Abstract
Previous work has shown that the transport of some small protein cargoes through the nuclear pore complex (NPC) can occur in vitro in the absence of nucleoside triphosphate hydrolysis. We now demonstrate that in the importin α/β and transportin import pathways, efficient in vitro transport of large proteins, in contrast to smaller proteins, requires hydrolyzable GTP and the small GTPase Ran. Morphological and biochemical analysis indicates that the presence of Ran and GTP allows large cargo to efficiently cross central regions of the NPC. We further demonstrate that this function of RanGTP at least partly involves its direct binding to importin β and transportin. We suggest that RanGTP functions in these pathways to promote the transport of large cargo by enhancing the ability of import complexes to traverse diffusionally restricted areas of the NPC.
Keywords: nuclear protein import; importin β; transportin; nucleoporin; Ran
Introduction
Macromolecular transport between the nucleus and cytoplasm is mediated by the nuclear pore complex (NPC),* a proteinaceous assembly of ∼125 MDa that spans the double membrane of the nuclear envelope (for reviews see Fahrenkrog et al., 2001; Rout and Aitchison, 2001; Vasu and Forbes, 2001). The framework of the NPC consists of eight central spokes flanked by nuclear and cytoplasmic rings, forming a ring–spoke assembly that surrounds a central transport channel. Extending outward from the ring–spoke assembly are ∼50–100-nm-long nuclear fibrils, which are joined in a basket-like structure, and ∼35–50-nm-long cytoplasmic fibrils. The NPC is composed of 30–50 different polypeptides called nucleoporins, many of which are characterized by the presence of multiple copies of the Phe-Gly (FG) repeat motif. Individual FG repeat nucleoporins have distinctive localizations within the three-dimensional structure of the NPC (see Fahrenkrog et al., 2001) and serve as binding sites for transport receptors via their FG repeat motifs (Bayliss et al., 2000).
Molecules of less than ∼20–40 kD can cross the NPC by passive diffusion, but transport of larger molecules requires interaction with components of the transport machinery (for reviews see Nakielny and Dreyfuss, 1999; Macara, 2001). Many proteins and RNAs are translocated through the NPC by shuttling transport receptors of the karyopherin family (importins and exportins), which recognize NLSs or nuclear export signals on the cargo. Some of the best-studied karyopherins are the importins transportin and importin β, and the exportin Crm1. Transportin recognizes its M9 transport signal directly. Importin β also directly associates with some cargos, although it often works in conjunction with the NLS binding adaptor protein importin α (for review see Görlich and Kutay, 1999; Nakielny and Dreyfuss, 1999). The importin β binding domain (IBB) of importin α itself behaves as a bona fide cargo for importin β (Görlich and Kutay, 1999).
The karyopherin–cargo complex is thought to cross the NPC by binding to and dissociating from a series of nucleoporins (for reviews see Macara, 2001; Stewart et al., 2001). This process appears to involve the initial docking of the transport complex to the fibrils on one side of the NPC, followed by movement through the central channel, which is the primary site of restriction for diffusional movement through the NPC (Feldherr and Akin, 1997), and then terminal association with the fibrils on the other side of the NPC. The GTPase Ran and its regulators are key determinants in the directionality of nuclear transport (Moore, 1998; for review see Macara, 2001). The GTP-bound form of Ran is concentrated in the nucleus, and the GDP-bound form predominates in the cytoplasm. This asymmetry is due to the nuclear compartmentalization of the Ran guanine nucleotide exchange factor RCC1 (RanGEF) and the cytoplasmic localization of the Ran GTPase-activating protein (RanGAP).
RanGTP directly binds to karyopherins, and this modulates the affinity of the receptors for cargo. When an importin–cargo complex encounters RanGTP in the nucleus, RanGTP promotes the dissociation of cargo from the receptor as well as the dissociation of the importin from nucleoporins, and the importin–RanGTP complex is recycled back to the cytoplasm. The converse is true for exportins: intranuclear RanGTP promotes the binding of cargo to exportins, and when the RanGTP-containing export complex encounters RanGAP in the cytoplasm, GTP hydrolysis results in release of the cargo and regeneration of the free exportin. In addition to the Ran system, another contribution to transport directionality could stem from the progressively higher affinity of certain receptors for nucleoporins along the transport pathway (Shah et al., 1998; Allen et al., 2001; Ben-Efraim and Gerace, 2001), which could promote directionally biased movement of the receptor complexes through the NPC.
Nuclear transport in vivo is an energy-requiring process involving GTP hydrolysis by Ran (for nuclear import; Schwoebel et al., 2002), which leads to cargo concentration up a chemical potential gradient (for review see Görlich and Kutay, 1999). However, with in vitro assays in which receptors are provided in excess and do not need to be recycled, Crm1-mediated protein export (Englmeier et al., 1999) and importin β–mediated protein import (Schwoebel et al., 1998) can be supported by Ran and a nonhydrolyzable analogue of GTP. Transportin-mediated protein import requires neither Ran nor GTP (Englmeier et al., 1999; Ribbeck et al., 1999), probably because of the apparent low affinity of transportin–nucleoporin interactions (Ribbeck and Görlich, 2001), which consequently would not require Ran-assisted dissociation.
Whereas the proteins used in these studies were all relatively small (∼70–120 kD), it is clear that the NPC is capable of translocating much larger cargos (Görlich and Kutay, 1999). Because getting such sizeable cargoes across the NPC could involve a more complex mechanism, we compared the Ran and energy requirement for nuclear import of large and small proteins in the importin α/β and transportin pathways. Intriguingly, we found that in both receptor pathways, the efficient import of large cargos, in contrast to that of small cargos, did require both Ran and hydrolyzable GTP. EM analysis showed that RanGTP promoted the movement of large cargos into the central regions of the NPC. Using receptor mutants defective for Ran binding, we determined that RanGTP acts to facilitate import of large cargo by directly binding to importin β and transportin, at least in part. We discuss these results in the context of a model whereby RanGTP promotes movement of karyopherin complexes through the diffusionally restricted central channel of the NPC.
Results
Distinct Ran and energy requirements for small versus large cargo import
We used in vitro transport reactions containing permeabilized HeLa cells to examine energy requirements for the NPC transit of small versus large cargo in two pathways of receptor-mediated protein transport: import of either classical, importin α–dependent NLS- or IBB-bearing cargo by importin β (both reflecting the importin α/β pathway), and import of M9-bearing cargo by transportin. To create cargo of different sizes (Table I), we fused the IBB or M9 signal either to the pentameric nucleoplasmin core (Npl; small) or to tetrameric β-galactosidase (βgal; large) and conjugated an NLS peptide to BSA (small) or to thyroglobulin (thyr; large). To ensure that the cargos were of the expected size, purification included a step of size selection. In addition, we verified that cargo retained its multimeric state under the conditions of the in vitro import assay by molecular sieving chromatography (unpublished data). Our experimental approach required that we compare the import efficiency of distinct import substrates under different reaction conditions. To do this, we routinely normalized import levels for a given cargo molecule to a control reaction containing Ran and hydrolyzable GTP.
Table I. Molecular mass and hydrodynamic (Stokes) radius of import cargos.
| Cargoa | Valency | Molecular mass | Stokes radiusb |
|---|---|---|---|
| kD | nm | ||
| GST-IBB | Dimer | 70 | 4.51 |
| IBB-nucleoplasmin | Decamerc | 280 | 6.27 |
| IBB-βgal | Tetramer | 500 | 7.56 |
| GST-M9 | Dimer | 65 | 3.13 |
| Nucleoplasmin-M9 | Decamerc | 250 | 5.72 |
| M9-βgal | Tetramer | 496 | 7.15 |
| BSA-colloidal gold | NA | NA | 10.35 |
| BSA-NLS | Monomerd | 70 | 3.79 |
| Thyroglobulin-NLS | Dimerd | 669 | 9.62 |
Net charge at pH 7.0 and frequency of hydrophobic residues: GST-IBB: +5.1 × 30.3%; GST-M9: +1.0 × 28.9%; IBB-βgal: −26.6 × 33.8%; IBB-Npl: −19.6 × 27.6%; M9-βgal: −28.8 × 32.9%; Npl-M9: −23.8 × 25.3%. These parameters could not be accurately determined for BSA-NLS and thyroglobulin-NLS, as the NLS-coupling density was not precisely known.
Note that the functional Stokes radius of a cargo will depend on the number of receptors bound to it during transport.
At the high concentration used for gel filtration, nucleoplasmin forms a decamer, whereas at the low concentration of our transport assay it forms a pentamer (Dutta et al., 2001).
