half the cultures for 10 min prior to extraction. Cellular lipids were extracted and analyzed as described in Materials and methods. The cpm in each peak was corrected for the total number of cells analyzed by expressing each as a percentage of total cpm eluted. Then these values were normalized to PtdIns(3)P with no salt added for the 4.5% perchloric acid extraction. This peak was 2.0% of the total cpm eluted (80,000 cpm of 4 × 106 total cpm). Values listed as ≤1.5 were areas where no peak above background was detected. The data (mean ± standard deviation) were calculated from results obtained in three independent experiments.
PtdIns(3,5)P2 levels rise 20-fold in response to osmotic stress
Using this improved method we found that osmotic stress results in much higher levels of PtdIns(3,5)P2 than reported previously (Table I). 10 min after exposure to osmotic stress, the levels of PtdIns(3,5)P2 and PtdIns(4,5)P2 are similar to PtdIns(3)P and PtdIns(4)P levels (Table I). Although the levels of each of the phosphorylated phosphatidylinositols underwent some change after exposure of the cells to osmotic stress, the change in PtdIns(3,5)P2 levels was the most dramatic, with the levels of this lipid rising 16–20-fold (Tables I and II). Quantification of the fold increase could not be performed on cells extracted with chloroform methanol HCl because with this method PtdIns(3,5)P2 was not detectable in cells grown in the absence of osmotic shock. Moreover, after a 10-min exposure to osmotic stress, ∼8-fold more PtdIns(3,5)P2 and 7-fold more PtdIns(4,5)P2 were detected in cells lysed with perchloric acid compared with cells extracted with chloroform methanol HCL (Table I).
Table II. Class III vac mutants fail to maintain normal levels of PtdIns(3,5)P2 .
| PtdIns(3)P | PtdIns(3,5)P2 | |||
|---|---|---|---|---|
| + | − | + | − | |
| Wild-type | 68 ± 7 | 100 ± 10 | 71 ± 18 | 3.5 ± 1.1 |
| fab1-Δ1 | 126 ± 16 | 91 ± 3 | ≤1.5 | ≤1.5 |
| vac7-Δ1 | 145 ± 12 | 105 ± 2 | ≤1.5 | ≤1.5 |
| vac14-Δ1 | 120 ± 10 | 97 ± 12 | 1.7 ± 0.6 | ≤1.5 |
| fab1-Δ1 vac14-Δ1 | 141 ± 5 | 101 ± 4 | ≤1.5 | ≤1.5 |
Cells were grown in inositol-free media in the presence of 0.01 mCi/ml 3H inositol. 0.9 M NaCl was added to half the cultures for 10 min prior to extraction. Values were obtained as described in Table I and normalized to PtdIns(3)P no salt in wild-type. This peak was 2.0% of the total cpm eluted (80,000 cpm of 4 ± 106 total cpm). Values listed as ≤1.5 were areas where no peak above background was detected. The data (mean ± standard deviation) were calculated from results obtained in six independent experiments.
Fab1p, Vac7p, and Vac14p are required for steady-state levels of PtdIns(3,5)P2 during vegetative growth
We next reevaluated PtdIns(3,5)P2 levels in both vac7-Δ1 and fab1-Δ1. As reported previously (Gary et al., 1998), there was no detectable PtdIns(3,5)P2 in fab1-Δ1, consistent with Fab1p being the sole 5-kinase that converts PtdIns(3)P to PtdIns(3,5)P2 (Table II). Likewise, no PtdIns(3,5)P2 was detected in the vac7-Δ1 mutant. We also observed an elevation in PtdIns(3)P levels in these mutants following osmotic stress (see below).
We next analyzed PtdIns(3,5)P2 levels in the vac14-Δ1 mutant. Like vac7 and fab1 mutants, in the absence of osmotic stress, no detectable PtdIns(3,5)P2 was observed (Table II). These results suggest that the Class III vac mutant phenotypes shared by vac7, fab1, and vac14 mutants are due to a deficiency in PtdIns(3,5)P2 levels.
vac14 mutants synthesize low levels of PtdIns(3,5)P2 after exposure to osmotic stress
Unlike vac7 and fab1 mutants, when vac14-Δ1 cells were exposed to 0.9 M NaCl for 10 min, a small amount of PtdIns(3,5)P2 was observed (1.7 U) (Table II). This level is similar to the basal levels detected during vegetative growth (3.5 U), but much lower than the osmotic stress–induced levels (71 U) observed in wild-type cells.
We suspect that low, yet undetectable, levels of PtdIns(3,5)P2 are synthesized in vac14-Δ1 cells in the absence of osmotic stress. First, vac14-Δ1 mutants produce PtdIns(3,5)P2 during osmotic shock, demonstrating that Fab1p, the phosphatidylinositol (3)P 5-kinase, retains partial activity in the absence of Vac14p. Second, the doubling time of vac14 cells is similar to that of wild-type cells (unpublished data), in contrast to the greatly reduced growth rate of fab1Δ and vac7Δ (Yamamoto et al., 1995; Bonangelino et al., 1997). The nearly normal doubling time of vac14 mutants is likely due to synthesis of small amounts of PtdIns(3,5)P2.
Vac14p is required for elevation of PtdIns(3,5)P2 in response to osmotic stress
Because overexpression of FAB1 suppresses the vacuolar defects of the vac14-Δ1 mutant (Fig. 2), we tested PtdIns(3,5)P2 levels in these strains. Expression of FAB1 from a low- or high-copy plasmid in the vac14-Δ1 mutant brought the levels of PtdIns(3,5)P2 from undetectable to nearly the basal levels detected in wild-type cells (2 U) (Table III). These observations are consistent with Vac14p functioning upstream of Fab1p.
Table III.
Vac14p is required for normal elevation of PtdIns(3,5)P 2 in response to osmotic stress
| PtdIns(3)P | PtdIns(3,5)P2 | |||
|---|---|---|---|---|
| + | − | + | − | |
| vac14-Δ1 with pRS426 | 112 ± 10 | 100 ± 2 | 2.4 ± 0.3 | ≤1.5 |
| vac14-Δ1 with FAB1 CEN | 100 ± 8 | 92 ± 16 | 3.2 ± 0.8 | 2.0 ± 0.3 |
| vac14-Δ1 with FAB1 2μ | 84 ± 8 | 84 ± 8 | 3.1 ± 0.4 | 2.1 ± 0.8 |
| vac14-Δ1 with VAC14 CEN | 55 ± 3 | 76 ± 3 | 28 ± 12 | 3.2 ± 0.8 |
| vac14-Δ1 with VAC14 2μ | 60 ± 16 | 80 ± 24 | 33 ± 0.4 | 3.2 ± 0.8 |
Cells were grown in inositol free media in the presence of 0.01 mCi/ml 3H inositol. 0.9 M NaCl was added to half the cultures for 10 min before extraction. The values were generated as described in Table II and normalized to wild-type (Table II). The data (mean ± standard deviation) were calculated from results obtained in four independent experiments.
