Abstract
Cells rapidly transduce forces exerted on extracellular matrix contacts into tyrosine kinase activation and recruitment of cytoskeletal proteins to reinforce integrin–cytoskeleton connections and initiate adhesion site formation. The relationship between these two processes has not been defined, particularly at the submicrometer level. Using talin1-deficient cells, it appears that talin1 is critical for building early mechanical linkages. Deletion of talin1 blocked laser tweezers, force-dependent reinforcement of submicrometer fibronectin-coated beads and early formation of adhesion sites in response to force, even though Src family kinases, focal adhesion kinase, and spreading were activated normally. Recruitment of vinculin and paxillin to sites of force application also required talin1. FilaminA had a secondary role in strengthening fibronectin–integrin–cytoskeleton connections and no role in stretch-dependent adhesion site assembly. Thus, force-dependent activation of tyrosine kinases is independent of early force-dependent structural changes that require talin1 as part of a critical scaffold.
Keywords: talin; integrin; actin cytoskeleton; tyrosine phosphorylation; mechano-sensing
Introduction
The generation of force on the ECM is required for cell viability, differentiation (Huang and Ingber, 2000), and migration (Lauffenburger and Horwitz, 1996). Force-dependent effects involve the spatial and temporal regulation of integrin interactions with both ECM proteins and the actin cytoskeleton. Forces applied to these nascent ECM–integrin–cytoskeleton connections induce strengthening of the integrin–cytoskeleton interactions (Choquet et al., 1997) leading to focal complex initiation and stabilization (Rottner et al., 1999; Galbraith et al., 2002). Further force generation is responsible for maturation of focal complexes to focal adhesions (Riveline et al., 2001), which also require sustained forces for their stabilization (Balaban et al., 2001). However, the proteins that compose the early mechanosensory system within precursors of focal complexes and the force-sensing phenomenon are poorly defined (Geiger and Bershadsky, 2002). At least two processes are known to be involved in early mechano-sensing: (1) the activation of signaling pathways including the Src family kinases (SFKs; von Wichert et al., 2003) and (2) the recruitment of structural proteins on a local scaffold leading to assembly of focal complexes at sites of cell–matrix adhesion (Galbraith et al., 2002).
The importance of signaling pathways in early mechano-sensing is shown by studies indicating that tyrosine phosphorylation and dephosphorylation are critical for rapid formation and turnover of adhesion sites (for review see Schoenwaelder and Burridge, 1999). Activations of SFKs and FAK are early, enzymatically linked events that immediately follow integrin engagement (Miyamoto et al., 1995; Cary et al., 2002), and tyrosine phosphorylation events are linked to the force applied on integrins (Pelham and Wang, 1997). FAK-deficient cells are unable to reorient their movement and form new adhesion sites in response to external forces on collagen-coated flexible substrates (Wang et al., 2001), and Src kinase activity weakens αvβ3/integrin–cytoskeletal linkages (Felsenfeld et al., 1999) and may stimulate focal adhesion turnover. Furthermore, the tyrosine phosphatase receptor-like protein phosphatase α (RPTPα) has been shown to act as a transducer of early mechanical force on fibronectin (FN)–integrin–cytoskeleton linkages through αvβ3/integrin-dependent activation of SFKs (von Wichert et al., 2003).
The second important aspect of mechano-sensing is the recruitment of proteins to sites of force generation mediated by binding to components of the adhesion site that are structurally altered by force. For example, when detergent-insoluble cytoskeletons are mechanically stretched, adhesion site-associated proteins will bind independent of kinase and phosphatase activity (Sawada and Sheetz, 2002). The molecular nature of the structural component involved in force sensing is still elusive, but it is logical to look first at proteins connecting integrins with the cytoskeleton because regions of contact between integrins and matrix are the sites of greatest stress, and there is local recruitment of cytoskeletal and adhesion site proteins. In vitro studies show that integrins can be coupled to the actin cytoskeleton through several connector proteins: α-actinin, tensin, filamin, and talin (Liu et al., 2000). Both filaminA and talin1 bind directly to integrins (Liu et al., 2000) and to the actin cytoskeleton (Hemmings et al., 1996; Stossel et al., 2001), and, therefore, might be involved in transmitting force on integrins to the cytoskeleton. Indeed, mechanical forces locally reinforce linkages between β1 integrins and the cytoskeleton through actin and filaminA recruitment, an effect not observed in filaminA-deficient melanoma cells (Glogauer et al., 1998). Deletion of talin1 inhibits adhesion site formation in mouse embryonic stem (ES) cells (Priddle et al., 1998) and formation of focal adhesion-like structures during Drosophila melanogaster embryogenesis (Brown et al., 2002). However, a talin1-deficient “fibroblast-like” cell line derived from talin1 (−/−) ES cells was able to assemble vinculin- and paxillin-containing adhesion structures (Priddle et al., 1998), suggesting that other actin-binding proteins such as filamin, α-actinin, tensin, or talin2 (Monkley et al., 2001) can compensate to a certain extent for talin1 deficiency.
We have focused here on the roles that talin1 and filaminA play in the reinforcement of integrin–cytoskeleton connections leading to initiation and stabilization of early adhesion sites in response to force. We have also addressed whether tyrosine kinase activation can be separated from the structural changes needed for reinforcement in response to matrix-generated forces. In the talin1-deficient cells, the force-dependent activation of SFKs and FAK were normal, whereas there was no reinforcement of integrin–actin connections at early times. The separation of enzymatic from structural changes induced by force provides the first evidence that these processes can be activated independently.