Although the NLS peptide was coupled to BSA and thyroglobulin at a similar molar ratio, the exact coupling density cannot be accurately determined.
Analysis of importin β–mediated import demonstrated that efficient transit of small, but not large, cargo could proceed without GTP hydrolysis (Fig. 1, A–D). All reactions contained Ran, and the standard reaction (β + GTP) contained IBB cargo at 500 nM or NLS cargo at 150 nM, and importin β at 62.5 nM. However, efficient import in the presence of the nonhydrolyzable analogue GMPPNP required a higher concentration of importin β (10 × β + GMPPNP). This higher receptor level was likely necessary both to obviate the need for receptor recycling and to balance the persistent dissociative action of RanGMPPNP on importin–cargo and importin–nucleoporin complexes (Rexach and Blobel, 1995). We found that GMPPNP supported substantial import of both of the small cargos tested; under optimal conditions, the average level of GMPPNP-supported import of IBB-Npl was equivalent to that of the control reaction (10 × β+ GTP), and that of BSA-NLS was ∼70% of the control reaction; the level of the latter is similar to that previously reported (Schwoebel et al., 1998). However, we found that GMPPNP was substantially less effective at supporting import of the large cargos IBB-βgal and thyr-NLS, with final import levels only ∼35–40% of those attained with GTP (Fig. 1, A–D). Although it was previously reported that import of IBB-βgal was supported by nonhydrolyzable GTP (Nachury and Weis, 1999), we suggest that the nuclear accumulation observed in that study likely reflects the low-efficiency import that we report here.
Figure 1.
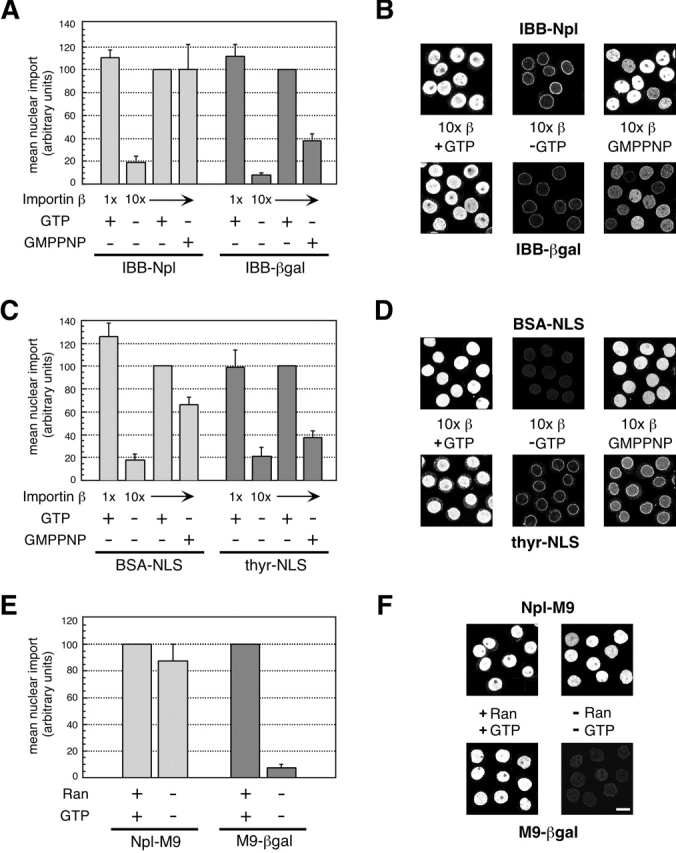
Import of large, but not small, cargo requires hydrolyzable GTP. Error bars represent the standard deviation of three independent in vitro import assays. Reactions lacking GTP and those containing GMPPNP were supplemented with hexokinase/glucose to deplete endogenous NTPs. (A and C) Import of small (IBB-Npl and BSA-NLS) or large (IBB-βgal and thyroglobulin-NLS) cargos. All reactions contained Ran; levels of importin β and the addition of nucleotide are indicated. NLS-cargo reactions also contained importin α. Confocal images are shown in (B and D). (E) Import of the small cargo M9-Npl or the large cargo M9-βgal. All reactions contained transportin; the addition of Ran and GTP is indicated. Confocal images are shown in F. For a given cargo, import levels were normalized either to the 10 × β + GTP reaction (A and C) or to the + Ran + GTP reaction (E). Bar, 10 μm.
It is formally possible that GMPPNP was unable to support efficient import of the large IBB cargos due to a nonspecific effect on the cytoplasmic fibrils, which was not seen with the small cargos. This is because the importin β–RanGMPPNP complex formed in these reactions, which is insensitive to disassembly by RanGAP, would be persistently bound to the Ran-binding domains of the cytoplasmic fibril protein Nup358/RanBP2 (Yaseen and Blobel, 1999). However, nonspecific inhibition by such a mechanism is unlikely, as we were unable to rescue large cargo import by adding an excess of RanBP1 (unpublished observations), a cytosolic protein that would efficiently compete for the binding of RanGMPPNP to the Ran binding domains of Nup358/RanBP2 (Villa Braslavsky et al., 2000). Moreover, the binding of large IBB cargo to the cytoplasmic fibrils clearly was not inhibited in the presence of Ran + GMPPNP (see Fig. 4).
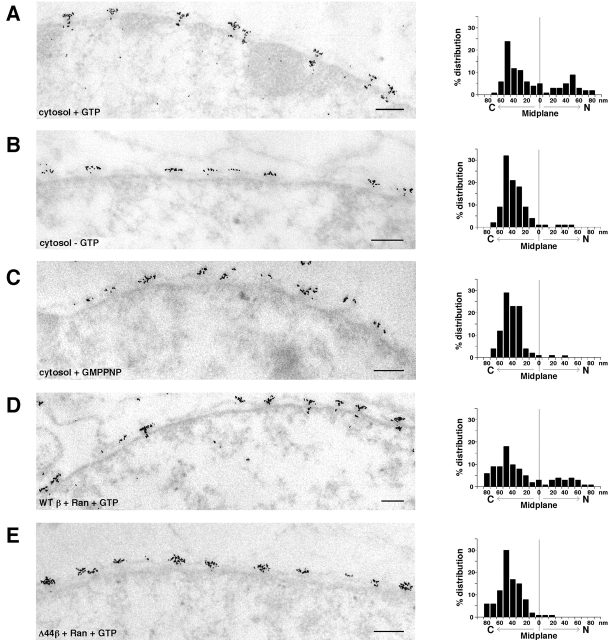
Figure 4.
EM analysis of large cargo import in the importin β pathway. Standard in vitro import reactions were processed for EM visualization (left). Reactions lacking GTP were supplemented with hexokinase/glucose to deplete endogenous NTPs. Note that in the presence of GTP and WT importin β there is clear intranuclear accumulation of gold. Distribution with respect to the NPC midplane (indicated by 0) is shown on the graphs (right). C, cytoplasm; N, nucleoplasm. (A–C) Reactions contained cytosol, supplemental importin β (565 nM), and GST-IBB colloidal gold. Nucleotide was absent or present as indicated. (D and E) Reactions contained GST-IBB colloidal gold, Ran, GTP, and either mutant or WT importin β, as indicated. Bars, 0.2 μm.
For the transportin-mediated import of M9-bearing cargo (Fig. 1, E–F), all reactions contained cargo at 250 nM and transportin at 250 nM; Ran and GTP were present or absent as indicated. Import of the small M9 cargo (Npl-M9) under these conditions, as reported previously (Englmeier et al., 1999; Ribbeck et al., 1999), did not require Ran or GTP. This was dramatically different from the large M9 cargo (M9-βgal), for which there was virtually no import in the absence of Ran and GTP. Addition of Ran alone, GTP alone, or Ran + GMPPNP did not rescue import of the M9-βgal (unpublished data). Furthermore, we found (unpublished data) that import of small but not large M9 cargo was supported by GTP + RanQ69L, a Ran mutant that is deficient in GAP-stimulated GTP hydrolysis (Klebe et al., 1995). Thus, for both the importin β and the transportin pathways, efficient passage of large protein cargo across the NPC, in contrast to small cargo, required Ran and hydrolyzable GTP.