Notably, FAB1 expression in vac14 mutants did not restore the elevation of PtdIns(3,5)P2 normally observed in response to osmotic shock. The levels of PtdIns(3,5)P2 after osmotic shock in vac14-Δ1, or vac14-Δ1 with either a low or high copy FAB1, were statistically identical (2.4, 3.2, and 3.1 U, respectively) (Table III). As a control, vac14-Δ1 with VAC14 expressed from a low copy plasmid produced 30 U of PtdIns(3,5)P2 in response to osmotic shock (Table III). Thus, Vac14p is required for the normal increase of PtdIns(3,5)P2 in response to hyperosmotic stress. The small increase in PtdIns(3,5)P2 observed after osmotic shock in the vac14-1 and vac14-Δ1 mutants is most likely due to an osmotic stress–induced elevation of the biosynthetic precursor, PtdIns(3)P (see below).
Vps34p activity increases in response to osmotic stress
As PtdIns(3,5)P2 levels rise, one would expect to see an equivalent decrease of the precursor, PtdIns(3)P. In fact, after exposure to osmotic stress, we observed a decrease in PtdIns(3)P in wild-type cells (Table II). However, our data suggests that the rate of PtdIns(3)P synthesis is elevated. First, in wild-type cells, the decrease in PtdIns(3)P by 32 U is not enough to account for the 71 U increase in PtdIns(3,5)P2 (Table II). Second, if the rate of PtdIns(3)P synthesis rises due to osmotic stress, then one would expect to detect this rise in cells that are blocked in PtdIns(3,5)P2 synthesis. Indeed, in fab1-Δ1 and vac7-Δ1 mutants, the levels of PtdIns(3)P after exposure to osmotic stress increased by 35 and 40 U, respectively (Table II). This suggests that after exposure to osmotic stress, PtdIns(3)P levels increase and are quickly depleted by the rapid production of PtdIns(3,5)P2. However, in mutants that cannot synthesize PtdIns(3,5)P2, the extra PtdIns(3)P accumulates.
In wild-type cells, the elevation of PtdIns(3)P in response to osmotic stress likely compensates for the rapid conversion of PtdIns(3)P to PtdIns(3,5)P2. Lack of a compensatory mechanism would deplete the levels of PtdIns(3)P, which are required for membrane trafficking to the yeast vacuole (Schu et al., 1993). In addition, a rise in PtdIns(3)P in response to osmotic shock might also be responsible for producing an increase in PtdIns(3,5)P2, as PtdIns(3)P could directly activate Fab1p or drive the reaction forward by mass action.
Although it is possible that Vac14p might indirectly affect PtdIns(3,5)P2 levels by regulating Vps34p activity, our data demonstrate that this is not the case. If Vac14p activated Vps34p, then in a vac14-Δ1, fab1-Δ1 double knockout there would be little or no PtdIns(3)P accumulation. However, in a vac14-Δ1, fab1-Δ1 double mutant, the levels of PtdIns(3)P were virtually identical to fab1-Δ1 mutants, with a large accumulation of PtdIns(3)P in the presence of osmotic stress (an elevation of 40 and 35 U, respectively) (Table II). Thus, while osmotic stress activates Vps34p, Vac14p is not involved in this activation. Moreover, the defects in PtdIns(3,5)P2 levels in vac14-Δ1 cells are not indirectly due to reduced PtdIns(3)P production.
Vac14p is an osmotic response activator of Fab1p
The observation that during osmotic stress vac14 mutants have low levels of PtdIns(3,5)P2 and accumulate PtdIns(3)P suggests either of two remaining hypotheses. First, Vac14p may function as an osmotic response activator of Fab1p. In the absence of its activator, Fab1p functions with reduced activity, leading to the accumulation of its substrate, PtdIns(3)P. Alternatively, Vac14p may negatively regulate a phosphatidylinositol 5-phosphatase required for PtdIns(3,5)P2 turnover which would also result in accumulation of PtdIns(3)P. Although turnover of PtdIns(3,5)P2 might also be achieved through the actions of a phosphatidylinositol 3-phosphatase, a polyphosphatidylinositol phosphatase, and/or lipases, none of these would result in the observed accumulation of PtdIns(3)P.
If Fab1p is activated in response to osmotic stress, then there might be a subset of fab1 mutants that produce basal levels of PtdIns(3,5)P2 but are incapable of further activation. The fab1-2 mutant displays this phenotype (Cooke et al., 1998; Gary et al., 1998). At the permissive temperature (24°C), fab1-2 had normal levels of PtdIns(3,5)P2 during vegetative growth (3.1 U), but after exposure to osmotic shock the elevation in PtdIns(3,5)P2 levels was greatly reduced (12 U) compared with wild-type levels (71 U) (Table II). In addition, overexpression of Vac14p in the fab1-2 mutant did not increase the levels of PtdIns(3,5)P2 produced in response to osmotic shock (8 U) (Table IV). Moreover, overexpression of Fab1p in fab1-2 did not fully restore levels of PtdIns(3,5)P2 to those seen in wild-type cells (27 instead of 71 U). This suggests that fab1-2 is incapable of full activation by Vac14p and is partially dominant, perhaps by sequestering Vac14p. Consistent with this hypothesis, simultaneous overexpression of wild-type Fab1p and Vac14p in fab1-2 resulted in much higher levels of PtdIns(3,5)P2 (40 U) (Table IV).
Table IV. Overexpression of VAC14 does not enable fab1-2 to produce elevated levels of PtdIns(3,5)P2 after exposure to osmotic stress.
| PtdIns(3)P | PtdIns(3,5)P2 | |||
|---|---|---|---|---|
| 0.9 M NaCl | + | − | + | − |
| fab1-2 with pRS426, 423, and 424 | 165 ± 1 | 150 ± 10 | 12 ± 2 | 3.1 ± 0.3 |
| fab1-2 with FAB1 2μ | 85 ± 1 | 89 ± 5 | 27 ± 1 | 3.1 ± 0.9 |
| fab1-2 with VAC14 2μ | 144 ± 9 | 122 ± 9 | 8 ± 2 | 2.4 ± 0.5 |
| fab1-2 with VAC14 and FAB1 2μ | 97 ± 1 | 100 ± 4 | 40 ± 4 | 2.8 ± 0.2 |
Cells were grown in inositol-free media in the presence of 0.01 mCi/ml 3H inositol. 0.9 M NaCl was added to half the cultures for 10 min prior to extraction. Cellular lipids were extracted and analyzed as described in Materials and methods. The cpm in each peak was corrected for the total number of cells analyzed by expressing each as a percentage of total cpm eluted. Then these values were normalized to PtdIns(3)P with no salt added in fab1-2 with VAC14 and FAB1 2μ (which was 2.1% of total cpm eluted; 84,000 cpm of 4 × 106 total cpm). The data (mean ± standard deviation) were calculated from results obtained in three independent experiments.
If Vac14p was the inhibitor of the phosphatidylinositol 5-phosphatase, an increase in PtdIns(3,5)P2 would be expected when VAC14 is overexpressed in fab1-2 cells. In fact, overexpression of VAC14 did not elevate the levels of PtdIns(3,5)P2 in this strain (Table IV). Since Vac14p is already present, it is possible that the phosphatidylinositol 5-phosphatase is fully inhibited and thus adding more Vac14p has no effect. However, if this were true, simultaneous overexpression of Vac14p and Fab1p should produce the same levels of PtdIns(3,5)P2 as overexpression of Fab1p alone. We observed that simultaneous overexpression of Vac14p and Fab1p in fab1-2 resulted in 48% more PtdIns(3,5)P2 than in fab1-2 overexpressing only Fab1p (Table IV). These results strongly suggest that Fab1p and Vac14p act in concert with each other and are consistent with Vac14p functioning as an activator of Fab1p rather than as the negative regulator of a phosphatidylinositol 5-phosphatase.