Results
Talin1 is not necessary for cell spreading and force-induced, integrin-mediated signaling in talin1 (−/−) cells
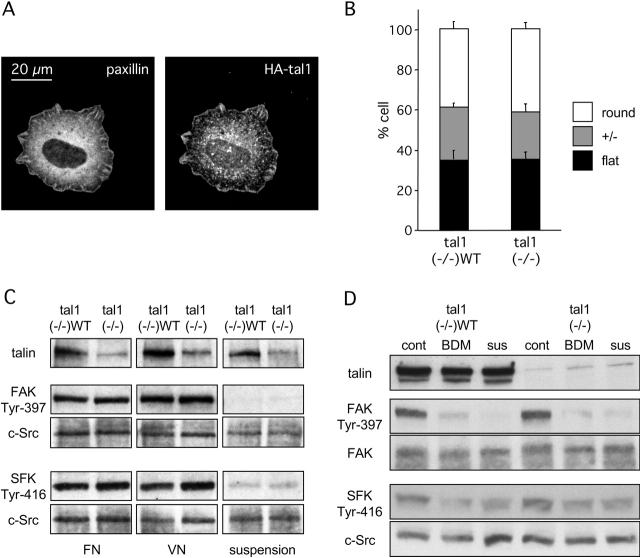
Because the talin1 head domain has been shown to interact with the cytoplasmic domains of integrin β1 and β3 subunits (Calderwood et al., 1999) and FAK (Critchley, 2000), we assayed a mouse talin1 (−/−) fibroblast-like cell line for ECM-activated integrin functions. For comparison, the cells were transiently transfected with an HA-tagged mouse talin1 cDNA (talin1 (−/−)WT cells). Efficient expression of talin1 (2,541 amino acids) was confirmed by Western blotting (Fig. 1, C and D); the residual talin immunoreactive protein in talin1 (−/−) cells is likely to be talin2, as determined using talin1- and talin2-specific antibodies (Craig, S.W., personal communication, unpublished data). The correct localization of HA-talin1 to adhesion sites was confirmed by immunostaining of talin1 (−/−)WT cells cotransfected with paxillin-GFP (Fig. 1 A). The early spreading efficiency of talin1 (−/−) cells and talin1 (−/−) WT cells on FN was similar (e.g., 10 min after plating; Fig. 1 B). The expression level of integrins α5, αv, β1, and β3, which are all involved in adhesion and spreading on FN, was comparable in deficient and rescued cells (for review see Priddle et al., 1998; unpublished data).
Figure 1.
Integrin- and force-dependent activation of SFKs and FAK is normal during spreading of talin1-deficient cells on FN. (A) After 30 min of spreading on FN 120 kD, talin1 (−/−) cells transiently cotransfected with HA-talin1 and paxillin-GFP were fixed; paxillin-GFP and HA-talin1 were visualized by fluorescence and immunofluorescence, respectively. (B) After 10 min of spreading on FN 120 kD, talin1 (−/−) cells or cells transiently cotransfected with talin1 and EGFP (talin1 (−/−)WT) cells were scored for flat, intermediary, or round morphology. Results represent the mean ± SD of three experiments. (C) Talin1 (−/−) and talin1 (−/−)WT cell suspension or cells allowed to spread for 10 min on either FN 120 kD or VN were lysed, and the protein was analyzed by Western blotting using a phosphospecific anti-SFK (SFK Tyr-416), a phosphospecific anti-FAK (FAK Tyr-397), and a talin antibody. The total amount of proteins was verified using an anti-Src antibody. (D) Talin1 (−/−) and talin1 (−/−)WT cells allowed to spread for 10 min on FN in the presence or absence (cont) of 20 mM of the myosin inhibitor BDM or in suspension (sus) were lysed; the protein was analyzed by Western blotting using a phosphospecific anti-SFK (SFK Tyr-416), a phosphospecific anti-FAK (FAK Tyr-397), and a talin antibody. The total amount of proteins was verified using an anti-Src and an anti-FAK antibody. (C and D) Results shown are representative of three independent experiments.
Integrin-dependent activation of tyrosine phosphorylation events (Pelham and Wang, 1997), and particularly FAK (Wang et al., 2001) and SFKs (Felsenfeld et al., 1999; von Wichert et al., 2003), has been linked to adhesion site formation during spreading and force-dependent signaling. Interestingly, in talin1 (−/−) cells, SFK and FAK activation appeared normal during the initial spreading (10 min) on FN or vitronectin (VN). With antibodies specific for autophosphorylation of SFKs (such as c-Src, Fyn, and c-Yes) on Tyr416, and for autophosphorylation of FAK on Tyr397 (Fig. 1 C), we observed a similar increase in phosphorylation after cell binding to FN- or VN-coated surfaces in both talin1 (−/−) and talin1 (−/−)WT cells. Next, we tested whether forces generated by talin1 (−/−) and talin1 (−/−) WT cells during the spreading are involved in SFK and FAK activation (Fig. 1 D). Inhibition of myosin-dependent contractility by 2,3-butanedione monoxime (BDM; 20 mM) significantly decreased both FAK and SFK activation during spreading, suggesting that tyrosine phosphorylation of SFK and FAK resulted from force-induced, integrin-mediated signaling. The normal spreading and SFK and FAK activation on FN for talin1 (−/−) cells suggest that early, force-dependent, matrix–integrin signaling processes do not directly involve talin1.