Import kinetics of large versus small cargo
To extend our analysis of the different energy requirements for the import of large versus small protein cargo, we also examined the kinetics of import (Fig. 2). First, to ensure that the exogenously added recombinant factors were not limiting in the in vitro import reaction, we analyzed the final level of nuclear accumulation of the substrate in the presence or absence of hydrolyzable GTP. Receptor was provided either at a sixfold molar excess over cargo or at levels that were equimolar to or in a slight excess over cargo (Fig. 2 A). For IBB cargo, results were essentially identical under both conditions, whereas for M9 cargo, the higher receptor levels supported a modest increase in the level of Ran-independent import for both small and large cargos. Further increases in the amount of receptor did not enhance import of either IBB or M9 cargo (unpublished data). We carried out a parallel analysis in which the amount of Ran was increased to 2×, 5×, or 10× over the standard level (unpublished data), and found that increases in Ran did not substantially alter the ratios of GTP- versus GMPPNP-supported import that we had previously observed with Ran (Figs. 1 and 2).
Figure 2.
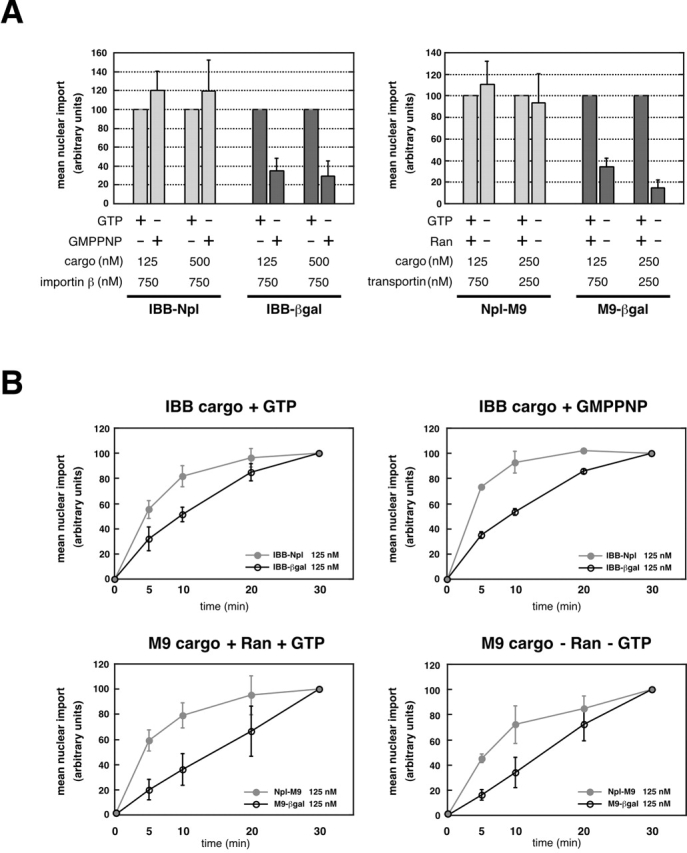
Rate analysis of the import of large and small protein cargo. Error bars represent the standard deviation of three independent in vitro import assays. Molar concentrations of receptor and cargo are indicated. (A) Import was analyzed at the standard 30-min time point; for a given receptor:cargo condition, import was normalized to the + Ran + GTP reaction. (B) Time course of nuclear protein import. Import levels were analyzed at 0, 5, 10, 20, and 30 min time points. For a given reaction, the 30-min time point was set at 100% and earlier time points were normalized as a fraction of the 100% level.
We then carried out a time course of import, with receptor provided in excess, in order to analyze the import rates of large versus small protein cargo under the different Ran/energy conditions examined in Fig. 1. To compare the rate profiles for nuclear accumulation of different cargos, the import level at a 30-min time point for a given cargo was set to 100%, and levels of nuclear fluorescence at 0–20-min time points for each reaction were normalized with reference to the 30-min time point. The results (Fig. 2 B) show that for both importin β– and transportin-mediated import, the rate profiles for each cargo were very similar regardless of the Ran/nucleotide conditions. For small IBB and M9 cargos, the rate profile showed a rapid burst followed by an eventual plateau, whereas the large IBB cargo displayed a much more gradual approach to a plateau, and the profile for the large M9 cargo was essentially linear. The more rapid approach to a plateau for the small cargo suggests that it may have more quickly consumed the available resources, so that some transport component became limiting sooner than it did for import of a large cargo. In this regard, the similarity of the rate profiles for the large cargos under the different energy/Ran conditions indicates that the differences in import levels obtained with the different conditions (Fig. 1) do not reflect a preferential depletion of a transport component in one of the cases.
Efficient import of large cargos requires Ran and hydrolyzable GTP
Although our data indicated that Ran and GTP were needed for efficient import of large IBB and M9 cargos, it wasn't clear if the only nucleoside triphosphate (NTP) involved was GTP, or if the only enzyme involved was Ran. To dissect the energy requirements for large cargo import, we utilized a mutant form of Ran (Sweet and Gerace, 1996; Weis et al., 1996) in which a single amino acid change (D125N) results in a switch of enzymatic specificity from GTP to xanthosine triphosphate (XTP), an NTP that does not occur naturally in cells. We examined import of the large cargo IBB-βgal (Fig. 3 A) or the large cargo M9-βgal (Fig. 3 B) in reactions containing either WT Ran + GTP or the D125N mutant of Ran (XRan) + XTP. For both the IBB and M9 cargos, the level of import supported by XRan + XTP was very similar to that supported by WT Ran + GTP, suggesting that no NTPases other than Ran were required for complete import.
Figure 3.
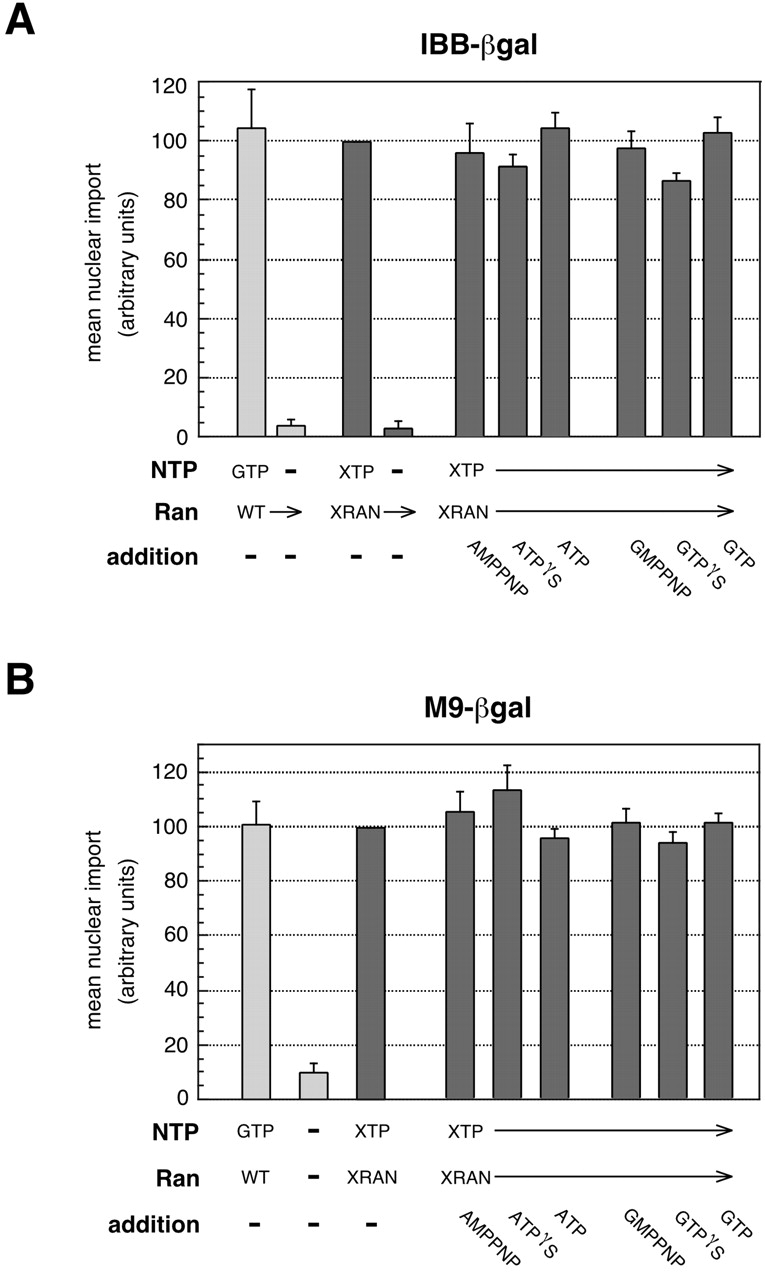
Import of large cargo requires no NTPs or NTPases other than Ran and GTP. Error bars represent the standard deviation of three independent in vitro import assays. ATP analogues or GTP analogues (2 mM final concentration), or ATP or GTP (0.5 mM final concentration) were added to the XRan reactions as indicated. Reactions lacking GTP were supplemented with hexokinase/glucose to deplete endogenous NTPs. (A) Import of IBB-βgal; (B) Import of M9-βgal. For a given cargo, import levels were normalized to the XRan + XTP reaction.
