Abstract
The Neisseria type IV pilus promotes bacterial adhesion to host cells. The pilus binds CD46, a complement-regulatory glycoprotein present on nucleated human cells (Källström et al., 1997). CD46 mutants with truncated cytoplasmic tails fail to support bacterial adhesion (Källström et al., 2001), suggesting that this region of the molecule also plays an important role in infection. Here, we report that infection of human epithelial cells by piliated Neisseria gonorrhoeae (GC) leads to rapid tyrosine phosphorylation of CD46. Studies with wild-type and mutant tail fusion constructs demonstrate that Src kinase phosphorylates tyrosine 354 in the Cyt2 isoform of the CD46 cytoplasmic tail. Consistent with these findings, infection studies show that PP2, a specific Src family kinase inhibitor, but not PP3, an inactive variant of this drug, reduces the ability of epithelial cells to support bacterial adhesion. Several lines of evidence point to the role of c-Yes, a member of the Src family of nonreceptor tyrosine kinases, in CD46 phosphorylation. GC infection causes c-Yes to aggregate in the host cell cortex beneath adherent bacteria, increases binding of c-Yes to CD46, and stimulates c-Yes kinase activity. Finally, c-Yes immunoprecipitated from epithelial cells is able to phosphorylate the wild-type Cyt2 tail but not the mutant derivative in which tyrosine 354 has been substituted with alanine. We conclude that GC infection leads to rapid tyrosine phosphorylation of the CD46 Cyt2 tail and that the Src kinase c-Yes is involved in this reaction. Together, the findings reported here and elsewhere strongly suggest that pilus binding to CD46 is not a simple static process. Rather, they support a model in which pilus interaction with CD46 promotes signaling cascades important for Neisseria infectivity.
Keywords: Neisseria type IV pili; CD46; tyrosine phosphorylation; c-Yes activation; adhesion
Introduction
The type IV pilus expressed by pathogenic Neisseria is a retractile structure that mediates bacterial motility on solid substrates (Brossay et al., 1994; Wall and Kaiser, 1999; Merz et al., 2000), DNA uptake (Wolfgang et al., 1998), and adhesion to host cells (Swanson, 1973; Nassif et al., 1993). Pili on live bacteria induce the formation of adhesion-promoting cortical plaques directly beneath the site of attachment. These plaques contain high concentrations of cortical actin and ezrin, membrane glycoproteins ICAM1, EGF receptor, and CD44, and unidentified tyrosine-phosphorylated proteins (Merz and So, 1997; Merz et al., 1999). Purified pili trigger the release of Ca2+ from intracellular stores (Källström et al., 1998) and a subsequent redistribution of Lamp1 compartments in target cells (Ayala et al., 1998). These activities of the type IV pilus suggest that the structure may promote attachment by stimulating signaling pathways in the host cell.
The Neisseria type IV pilus binds CD46, or membrane cofactor protein (Källström et al., 1997), a complement-regulatory membrane glycoprotein that prevents cell damage by autologous complement components (Liszewski et al., 1991; Seya, 1995). CD46 consists of an ectodomain with four short consensus repeats (SCR1–4) followed by a segment of serine-threonine-proline–rich domains (STP-A, -B, and -C), a short region of unknown function, a transmembrane anchor, a juxtamembrane cytoplasmic segment (JxM), and one of two cytoplasmic tails (Cyt1 or Cyt2). CD46 isoforms, the result of differential RNA splicing, differ in the number of SCR domains and their cytoplasmic tail. Most cell types express all CD46 isoforms, though in different ratios. Efficient adherence of Neisseria gonorrhoeae (GC)* requires SCR-3 and the STP domain (Källström et al., 2001). CD46 serves as a receptor for numerous pathogens, including group A strains of measles virus (Dorig et al., 1993), Streptococcus pyogenes (Okada et al., 1995), and human herpesvirus 6 (Santoro et al., 1999).
The CD46 cytoplasmic tails are potential substrates for cellular kinases and therefore are likely to have signaling functions. In the RAW264.7 mouse macrophage cell line, the CD46 tail interacts with multiple kinases, and this interaction correlates with tyrosine phosphorylation of the CD46 cytoplasmic domains (Wong et al., 1997). In Jurkat cells, the CD46 Cyt2 isoform is tyrosine phosphorylated by the Src kinase Lck after antibody ligation (Wang et al., 2000).
Cells expressing CD46 with truncated tails fail to support bacterial adhesion (Källström et al., 2001), suggesting that the tail fulfills an important function in this process. Furthermore, GC adhesion is inversely proportional to the level of expression of CD46 (Tobiason and Seifert, 2001), indicating that pilus-mediated adhesion does not require high levels of CD46. These observations strongly suggest that pilus-mediated cellular adhesion may occur through signaling cascade(s) generated through the CD46 cytoplasmic tail.
To better understand the role of CD46 in Neisseria adhesion, we studied the fate of its cytoplasmic COOH terminus upon infection. In particular, we tested the hypothesis that GC infection would trigger tyrosine phosphorylation of the CD46 COOH terminus. We report that GC infection of human epithelial cells triggers rapid tyrosine phosphorylation of CD46 and exogenous Src kinase phosphorylates the Cyt2 tail isoform at tyrosine 354 in vitro. In support of these findings, treatment of epithelial cells with PP2, a specific Src family kinase inhibitor, but not PP3, an inactive variant of this drug, reduces GC adherence. Several lines of evidence strongly suggest that the Src kinase c-Yes is involved in this reaction. GC infection rapidly leads to the clustering of c-Yes in the cell cortex beneath adherent bacteria, increases binding of c-Yes to the CD46 tail, and results in c-Yes activation. Finally, c-Yes isolated from epithelial cells preferentially phosphorylates the CD46 Cyt2 tail at tyrosine 354. We conclude that GC infection triggers rapid phosphorylation of tyrosine residue 354 of the Cyt2 tail of CD46 and c-Yes participates in these reactions. The results reported here and elsewhere support the notion that the GC type IV pilus promotes bacterial adherence through signaling cascade(s) via the CD46 tail.