Levels of PtdIns(3,5)P2 regulate vacuolar morphology
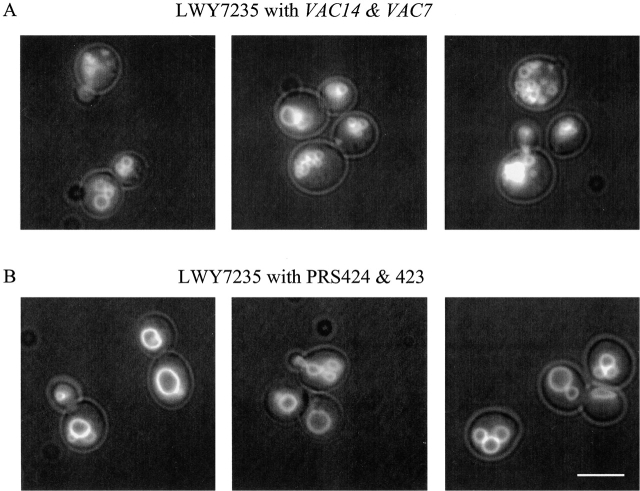
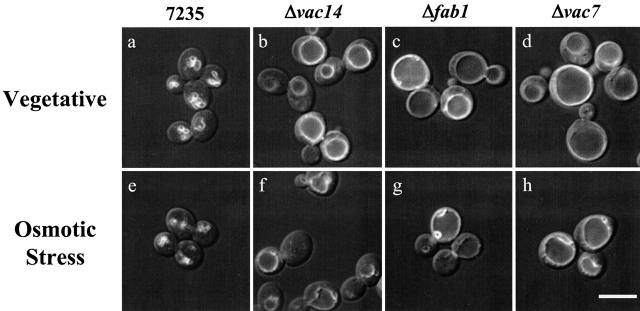
Observations of the dramatic increase in PtdIns(3,5)P2 levels combined with our identification of a molecule required for activation of Fab1p strongly suggest that PtdIns(3,5)P2 protects yeast from rapid increases in osmolarity. As described below, it is likely that this protection occurs via PtdIns(3,5)P2 regulation of vacuole volume. Overproduction of PtdIns(3,5)P2 resulted in both an increase in the number and a decrease in size of vacuole lobes (Fig. 7). In this case, elevation of PtdIns(3,5)P2 was achieved in the absence of osmotic stress by simultaneous overexpression of both Vac14p and Vac7p. This resulted in a 70% increase in basal levels of PtdIns(3,5)P2, 12 U, instead of 7 U (wild-type expressing the vectors alone). Conversely, a pronounced phenotype in cells with defects in steady-state levels of PtdIns(3,5)P2 is an enlarged unlobed vacuole. These observations suggest that PtdIns(3,5)P2 levels regulate the surface area to volume ratio of the vacuole by changing the number and size of the vacuole lobes. Note that a sphere of a given diameter can create four spheres with a combined total of half the volume using the same total membrane surface area. Thus, increasing PtdIns(3,5)P2 levels in response to osmotic stress may serve to regulate vacuole morphology under conditions when the vacuole loses volume due to loss of water. Consistent with this hypothesis, we found that after a short exposure to hyperosmotic stress, the vacuole morphology of wild-type cells changed to smaller, more highly fragmented vacuoles (Fig. 8, a and e). However, the vacuole morphology of mutants unable to produce PtdIns(3,5)P2 remained largely unchanged (Fig. 8). This demonstrates that changes in vacuole morphology induced by exposure to osmotic stress are regulated via a molecular mechanism that requires the synthesis of more PtdIns(3,5)P2. Moreover, this observation provides the first insight into a physiological consequence of increased PtdIns(3,5)P2 production in response to osmotic stress.
Figure 7.
Simultaneous overexpression of VAC7 and VAC14 results in cells with a larger number of vacuole lobes and lobes of smaller size. Wild-type cells simultaneously expressing VAC7 and VAC14 from multicopy plasmids (A) or containing empty vectors, pRS424 and pRS423 (B). Vacuoles were labeled with 80 μM FM4-64. Several fields are shown for each. Bar, 8 μm.
Figure 8.
PtdIns(3,5)P 2 is required for osmotic stress induced fragmentation of the vacuole. Wild-type (a and e), vac14-Δ1 (b and f), fab1-Δ1 (c and g), vac7-Δ1 (d and h) cells were labeled with FM4-64 to visualize vacuole membranes. Cells were incubated in YEPD alone (a–d) or YEPD containing 0.4 M NaCl (e–h) for 10 min. Bar, 8 μm.
Discussion
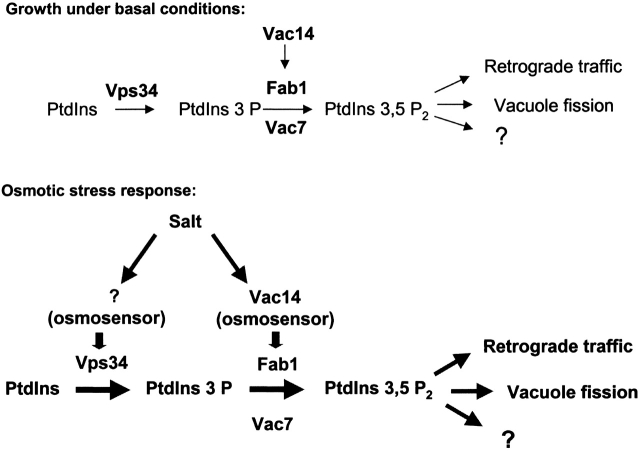
The discovery of a new biological phosphatidylinositol, PtdIns(3,5)P2, and the observation that PtdIns(3,5)P2 levels are acutely regulated by osmotic stress poses questions as to how this phospholipid is synthesized, how its cellular levels are regulated, where PtdIns(3,5)P2 production and turnover occur, and what functions PtdIns(3,5)P2 serves. Biosynthesis of PtdIns(3,5)P2 occurs through phosphorylation of PtdIns(3)P by Fab1p, the phosphatidylinositol(3)P 5-kinase (Huang et al., 1998; Sui et al., 1998). Its production also requires Vac7p, a novel vacuolar protein, whose function remains unknown (Bonangelino et al., 1997; Gary et al., 1998). Here we identify Vac14p, which functions in PtdIns(3,5)P2 synthesis by regulating Fab1p activity. Localization of Vac14p, Fab1p, and Vac7p, along with observations of the defects exhibited in strains lacking PtdIns(3,5)P2, suggests that this lipid is produced and functions at the late endosome and vacuole. The fact that the lipid levels rise 20-fold within 10 min of osmotic stress and cause the vacuole lobes to decrease in size and increase in number suggests that the increasing PtdIns(3,5)P2 levels and the concomitant change in vacuole volume may be a first line of defense against a sudden increase in osmolarity of the environment.