However, cellular analysis of nucleotide requirements is complicated by a nucleotide diphosphokinase-like activity that is able to transfer a terminal phosphate from an NTP to an NDP (Lazarowski et al., 2000). Thus, it is possible that XTP, in concert with the diphosphokinase activity that is retained in the permeabilized cells, could generate ATP and GTP from small intranuclear pools of ADP and GDP remaining in the cells. To validate that there was no significant contribution from an ancillary ATPase or GTPase, we added nonhydrolyzable ATP or GTP analogues to the XRan + XTP reaction (Fig. 3, A and B). Import was not substantially inhibited by the analogues, nor was it stimulated by the addition of ATP or GTP, suggesting that XRan and XTP alone fulfilled the NTP hydrolysis requirement for efficient large cargo import.
Similar to the above results, a previous study suggested that Ran is the only GTPase involved in nuclear import (Weis et al., 1996), whereas another study suggested a role for a second GTPase, distinct from Ran in import (Sweet and Gerace, 1996). The reason for this disparity is not clear, but it is possible that in the latter experiments, the permeabilized cells preferentially retained a factor sensitive to GTP analogues, such as a GTPase involved in a signaling cascade that negatively regulates import by phosphorylation (Kehlenbach and Gerace, 2000). A GTP analogue could constitutively activate such a factor, resulting in the inhibition of XTP-supported import.
Large cargo requires RanGTP to traverse the central channel of the NPC
To directly investigate the point in NPC passage at which RanGTP became rate limiting for large cargo import, we carried out EM analysis of transport reactions containing cargo-coated colloidal gold particles (Table I) under different Ran and energy conditions (Figs. 4 and 5). We attempted to carry out a parallel EM analysis of small gold cargo, but smaller versions of the colloidal gold substrate were still similar to a large cargo in hydrodynamic and biochemical behavior, and tagging of protein substrates with chemically reactive Nanogold™ resulted in functional inactivation (unpublished data).
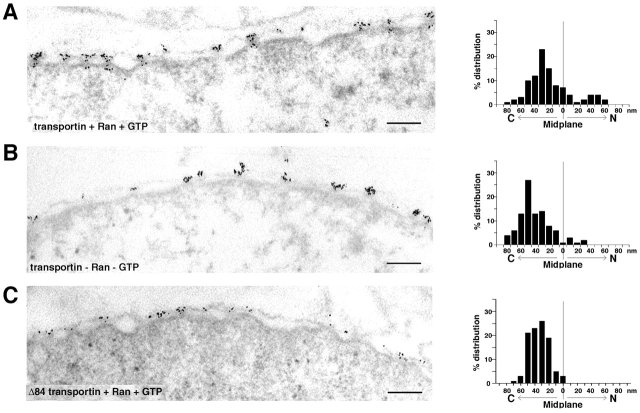
Figure 5.
EM analysis of large cargo import in the transportin pathway. In vitro import reactions were performed and analyzed as in Fig. 4. Representative EM images (left) and graphs presenting distribution of gold with respect to the NPC midplane (right) are shown. C, cytoplasm; N, nucleoplasm. Reactions contained GST-M9 colloidal gold, Ran, GTP, and either WT (A and B) or mutant (C) transportin, as indicated. Bars, 0.2 μm.
Fig. 4 (A–C) shows thin section electron micrographs (left) of in vitro import reactions containing cytosol, IBB-gold, and 565 nM additional importin β (so that total importin β would be equivalent to that in the high β import reactions carried out with recombinant factors). In the right panels are plots of the distribution of the gold cargo with respect to the NPC midplane (i.e., a plane bisecting the NPC into nuclear and cytoplasmic halves). These data clearly demonstrate that in the presence of GTP (Fig. 4 A), cargo was able to enter central and nucleoplasmic regions of the NPC, whereas in the absence of hydrolyzable GTP, the cargo was largely restricted to the cytoplasmic side of the NPC (Fig. 4, B and C). Control reactions showed that this binding was both specific and physiological (unpublished data).
An analysis of M9-gold in reactions containing recombinant transportin (Fig. 5, A and B) showed a similar dependence on Ran and GTP for significant access to central and nucleoplasmic areas. Note also the Ran-dependent shift in the cytoplasmic distribution of the M9-gold from a mode of ∼50–60 nm (−Ran; Fig. 5 B) to a more centrally located mode of ∼20–30 nm (+Ran; Fig. 5 A), which suggests a role for Ran in facilitating entry into the central channel. Taken together with our previous results, these data indicate that efficient movement of large cargo into central and nucleoplasmic regions of the NPC is obligately coupled to the presence of Ran and hydrolyzable GTP.
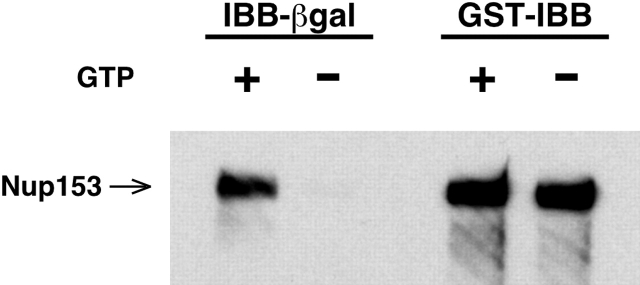
To complement this EM analysis with cargo-coated gold, we used a biochemical assay to investigate the ability of cargo to reach the nucleoplasmic side of the NPC under different Ran and energy conditions. We used Nup153, which is localized to the nucleoplasmic basket of the NPC, as a focal point for this analysis, as this protein is predicted to be a terminal binding site for the receptor–cargo complex in importin β–mediated import (Görlich et al., 1996; Ben-Efraim and Gerace, 2001). To examine the ability of cargo to reach Nup153 under different energy conditions, we carried out import reactions containing permeabilized cells, cytosol, and IBB cargo, and then solubilized the reactions with a buffer containing nonionic detergent. Nup153 association was analyzed in a cargo pulldown assay. It was not possible to test either M9 cargo or the small cargo IBB-Npl, as we detected only inconsistent, low-level association of these with Nup153 under our conditions (unpublished data). Nucleoporin interactions in the former case are suggested to be of low affinity (Ribbeck and Görlich, 2001), and in the latter case, the presence of strong intranuclear binding sites for the Npl core might have shifted the equilibrium to favor intranuclear binding of cargo rather than association with Nup153.
Therefore, we utilized GST-IBB (∼70 kD; Table I) as an alternate small cargo. For GST-IBB, as for the small cargos IBB-Npl and BSA-NLS, GMPPNP supported substantial import (unpublished data). Fig. 6 shows that in the solubilized cells, GST-IBB associated with Nup153 both in the presence and absence of GTP, whereas the large cargo IBB-βgal showed significant association with Nup153 only in the presence of GTP. This association was specific, as neither GST nor β galactosidase lacking the IBB moiety coprecipitated either receptor or nups (unpublished data). These results suggest that small, but not large, cargo was able to move to the nucleoplasmic side of the NPC without the benefit of GTP, and support the results of the EM analysis (Figs. 4 and 5).
Figure 6.
Association of large cargo with the nucleoplasmic protein Nup153 requires GTP. In vitro import assays were solubilized with NP-40 and cargo was precipitated in a pulldown assay. Coprecipitating Nup153 was analyzed by immunoblotting.
Import of large cargo requires a functional Ran binding domain on the receptor
RanGTP could promote the translocation of large cargos through the NPC by interacting directly with a component(s) of the NPC to allow movement across the central channel, or alternatively, RanGTP could act by directly associating with a receptor–cargo complex. Although we could not address the first possibility because of the lack of information on potential nucleoporin targets for RanGTP in the central channel, we were able to investigate the second possibility by utilizing receptor mutants defective for Ran binding.