Results and discussion
CD46 is rapidly tyrosine phosphorylated upon GC infection
We tested the hypothesis that GC infection triggers tyrosine phosphorylation of CD46. A431 human endocervical epithelial cells were infected with GC strain MS11 N400 (Wolfgang et al., 1998) (P+, Opa−), and CD46 was immunoprecipitated from cell lysates with a monoclonal antibody that recognizes the SCR repeats (Mohler et al., 1999). The presence of phosphorylated tyrosine residues in the precipitate was determined by immunoblotting with a monoclonal antibody that recognizes phosphotyrosine (Fig. 1 A, top). Infected cells had noticeably higher levels of tyrosine phosphorylated CD46 than uninfected cells (0 time point). The level of tyrosine phosphorylated CD46 peaked within 5 min after infection and was undetectable by 30 min postinfection. Total levels of CD46 in the precipitate were determined by reprobing the same blot with polyclonal anti-CD46 antibodies recognizing all isoforms of the protein (Wang et al., 2000; Källström et al., 2001). These controls show that the same amount of CD46 had been immunoprecipitated from each sample (Fig. 1 A, bottom). Thus, the increase in tyrosine-phosphorylated CD46 occurred specifically in response to bacterial infection. Tyrosine-phosphorylated CD46 was not detectable in infected cultures treated with Src kinase inhibitor PP2 (unpublished data). These and all other experiments have been repeated at least twice with identical results. Finally, the same results were obtained with GC-infected T84 epithelial cells (unpublished data). Thus, GC infection induces CD46 tyrosine phosphorylation in more than one cell line.
Figure 1.
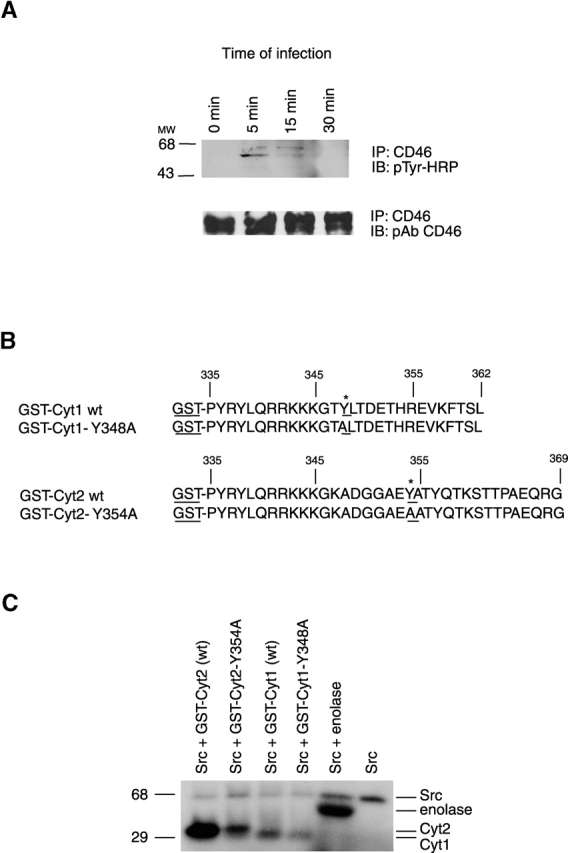
Identification of the site of CD46 tyrosine phosphorylation. (A) Presence of phosphotyrosine in CD46 immunoprecipitated from GC-infected cells. CD46 was immunoprecipated from A431 cells infected with MS11 N400 for various lengths of time using an anti-CD46 monoclonal antibody (Immunotech). The presence of phosphorylated tyrosine residues in the precipitate (top) was determined by immunoblotting with HRP-labeled monoclonal antibodies to phosphotyrosine (pTyr-HRP). Total levels of CD46 in the precipitates (bottom) were determined by reprobing the same blot with polyclonal antibodies (pAb) to CD46 (a gift from J. Atkinson, Washington University). (B) Sequences of the CD46 tails fused to GST. The Cyt1 and Cyt2 isoforms of the CD46 tail were fused to GST at Pro-335. In the GST-Cyt1 Y348A fusion protein, tyrosine at position 348 was changed to alanine. In the GST-Cyt2 Y354A fusion, tyrosine at position 354 was changed to alanine. (C) Phosphorylation of GST-Cyt2 by c-Src. GST fusion proteins were incubated with exogenous c-Src (Upstate Biotechnology) and [γ-32P]ATP in an in vitro kinase assay with enolase (Sigma-Aldrich) as the control substrate for Src activity.
The migration of immunoprecipitated CD46 as two major bands in SDS-PAGE (Fig. 1 A, bottom) reflects the polymorphic nature of the receptor. It is not clear how many CD46 isoforms are in these bands or how the proteins in these bands differ with regard to their SCR repeats, STP regions, and cytoplasmic tails. At the moment, there is no reagent that can distinguish between isoforms containing Cyt1 and Cyt2 tails.
Src kinase phosphorylates CD46 at Y354 of the Cyt2 isoform in vitro
The CD46 Cyt1 and Cyt2 COOH termini contain tyrosine residues (Y348 in Cyt1 and Y354 in Cyt2) that are potential substrates for tyrosine kinases (Wang et al., 2000) (Fig. 1 B). We determined whether Src kinase is able to phosphorylate these tails in vitro. Wild-type and mutant Cyt1 and Cyt2 tails (Fig. 1 B) were fused to glutathione S-transferase (GST) and purified from Escherichia coli. Fusion proteins were incubated with Src and [γ-32P]ATP and analyzed by SDS-PAGE and autoradiography. As expected, Src phosphorylated itself and enolase, a commonly used substrate for Src family kinases. Src also robustly phosphorylated wild-type GST-Cyt2 (Fig. 1 C). In contrast, it phosphorylated the GST-Cyt2Y354A mutant, and the wild-type and mutant GST-Cyt1 tails at very low levels.