Changes in PtdIns(3,5)P2 levels could occur either via increased PtdIns(3,5)P2 synthesis, negative regulation of PtdIns(3,5)P2 turnover, or regulation of both (Fig. 9). However, analysis of selected fab1 mutants indicates that up-regulation of PtdIns(3,5)P2 synthesis, rather than negative regulation of its turnover, is the major contributor to the osmotic stress–induced increase in PtdIns(3,5)P2 levels. First, the fab1-2 mutant produces relatively normal levels of PtdIns(3,5)P2 under basal conditions, but is specifically defective in elevation of PtdIns(3,5)P2 synthesis in response to osmotic stress (Cooke et al., 1998, and Table IV). Second, when expressed in fab1Δ, Fab1p homologues from both Schizosaccharomyces pombe and mouse produce normal basal levels of PtdIns(3,5)P2 (McEwen et al., 1999). However, strains expressing these homologues as the sole copy of Fab1p are defective in the osmotic stress–induced elevation of PtdIns(3,5)P2. The only difference in these cells is the lack of wild-type Fab1p, and therefore the enzymes responsible for regulating PtdIns(3,5)P2 turnover should not be affected. This demonstrates that the dramatic elevation in PtdIns(3,5)P2 occurs via activation of Fab1p by accessory factors that are unable to act on the Fab1p homologues.
Figure 9.
Proposed model for regulation of PtdIns(3,5)P2 levels in yeast. PtdIns(3)P is produced by Vps34p (Schu et al., 1993; Stack et al., 1993) and is phosphorylated by Fab1p to produce PtdIns(3,5)P2 (Cooke et al., 1998; Gary et al., 1998). Both Vac7p (Gary et al., 1998) and Vac14p are required for normal levels of PtdIns(3,5)P2. Although Vac7p is required for conversion of PtdIns(3)P to PtdIns(3,5)P2, it is unclear whether Vac7p plays a specific role in the osmotic stress response. Vac14p regulates Fab1p to produce basal levels of PtdIns(3,5)P2 during vegetative growth and further stimulates Fab1p activity during osmotic stress. Although osmotic stress activates both Vsp34p and Fab1p, Vac14p activates Fab1p but not Vps34p. Increased levels of PtdIns(3,5)P2 may protect cells from osmotic stress by modulating water efflux and ion influx across the vacuole membrane, regulating vacuole membrane fission and retrograde traffic, and possibly via modulation of other downstream targets of PtdIns(3,5)P2.
Vps34p activity is also regulated in response to osmotic stress in yeast. First, in wild-type cells there is a relatively small decrease of precursor, PtdIns(3)P, compared with the very large increase in product, PtdIns(3,5)P2. Moreover, in fab1-Δ1 and vac7-Δ1 mutants, exposure to osmotic stress produces elevated levels of PtdIns(3)P. Vps34p activity is not regulated by Vac14p, as levels of PtdIns(3)P rise in fab1-Δ1, vac14-Δ1 double mutants; however, conversion of the additional PtdIns(3)P to PtdIns(3,5)P2 is dependent on the presence of Vac14p. Thus, while elevated Vps34p activity may be a prerequisite for elevated PtdIns(3,5)P2 levels, the activation of Fab1p via Vac14p is required for the 20-fold increase of PtdIns(3,5)P2.
Regulated turnover may also contribute to the control of PtdIns(3,5)P2 levels. PtdIns(3,5)P2 increases rapidly after osmotic shock and then disappears after 30–60 min (Dove et al., 1997). A concerted increase in the activity of the kinases combined with a delayed increase in the activity of the phosphatase(s) and/or lipase could cause the rapid disappearance of PtdIns(3,5)P2 that occurs within such a short period of time. Alternatively, it is possible that regulation of PtdIns(3,5)P2 levels may occur solely via regulating Fab1p activity. Identification and characterization of enzymes that turnover PtdIns(3,5)P2 will be necessary to determine whether turnover of PtdIns(3,5)P2 is regulated as well.
Given that PtdIns(3,5)P2 levels rise so dramatically after a short exposure to osmotic stress leads to questions of how a rise in PtdIns(3,5)P2 levels could mitigate problems caused by a sudden change in osmolarity. One of the first consequences of exposing yeast to osmotic stress is the loss of water from the cells, producing a hypertonic cytoplasm. This in turn results in loss of water from the vacuole. It is possible that this water release is regulated by PtdIns(3,5)P2 through control of a vacuolar-specific water channel. Although a vacuolar aquaporin has not yet been demonstrated in S. cerevisiae, at least two aquaporins have been identified, AQY1 and AQY2 (Carbrey et al., 2001; Meyrial et al., 2001). Either of these may localize, in part, to the vacuole membrane. Further, most plant cells contain both plasma membrane and vacuolar aquaporins (Martinoia et al., 2000; Maurel and Chrispeels, 2001). These prevent the rapid dehydration and cell lysis that could occur when the osmolarity of the external environment changes. As yeast are exposed to similar fluctuations in their external environment, it is likely that they also have similar mechanisms to regulate both water and ion uptake and exit.
PtdIns(3,5)P2 may also regulate a vacuolar ion channel and thereby control the internal osmotic strength of the vacuole lumen. A decrease in ion flux out of the vacuole would help retain water that would otherwise be lost to the cytoplasm. Notably, other phosphatidylinositols have been implicated in the regulation of several types of channels. For instance, PtdIns(4,5)P2 serves as a positive regulator of inward-rectifying potassium channels (Huang et al., 1998; Sui et al., 1998). PtdIns(4,5)P2 also plays a role in the regulation of TRP channel in Drosophila photoreceptors (Hardie et al., 2001). Studies on the purified yeast vacuolar ATPase have suggested that this enzyme requires phospholipids for maximal activity (Uchida et al., 1988). In addition, the yeast phosphatidylinositol–specific phospholipase C (Plc1p) is required for activation of a plasma membrane ATPase, thus implicating phosphatidylinositol phospholipid metabolism in its regulation (Coccetti et al., 1998). Importantly, all of the yeast mutants defective in PtdIns(3,5)P2 levels are also defective in vacuolar acidification, despite the normal localization of the vacuolar ATPase to the vacuole membrane (Bonangelino et al., 1997). This suggests that PtdIns(3,5)P2 may regulate the vacuolar ATPase.
PtdIns(3,5)P2 also regulates vacuole membrane fission. In the absence of PtdIns(3,5)P2, vacuoles are extremely large and unlobed. In contrast, wild-type vacuoles are complex and contain multiple lobes. In addition, production of elevated levels of PtdIns(3,5)P2 in the absence of osmotic stress, by simultaneous overexpression of Vac14p and Vac7p, results in a larger number of smaller vacuolar lobes, consistent with an increase in vacuole membrane fission.
Allowing the vacuole membrane surface area to remain constant during a rapid change in vacuole volume enables cells to adjust to a dynamic environment. Hypo-osmotic stress induced vacuole fusion maintains vacuole membrane surface area in the face of a rapid influx of water into the vacuole (Wang et al., 2001). Conversely, exposure to high osmolarity causes vacuoles in wild-type cells to become smaller and more highly fragmented. In addition, an increase in retrograde vesicle traffic, which requires PtdIns(3,5)P2 (Bryant et al., 1998), removes a higher proportion of vacuole membranes than vacuole volume. By simultaneously regulating water and ion flux, and increasing vacuolar fission and retrograde traffic, PtdIns(3,5)P2 may provide a concerted mechanism to prevent vacuole lysis during osmotic stress.