To study the importin β pathway, we utilized a previously described importin β mutant lacking its 44 NH2-terminal residues. This mutant can bind IBB but is unable to bind RanGTP (Görlich et al., 1996). EM analysis of IBB-gold in import reactions reconstituted with recombinant factors and containing both Ran and GTP showed that Δ44 importin β (Fig. 4 E), in contrast to WT importin β (Fig. 4 D), was unable to support entry of IBB-gold into the central and nucleoplasmic regions of the NPC. This result is at variance with the conclusions of a previous study (Görlich et al., 1996), in which Δ44 importin β appeared to support accumulation of IBB-gold at the nucleoplasmic face of the NPC. However, there are two significant methodological differences that may explain the disparity: First, we reconstituted import with recombinant factors in permeabilized HeLa cells, whereas Görlich et al. (1996) examined import by microinjecting IBB-gold preincubated with Δ44 importin β into the cytoplasm of Xenopus oocytes. Because cytoplasm is predicted to contain substantial levels of WT importin β, this WT receptor rather than the mutant could have supported import. Second, we carried out the in vitro import reaction for 30 min, whereas the microinjection experiment was analyzed after 2 h. If the transport block that we observe with Δ44 importin β reflects a kinetic lag rather than an absolute block to passage, a prolonged incubation might result in the advancement of some cargo molecules across the NPC. However, it is clear that in our analysis of parallel reactions comparing mutant and WT importin β, Δ44 importin β was deficient in promoting movement of IBB-gold to central and distal regions of the NPC.
To carry out an analysis of the transportin pathway, we generated a transportin mutant that was deficient in Ran binding by deleting the NH2-terminal 84 residues of transportin. To demonstrate this deficiency, we utilized the known ability of importin β-like receptors to prevent the GAP-mediated stimulation of GTP hydrolysis by Ran (Floer and Blobel, 1996; Bischoff and Görlich, 1997; Lounsbury and Macara, 1997). Fig. 7 A shows that WT transportin presented a substantial block to GAP-stimulated hydrolysis. In contrast, Δ84 transportin had no detectable ability to inhibit GAP-stimulated hydrolysis, even at an approximately sixfold molar excess over Ran. Thus, the deletion effectively abolished the ability of transportin to bind Ran. Transportin-mediated import lends itself particularly well to an analysis of RanGTP function, as the import of small M9 cargos is independent of both Ran and GTP (Englmeier et al., 1999; Ribbeck et al., 1999). Thus, Δ84 transportin is predicted to be fully functional for small cargo import, and whether or not it can support large cargo import will hinge on the mechanism by which RanGTP facilitates transport.
Figure 7.
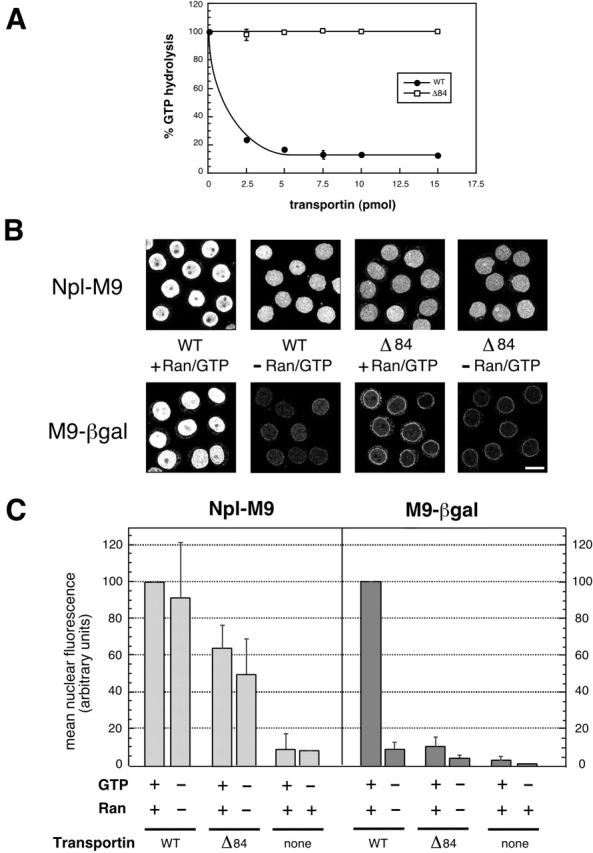
Transportin-mediated import of large cargo requires a functional Ran binding domain. (A) Δ84 transportin is defective in Ran binding; error bars represent the standard deviation of two independent GAP assays which contained ∼2.5 pmol 32P-GTP-Ran and increasing amounts of either WT transportin or Δ84 transportin, as indicated. A 10-min time point is shown. (B and C) Error bars represent the standard deviation of three independent in vitro import assays. Reactions lacking GTP were supplemented with hexokinase/glucose to deplete endogenous NTPs. (B) Reactions contained Npl-M9 or M9-βgal, WT or Δ84 transportin, and Ran and GTP as indicated. (C) Quantification of reactions containing Npl-M9 or M9-βgal and either WT or Δ84 transportin. Ran and GTP were added, as indicated. None indicates that no transportin was added; the observed import is presumably due to incomplete depletion of endogenous WT transportin. For a given cargo, import levels were normalized to the WT transportin + Ran + GTP reaction. Bar, 10 μm.
Our analysis (Fig. 7, B and C) showed that as predicted, Δ84 transportin was able to support substantial import of the small cargo Npl-M9. However, it did not support import of the large cargo M9-βgal. Although the level of Npl-M9 import mediated by Δ84 transportin was less than that achieved with WT, it was clearly substantial. We hypothesize that this lower import competence may be due to a slight deficiency in the mutant's ability to bind to cargo or to nucleoporins. The import of Npl-M9 in reactions containing Δ84 transportin, Ran, and GTP was consistently somewhat greater than that of a parallel reaction lacking Ran and GTP. This was likely due in part to a contribution from low levels of endogenous WT transportin, and possibly also to an unknown mechanism by which RanGTP facilitates import by interacting directly with NPC components. To determine the point where transit of large cargo through the NPC is inhibited with Δ84 transportin, we carried out an EM analysis (Fig. 5 C). This work demonstrated that the mutant transportin, like the mutant importin β, was unable to support entry of cargo-coated gold into the central and nucleoplasmic regions of the NPC. Considered together, our EM and biochemical results suggest that the binding of RanGTP to both importin β and transportin is required to support movement of large cargo through the central channel of the NPC. Nonetheless, it remains possible that facilitation of large cargo import by RanGTP might involve its direct interaction with the NPC, in addition to its association with the import receptor.
Discussion
In a comparison of the nuclear import of model small and large protein cargos by the importin α/β and transportin pathways, we have made the striking observation that the large cargos require both Ran and hydrolyzable GTP for nuclear import. This contrasts with the import requirements for small cargos that were observed previously and also that were noted in this study: Transportin-mediated import requires neither Ran nor GTP (Englmeier et al., 1999; Ribbeck et al., 1999; Fig. 1), and importin α/β-mediated import requires Ran and a GTP analogue but not GTP hydrolysis (Schwoebel et al., 1998; Fig. 1). We determined that RanGTP is required for the large cargos to traverse the central channel of the NPC, but is not required for association with the cytoplasmic side of the NPC. Furthermore, our data indicate that RanGTP acts to promote large cargo import by directly binding to importin β and transportin, at least in part. These findings are most easily explained by a model in which RanGTP facilitates the movement of the cargo–receptor complex through the diffusionally restricted central channel of the NPC by modulating the interaction of the receptor with nucleoporins (discussed below). However, our results do not exclude an additional role for RanGTP in altering the conformation of the NPC (Goldberg et al., 2000).
It recently was reported that the rate of nuclear import by certain receptors is influenced by cargo size, hydrophobicity, and the number of receptors bound to a specific cargo (Ribbeck and Gorlich, 2002). However, several lines of evidence indicate that in each of the receptor/signal pathways we analyzed, the differences that we observed in the Ran and energy requirements for import are primarily related to cargo size, although other properties of the cargos (such as shape; see below) also may have an influence. First, in the importin α/β pathway, we found that efficient import of three different small proteins (all between 60 and 125 kD) was supported by Ran and a nonhydrolyzable GTP analogue, whereas efficient import of two different large proteins (500 and 669 kD) required both Ran and GTP. Second, in our analysis of both the importin α/β and transportin pathways, the Npl-based small cargos have a similar net charge and frequency of hydrophobic residues as compared with the βgal-based large cargos. Third, βgal fused to the IBB domain of snurportin can be imported without either Ran or GTP (Huber et al., 2002), clearly showing that the properties of the receptor pathway, rather than the intrinsic properties of βgal, define the energy and Ran requirements for this cargo (see below).
The observation that certain karyopherin–cargo complexes can cross the NPC without NTP hydrolysis has supported the model that the complexes are transported by a facilitated diffusion mechanism, involving the repeated binding and dissociation from nucleoporins (for reviews see Görlich and Kutay, 1999; Macara, 2001). This facilitated diffusion may be stochastic (Rexach and Blobel, 1995), or may have biased directionality due to a nucleoporin affinity gradient (Ben-Efraim and Gerace, 2001). The primary restriction of free diffusional movement through the NPC occurs in the central channel (Feldherr and Akin, 1997). This area must be able to accommodate a high level of cargo flux, while simultaneously maintaining stringent selectivity for signal-bearing cargos. Several models have been proposed to explain these properties of the NPC (for reviews see Macara, 2001; Stewart et al., 2001). Although the detailed structural characteristics of the central channel remain obscure, models have proposed that it has a high concentration of nucleoporins, which may form a dense, flexible protein meshwork (Ribbeck and Görlich, 2001; Rout and Aitchison, 2001).