Initiation of adhesion site assembly is delayed in talin1 (−/−) cells
Focal complex/adhesion formation is another major function involving integrins and force generation, and was previously reported to be decreased in undifferentiated talin1 (−/−) ES cells deficient for talin1. However, upon differentiation of these cells into the fibroblast-like cells used here, focal adhesions appeared normal at least at later times (Priddle et al., 1998). The dynamic reorganization of integrin-associated protein complexes during focal complex formation (Galbraith et al., 2002) prompted us to characterize the temporal dependence of adhesion site formation in talin1 (−/−) cells, and in cells expressing either the full-length mouse talin1 cDNA (talin1 (−/−)WT cells) or a talin1 polypeptide (residues 1–2299) lacking the highly conserved COOH-terminal actin-binding site (talin1 (−/−) ABS; Hemmings et al., 1996). Visualization of adhesion sites was performed 1 and 24 h after plating on FN using paxillin-GFP as a marker. Although after 24 h there appeared to be little difference, after 1 h there was a dramatically lower percentage of cells displaying paxillin-GFP containing contacts in talin1(−/−) and talin1(−/−)ABS cells than in the talin1 (−/−)WT cells (Fig. 2, A and B), implying that formation of linkages between talin1 and the actin cytoskeleton are critical for the recruitment of paxillin-GFP and formation of adhesion sites.
Figure 2.
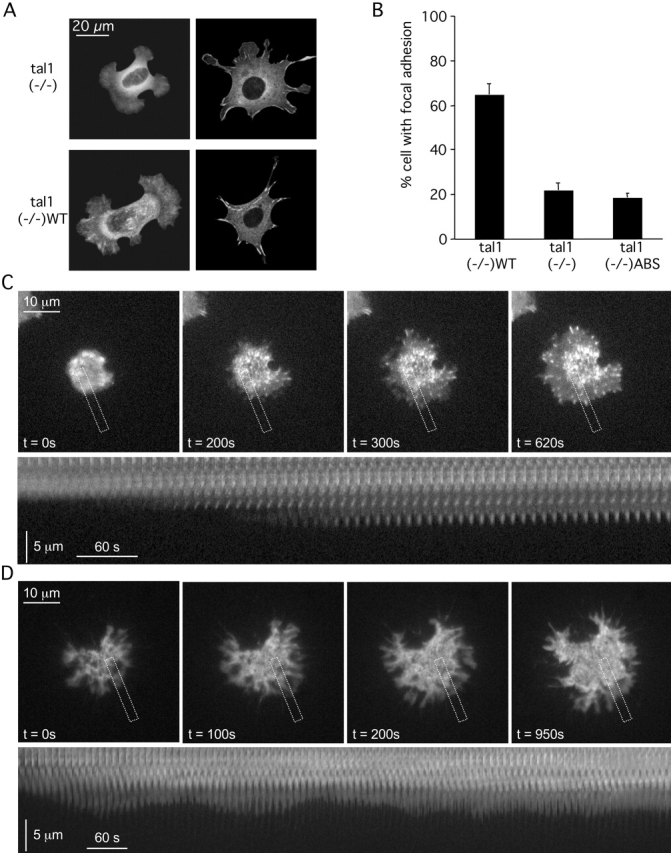
The initiation and stabilization of focal complexes to focal adhesions is delayed in talin1-deficient cells. Talin1 (−/−), talin1 (−/−)WT, and talin1 (−/−)ABS were transiently transfected with paxillin-GFP. After 1 h of spreading on FN 120 kD–coated coverslips, cells were fixed, and paxillin-GFP was visualized (A) to quantify the percentage of cells with focal adhesions (B). Approximately 65% of talin1 (−/−)WT cells expressing full-length talin1 displayed paxillin-positive adhesions (A, bottom right) compared with only 20% in talin1 (−/−) (A, top right) and talin1 (−/−)ABS cells (B). Results represent the mean ± SD of at least three experiments. (C) Formation of adhesion sites was observed in talin1 (−/−)WT cells transfected with paxillin-GFP spreading on FN 120 kD–coated coverslips using TIRF microscopy. The bottom kymograph of the dotted box displayed in the upper sequence showed that the initiation and stabilization of focal complexes is fast and simultaneous with protrusion/retraction of the lamellipodia in talin1 (−/−)WT cells. (D) In talin1 (−/−) cells transfected with paxillin-GFP spreading, no distinct adhesion sites were formed during >30-min acquisition time. The bottom kymograph of the dotted box displayed in the upper sequence showed that despite extensive protrusion/retraction events of the lamellipodia, no initiation of focal complexes was observed.