Materials and methods
Media, strains, and molecular biology techniques
Yeast extract-peptone-dextrose (YEPD) media, synthetic minimal media with the necessary nutritional auxotrophic supplements, sporulation media, synthetic complete media without inositol, and synthetic media without uracil or inositol (Sherman, 1991; Schu et al., 1993) were prepared as described. High pH YEPD plates with 1.8 M ethylene glycol contained: 1% yeast extract, 2% Bacto-peptone, 1.5% agar, 2% glucose, 1.8 M ethylene glycol, and 100 mM potassium phosphate (pH 7.6). The last three ingredients were not autoclaved and added separately. The strains used in this study are listed in Table V.
Table V. Strains used in this study.
| Strains | Genotype | Source |
|---|---|---|
| RHY6210 | MATα leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 pep4-Δ1137 | Gomes de Mesquita et al., 1996 |
| JBY007 | RHY6210 vac7-1 | Gomes de Mesquita et al., 1996 |
| LWY7217 | MATa leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | Bonangelino et al., 1997 |
| LWY7213 | MATa leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 ade8Δ::HIS3 | Wang et al., 1996 |
| LWY3143 | MATa/α leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | This study |
| pep4-Δ1137/PEP4 ade8Δ::HIS3/ADE8 | ||
| EMY119 | MATa leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 fab1-2 | Yamamoto et al., 1995 |
| LWY1527 | MATα leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 vac7Δ::HIS3 | Bonangelino et al., 1997 |
| LWY2365 | LWY7217 vac14-1 | Bonangelino et al., 1997 |
| LWY7235 | MATa leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | Bonangelino et al., 1997 |
| LWY7239 | MATα leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | This study |
| ade8Δ::HIS3 pep4-Δ1137 | ||
| LWY2614 | MATa leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 fab1-2 vac14-1 | Bonangelino et al., 1997 |
| LWY2310 | MATα leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 fab1-2 vac7-1 | Bonangelino et al., 1997 |
| LWY4679 | MATα leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 vac7-1 vac14-1 | Bonangelino et al., 1997 |
| LWY4544 | MATα leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 ade8Δ::HIS3 vac14-1 | This study |
| LWY4552 | MATa leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | This study |
| ade8Δ::HIS3 pep4-Δ1137 vac14-1 | ||
| LWY5177 | LWY7235 vac14Δ::TRP1 | This study |
| LWY5178 | MATα leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 vac14Δ::TRP1 | This study |
| LWY2054 | LWY7217 vac7Δ::HIS3 | This study |
| LWY2055 | LWY7217 fab1Δ::LEU2 | This study |
| LWY5956 | LWY7235 fab1Δ::LEU2 vac14Δ::HIS3 | This study |
| LWY5942 | MATα leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | This study |
| pep4-Δ1137 vac7Δ::HIS3 vac14Δ::TRP1 |
Labeling yeast vacuoles with FM4-64 or quinacrine
Yeast vacuoles were visualized in vivo by labeling log-phase cells with 80 μM N-(3-triethylammoniumpropyl)-4-(p-diethyl-aminophenylhexatrienyl) (FM4-64) (Bonangelino et al., 1997; Vida and Emr, 1995) or with quinacrine (Weisman et al., 1987). Cells were viewed with a 100× objective lens on an Olympus BX-60 fluorescence microscope (FM4-64: excitation, 560 nm, dichroic mirror at 595 nm, emission, 630 nm; quinacrine; excitation, 470 nm; dichroic mirror at 495 nm; emission, 425 nm) combined with a low level of transmitted light to reveal cell outlines. Images were captured digitally with a Hamamatsu ORCA CCD camera controlled with IP spectrum software (Scanalytics). Images were processed using Adobe Photoshop™.
Cloning VAC14
VAC14 was cloned by complementing an inability of vac14-1 cells to grow in high pH media with ethylene glycol at 33°C (Bonangelino et al., 1997). 12 overlapping clones that complemented the growth and vacuolar morphology defects of vac14-1 cells were obtained. A 3.9-kb SpeI fragment containing a single complementing open reading frame was identified and partially sequenced. Confirmation that YLR386W (sequence deposited by the Yeast Genome Sequencing Project) is VAC14 was demonstrated by creating a strain with LEU2 linked to the wild-type YLR386W locus (see below).
Construction of a strain with LEU2 integrated adjacent to the VAC14 locus and a strain with a chromosomal deletion of VAC14
To construct a LEU2-marked strain, a 3.9-kb SpeI fragment containing VAC14 was subcloned into pRS305 (Sikorski and Hieter, 1989) (pCB48). This plasmid was linearized with XhoI and transformed into wild-type (LWY7235). PCR with genomic DNA isolated from the transformants (Davis et al., 1980) confirmed that integration occurred at the correct locus. The primers used were 5′-CGCGGCAGTATTGAGGG-3′ (v14A) and the T7 universal primer. Diploid cells were generated by crossing the LEU2-marked strain (VAC14) with LWY4552 (leu2,3-112, vac14-1). Of 16 tetrads analyzed, all LEU2 + colonies were wild-type, whereas all leu2 − cells displayed vac14-1 phenotypes.
To create a chromosomal deletion of VAC14, the 3.9-kb SpeI fragment, containing VAC14 and both 5′ and 3′ untranslated regions, was subcloned into pRS424 (pCB49). VAC14 was removed with StyI and BclI and replaced by the PCR-amplified TRP1 gene (digested with StyI and BamHI). The primers used to amplify TRP1 were V41 (5′-GCTACCCCTTGGGTC ACCTTACGTACAATCTTG-3′) and V42 (5′-CGGGATCCCACTCAACCCTATCTCGGTC-3′). The resulting plasmid (pCB52) was digested with SpeI and the 2.5-kb fragment (containing the VAC14 knockout cassette) was transformed into LWY3143. Colony PCR (with the primers v14A; see above) and V43 5′-GACTTGAAATTTTCCTTGC-3′) identified transformants containing TRP1 at the VAC14 locus.
Preparation of antibodies to Vac14p
A GST-VAC14 chimera was created by subcloning VAC14 into the IPTG inducible GST vector pET21a (Marshall et al., 1996). To introduce the appropriate restriction sites (XmaI at the 5′ end and XhoI at the 3′ end), VAC14 was amplified using the primers 5′-CGCGCCCCGGGGACCATGGAAAAATCGATTGCC-3′ and 5′-GGGCTCGAGGGGTTATTTTTTTAATTTATCGG-3′. The PCR product was digested and ligated to pET21a and digested with XmaI and SalI. The resulting plasmid (pCB69) was transformed into Escherichia coli DE3 cells. The soluble GST–Vac14 fusion protein was isolated on glutathione-Sepharose beads and antibodies were raised in goats (Elmira Biological). Serum was depleted of GST antibodies by passaging over a GST-Affi gel column (Bio-Rad Laboratories) and further purified by passage over a total yeast protein column prepared from vac14Δ cells.