Our data show that the Ran and energy requirements for nuclear import in a specific import pathway (i.e., involving a particular receptor/signal combination) can change in relation to cargo size. However, it also is evident that the Ran and energy requirements can differ when a specific cargo protein is targeted to different import pathways. For example, the import of nucleoplasmin fused to IBB is supported by Ran and nonhydrolyzable GTP (Fig. 1), whereas nucleoplasmin fused to M9 requires neither Ran nor GTP (Englmeier et al., 1999; Ribbeck et al., 1999; Fig. 1). Thus, both cargo size and the receptor/signal pathway impact significantly on the Ran and energy requirements for import.
In view of these results, we suggest that receptor–cargo movement through the NPC (and in particular, through the diffusionally restricted central channel) can be explained by a modified facilitated diffusion model that includes a role for RanGTP. We propose that the Ran and energy requirement for import is dictated largely by a combination of two properties of the receptor–cargo complex: its diffusion coefficient, which is related to its molecular mass and shape (Creighton, 1993), and its avidity for nucleoporins, which is determined by the specific receptor/signal pathway and the number of receptors bound to the cargo. Smaller receptor–cargo complexes, with a larger diffusion coefficient, would have a higher probability of diffusionally escaping the immediate vicinity of one nucleoporin after dissociating from it. This feature would allow them to bind to a different nucleoporin, rather being recaptured by original binding site, and thus to transit the NPC by multiple binding/dissociation cycles. In contrast, larger complexes, with a smaller diffusion coefficient and reduced mobility, would be more likely to rebind to the same nucleoporin after dissociating from it and thereby could be preferentially stalled in a particular region of the NPC.
This diffusion-driven movement would be strongly influenced by the avidity of the receptor–cargo complex for nucleoporins, which can vary widely for different receptor/signal complexes (see below). Importin–cargo complexes with a low avidity for nucleoporins would be able to migrate through the NPC even if they are large and more diffusionally restricted, as rebinding to the same nucleoporin would be limited by a low on-rate. In contrast, importin–cargo complexes with a high avidity for nucleoporins might be stalled in a particular region of the NPC, even if they are relatively small, due their fast on-rate for nucleoporin binding and to the presence of multiple FG repeat binding sites in individual nucleoporins.
The binding of RanGTP to importins has been shown to substantially weaken their affinity for FG repeat nucleoporins (Rexach and Blobel, 1995; for review see Conti and Izaurralde, 2001). Based on our finding that mutants of importin β and transportin that are deficient in Ran binding do not support the movement of large cargo through the central channel of the NPC, we propose that RanGTP binding to the importins plays a pivotal role in dissociating receptor/nucleoporin interactions in the import of certain types of cargo. Modulation of importin/nucleoporin binding interactions by RanGTP would be important for the transport of importin–cargo complexes with a high avidity for nucleoporins, or for those with a small diffusion coefficient (i.e., large cargos). In this manner, RanGTP would effectively increase the nucleoporin off-time of an importin–cargo complex in the vicinity of a particular nucleoporin, and promote its diffusional movement to other nucleoporin binding sites.
It is noteworthy that the relative requirement of RanGTP for import, as seen in this study and previous work, is correlated with the affinity of importin–cargo complexes for nucleoporins. The importin α/β complex (with the most stringent Ran/energy requirement) binds to nucleoporins with relatively high affinity (Kd, 1-200 nM; Ben-Efraim and Gerace, 2001), whereas transportin/M9 (with a lower Ran/energy requirement) apparently binds with a Kd in the low μM range (Ribbeck and Görlich, 2001), and snurportin IBB/importin β (with no detectable Ran/energy requirement) may bind with an even lower affinity (Huber et al., 2002).
The RanGTP involved in promoting cargo dissociation from nucleoporins by the above model would likely enter the NPC by passive diffusion from the nuclear interior. We suggest that the requirement for hydrolyzable GTP to support large cargo import reflects a need to maintain the local concentration of RanGTP in the NPC sufficiently low by the action of cytosolic and NPC-associated RanGAP so as to prevent persistent dissociative effects of RanGTP on the receptor–cargo interaction (see below). Our finding that efficient import of large IBB cargo requires Ran and hydrolyzable GTP, whereas efficient import of small IBB cargo is supported by Ran and nonhydrolyzable GTP, is consistent with this model: small receptor–cargo complexes, unlike larger complexes, would be able to migrate through the NPC sufficiently rapidly to escape the dissociative effects of RanGMPPNP and to maintain their integrity during transit.
Our gel filtration analysis of receptor–cargo complexes for the Npl and βgal cargos (with receptor provided in excess) suggested that each cargo can bind the theoretically predicted number of receptors (i.e., five receptors for Npl and four receptors for βgal; unpublished data). Although we cannot ascertain the exact number of receptors bound to the cargo molecules that were imported in our assays, it is reasonable to expect that cargos with the maximal numbers of bound receptors would preferentially be imported, as increased receptor number increases the efficacy of import (Ribbeck and Gorlich, 2002). It should be noted that for diffusion through a confined space, such as the central channel of the NPC, relatively small changes in particle size can have large effects on the rate of diffusion as the particle begins to approach the size of the channel (Paine et al., 1975). Thus, even though the differences in molecular weights of our small versus large receptor cargo complexes are relatively modest (e.g., Npl-M9 with five transportins, 631 kD; M9-βgal with four transportins, 905 kD), their differences in Ran/energy requirements could easily be explained by this phenomenon.
RanGTP has been shown to dissociate cargo as well as nucleoporins from importins in solution binding studies (for review see Macara, 2001). Because cargo dissociation from the receptor could abort the import process, we suggest that the binding of RanGTP to a nucleoporin–receptor–cargo complex may selectively dissociate the nucleoporin and not the cargo from the receptor, by forming a kinetically transient RanGTP: receptor–cargo complex. In some conditions, this intermediate could generate a stable RanGTP–receptor complex + free cargo. However, we suggest that because the environment of the NPC contains a very high concentration of nucleoporins (Stewart et al., 2001), nucleoporins would preferentially bind to this complex and displace the RanGTP, thereby creating a new nucleoporin–receptor–cargo complex.
A Kd of ∼10 nM was measured for the importin α IBB/importin β complex (Catimel et al., 2001), whereas the Kd of importin β for all known nucleoporins of the central channel is ∼100 nM (Ben-Efraim and Gerace, 2001). The substantially lower affinity of importin β for nucleoporins than for cargo might favor a more rapid disruption of nucleoporin binding than cargo binding during the process of RanGTP association. In addition, as the binding of RanGTP to importins may be a progressive process (discussed by Conti and Izaurralde, 2001), it is plausible that during the process of RanGTP binding, importins may exist in transient conformational intermediates in which the nucleoporin binding sites on the receptors, but not the cargo binding sites, are preferentially disrupted.
In conclusion, our data demonstrate for the first time that RanGTP plays a pivotal role in facilitating movement of certain large cargos through the diffusionally restricted central channel of the NPC, and suggest that the transient binding of RanGTP to these import complexes is a key feature of translocation through the NPC. More extensive experimental analysis of this hypothesis will become feasible with the development of new biophysical and biochemical means of analyzing intermediates in the transit of cargo through the NPC.
Materials and methods
Plasmids
High-fidelity PCR amplification was used to amplify the DNA fragments used to generate the following constructs. All fragments were verified by DNA sequencing. IBB-βgal: residues 1–65 of importin α (BglII-PstI) were cloned into pKW319, provided by Karsten Weis (University of California, Berkeley, CA), excised (with βgal from pKW319) as a BglII-HindIII fragment, and cloned into the BamHI-HindIII sites of pET30a (Novagen). IBB-Npl: residues 1–65 of importin α (EcoRI-BamHI) and the nucleoplasmin (Npl) core (residues 1–149; BamHI-HindIII) were cloned into the EcoRI-HindIII sites of pET30a. GST-IBB: residues 1–65 of importin α (BglII-EcoRI) were cloned into the BamHI-EcoRI sites of pGEX2T (Amersham Biosciences). M9-βgal: residues 255–320 of hnRNPA1 (BamHI-KpnI) and βgal (KpnI-HindIII) were cloned into the BamHI-HindIII sites of pET30a. Npl-M9: the Npl core (residues 1–149; BamHI-EcoRI) and residues 263–306 of hnRNPA1 (EcoRI-XhoI) were cloned into the BamHI-XhoI sites of pET30a. Δ84 transportin was generated by replacing the NH2 terminus of transportin in pGEX-Tev-β2, provided by Yuh Min Chook (University of Texas Southwestern Medical Center, Dallas, TX) with a BamHI-NsiI fragment comprising residues 253–764 of transportin. WT and Δ44 importin β were expressed using pTYB4 vector (New England Biolabs) and purified according to the manufacturer's instructions.