The low level Src activity on the latter three GST tail fusions is unlikely to be due to contaminants copurifying with the fusions, since these constructs were purified from E. coli, which does not make tyrosine kinase. The background activity is most likely to be due to low level phosphorylation of tyrosine residue 336 at the NH2-terminal end of the tail fusions (Fig. 1 B). This residue is in the transmembrane domain of CD46 (Liszewski et al., 1991) and would therefore be inaccessible to kinases in vivo. However, because our purified fusion proteins are free of membrane Src may be able to phosphorylate Y336 at low levels, even though the flanking sequences are not consensus tyrosine kinase sites. The difference in phosphorylation levels between CD46 wtCyt1 and CD46 Y348ACyt1 is most likely due to background phosphorylation on the tyrosine at 348. However, this difference is slight compared with that seen between the wild-type Cyt2 tails. When we compare phosphorylation levels of CD46 Cyt2wt and Cyt1wt, it is clear that the tyrosine residue at 348 is not phosphorylated above background.
Together, results from this experiment indicate that the Cyt2 tail of CD46 is a substrate for Src kinase in vitro, and phosphorylation occurs at Y354. These data lend additional support to our previous findings of the presence of tyrosine phosphorylated CD46 in GC-infected cells.
Src family tyrosine kinases play a role in bacterial adherence
We next examined the importance of Src family tyrosine kinases in GC adherence. A431 cultures were infected with MS11 N400 in the presence of PP2, an inhibitor specific for Src family kinases (Hanke et al., 1996), or PP3, an inactive variant of this drug, and bacterial adhesion to these cells were determined. PP2 inhibits Src kinase activity by competing with ATP for binding at the catalytic domain of the target enzyme. PP3, a chemical variant of PP2, does not inhibit Src kinases. Bacterial adhesion to PP2-treated cultures was reduced by 47.5% compared with adhesion to untreated cultures (adhesion index for PP2-treated cultures, 16.3 ± 5.0% SD; adhesion index for untreated cultures, 31 ± 5.1% SD). In contrast, adhesion to PP3 cultures treated was unaffected (31.5 ± 6.7% SD adhesion index). These results strongly suggest that GC adhesion to epithelial cells requires the participation of Src family tyrosine kinases. They are consistent with a recent report demonstrating that Neisseria meningitidis infection of endothelial cells is reduced in the presence of Src kinase inhibitors (Hoffmann et al., 2001). It is likely that the activation of other kinases by Neisserial adhesins contributes to the overall infection process (Chen et al., 1997; Hauck et al., 1998; Hoffmann et al., 2001). Other GC adhesins trigger signaling cascades and cross-talk is likely to occur (Merz and So, 2000; Pujol et al., 2000), making the complete abrogation of adhesion by a Src family–specific inhibitor unlikely. However, the significant reduction in adhesion by PP2 indicates that Src family kinases play an important role in infection. Indeed, these findings are consistent with our hypothesis that signaling through CD46 activation contributes signaling cascades that are important for Neisseria pathogenesis.
c-Yes aggregates beneath adherent GC microcolonies
Piliated Neisseria triggers the formation of cortical plaques, structures beneath sites of bacterial attachment that are enriched in membrane proteins, signaling proteins, and cytoskeletal components (Merz and So, 1997; Merz et al., 1999). One constituent of these plaques is ezrin, whose function is to tether proteins, directly or indirectly, to the actin cytoskeleton (Bretscher et al., 1997). The Src kinase p62c-Yes associates with ezrin in MDCK cells (Crepaldi et al., 1997) and is enriched in many epithelial cell types (Zhao et al., 1990; Mohler et al., 1999). Furthermore, c-Yes and Src have overlapping substrate specificities. Therefore, c-Yes is a candidate kinase for CD46 activation.
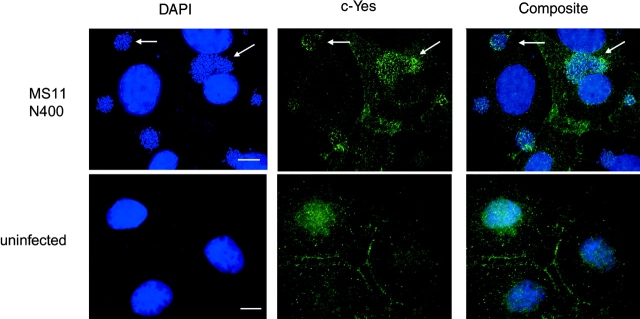
We used several approaches to investigate the participation of c-Yes in CD46 phosphorylation. First, the location of c-Yes in infected and uninfected cells was compared by immunofluorescence deconvolution microscopy. c-Yes visibly accumulated beneath GC microcolonies in infected cells (Fig. 2). In contrast, the kinase was diffusely distributed throughout the cytosol of uninfected cells. c-Yes was also observed to cluster beneath GC in infected T84 cells (unpublished data).
Figure 2.
c-Yes clustering beneath GC microcolonies in infected epithelial cells. A431 endocervical epithelial cells were infected with MS11 N400 for 3 h (top) or left uninfected (bottom), and then fixed and processed for immunofluorescence deconvolution microscopy using anti–c-Yes antibodies to visualize the kinase (middle) and DAPI to visualize nuclei and GC microcolonies (left). The clustering of c-Yes beneath the microcolonies can be seen in the composite image (right). Arrows denote the location of some microcolonies. Images are 0.2-μm-thick optical sections of the apical surfaces of cells obtained using a Deltavision restoration microscope (API) with a 60× oil objective.
c-Yes is activated and associates with CD46 after infection
Using a coimmunoprecipitation approach, we next examined the ability of c-Yes to associate with the CD46 tail. CD46 was immunoprecipitated from GC-infected A431 cells and immunoblotted with c-Yes antisera to determine the presence of the kinase in the precipitate (Fig. 3 A). A large amount of c-Yes coimmunoprecipitated with CD46 within 5 min of infection. The amount of coimmunoprecipitated c-Yes decreased with longer periods of infection. Very little of the kinase was found in association with CD46 in uninfected cells (0 min). Control immunoblots of c-Yes in total cell lysates showed that equal amounts of sample had been loaded into the gel. Densitometric analyses of the c-Yes signal from the 0 and 5 min time points indicate that the amount of c-Yes associated with CD46 tail increased fourfold upon infection. EGF receptor, a membrane protein present in the cortical plaques, did not coimmunoprecipitate with CD46 from infected or uninfected cells (unpublished data). These results strongly suggest that GC infection of epithelial cells results in a rapid but brief physical association of c-Yes with CD46.