Subcellular fractionation and Western blot Analysis
Yeast cell extracts and subcellular fractionations were performed as described (Bonangelino et al., 1997). Briefly, yeast strains were grown in YEPD media at 24°C to an OD600 of 0.6. Cells were harvested, washed with cytosol buffer (20 mM Hepes, pH 6.8, 0.15 M potassium acetate, 10 mM MgCl2, and 0.25 M sorbitol), resuspended to ∼100 OD/ml and lysed with glass beads. Cell extracts were centrifuged at 500 g at 4°C for 4 min. The S5 supernatants were centrifuged at 13,000 g at 4°C for 10 min and the resulting S13 supernatants were centrifuged at 100,000 g at 4°C for 1 h. The pellets (P13 and P100) were resuspended in an equal amount of cytosol cocktail as the supernatants. Laemmli sample loading buffer was added to equivalent OD units of each fraction. Samples were heated to 80°C for 10 min before loading onto a 7.5% SDS-polyacrylamide gel. The proteins were transferred to nitrocellulose (ECL High Bond; Amersham Life Science, Piscataway, NJ) in Tris-glycine-methanol transfer buffer at 4°C at 34 V for 20 h.
Detection of Vac14p was performed with goat anti-Vac14 antibodies at a 1:5,000 dilution. Donkey anti–goat IgG-horseradish peroxidase (Jackson ImmunoResearch Laboratories) at 1:5,000 dilution was the secondary antibody. Detection of Pho8p was performed with rabbit anti-Pho8 antibodies at 1:1,000 dilution and of Kar2p with rabbit anti-Kar2 antibodies at 1:1,000 dilution (provided by Dr. R. Piper, University of Iowa, Iowa City, IA). The secondary antibody, goat anti–rabbit IgG-horseradish peroxidase (Bio-Rad Laboratories) was used at 1:5,000 dilution.
Vacuole isolation on a Ficoll gradient
Yeast vacuoles were isolated as described previously on discontinuous Ficoll gradients (Conradt et al., 1992). Equal micrograms of vacuoles (found as a band at the interface between the 4% and 0% Ficoll) from each strain were separated on a 7.5% SDS polyacrylamide gel, transferred to nitrocellulose, and immunoblotted as described above.
Extraction of Vac14p from the membrane
Vacuoles from wild-type (LWY7235) cells were diluted to 1 μg/μl with 0% Ficoll buffer (10 mM Pipes, pH 6.8, 200 mM Sorbitol) and 100 μl aliquots were treated with one of the following conditions: 2% Triton X-100, 1.4 M urea, 0.1 M Na2CO3, 1 M NaCl, 0.8 M NH2OH, or left untreated (buffer added to the appropriate volume). Samples were incubated on ice for 30 min and centrifuged at 100,000 g at 4°C for 30 min and the pellets were resuspended with an equal volume as the corresponding supernatant fraction. Equal amounts were analyzed by SDS-PAGE and immunoblotting as described above.
Analysis of GFP-Fab1p in vivo
GFP was inserted into the FAB1 ORF in pRS426 by homologous recombination. To introduce FAB1 sequences at both the 5′ and 3′ ends, GFP was amplified by PCR from pGOGFP (Cowles et al., 1997) using the primers FABGFP-N (5′-GCT CAC ATG TCC GGT CGTCCT CCA CTG GTA CTT CAT CTG TGA TGG GTA AAG GAG AAG AAC TTT TC-3′) and FABGFP-C2 (5′-GCG ACG CAG TGC CGG TCA CGT GAC TTG TTG ATG TCG CTG TTG CGG ATC CCG GGC CCG CGG TAC CGT C-3′). The PCR product and AatII-linearized pRS426-FAB1 were cotransformed into yeast and resultant colonies were screened for GFP fluorescence. The pRS426-GFP-FAB1 plasmid was then transformed into various yeast strains and fluorescence was visualized with the same filter set used for quinacrine.
Analysis of phosphatidylinositols
Cells were grown in YEPD or synthetic media to an OD600 between 0.6 and 0.8. 1 OD600 U of cells was harvested, washed, and resuspended in synthetic media lacking inositol. 0.14–0.4 OD600 U were used to inoculate 5 ml of media lacking inositol containing 50 μCi of myo-[2-3H]-inositol. Cells were labeled for 12 h at 24°C, harvested by centrifugation, washed, and resuspended in 100 μl of inositol-free media. For hyperosmotic shock, an equal volume of 1.8 M NaCl was added to cells (for a final concentration of 0.9 M NaCl) and the resulting suspension was incubated at 24°C for 10 min. Then, 800 μl of ice cold 4.5% perchloric acid (Whiteford et al., 1996) was add to the cells and transferred to 15 ml Falcon tubes containing 0.5 g acid-washed glass beads. Cells were lysed by vortexing on high for 30 s with at least a 30 s rest (repeated 10 times for each). The cell extracts were centrifuged at 14,000 rpm for 10 min at 4°C. The pellets were washed with 1 ml of 100 mM EDTA, centrifuged as described above, and resuspended in 50 μl of sterile distilled deionized water.
The lipids were deacylated by treatment with methylamine (Hawkins et al., 1986). Briefly, 1 ml of methylamine reagent (10.7% methylamine, 45.7% methanol, 11.4% n-butanol) was added to each and incubated in a 55°C heat block for 1 h. The samples were dried in a SpeedVac and the pellets were resuspended in 300 μl of sterile water. The samples were centrifuged at 14,000 rpm for 2 min and the supernatants were transferred to new Eppendorf tubes. 300 μl of butanol/ethyl ether/formic acid ethyl ester (20:4:1) was added to each. The samples were vortexed and centrifuged at 14,000 rpm for 2 min. The aqueous phase (bottom layer) was transferred to new tubes and the extraction was repeated. At the end of the second extraction the aqueous phase was dried in a SpeedVac. Samples were resuspended in 20 μl of sterile water and 10 μl of each was analyzed by HPLC using an anion exchange, PartisphereSAX (Whatman), column. The column was developed with a gradient of 1 M (NH4)2HPO4, pH 3.8 (pH adjusted with phosphoric acid): 1% for 5 min, 1–20% over 44 min, 20–50% over 3.75 min, and remained at 50% for 8 min; the flow rate used was 1.0 ml/min (Schu et al., 1993; Stack et al., 1995).
For comparison of phosphatidylinositol polyphosphate levels, the raw cpms in each peak were expressed as a percentage of the total cpms eluted (phosphatidylinositol is ∼90% of the total 3H phosphatidylinositols, whereas phosphatidylinositol polyphosphates are a small percentage, 0.1–3%). The level of PtdIns(3)P identified in the absence of osmotic stress was assigned a value of 100 U; the levels of the other phosphatidylinositol polyphosphates were expressed relative to this value.
Acknowledgments
We would like to thank Terry Kaduce, Shawn Harmon, Dr. Arthur Spector, and Dr. Robert Cohen for use of their HPLCs and their generous assistance in their repair. We thank Dr. Brian Schutte, Dr. Jeff Murray, Bryan Bjork, and Charles Huppmann for their help in building a contig of the mouse ESTs and their advice and insightful discussion. We thank Dr. Robert Piper for antibodies to Pho8p and Kar2p. Finally, we thank Drs. Yong-Xu Wang, Natalie Catlett, Robert Piper, Peter Rubenstein, and Robert Cohen for helpful discussions.