We tested if the lower percentage of adhesion sites in talin1 (−/−) cells after 1 h spreading is due to a slower formation of focal adhesions or to a delay in the initiation of focal complexes slowing down the formation of focal adhesions. We performed total internal reflection fluorescence (TIRF) microscopy to detect the initiation of paxillin-GFP clusters in talin1 (−/−)WT (Fig. 2 C) and talin1 (−/−) cells (Fig. 2 D). In talin1 (−/−)WT, focal complexes were initiated in concert with protrusions and retractions of the lamellipodia (Fig. 2 C). The mean time necessary for initiation and stabilization of the paxillin-GFP clusters was 123 ± 55 s (n = 31 adhesion sites; seven cells). In talin1 (−/−) cells, despite the presence of active lamellipodia, no initiation of distinct adhesion sites was detected during >30 min of data acquisition (Fig. 2 D) in 80% of cells. In ∼20% of the cells, adhesions were observed, and the rate of adhesion site formation in those talin1 (−/−) cells was similar to talin1 (−/−)WT cells (116 ± 54 s; n = 17 adhesion sites; three cells). This suggests that the adhesion site formation was an all or none process perhaps triggered by compensation of a talinlike gene (e.g., talin2 or tensin). Nevertheless, talin1 (−/−) cells were able to apply force on ECM–integrin contacts as indicated by (a) the force-dependent activation of FAK and SFK (Fig. 1 D); (b) the ruffling of protrusions; and (c) the movement of FN-coated beads out of the laser trap (Fig. 3). Thus, early focal complex formation appears to depend on talin1, which raises the possibility that talin1 is involved in the initial linkages between integrin and the actin cytoskeleton, but is not essential for integrin signaling or cell spreading.
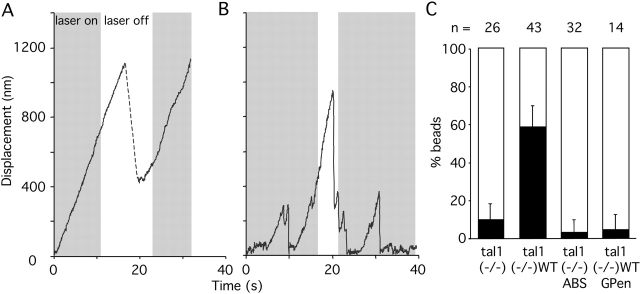
Figure 3.
FNIII7–10–integrin–talin1–actin connections are essential for the force-dependent strengthening of integrin–cytoskeleton linkages. (A) A representative trace showing displacement of trimeric FNIII7–10-coated beads from their initial position over time on the surface of talin1 (−/−)WT cells. Restrained beads (gray strips, turn on of the laser trap) able to escape the laser trap (movement outside the first gray strip) were tested with a second pulse (second gray strip) after turning off (white strip) and repositioning the laser trap (<0.5 μm behind the bead). Beads were scored reinforced if they were unable to be dislocated (no changes of the bead trajectory >100 nm, just after the beginning of the second gray strip, test pulse). Most beads were not displaced by the test pulse (retrap [reinforced]), suggesting that the cell regulates, in response to the rigidity of the laser trap, the strength of integrin–cytoskeleton linkages. (B) Representative trace showing displacement of trimeric FNIII7–10-coated beads from its initial position over time on the surface of talin1 (−/−) cells. Trimeric FNIII7–10-coated beads, which were not reinforced, were displaced by the laser trap test pulse (second gray strip; retrap [loose]) after initial escape from the laser trap (movement outside the first gray strip). (C) Summary of reinforcement assay results, showing the percentage of experiments in which beads were reinforced (black bars) or loose (white bars). Transfected cells were identified by EGFP cotransfection. Results represent the mean ± SD of at least three experiments.
Talin1 is required for reinforcement of integrin–cytoskeleton linkages
Forces exerted on integrin–cytoskeleton connections result in their strengthening (Choquet et al., 1997) and are associated with initiation and stabilization of focal complexes (Galbraith et al., 2002). We used laser tweezers to assess the role of talin1 in regulating the strength of early connections between FN and the cytoskeleton in a reinforcement assay (Fig. 3). To specifically focus on focal complex initiation and stabilization, we used a fragment of FN type III domains 7–10 (FNIII7–10), which contains the cell-binding domain (RGD) and the synergy site. FNIII7–10-coated beads bound to cell surface integrins, and the retrograde moving actin cytoskeleton normally pulled the beads out of the trap by applying a significant force to the FNIII7–10–integrin–cytoskeleton linkages (Choquet et al., 1997). Later, when the force of the tweezers was applied again to the bound bead, reinforced beads could not be moved back toward the leading edge, whereas unreinforced beads were moved (Choquet et al., 1997).
Talin1 (−/−) and talin1 (−/−)ABS cells showed abnormal behavior in the reinforcement assay at several levels. Initially, a lower fraction of beads was able to escape the laser trap (56 ± 19% and 55 ± 18%) compared with talin1 (−/−)WT cells (79 ± 10%). Furthermore, the time required for a bead to move 50 nm from the trap center in talin1 (−/−) cells (10 ± 8 s) was significantly longer than in talin1 (−/−)WT (3 ± 5 s) cells (Fig. 3, compare A with B), suggesting that talin1 is involved in strengthening initial integrin connections with the cytoskeleton. When tested for reinforcement, there was an even greater difference. In talin1 (−/−)WT cells, 58 ± 11% of the beads that were able to escape the trap were reinforced and did not move in response to the tweezers' force, whereas in talin1 (−/−) cells only 10 ± 8% of the escaped FNIII7–10-coated beads were reinforced (Fig. 2 C). Transient expression of a truncated talin1 lacking the COOH-terminal actin-binding site was unable to restore normal reinforcement (10 ± 6%), suggesting that the interaction between talin1 and actin filaments is necessary for the reinforcement process. Although integrin coupling to the actin cytoskeleton is possible without talin1, it occurs much more slowly, and the integrin–cytoskeleton connections cannot be strengthened in response to force.