Figure 3.
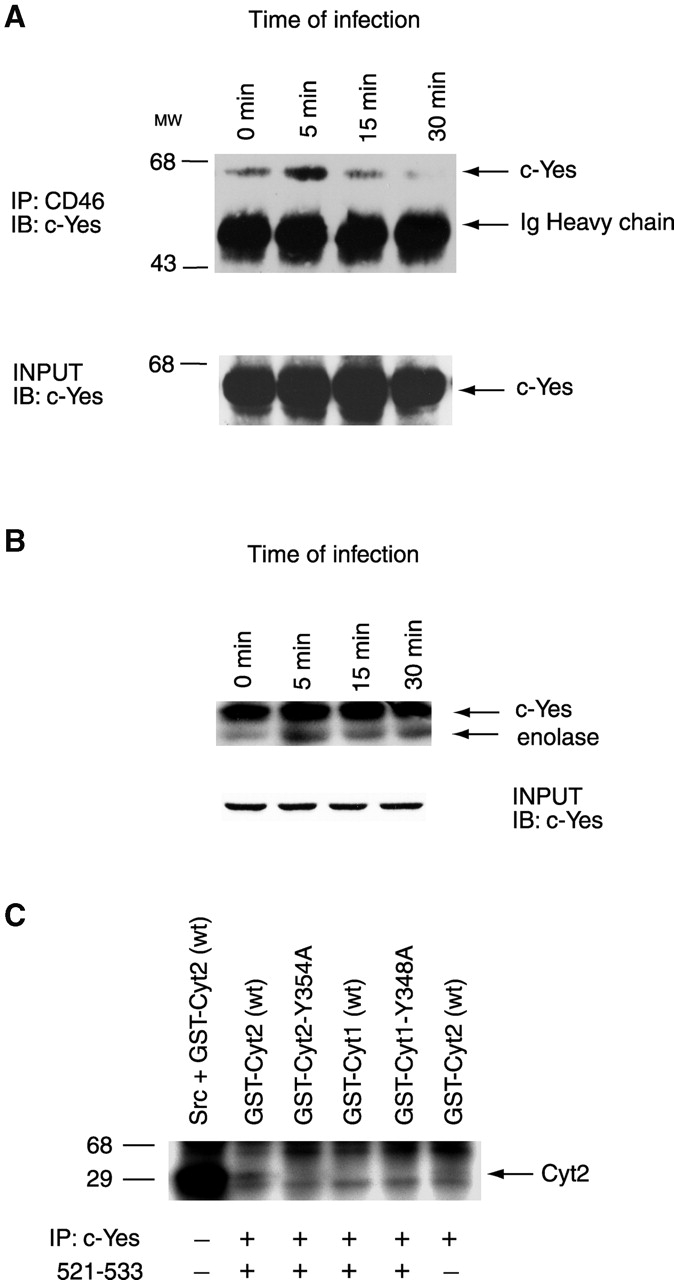
Association of c-Yes tyrosine kinase with CD46 and its activation upon infection. (A) Coimmunoprecipitation of c-Yes with CD46. CD46 was immunoprecipitated from A431 cells infected with MS11 N400. The presence of c-Yes in the precipitates was determined by immunoblotting with monoclonal anti–c-Yes antibodies (top). Inputs (total cell lysates from each time point) were immunoblotted with anti–c-Yes antibodies to confirm uniform levels of the kinase in the samples (bottom). (B) Activation of c-Yes upon GC infection. c-Yes was immunoprecipitated from MS11 N400-infected A431 cells and used in an in vitro kinase assay with enolase as the substrate (top). Inputs (total cell lysates from each time point) were immunoblotted with anti–c-Yes antibodies to confirm uniform levels of the kinase in the samples (bottom). (C). c-Yes phosphorylation of CD46 Cyt2 Tyr354. c-Yes was immunoprecipitated from uninfected A431 cells and activated with Src peptide 521–533 before incubation with GST-CD46 tail fusion proteins in the presence of [γ-32P]ATP. (Lane 1) GST-Cyt2 wild-type incubated with Src. (Lanes 2–5) Other GST-CD46 tail isoforms as indicated incubated with activated c-Yes. (Lane 6) GST- Cyt2 wild-type incubated with c-Yes in the absence of activating peptide.
We also determined whether GC infection stimulates c-Yes kinase activity. c-Yes was immunoprecipitated from GC-infected A431 cells, and the precipitate was incubated with enolase and [γ-32P]ATP. Phosphorylation of enolase was detected by SDS-PAGE and autoradiography (Fig. 3 B). As expected, c-Yes autophosphorylated. c-Yes from infected cells phosphorylated enolase to a greater degree than c-Yes from uninfected cells (0 time point). Cells infected for 5 min had the highest c-Yes activity. A parallel control experiment in which total cell lysates were immunoblotted for c-Yes demonstrated that equal amounts of the kinase were present in each sample. These results show that c-Yes is rapidly but briefly activated upon GC infection.
c-Yes isolated from epithelial cells phosphorylates CD46 Cyt2 at Tyr354
Finally, we tested the ability of c-Yes to phosphorylate the CD46 tail. c-Yes was immunoprecipitated from uninfected A431 cells and incubated with CD46 GST tail fusion proteins (GST-Cyt1, GST-Cyt2, GST-Cyt1-Y348A, and GST-Cyt2 Y354A) in the presence of [γ-32P]ATP. The majority of c-Yes in uninfected cells was expected to be in the inactive form. Therefore, the immunoprecipitates were incubated with phosphoSrc peptide 521–533 (Calbiochem) before the kinase assay. This peptide binds to the Src homology (SH)2 domain of c-Yes and relieves inhibitory intra- or intermolecular interactions with a phosphotyrosine in the COOH terminus of the enzyme. Activated c-Yes robustly phosphorylated GST-Cyt2 (Fig. 3, lane 2). As expected, c-Yes phosphorylated itself (upper 62-kD band) and a protein migrating immediately below all the GST tail fusions. The identity of this lower band in unknown but is likely to be a protein that coprecipitated with cYes, since it is absent from samples lacking c-Yes (unpublished data). The fact that c-Yes phosphorylated GST-Cyt2 but not GST-Cyt2Y354A demonstrates the specificity of the kinase for the tyrosine residue at position 354 of the Cyt2 tail. The fact that c-Yes did not phosphorylate the Cyt1 tails demonstrates its specificity for Cyt2.