C.J. Bonangelino was supported by an National Institute of General Medical Sciences predoctoral fellowship 1 F31 GM18506-01. J.E. Deux was supported by the National Institutes of Health/National Institute on Aging grant T32 AG00214 to the interdisciplinary Research Training Program on Aging, University of Iowa. This work was supported by National Institutes of Health grant GM50403 to L.S. Weisman.
Footnotes
Abbreviations used in this paper: FM4-64, N-(3-triethylammoniumpropyl)-4-(p-diethyl-aminophenylhexatrienyl); GFP, green fluorescent protein; GST, glutathione S-transferase; PtdIns(4,5)P2, phosphatidylinositol 4,5 bisphosphate.
References
- Altschul, S.F., W. Gish, W. Miller, E.W. Myers, and D.J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410. [DOI] [PubMed] [Google Scholar]
- Barylko, B., D. Binns, K.M. Lin, M.A. Atkinson, D.M. Jameson, H.L. Yin, and J.P. Albanesi. 1998. Synergistic activation of dynamin GTPase by Grb2 and phosphoinositides. J. Biol. Chem. 273:3791–3797. [DOI] [PubMed] [Google Scholar]
- Beck, K.A., and J.H. Keen. 1991. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J. Biol. Chem. 266:4442–4447. [PubMed] [Google Scholar]
- Berridge, M.J. 1993. Inositol trisphosphate and calcium signalling. Nature. 361:315–325. [DOI] [PubMed] [Google Scholar]
- Bonangelino, C.J., N.L. Catlett, and L.S. Weisman. 1997. Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol. Cell. Biol. 17:6847–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley, M.J., P. Lo Surdo, and P.C. Driscoll. 1999. Endocytosis: how dynamin sets vesicles PHree! Curr. Biol. 9:R301–R304. [DOI] [PubMed] [Google Scholar]
- Brown, W.J., D.B. DeWald, S.D. Emr, H. Plutner, and W.E. Balch. 1995. Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J. Cell Biol. 130:781–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, N.J., R.C. Piper, S.R. Gerrard, and T.H. Stevens. 1998. Traffic into the prevacuolar/endosomal compartment of Saccharomyces cerevisiae: a VPS45-dependent intracellular route and a VPS45-independent, endocytic route. Eur. J. Cell Biol. 76:43–52. [DOI] [PubMed] [Google Scholar]
- Burd, C.G., and S.D. Emr. 1998. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell. 2:157–162. [DOI] [PubMed] [Google Scholar]
- Carbrey, J.M., M. Bonhivers, J.D. Boeke, and P. Agre. 2001. Aquaporins in Saccharomyces: characterization of a second functional water channel protein. Proc. Natl. Acad. Sci. USA. 98:1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccetti, P., R. Tisi, E. Martegani, L. Souza Teixeira, R. Lopes Brandao, I. de Miranda Castro, and J.M. Thevelein. 1998. The PLC1 encoded phospholipase C in the yeast Saccharomyces cerevisiae is essential for glucose-induced phosphatidylinositol turnover and activation of plasma membrane H+-ATPase. Biochim. Biophys. Acta. 1405:147–154. [DOI] [PubMed] [Google Scholar]
- Conradt, B., J. Shaw, T. Vida, S. Emr, and W. Wickner. 1992. In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. J. Cell Biol. 119:1469–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, F.T., S.K. Dove, R.K. McEwen, G. Painter, A.B. Holmes, M.N. Hall, R.H. Michell, and P.J. Parker. 1998. The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Curr. Biol. 8:1219–1222. [DOI] [PubMed] [Google Scholar]
- Cowles, C.R., G. Odorizzi, G.S. Payne, and S.D. Emr. 1997. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 91:109–118. [DOI] [PubMed] [Google Scholar]
- Davidson, H.W. 1995. Wortmannin causes mistargeting of procathepsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J. Cell Biol. 130:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, R.W., M. Thomas, J. Cameron, T.P. St. John, S. Scherer, and R.A. Padgett. 1980. Rapid DNA isolation for enzymatic and hybridization analysis. Methods Enzymol. 65:404–411. [DOI] [PubMed] [Google Scholar]
- De Camilli, P., S.D. Emr, P.S. Mcpherson, and P. Novick. 1996. Phosphoinositides as regulators in membrane traffic. Science. 271:1533–1539. [DOI] [PubMed] [Google Scholar]
- Dove, S.K., F.T. Cooke, M.R. Douglas, L.G. Sayers, P.J. Parker, and R.H. Michell. 1997. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature. 390:187–192. [DOI] [PubMed] [Google Scholar]
- Ford, M.G., B.M. Pearse, M.K. Higgins, Y. Vallis, D.J. Owen, A. Gibson, C.R. Hopkins, P.R. Evans, and H.T. McMahon. 2001. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 291:1051–1055. [DOI] [PubMed] [Google Scholar]
- Gary, J.D., A.E. Wurmser, C.J. Bonangelino, L.S. Weisman, and S.D. Emr. 1998. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J. Cell Biol. 143:65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaullier, J.M., E. Ronning, D.J. Gillooly, and H. Stenmark. 2000. Interaction of the EEA1 FYVE Finger with phosphatidylinositol 3-phosphate and early endosomes. J. Biol. Chem. 275:24595–24600. [DOI] [PubMed] [Google Scholar]
- Gomes de Mesquita, D.S. B. van den Haazel, J. Bouwman, and C.L. Woldringh. 1996. Chracterization of new vacuolar segregation mutants, isolated by screening for loss of proteinase B self-activation. Eur. J. Cell Biol. 71:237–247. [PubMed] [Google Scholar]
- Hardie, R.C., P. Raghu, S. Moore, M. Juusola, R.A. Baines, and S.T. Sweeney. 2001. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron. 30:149–159. [DOI] [PubMed] [Google Scholar]
- Hawkins, P.T., L. Stephens, and C.P. Downes. 1986. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem. J. 238:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, J.C., P.L. Fisette, G.H. Jenkins, K. Fukami, T. Takenawa, R.A. Anderson, and T.F.J. Martin. 1995. ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature. 374:173–177. [DOI] [PubMed] [Google Scholar]
- Herman, P.K., J.H. Stack, and S.D. Emr. 1992. An essential role for a protein and lipid kinase complex in secretory protein sorting. Trends Cell Biol. 2:363–368. [DOI] [PubMed] [Google Scholar]
- Huang, C.L., S. Feng, and D.W. Hilgemann. 1998. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 391:803–806. [DOI] [PubMed] [Google Scholar]
- Itoh, T., S. Koshiba, T. Kigawa, A. Kikuchi, S. Yokoyama, and T. Takenawa. 2001. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 291:1047–1051. [DOI] [PubMed] [Google Scholar]
- Joly, M., A. Kazlauskas, and S. Corvera. 1995. Phosphatidylinositol 3-kinase activity is required at a postendocytic step in platelet-derived growth factor receptor trafficking. J. Biol. Chem. 270:13225–13230. [DOI] [PubMed] [Google Scholar]
- Jost, M., F. Simpson, J.M. Kavran, M.A. Lemmon, and S.L. Schmid. 1998. Phosphatidylinositol-4,5-bisphosphate is required for endocytic coated vesicle formation. Curr. Biol. 8:1399–1402. [DOI] [PubMed] [Google Scholar]