The nearly sixfold difference in reinforcement was not related to a difference in FNIII7–10 bead binding. In the bead binding assay, FNIII7–10-coated beads were placed for 3 s on the upper surface of lamellipodia (<0.5 μm from the leading edge of the cell) and the trap was turned off. When beads were coated with high levels of FNIII7–10, little difference was found in the binding frequency between talin1 (−/−) cells (86 ± 5%) and talin1 (−/−) WT cells (97 ± 3%). We compared the reinforcement process in talin1 (−/−) and talin1 (−/−)WT at similar adhesion strength. To do this comparison, we reduced the percentage binding of FNIII7–10-coated beads on talin1 (−/−)WT cells to 75 ± 8%, (by adding BSA with FN, FNIII7–10/BSA 1/1). Under these conditions, the reinforcement in talin1 (−/−)WT was not impaired (57 ± 8%, n = 51 beads), which demonstrated that at the same adhesion strength between the ECM and integrins, integrin–cytoskeleton strengthening was dependent on talin1.
Interestingly, we observed that after >25 passages, the talin1 (−/−) cells lost their severe impairment in the reinforcement process (35 ± 16% showed reinforcement). Later passages of talin1 (−/−) cells were characterized by up-regulation of expression of filaminA and talin2 (unpublished data). From the analysis of the role of filaminA in reinforcement (see Fig. 6), it appears that the up-regulation of talin2 restored the reinforcement process.
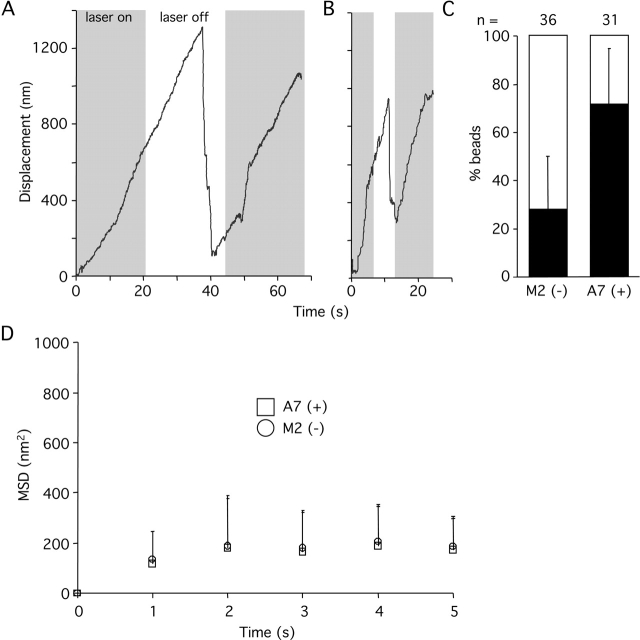
Figure 6.
FilaminA is moderately involved in fast stabilization of integrin–cytoskeleton linkages. (A) A representative trace showing displacement of trimeric FNIII7–10-coated beads from its initial position over time on the surface of filaminA-expressing A7 cells. (B) Representative trace showing displacement of trimeric FNIII7–10-coated beads from its initial position over time on the surface of filaminA-null M2 cells. (A and B) Gray and white strips represent turn on and off of the laser trap, respectively (Fig. 3). (C) Summary of reinforcement assay results showing the percentage of experiments in which beads were reinforced (black bars) or loose (white bars). FilaminA-deficient M2 melanoma cells displayed a relatively weak impairment of the reinforcement process compared with A7 cells expressing the filaminA cDNA. Results represent the mean ± SD of at least three experiments. (D) Summary of MSD quantification after beads left the trap field (500 nm; see Materials and methods). Results shown are the mean ± SD of at least five independent experiments.
The talin1 force-dependent reinforcement of FN–integrin–cytoskeleton linkages involves αvβ3/integrin
The αvβ3/integrin, originally described as the VN receptor, binds to a variety of plasma and ECM proteins including VN and FN (Boettiger et al., 2001). Because αvβ3/integrin-dependent activation of SFK is involved in early adhesion site formation on FN and reinforcement of FNIII7–10–integrin–cytoskeleton connections (von Wichert et al., 2003), we tested if αvβ3/integrin was implicated in the reinforcement process in talin1 (−/−)WT cells by adding the cyclic peptide GPenGRGDSPCA (GPen; 0.5mM; Fig. 3 C). At this concentration, the peptide was shown to be a selective, competitive inhibitor of the αvβ3/integrin, and did not block binding of FN to its receptor (α5β1/integrin; Pierschbacher and Ruoslahti, 1987). GPen treatment reduced the binding of FNIII7–10-coated beads on the surface of talin1 (−/−)WT cells from 97 ± 3% to 55 ± 5%. Of the beads bound in the presence of GPen, 55 ± 16% escaped from the laser trap and only 4 ± 8% were reinforced (Fig. 3 C). Because we had found no significant effect of αvβ3/integrin inhibition by GPen under different conditions (c-Src(+/+) cells after 24 h spreading with FNIII7–10 monomer; Felsenfeld et al., 1999), we tested the effect of GPen in our experimental conditions. In c-Src (+/+) cells, we found that our current FNIII7–10-coated beads show inhibition of reinforcement in the presence of GPen inhibitor. These results indicated that binding of FN to αvβ3/integrin and recruitment of talin1 were both essential for reinforcement of FN–integrin–cytoskeleton connections.