In this report, we presented evidence that GC infection results in rapid tyrosine phosphorylation of CD46 (Fig. 1 A). Exogenous Src robustly phosphorylates wild-type GST-Cyt2 fusion protein in contrast to its low level activity on the mutant GST-Cyt2Y354A fusion, the wild-type GST-Cyt1 fusion, and the mutant GST-Cyt1Y348A fusion (Fig. 1 C). PP2 inhibitor studies are consistent with these findings and indicate the importance of Src family kinases in promoting GC adherence to epithelial cells. Many bacterial pathogens trigger cellular phosphorylation events through Src family tyrosine kinases (Isberg and Leong, 1990; Bliska et al., 1993; Rosenshine et al., 1994; Van Putten et al., 1994; Dehio et al., 1995; Finlay and Cossart, 1997; Van Langendonck et al., 1998). Inhibiting Src kinases reduces the infectivity of Pseudomonas aeruginosa for epithelial cells (Esen et al., 2001) and N. meningitidis for endothelial cells (Hoffmann et al., 2001). Our inhibitor studies are also consistent with these findings.
Several lines of evidence strongly suggest that c-Yes participates in the phosphorylation of the CD46 tail. c-Yes is abundant in the apical regions of human epithelial cells, the site of Neisseria infection. c-Yes is recruited to the cell cortex beneath adherent GC (Fig. 2). Infection rapidly activates c-Yes and increases its association with CD46 (Fig. 3). The timing of CD46 phosphorylation upon GC infection is exactly coincident with the timing of c-Yes activation and binding to CD46. Finally, c-Yes isolated from epithelial cells is able to phosphorylate the wild-type GST-Cyt2 tail fusion but has little activity on the mutant GST-Cyt2Y354A tail or the GST-Cyt1 tails (Fig. 3).
The Cyt2 tail is found in association with many CD46 isoforms. Since there is no reagent that will distinguish the two tail isoforms from each other, we are unable to determine which CD46 ectodomain and STP region(s) are associated with the phosphorylated Cyt2 tail. The cytoplasmic tails of many signaling proteins are often associated with complexes of kinases, phosphatases, and anchoring proteins; in some cases, receptor activation requires the serial activation of these enzymes. Indeed, the CD46 tail has been shown to interact with several unidentified kinases from a macrophage cell line (Wong et al., 1997). Moreover, recent studies demonstrate a functional interaction between CD46 and Dlg4, an important scaffolding protein containing protein–protein interaction domains and an SH3 domain (Ludford-Menting et al., 2002). It is unclear whether c-Yes directly phosphorylates Cyt2 in GC-infected cells. Nevertheless, our results indicate that c-Yes has the capability of phosphorylating the Cyt2 tail, either directly or through an intermediary. Further experiments will clarify this issue.
CD46 is a substrate for Src kinase in Jurkat cells (Wang et al., 2000). Upon antibody ligation of CD46, Cyt2 is rapidly phosphorylated by Lck, a Src kinase family member enriched in immune cells. As in GC-infected epithelial cells, CD46 phosphorylation occurs rapidly in Jurkat cells, reaching a peak within minutes after ligation and declining thereafter. The rise and fall of phosphorylated protein levels is a common theme in signal transduction, where cycles of phosphorylation are rapidly followed by cycles of dephosphorylation.
How does CD46 phosphorylation promote GC infectivity? One hypothesis is that CD46 activation triggers the formation of cortical plaques. These plaques, which promote Neisseria adhesion, contain high concentrations of cytoskeletal components, tyrosine-phosphorylated proteins, signaling molecules, and Opa receptors (Merz and So, 1997; Merz et al., 1999; Hoffmann et al., 2001 ). Both CD46 phosphorylation and cortical plaque formation are triggered by infection with piliated organisms. It is reasonable then to speculate that the rapid association of activated c-Yes with the CD46 tail upon infection may serve to localize signaling machinery to the sites of bacterial attachment and that these reactions in turn trigger plaque formation. A similar process occurs in the formation of focal adhesion complexes, where attachment to substrates or other cells triggers signal transduction cascades that result in the clustering of cytoskeletal components, tyrosine-phosphorylated proteins, and signaling molecules to the cell cortex at sites of adhesion (Singer, 1992; Yamada and Geiger, 1997). Thus, bacteria may engage signaling pathways that are normally used for cell–cell or cell–substrate adhesion.
Neisseria pili also trigger the release of Ca2+ from intracellular stores and lysosome exocytosis (Ayala et al., 1998; Källström et al., 1998). Together, the activities of the Neisseria type IV pilus argue against the traditional model for pilus function in which the structure serves to bind bacteria passively to host cells. Rather, they support a model in which pilus interaction with CD46 promotes signaling cascades important for Neisseria infectivity.
Materials and methods
Cell lines, bacterial strains, and infections
A431 cells were maintained in DME with 10% heat-inactivated FBS at 37°C (Life Technologies) and 5% CO2. GC strain MS11 N400 (Wolfgang et al., 1998) was used for all infection experiments. Piliation and Opa phenotypes were monitored by colony morphology. For infection experiments, bacteria were resuspended and diluted into unsupplemented DME, and then added to epithelial cells at a ratio of 500 colony forming units per cell or as specified. E. coli were grown on Luria-Bertani agar.