- Kearns, B.G., J.G. Alb, Jr., and V. Bankaitis. 1998. Phosphatidylinositol transfer proteins: the long and winding road to physiological function. Trends Cell Biol. 8:276–282. [DOI] [PubMed] [Google Scholar]
- Lanier, L.M., and F.B. Gertler. 2000. Actin cytoskeleton: thinking globally, actin' locally. Curr. Biol. 10:R655–R657. [DOI] [PubMed] [Google Scholar]
- Liscovitch, M., and L.C. Cantley. 1995. Signal transduction and membrane traffic: the PITP/phosphoinositide connection. Cell. 81:659–662. [DOI] [PubMed] [Google Scholar]
- Marshall, N.F., J. Peng, Z. Xie, and D.H. Price. 1996. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J. Biol. Chem. 271:27176–27183. [DOI] [PubMed] [Google Scholar]
- Martinoia, E., A. Massonneau, and N. Frangne. 2000. Transport processes of solutes across the vacuolar membrane of higher plants. Plant Cell Physiol. 41:1175–1186. [DOI] [PubMed] [Google Scholar]
- Maurel, C., and M.J. Chrispeels. 2001. Aquaporins. A molecular entry into plant water relations. Plant Physiol. 125:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, R.K. S.K. Dove, F.T. Cooke, G.F. Painter, A.B. Holmes, A. Shissheva, Y. Ohya, P.J. Parker, and R.H. Michell. 1999. Complementation analysis in PtdInsP kinase-deficient yeast mutants demonstrates that Schizosaccharomyces pombe and murine Fab1p homologues are phosphatidylinositol 3-phosphate 5-kinases. J. Biol. Chem. 274:33905–33912. [DOI] [PubMed] [Google Scholar]
- Meyrial, V., V. Laize, R. Gobin, P. Ripoche, S. Hohmann, and F. Tacnet. 2001. Existence of a tightly regulated water channel in Saccharomyces cerevisiae. Eur. J. Biochem. 268:334–343. [DOI] [PubMed] [Google Scholar]
- Michell, R.H. 1997. The multiplying roles of inositol lipids and phosphates in cell control processes. Essays Biochem. 32:31–47. [PubMed] [Google Scholar]
- Odorizzi, G., M. Babst, and S.D. Emr. 2000. Phosphoinositide signaling and the regulation of membrane trafficking in yeast. Trends Biochem. Sci. 25:229–235. [DOI] [PubMed] [Google Scholar]
- Patki, V., D.C. Lawe, S. Corvera, J.V. Virbasius, and A. Chawla. 1998. A functional PtdIns(3)P-binding motif. Nature. 394:433–434. [DOI] [PubMed] [Google Scholar]
- Peterson, M.R., C.G. Burd, and S.D. Emr. 1999. Vac1p coordinates Rab and phosphatidylinositol 3-kinase signaling in Vps45p-dependent vesicle docking/fusion at the endosome. Curr. Biol. 9:159–162. [DOI] [PubMed] [Google Scholar]
- Rameh, L.E., K.F. Tolias, B.C. Duckworth, and L.C. Cantley. 1997. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature. 390:192–196. [DOI] [PubMed] [Google Scholar]
- Roth, M.G. 1999. Lipid regulators of membrane traffic through the Golgi complex. Trends Cell Biol. 9:174–179. [DOI] [PubMed] [Google Scholar]
- Schu, P.V., K. Takegawa, M.J. Fry, J.H. Stack, M.D. Waterfield, and S.D. Emr. 1993. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 260:88–91. [DOI] [PubMed] [Google Scholar]
- Sherman, F. 1991. Getting started with yeast. Methods in Enymol. Vol. 194. C. Guthrie and G.R. Fink, editors. Academic Press, Inc., San Diego. 281–301.
- Sikorski, R.S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulations of DNA in Saccharomyces cerevisiae. Genetics. 122:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack, J.H., D.B. DeWald, K. Takegawa, and S.D. Emr. 1995. Vesicle-mediated protein transport: regulatory interactions between the Vps15 protein kinase and the Vps34 PtdIns 3-kinase essential for protein sorting to the vacuole in yeast. J. Cell Biol. 129:321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack, J.H., P.K. Herman, P.V. Schu, and S.D. Emr. 1993. A membrane-associated complex containing the Vps15 protein kinase and the Vps34 PI 3-kinase is essential for protein sorting to the yeast lysosome-like vacuole. EMBO J. 12:2195–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark, H., and R. Aasland. 1999. FYVE-finger proteins - effectors of an inositol lipid. J. Cell Sci. 112:4175–4183. [DOI] [PubMed] [Google Scholar]
- Sui, J.L., J. Petit-Jacques, and D.E. Logothetis. 1998. Activation of the atrial KACh channel by the βγ subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc. Natl. Acad. Sci. USA. 95:1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall, G.G., H. Hama, D.B. DeWald, and B.F. Horazdovsky. 1999. The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol. Biol. Cell. 10:1873–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker, A., and L.C. Cantley. 1997. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 387:673–676. [DOI] [PubMed] [Google Scholar]
- Uchida, E., Y. Ohsumi, and Y. Anraku. 1988. Purification fo yeast vacuolar membrane H+-ATPase and enzymological discrimination of three ATP-driven proton pumps in Saccharomyces cerevisiae. Methods Enzymol. 157:544–563. [DOI] [PubMed] [Google Scholar]
- Vida, T.A., and S.D. Emr. 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128:779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.-X., H. Zhao, T. Harding, D.S. Gomes de Mesquita, C.L. Woldringh, D.J. Klionsky, A.L. Munn, and L.S. Weisman. 1996. Multiple classes of yeast mutants are defective in vacuole partitioning yet target vacuole proteins correctly. Mol. Biol. Cell. 7:1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.X., N.L. Catlett, and L.S. Weisman. 1998. Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasm to vacuole. J. Cell Biol. 140:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.X., E.J. Kauffman, J.E. Duex, and L.S. Weisman. 2001. Fusion of docked membranes requires the armadillo repeat protein Vac8p. J. Biol. Chem. 276:35133–35140. [DOI] [PubMed] [Google Scholar]
- Weisman, L.S., R. Bacallao, and W. Wickner. 1987. Multiple methods of visualizing the yeast vacuole permit evaluation of its morphology and inheritance during the cell cycle. J. Cell Biol. 105:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford, C.C., C. Best, A. Kazlauskas, and E.T. Ulug. 1996. D-3 phosphoinositide metabolism in cells treated with platelet-derived growth factor. Biochem. J. 319:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford, C.C., C.A. Brearley, and E.T. Ulug. 1997. Phosphatidylinositol 3,5-bisphosphate defines a novel PI 3-kinase pathway in resting mouse fibroblasts. Biochem. J. 323:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, A., D.B. DeWald, I.V. Boronenkov, R.A. Anderson, S.D. Emr, and D. Koshland. 1995. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol. Biol. Cell. 6:525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]