Talin1 causes tighter integrin–cytoskeleton connections
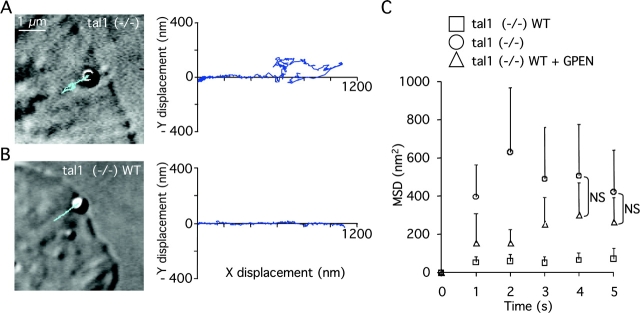
Without reinforcement, FNIII7–10-coated beads on talin1 (−/−) cells may be less rigidly attached to the actin cytoskeleton and may show a greater diffusion rate perpendicular to the direction of the rearward movement. We used the mean square displacement (MSD) of the bead diffusion (Qian et al., 1991) as a reporter of integrin diffusion after the bead escaped the laser trap (Fig. 4). Although beads moved toward the nucleus in all cases, on talin1 (−/−) cells there was a high MSD perpendicular to the direction of movement (395 ± 170 nm2; Fig. 4, A and C) immediately after escaping the laser trap compared with reinforced beads in talin1 (−/−)WT cells (52 ± 29 nm2; Fig. 4, B and C). Interestingly, when reinforcement was inhibited by GPen treatment, the MSD (157 ± 154 nm2) in talin1 (−/−)WT cells was increased compared with nontreated talin1 (−/−)WT cells, but reached talin1 (−/−) cell values only after a period outside the trap (4 s; Fig. 4 C). This suggested that although αvβ3/integrin binding to FN was required for the reinforcement process, talin1 could link other integrins, probably β1 to the actin cytoskeleton (Felsenfeld et al., 1996). These data define talin1 as a key component in establishing a stable connection between integrins and the cytoskeleton, even in the absence of reinforcement (in the presence of GPen).
Figure 4.
Talin1 is necessary for fast stabilization of integrin–cytoskeleton linkages after application of force on FNIII7–10–integrin–cytoskeleton linkages. (A) Representative DIC image of trimeric FNIII7–10-coated beads restrained by the laser trap just after positioning on the surface of the lamellipodia of a talin1 (−/−) cell, and superimposed are the x and y coordinates (blue trace) of the bead movement. The graph shows the parallel and perpendicular movement of the bead while escaping from the laser trap (x and y coordinates, respectively). The movement pattern is characterized by first, a stabilization of the bead in the laser trap center, followed by a linear movement until the bead escapes the laser force field where it undergoes a more diffuse movement. (B) Representative DIC image of trimeric FNIII7–10-coated beads restrained by the laser trap just after positioning on the surface of the lamellipodia of a talin1 (−/−)WT cell and the superimposed x and y coordinates (blue trace) of the bead. The graph shows that compared with talin1 (−/−) cells, coated beads displayed a direct linear movement out of the laser trap, and that the movement remained linear after the bead escaped the laser force field. (C) Summary of mean square displacement (MSD) quantification after beads left the trap field (500 nm; see Materials and methods). Results shown are the mean ± SD of at least eight independent experiments (ANOVA, P > 0.05).
Talin1 induces recruitment of paxillin and vinculin at sites of force generation
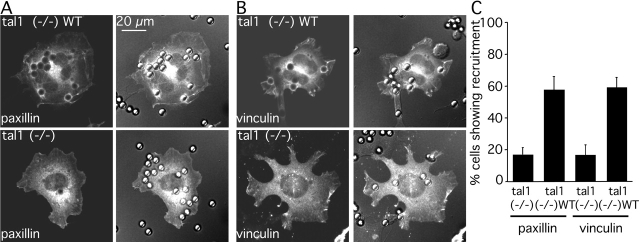
Accumulation of paxillin and vinculin at matrix contacts on large beads is associated with the force-dependent reinforcement of integrin–cytoskeleton connections and focal complex initiation and stabilization (Galbraith et al., 2002; von Wichert et al., 2003). Thus, we tested if talin1 was required for paxillin and vinculin recruitment to sites of force generation (Fig. 5). We centrifuged large beads (5.9-μm diam) coated with FNIII7–10 onto talin1 (−/−) and talin1 (−/−)WT cells. In contrast to small beads used for laser trap experiments, large beads do not require generation of external forces to induce accumulation of paxillin and vinculin, but rather myosin activity is important (Galbraith et al., 2002). The percentage of cells displaying paxillin-EGFP (Fig. 5 A) and EGFP-vinculin (Fig. 5 B) assembly underneath and around beads (bound within 10 μm from the edge) was more than threefold higher in talin1 (−/−)WT cells (∼60%) than in talin1 (−/−) cells (∼17%; Fig. 5 C). To exclude the possibility of a volume-effect around the beads, we transfected cells with EGFP alone, which did not cause an increase in signal intensity around the beads in any case (unpublished data). Therefore, talin1 is involved in the recruitment of paxillin and vinculin, events correlated with strengthening of linkages between integrins and the cytoskeleton and focal complex initiation and stabilization.