Construction and purification of GST-CD46 tail fusion proteins
Sequences of the Cyt1 and Cyt2 isoforms of the CD46 tails have been published (Lublin et al., 1988; Wong et al., 1997). CD46 Cyt1 and Cyt2 isoforms encoding amino acids 335–362 of the Cyt1 tail or 335–369 of the Cyt2 tail were amplified by PCR using CD46 BC-Cyt1 or CD46 BC-Cyt2 cDNA as template and the following primers: Cyt2 wild-type, upstream, 5′-AGGATCCCGTACAGATATCTTCAAAGG-3′ and downstream 5′-TCTCGAGTCAGCCTCTCTGCTCTGCTGGAGT-3′; Cyt2 Y354A, downstream, 5′-GCCTCTCTGCTCTGCTGGAGTGGTTGATTTAGTCTGGTAAGTGGCAGCTTTCAGC-TCC-3′; Cyt1 wild-type, upstream, 5′-TAGCAATTTGGAGCGGTAAGCC-CCCAATAT-3′ and downstream, 5′-TCTCGAGTCAGAGAGAAGTAAAA-TTTTACTTC-3′; and Cyt1 Y348A, 5′-TCTCGAGTCAGAGAGAAGTAAA-TTTTACTTCTCTGTGGGTCTCATCAGTTAGGGCTGTGCCTTTC-3′. Primers were used to create a BamH1 site proximal to the tail and an Xho1 site immediately after the stop codon. Y to A point mutations in the cytoplasmic tails were introduced by primer-directed PCR mutagenesis as described (Hirano et al., 1996; Yant et al., 1997). PCR products were cloned into the pGEX-5x-2 vector (Amersham Pharmacia Biotech) to generate the GST-CD46 tail constructs encoding amino acids 335–362 of the Cyt1 tail or 335–369 of the Cyt2 tail fused at the beginning of the juxtamembrane region to GST. The sequences of all of the fusion proteins were confirmed by DNA sequencing.
The BL-21 strain of E. coli harboring the various GST-CD46 DNA recombinant plasmids were induced with 100 mM IPTG at 30°C for 2 h. The GST fusion proteins were affinity purified with Glutathione-Sepharose as directed by the manufacturer (Amersham Pharmacia Biotech). The purified proteins were quantitated using a Bicinchoninic assay (Pierce Chemical Co.) and visualized by Coomassie blue staining of the gel bands.
Adhesion assay
Confluent A431 cells on 6-well plates were serum starved for 18 h before infection to lower basal levels of tyrosine phosphorylation. Cells were treated for 2 h with Src family kinase inhibitor PP2 (Calbiochem) or the inactive analogue PP3 (Calbiochem) at a concentration of 5 μM before infection. Adhesion was measured as described previously (Shaw and Falkow, 1988; Waldbeser et al., 1994). Cells were inoculated with MS11 N400 at a multiplicity of infection of 50 for 2 h. After washing five times in PBS to remove nonadherent bacteria, cells were lysed and collected in GCB plus 0.5% (wt/vol) saponin, serially diluted and spread onto GCB plates, and grown for 48 h, and colony forming U were counted. Adhesion index was measured as the number of cell-associated bacteria divided by the total number of bacteria in the well at the end of the infection.
In vitro kinase assay
Purified GST fusion proteins containing the CD46 cytoplasmic tails were incubated in kinase buffer, (100 mM Tris-HCl, pH 7.2, 125 mM MgCl2, 25 mM MnCl2, 2 mM EGTA, 0.25 mM sodium orthovanadate, 2 mM dithiothreitol, and a protease inhibitor cocktail [2 μg/ml leupeptin, 1.6 μg/ml benzamidine, 0.3 μg/ml PMSF]) (Trotter et al., 1999). 2 μCi of [γ-32P]ATP and 1 μl of purified c-Src kinase (Upstate Biotechnology) were added, and the mixture was incubated for 30 min at 30°C. All reactions were terminated by the addition of 6× Laemmli buffer containing 0.6% β-mercaptoethanol, and proteins were resolved by electrophoresis in 10% SDS polyacrylamide gels under reducing conditions. Gels were dried at 80°C for 45 min and exposed to Eastman Kodak Co. x-ray film.
Immunoprecipitation
A431 cells were infected with MS11 N400 for different periods, washed three times with PBS, and incubated for 20 min at 4°C in lysis buffer containing a 3 to 1 ratio of RIPA (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% deoxycholic acid, 5 mM EDTA) to TEE (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, 150 mM NaCl, 1% Triton X-100) containing 0.25 mM sodium orthophosphate and a protease inhibitor cocktail (2 μg/ml leupeptin, 1.6 μg/ml benzamidine, 0.3 μg/ml PMSF) (Mohler et al., 1999). Nuclei and insoluble materials were removed by centrifugation at 13,000 g for 20 min at 4°C, and cell homogenates were incubated overnight in lysis buffer with either 1 μg of mouse monoclonal antibody directed against CD46, clone J4–48 Lot #4 (Immunotech) or c-Yes kinase (BD Transduction Labs). Protein G–agarose beads (Oncogene Research Products) were added to the cell preparations for 1 h. The beads were then washed carefully five times with lysis buffer (TEE) and pelleted at 7,000 rpm for 60 s. The pellets were then resuspended in 40 μl Laemmli buffer containing 0.6% β-mercaptoethanol and boiled for 5 min at 100°C. Proteins were separated by SDS–10% polyacrylamide gels and transferred onto nitrocellulose sheets. Membranes were probed, stripped, and reprobed as necessary (Ausubel et al., 1990). For kinase assays using immunoisolated c-Yes, the immunoprecipitates were resuspended in kinase buffer after the last wash, and kinase assays were performed as described earlier. Western blot analysis was performed as described (Ausubel et al., 1990) using rabbit anti-CD46 (a gift from J. Atkinson, Washington University) at a dilution of 1:3,000. Peroxidase-conjugated monoclonal antibody against phosphotyrosine (Ab-4) was used per manufacturer's recommendations (Oncogene Research Products). Monoclonal antibody against c-Yes was used at a concentration of 1:3,000 for immunoblotting and 1:500 for immunofluorescence microscopy.