Protein purification
Protein expression and cell lysis were generally by the following protocol; exceptions are indicated. Cultures were inoculated with freshly transformed Escherichia coli BL21− cells and grown at 30°C to an OD600 of ∼0.6–0.8; expression was induced by the addition of 15 μM IPTG for 12–16 h at 20°C. Cells were resuspended in buffer S (50 mM Tris, pH 8.0, 500 mM NaCl, 1 mM MgOAc, 2 mM DTT or BME, 5% glycerol), 1 mg/ml lysozyme, 10 μg/ml DNase, a protease inhibitor cocktail, and 0.1% Triton X-100. The suspension was frozen, thawed, sonicated (3 × 15 s), and centrifuged at 100,000 g for 20 min. IBB-Npl, IBB-βgal, Npl-M9, and M9-βgal were purified over Talon Sepharose (CLONTECH Laboratories, Inc.). Expression of GST-IBB and GST-M9 was induced with 50 μM IPTG for 2 h at 37°C. Proteins were purified on glutathione-Sepharose (Amersham Biosciences). The lysis buffer for WT and Δ84 transportin contained 2 mM CHAPS (Calbiochem) instead of Triton. Protein was purified on glutathione-Sepharose, and GST was cleaved by TEV protease (Invitrogen). IBB-Npl, IBB-βgal, Npl-M9, M9-βgal, GST-IBB, GST-M9, and WT and Δ84 transportin were all further purified on a Superdex 200 column. The lysis buffer for WT and Δ44 importin β lacked lysozyme, and contained 10 mM CHAPS in place of Triton and 1 mM TCEP (Pierce Chemical Co.) in place of DTT. The lysate was purified over chitin beads (New England Biolabs). Ran, RanQ69L, and XRan were purified essentially as described for WT Ran (Melchior et al., 1993), except that expression of XRan was induced with 20 μM IPTG at 20°C for 16 h, and 5 μM XDP (JenaBioScience) was included in the lysate. Importin α (Kehlenbach et al., 2001), NTF2 (Paschal et al., 1996), and RanGAP (Mahajan et al., 1997) were purified essentially as described. All proteins were dialyzed into transport buffer (TB; 20 mM Hepes, pH 7.4, 110 mM KOAc, 2 mM MgOAc, 2 mM DTT) and stored at −80°C.
Stokes radius analysis
The hydrodynamic radius of the cargo molecules was determined by gel filtration as described by Ribbeck and Görlich (2001). It was not possible to directly determine the radius of the IBB-gold and M9-gold, as a portion of the gold bound to the column prefilter and nucleated aggregation. Therefore, we used BSA-coated colloidal gold to approximate the radius of the cargo-coated gold.
Nuclear import assay
For analysis of nuclear import in digitonin-permeabilized adherent HeLa cells, in vitro assays, substrate visualization, and preparation of HeLa cytosol (by digitonin lysis) were carried out essentially as in Kehlenbach et al. (2001), except that cells were preincubated in TB for 15 min at 30° before import. FITC labeling and NLS peptide conjugation to carrier proteins was performed as in Melchior et al. (1993). Unlabeled cargo was detected either with a monoclonal antibody against βgal (Promega) or with an affinity-purified polyclonal antibody that we generated against nucleoplasmin. For each reaction condition, 10–12 different microscope fields were quantified with NIH Image, for a total cell count of 100–200. Import reactions contained either 2.5 mg/ml HeLa cytosol or recombinant factors (the levels of which were optimized for each class of import signal): WT or Δ44 importin β at 62.5 nM or 625–750 nM (as indicated); WT or Δ84 transportin at 250 nM or 750 nM; Ran, RanQ69L, or XRan at 300 nM (450 nM for NLS cargo reactions, which also contained 330 nM importin α), NTF2 at 500 nM; IBB cargo at 125 nM or 500 nM; M9 cargo at 125 nM or 250 nM; NLS cargo at 150 nM. The final concentration of GTP (Sigma-Aldrich) was 1 mM, and XTP (JenaBioScience) was 0.5 mM. For those reactions containing cytosol, GTP was added at 200 μM in conjunction with an energy-regenerating system. In the absence of added NTP, hexokinase/glucose was added to deplete endogenous cellular NTPs.
Coprecipitation assay
Trypsinized adherent HeLa cells were collected and used for in vitro import assays. After import, cells were washed and then solubilized in TB containing 1% NP40, 5% glycerol, and 300 mM NaCl (in place of KOAc). Lysates were cleared by centrifugation, and cargo was precipitated with S-protein agarose (Novagen) or glutathione Sepharose. Associated proteins were detected by SDS-PAGE (105 cells/lane) and immunoblotting as in (Kehlenbach et al., 2001).
GAP assay
Ran was loaded with γ32P GTP (Perkin-Elmer) as in (Delphin et al., 1997). GAP assays (20 μl in 50 mM Hepes, pH 7.4, 5 mM MgCl2, 1 mM DTT, 0.1 mg/ml BSA, 0.005% Tween 20) contained 0.1 pmol RanGAP and 0–15 pmol WT or Δ84 transportin. Reactions were initiated by the addition of ∼2.5 pmol 32P-GTP Ran, and reaction products were separated by chromatography on PEI-cellulose plates (J.T. Baker) in 1 M formic acid, 0.5 M LiCl. Quantitation was by PhosphorImager (Molecular Dynamics).
Electron microscopy
For EM analysis, HeLa cells were grown on pieces of Aclar embedding film (Ted Pella Inc.), and in vitro import reactions contained gold cargo that was freshly prepared as in Slot and Geuze (1985). For import assays lacking GTP, cytosol was pretreated (or mock treated) with an energy depletion system, which was also added to the reaction mix. The fixation, dehydration, and embedding procedure was essentially as in (Delphin et al., 1997) except that samples were stained with 1% uranyl acetate for 20 min. Micrographs were recorded with a Philips 208 electron microscope at 80 kV. For distribution analysis, ∼200–300 gold particles were counted for each reaction condition. We also analyzed import in reactions containing cytosol, importin β, and our standard protein (i.e., nongold) cargos (unpublished data) in order to ensure that import under these conditions recapitulated the trends we previously observed (Fig. 1) with recombinant factors.
Acknowledgments
We thank Gino Cingolani for helpful discussions and Jeff Brodsky for critical reading of the manuscript. We are grateful for DNA constructs provided by Karsten Weis, Yuh Min Chook, and Dirk Görlich (ZMBH, Heidelberg, Germany), and also for advice from Yuh Min Chook on generating transportin mutants.
This work was supported by National Institutes of Health grant GM41955 (to L. Gerace). Postdoctoral fellowships supported S.K. Lyman (Damon Runyon Cancer Research Foundation; DRG 1407), H. Wodrich (Deutsche Forschungsgemeinschaft; WO 805/1-1), and J. Bednenko (Leukemia and Lymphoma Society; LSA5046-00).
Footnotes
Abbreviations used in this paper: βgal, β-galactosidase; FG, Phe-Gly; IBB, importin β binding domain; NPC, nuclear pore complex; Npl, nucleoplasmin core; NTP, nucleoside triphosphate; thyr, thyroglobulin; XTP, xanthosine triphosphate
References
- Allen, N.P., L. Huang, A. Burlingame, and M. Rexach. 2001. Proteomic analysis of nucleoporin interacting proteins. J. Biol. Chem. 276:29268–29274. [DOI] [PubMed] [Google Scholar]
- Bayliss, R., T. Littlewood, and M. Stewart. 2000. Structural basis for the interaction between FxFG nucleoporin repeats and Importin β in nuclear trafficking. Cell. 102:99–108. [DOI] [PubMed] [Google Scholar]
- Ben-Efraim, I., and L. Gerace. 2001. Gradient of increasing affinity of importin β for nucleoporins along the pathway of nuclear import. J. Cell Biol. 152:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, F.R., and D. Görlich. 1997. RanBP1 is crucial for the release of RanGTP from Importin β-related nuclear transport factors. FEBS Lett. 419:249–254. [DOI] [PubMed] [Google Scholar]
- Catimel, B., T. Teh, M.R. Fontes, I.G. Jennings, D.A. Jans, G.J. Howlett, E.C. Nice, and B. Kobe. 2001. Biophysical characterization of interactions involving importin α during nuclear import. J. Biol. Chem. 276:34189–34198. [DOI] [PubMed] [Google Scholar]
- Creighton, T.E. 1993. Proteins. Structures and Molecular Properties. W.H. Freeman and Company, New York. 261–328.