Figure 5.
Force-dependent stabilization of integrin–cytoskeleton linkages involves recruitment of paxillin and vinculin by talin1. (A) Talin1 (−/−)WT (top) and talin1 (−/−) (bottom) cells were transfected with paxillin-GFP and were allowed to spread for 30 min. Large FNIII7–10-coated beads (5.9-μm diam) were spun onto the cells and incubated for 30 min. (left) Paxillin-GFP; (right) merged fluorescence and DIC images showing bead-induced accumulation of paxillin-GFP. (B) Talin1 (−/−)WT (top) and talin1 (−/−) (bottom) cells were transfected with EGFP-vinculin, and the cells were treated with FNIII7–10-coated beads as in A. (left) EGFP-vinculin; (right) merged fluorescence and DIC images, showing bead-induced accumulation of EGFP-vinculin. (C) Percentage of cells displaying accumulation of paxillin or vinculin at the bead interface. Cells were scored positive if accumulation of paxillin-GFP or EGFP-vinculin was detected beneath any bead bound within 10 μm from the edge. Results shown are the mean ± SD of three independent experiments.
FilaminA is not involved in force-dependent reinforcement of integrin–cytoskeleton connections
Filamin, like talin, binds to both integrins and actin filaments. Force-dependent rigidification of collagen-coated bead contacts was decreased in filaminA-deficient human melanoma (M2) cells compared with M2 cells rescued by stable expression of filaminA (A7 cells; Glogauer et al., 1998). Therefore, we tested reinforcement in M2 and A7 cells (Fig. 6). A lower level of reinforcement of FNIII7–10-coated beads was found in M2 (28 ± 22%) cells than in A7 cells (71 ± 23%; Fig. 6, A–C), which is consistent with previous observations (Glogauer et al., 1998). However, a substantial fraction of M2 cells (28%) displayed a normal reinforcement, unlike talin1-deficient cells, which had only background levels of reinforcement (10%; Fig. 3 C). Moreover, unlike talin1-deficient cells (Fig. 4 C), the average MSD of nonreinforced beads in filaminA-deficient M2 cells just after escaping from the laser trap (135 ± 111 nm2) was not significantly different from that of filaminA-expressing M7 cells (118 ± 127 nm2; Fig. 6 D). No difference in spreading on FN or VN was observed between M2 and A7 cells (unpublished data), suggesting that interaction of integrins α5β1 and αvβ3 with ligand is normal. Despite the participation of the full-length filaminA in the reinforcement process, it seems that the filaminA linkage with the actin cytoskeleton is not as critical as talin1. Therefore, this rules out a simple mechanism where integrins are bridged by filaminA to the actin cytoskeleton forming a force-sensing module.
Talin1 is involved in stretch-dependent adhesion site formation at early, but not late, times
Another role for talin1 may be in the response to stretching forces, where the recruitment of proteins such as paxillin into adhesion sites is promoted by force on the cytoskeleton (Sawada and Sheetz, 2002). To test this hypothesis, we used a stretchable FN-like–coated (pronectin) silicon substrate to apply external forces to spreading cells. After 10 min of spreading, talin1 (−/−) and talin1 (−/−)WT cells expressing paxillin-GFP displayed no visible adhesion sites, and variation of the fluorescence at the cell edge corresponded to ruffling of the lamellipodia (Fig. 7, A and B, before stretch). Stretching of talin1 (−/−)WT cells for 2 min induced the formation of new adhesion sites (55 ± 8%; Fig. 7 B, during stretch; and Fig. 7 D); however, the formation of new adhesion sites in talin1 (−/−) cells was greatly reduced (11 ± 3%; Fig. 7 A, during stretch, and Fig. 7 D). Moreover, inhibition of αvβ3/integrin with GPen prevented the formation of these stretch-induced adhesion sites in talin1 (−/−)WT cells (Fig. 7 D). In contrast, filaminA null M2 cells showed no defect in early stretch-dependent formation of adhesion sites compared with rescued A7 cells (Fig. 7, C and D). Thus, talin1, but not filaminA, has a significant role in the force-sensing mechanisms leading to rapid stabilization of nascent integrin–cytoskeleton connections necessary for initiation of focal complexes and their stabilization.
Figure 7.