Immunofluorescence microscopy
A431 cells were grown on coverslips to 30–50% confluence and infected for 3 h with MS11 N400 (Wolfgang et al., 1998) at a multiplicity of infection of 100. Coverslips were washed three times in PBS, fixed for 20 min at room temperature in 4% paraformaldehyde, and blocked for 1 h in isotonic PBS containing 3% (vol/vol) normal goat serum (GIBCO BRL) and 0.02% saponin (Sigma-Aldrich). Primary antibody was diluted as specified above in blocking buffer, added to samples, and incubated overnight at 4°C in a moist chamber. The coverslips were rinsed in PBS and incubated with an Alexa 488–conjugated secondary antibody (Molecular Probes) diluted 1:250 in blocking buffer for 1 h at 25°C. The cells were also incubated with DAPI at 1:1,000 for 10 min at 25°C to visualize the bacteria and nuclei. Samples were rinsed extensively in PBS before mounting in Fluromount-G (Fisher Scientific). Omission of primary antibodies was used as a negative control. 0.2-μm optical sections in the z-axis plane were obtained with a Nikon 60× oil immersion objective, and the images were processed using a Deltavision Restoration Microscope (Applied Precision Instruments, Inc.) and Silicon graphics workstation with accompanying API software. The images were subsequently exported to Adobe Photoshop® and Adobe Illustrator® for manuscript preparation.
Acknowledgments
We thank Patricia Ayala, Michael Howell, Aurelie Snyder, and Brandi Vasquez for their expert technical assistance, and J. Scott Wilbur and Chris Langford for their help in preparing the article.
This work was funded by National Institutes of Health grants AI32493 and AI4997301 to M. So and HL63755 to S.L. Milgram, and an Oregon Health Science University N.L. Tartar research fellowship to S.W. Lee.
Footnotes
Abbreviations used in this paper: GC, Neisseria gonorrhoeae; GST, glutathione S-transferase. SH, Src homology.
References
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1990. Current Protocols in Molecular Biology. Publishing Associates and Wiley Interscience, New York.
- Ayala, P., L. Lin, S. Hopper, M. Fukuda, and M. So. 1998. Infection of epithelial cells by pathogenic neisseriae reduces the levels of multiple lysosomal constituents. Infect. Immunol. 66:5001–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska, J.B., J.E. Galán, and S. Falkow. 1993. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 73:903–920. [DOI] [PubMed] [Google Scholar]
- Bretscher, A., D. Reczek, and M. Berryman. 1997. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J. Cell Sci. 110:3011–3018. [DOI] [PubMed] [Google Scholar]
- Brossay, L., G. Paradis, R. Fox, M. Koomey, and J. Hebert. 1994. Identification, localization, and distribution of the PilT protein in Neisseria gonorrhoeae. Infect. Immun. 62:2302–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T., F. Grunert, A. Medina-Marino, and E.C. Gotschlich. 1997. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J. Exp. Med. 185:1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepaldi, T., A. Gautreau, P.M. Comoglio, D. Louvard, and M. Arpin. 1997. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J. Cell Biol. 138:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehio, C., M.C. Prevost, and P.J. Sansonetti. 1995. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signalling pathway. EMBO J. 14:2471–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorig, R.E., A. Marcil, A. Chopra, and C.D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 75:295–305. [DOI] [PubMed] [Google Scholar]
- Esen, M., H. Grassmé, J. Riethmüller, A. Riehle, K. Fassbender, and E. Gulbins. 2001. Invasion of human epithelial cells by Pseudomonas aeruginosa involves src-like tyrosine kinases p60Src and p59Fyn. Infect. Immun. 69:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay, B.B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 276:718–725. [DOI] [PubMed] [Google Scholar]
- Hanke, J.H., J.P. Gardner, R.L. Dow, P.S. Changelian, W.H. Brisette, E.J. Weringer, B.A. Pollok, and P.A. Connelly. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and Fyn-T dependent T cell activation. J. Biol. Chem. 271:695–701. [DOI] [PubMed] [Google Scholar]
- Hauck, C.R., T.F. Meyer, F. Lang, and E. Gulbins. 1998. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 17:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano, A., S. Yant, K. Iwata, J. Korte-Sarfaty, T. Seya, S. Nagasawa, and T.C. Wong. 1996. Human cell receptor CD46 is down regulated through recognition of a membrane-proximal region of the cytoplasmic domain in persistent measles virus infection. J. Virol. 70:6929–6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, I., E. Eugene, X. Nassif, P.O. Courad, and S. Bourdolous. 2001. Activation of ErbB2 receptor tyrosine kinase supports invasion of endothelial cells by Neisseria meningitidis. J. Cell Biol. 155:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg, R.R., and J.M. Leong. 1990. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 60:861–871. [DOI] [PubMed] [Google Scholar]
- Källström, H., M.K. Liszewski, J.P. Atkinson, and A.B. Jonsson. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25:639–647. [DOI] [PubMed] [Google Scholar]
- Källström, H., M.S. Islam, P.O. Berggren, and A.B. Jonsson. 1998. Cell signaling by the type IV pili of pathogenic Neisseria. J. Biol. Chem. 273:21777–21782. [DOI] [PubMed] [Google Scholar]
- Källström, H., D. Blackmer-Gill, B. Albiger, M.L. Liszewski, J.P. Atkinson, and A.B. Jonsson. 2001. Attachment of Neisseria gonorrhoeae to the cellular pilus receptor CD46: identification of domains important for bacterial adherence. Cell. Microbiol. 3:133–143. [DOI] [PubMed] [Google Scholar]