- Conti, E., and E. Izaurralde. 2001. Nucleocytoplasmic transport enters the atomic age. Curr. Opin. Cell Biol. 13:310–319. [DOI] [PubMed] [Google Scholar]
- Delphin, C., T. Guan, F. Melchior, and L. Gerace. 1997. RanGTP targets p97 to RanBP2, a filamentous protein localized at the cytoplasmic periphery of the nuclear pore complex. Mol. Biol. Cell. 8:2379–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, S., I.V. Akey, C. Dingwall, K.L. Hartman, T. Laue, R.T. Nolte, J.F. Head, and C.W. Akey. 2001. The crystal structure of nucleoplasmin-core: implications for histone binding and nucleosome assembly. Mol. Cell. 8:841–853. [DOI] [PubMed] [Google Scholar]
- Englmeier, L., J.-C. Olivo, and I.W. Mattaj. 1999. Receptor-mediated substrate translocation through the nuclear pore complex without nucleotide triphosphate hydrolysis. Curr. Biol. 9:30–41. [DOI] [PubMed] [Google Scholar]
- Fahrenkrog, B., D. Stoffler, and U. Aebi. 2001. Nuclear pore complex architecture and functional dynamics. Curr. Top. Microbiol. Immunol. 259:95–117. [DOI] [PubMed] [Google Scholar]
- Feldherr, C.M., and D. Akin. 1997. The location of the transport gate in the nuclear pore complex. J. Cell Sci. 110:3065–3070. [DOI] [PubMed] [Google Scholar]
- Floer, M., and G. Blobel. 1996. The nuclear transport factor karyopherin β binds stoichiometrically to RanGTP and inhibits the Ran GTPase activating protein. J. Biol. Chem. 271:5313–5316. [DOI] [PubMed] [Google Scholar]
- Goldberg, M., S.A. Rutherford, M. Hughes, L.A. Cotter, S. Bagley, E. Kiseleva, T.D. Allen, and P.R. Clarke. 2000. Ran alters nuclear pore complex conformation. J. Mol. Biol. 300:519–529. [DOI] [PubMed] [Google Scholar]
- Görlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15:607–660. [DOI] [PubMed] [Google Scholar]
- Görlich, D., N. Pante, U. Kutay, U. Aebi, and F.R. Bischoff. 1996. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- Huber, J., A. Dickmanns, and R. Luhrmann. 2002. The importin-beta binding domain of snurportin1 is responsible for the Ran- and energy-independent nuclear import of spliceosomal U snRNPs in vitro. J. Cell Biol. 156:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlenbach, R.H., and L. Gerace. 2000. Phosphorylation of the nuclear transport machinery down-regulates nuclear protein Import in vitro. J. Biol. Chem. 275:17848–17856. [DOI] [PubMed] [Google Scholar]
- Kehlenbach, R.H., R. Assheuer, A. Kehlenbach, J. Becker, and L. Gerace. 2001. Stimulation of nuclear export and inhibition of nuclear import by a Ran mutant deficient in binding to Ran-binding protein 1. J. Biol. Chem. 276:14524–14531. [DOI] [PubMed] [Google Scholar]
- Klebe, C., F.R. Bischoff, H. Ponstingl, and A. Wittinghofer. 1995. Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry. 34:639–647. [DOI] [PubMed] [Google Scholar]
- Lazarowski, E.R., R.C. Boucher, and T.K. Harden. 2000. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J. Biol. Chem. 275:31061–31068. [DOI] [PubMed] [Google Scholar]
- Lounsbury, K.M., and I.G. Macara. 1997. RanBP1 forms a ternary complex with Ran and karyopherin β and reduces RanGAP inhibition by karyopherin β. J. Biol. Chem. 272:551–555. [DOI] [PubMed] [Google Scholar]
- Macara, I.G. 2001. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 65:570–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan, R., C. Delphin, T. Guan, L. Gerace, and F. Melchior. 1997. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 88:97–107. [DOI] [PubMed] [Google Scholar]
- Melchior, F., B. Paschal, J. Evans, and L. Gerace. 1993. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J. Cell Biol. 123:1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, M.S. 1998. Ran and nuclear transport. J. Biol. Chem. 273:22857–22860. [DOI] [PubMed] [Google Scholar]
- Nachury, M.V., and K. Weis. 1999. The direction of transport through the nuclear pore can be inverted. Proc. Natl. Acad. Sci. USA. 96:9622–9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny, S., and G. Dreyfuss. 1999. Transport of proteins and RNAs in and out of the nucleus. Cell. 99:677–690. [DOI] [PubMed] [Google Scholar]
- Paine, P.L., L.C. Moore, and S.B. Horowitz. 1975. Nuclear envelope permeability. Nature. 254:109–114. [DOI] [PubMed] [Google Scholar]
- Paschal, B., C. Delphin, and L. Gerace. 1996. Nucleotide-specific interaction of Ran/TC4 with nuclear transport factors NTF2 and p97. Proc. Natl. Acad. Sci. USA. 93:7679–7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach, M., and G. Blobel. 1995. Protein import into nuclei: Association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 83:683–692. [DOI] [PubMed] [Google Scholar]
- Ribbeck, K., and D. Görlich. 2001. Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 20:1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbeck, K., and D. Gorlich. 2002. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 21:2664–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbeck, K., U. Kutay, E. Paraskeva, and D. Görlich. 1999. The translocation of transportin-cargo complexes through nuclear pores is independent of both Ran and energy. Curr. Biol. 9:47–50. [DOI] [PubMed] [Google Scholar]
- Rout, M.P., and J.D. Aitchison. 2001. The nuclear pore complex as a transport machine. J. Biol. Chem. 276:16593–16596. [DOI] [PubMed] [Google Scholar]
- Schwoebel, E.D., B. Talcott, I. Cushman, and M.S. Moore. 1998. Ran-dependent signal-mediated nuclear import does not require GTP hydrolysis by Ran. J. Biol. Chem. 273:35170–35175. [DOI] [PubMed] [Google Scholar]
- Schwoebel, E.D., T.H. Ho, and M.S. Moore. 2002. The mechanism of inhibition of Ran-dependent nuclear transport by cellular ATP depletion. J. Cell Biol. 157:963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, S., S. Tugendreich, and D.J. Forbes. 1998. Major binding sites for the nuclear import receptor are the internal nucleoporin Nup153 and the adjacent nuclear filament protein Tpr. J. Cell Biol. 141:31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot, J.W., and H.J. Geuze. 1985. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur. J. Cell Biol. 38:87–93. [PubMed] [Google Scholar]
- Stewart, M., R.P. Baker, R. Bayliss, L. Clayton, R.P. Grant, T. Littlewood, and Y. Matsuura. 2001. Molecular mechanism of translocation through nuclear pore complexes during nuclear protein import. FEBS Lett. 498:145–149. [DOI] [PubMed] [Google Scholar]
- Sweet, D.J., and L. Gerace. 1996. A GTPase distinct from Ran is involved in nuclear protein import. J. Cell Biol. 133:971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasu, S.K., and D.J. Forbes. 2001. Nuclear pores and nuclear assembly. Curr. Opin. Cell Biol. 13:363–375. [DOI] [PubMed] [Google Scholar]
- Villa Braslavsky, C., C. Nowak, D. Gorlich, A. Wittinghofer, and J. Kuhlmann. 2000. Different structural and kinetic requirements for the interaction of Ran with the Ran-binding domains of RanBP2 and importin β. Biochemistry. 39:11629–11639. [DOI] [PubMed] [Google Scholar]
- Weis, K., C. Dingwall, and A. Lamond. 1996. Characterization of the nuclear protein import mechanism using Ran mutants with altered nucleotide binding specificities. EMBO J. 15:7120–7128. [PMC free article] [PubMed] [Google Scholar]
- Yaseen, N.R., and G. Blobel. 1999. GTP hydrolysis links initiation and termination of nuclear import on the nucleoporin Nup358. J. Biol. Chem. 274:26493–26502. [DOI] [PubMed] [Google Scholar]