- Liszewski, M.K., T.W. Post, and J.P. Atkinson. 1991. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9:431–455. [DOI] [PubMed] [Google Scholar]
- Lublin, D.M., M.K. Liszewski, T.W. Post, M.A. Arce, M.M. Le Beau, M.B. Rebentisch, L.S. Lemons, T. Seya, and J.P. Atkinson. 1988. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J. Exp. Med. 168:181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludford-Menting, M.J., S.J. Thomas, B. Crimeen, L.J. Harris, B.E. Loveland, M. Bills, S. Ellis, and S.M. Russel. 2002. A functional interaction between CD46 and Dlg4: a role for Dlg4 in epithelial polarization. J. Biol. Chem. 277:4477–4784. [DOI] [PubMed] [Google Scholar]
- Merz, A.J., and M. So. 1997. Attachment of piliated, Opa- and Opc-gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect. Immun. 65:4341–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz, A.J., and M. So. 2000. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423–457. [DOI] [PubMed] [Google Scholar]
- Merz, A.J., C.A. Enns, and M. So. 1999. Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Mol. Microbiol. 32:1316–1332. [DOI] [PubMed] [Google Scholar]
- Merz, A.J., M. So, and M.P. Sheetz. 2000. Pilus retraction powers bacterial twitching motility. Nature. 407:98–102. [DOI] [PubMed] [Google Scholar]
- Mohler, P.J., S.M. Kreda, R.C. Boucher, M. Sudol, M.J. Stutts, and S.L. Milgram. 1999. Yes-associated protein 65 localizes p62c-Yes to the apical compartment of airway epithelia by association with EBP50. J. Cell Biol. 147:879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif, X., J. Lowy, P. Stenberg, P. O'Gaora, A. Ganji, and M. So. 1993. Antigenic variation of pilin regulates adhesion of Neisseria meningitidis to human epithelial cells. Mol. Microbiol. 8:719–725. [DOI] [PubMed] [Google Scholar]
- Okada, N., M.K. Liszweski, J.P. Atkinson, and M. Caparon. 1995. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc. Natl. Acad. Sci. USA. 92:2489–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol, C., E. Eugene, P. Morand, and X. Nassif. 2000. Do pathogenic neisseriae need several ways to modify the host cell cytoskeleton? Microbes Infect. 2:821–827. [DOI] [PubMed] [Google Scholar]
- Rosenshine, I., S. Ruschkowski, V. Foubister, and B.B. Finlay. 1994. Salmonella typhimurium invasion of epithelial cells: role of induced host cell tyrosine protein phosphorylation. Infect. Immun. 62:4969–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro, F., P.E. Kennedy, G. Locatelli, M.S. Malnati, E.A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell. 99:817–827. [DOI] [PubMed] [Google Scholar]
- Seya, T. 1995. Human regulator of complement activation (RCA) gene family proteins and their relationship to microbial infection. Microbiol. Immunol. 39:295–305. [DOI] [PubMed] [Google Scholar]
- Shaw, J.H., and S. Falkow. 1988. Model for invasion of human tissue culture cells by Neisseria gonnorhoeae. Infect. Immun. 56:1625–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, S.J. 1992. Intercellular communication and cell-cell adhesion. Science. 255:1671–1677. [DOI] [PubMed] [Google Scholar]
- Swanson, J. 1973. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J. Exp. Med. 137:571–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiason, D.M., and H.S. Seifert. 2001. Inverse relationship between pilus-mediated gonococcal adherence and surface expression of the pilus receptor, CD46. Microbiology. 147:2333–2340. [DOI] [PubMed] [Google Scholar]
- Trotter, K.W., I.D. Fraser, G.K. Scott, M.J. Stutts, J.D. Scott, and S.L. Milgram. 1999. Alternative splicing regulates the subcellular localization of A-kinase anchoring protein 18 isoforms. J. Cell Biol. 147:1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Langendonck, N., P. Velge, and E. Bottreau. 1998. Host cell protein tyrosine kinases are activated during the entry of Listeria monocytogenes. Possible role of pp60c-src family protein kinases. FEMS Microbiol. Lett. 162:169–176. [DOI] [PubMed] [Google Scholar]
- Van Putten, J.P., J.F. Weel, and H.U. Grassme. 1994. Measurements of invasion by antibody labeling and electron microscopy. Methods Enzymol. 236:420–437. [DOI] [PubMed] [Google Scholar]
- Waldbeser, L.S., R.S. Ajioka, A.J. Merz, D. Puaoi, L. Lin, M. Thomas, and M. So. 1994. The opaH locus of Neisseria gonnorhoeae MS11A is involved in epithelial cell invasion. Mol. Microbiol. 13:919–928. [DOI] [PubMed] [Google Scholar]
- Wall, D., and D. Kaiser. 1999. Type IV pili and cell motility. Mol. Microbiol. 32:1–10. [DOI] [PubMed] [Google Scholar]
- Wang, G., M.K. Liszweski, A.C. Chan, and J.P. Atkinson. 2000. Membrane cofactor protein(MCP; CD46): isoform-specific tyrosine phosphorylation. J. Immunol. 164:1839–1846. [DOI] [PubMed] [Google Scholar]
- Wolfgang, M., P. Lauer, H.S. Park, L. Brossay, J. Hebert, and M. Koomey. 1998. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 29:321–330. [DOI] [PubMed] [Google Scholar]
- Wong, T.C., S. Yant, B.J. Harder, J. Korte-Sarfaty, and A. Hirano. 1997. The cytoplasmic domains of complement regulatory protein CD46 interact with multiple kinases in macrophages. J. Leukoc. Biol. 62:892–900. [DOI] [PubMed] [Google Scholar]
- Yamada, K.M., and B. Geiger. 1997. Molecular interactions in cell adhesion complexes. Curr. Opin. Cell Biol. 9:76–85. [DOI] [PubMed] [Google Scholar]
- Yant, S., A. Hirano, and T.C. Wong. 1997. Identification of a cytoplasmic Tyr-X-X-Leu motif essential for down regulation of the human cell receptor CD46 in persistent measles virus infection. J. Virol. 71:766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y.H., J.G. Krueger, and M. Sudol. 1990. Expression of cellular-yes protein in mammalian tissues. Oncogene. 5:1629–1635. [PubMed] [Google Scholar]