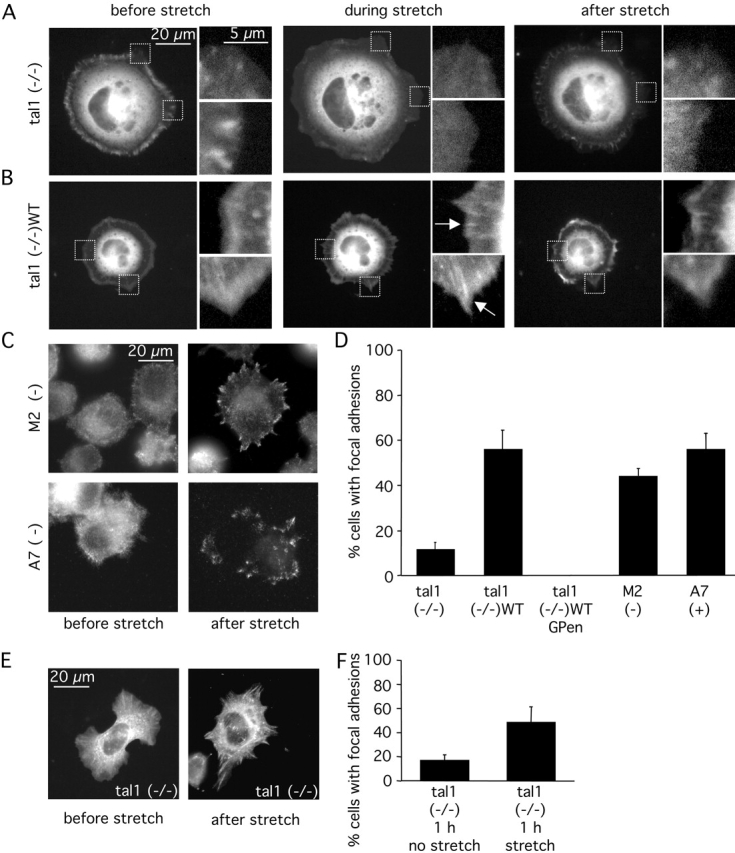
Talin1 is critical for the early integrin- and stretch-dependent formation of adhesion sites. (A) Representative stretching (10%) of talin1 (−/−) cells transiently transfected with paxillin-GFP. After 10 min of spreading, cells were stretched, held for 2 min, and subsequently relaxed by allowing the stretched silicone membrane to return to its original size. Before stretch (left), 2 min after stretch (middle), and after relaxation of stretch (right). Boxed areas are shown enlarged (right panels). (B) Representative stretching of talin1 (−/−) cells transiently transfected with the full-length talin1 and paxillin-GFP. As for talin1 (−/−) cells, no adhesion sites were visible in talin1 (−/−) WT cells before stretch (left). However, after 2-min stretch, adhesion sites were formed at the cell periphery visualized by paxillin-GFP fluorescence (arrows). Boxed areas are shown enlarged (right panels). (C) M2 (filaminA null) or A7 cells (stably expressing filaminA) were allowed to spread for 10 min and stretched for 2 min before fixation with 3.7% formaldehyde/PBS. M2 and A7 cells were subjected to immunostaining using antipaxillin antibody. (D) Summary of stretch-dependent formation of adhesion sites experiments during cell spreading. (E) Talin1 (−/−) cells transiently transfected with paxillin-GFP were allowed to spread for 1 h on pronectin-coated silicone membranes, and were stretched or not for 2 min before fixation. Representative localization of paxillin-GFP in talin1 (−/−) cells before stretch (left) and after stretch (right). (F) Summary of stretch-dependent formation of adhesion sites experiments after 1 h of cell spreading. (D and F) Results shown are the mean ± SD of at least three independent experiments.
After 1 h of spreading on the pronectin-coated stretchable substrate, ∼17% of talin1 (−/−) cells had detectable adhesion sites without stretching (Fig. 7 F), which was similar to FN-coated glass (Fig. 2 B). Unlike the 10-min time point, stretching induced the formation of adhesion sites in ∼48% of the cells (Fig. 7 E, after stretch; and Fig. 7 F), similar to the control (talin1 (−/−)WT) cells after 10 min of spreading. The percentage of talin1 (−/−)WT cells having adhesion sites after 1 h of spreading on the pronectin-coated substrate was already high (∼60%); after stretching, 86% of talin1 (−/−)WT cells displayed adhesions sites. That talin1 was not required for stretch responsiveness after 1 h was consistent with the fact that a similar percentage of talin1 (−/−) and talin1 (−/−)WT cells had adhesion sites after 24-h plating. Therefore, talin1 was critical in rapid force sensing by the early adhesions, but might be replaced by a slower accumulation of other actin-binding proteins around nascent ECM–integrin–cytoskeleton connections over time.
Discussion
Talin1 (−/−) fibroblast-like cells appear normal in their spreading behavior and the activation of SFKs and FAK on FN. Furthermore, inhibition of contractility inhibited activation of SFKs and FAK during spreading of both talin1 (−/−) and talin1 (−/−)WT cells, suggesting that talin1 is not necessary for integrin-dependent signaling in response to force. However, force-dependent reinforcement of FNIII7–10–integrin–cytoskeleton connections, and stretch-induced initiation of focal complex assembly are decreased to nearly background levels. The normal phenotype is rescued by expression of full-length talin1, but not a talin1 polypeptide lacking the COOH-terminal actin-binding site. In contrast, the depletion of filaminA has a milder effect on reinforcement and no effect on the early stretch response. Reinforcement of submicrometer bead contacts has been linked to the recruitment of paxillin and vinculin, a response which is also missing in talin1 (−/−) cells. Because of its ability to bind integrins, F-actin, vinculin, and FAK, and the lack of known enzyme functions, we suggest that talin1 plays a structural scaffolding role in focal complex initiation and assembly in response to force.
Although it has been suggested that the formation of focal complexes is independent of force (Geiger and Bershadsky, 2002), recent studies have demonstrated that forces are required for the initiation and stabilization of focal complexes (Galbraith et al., 2002; von Wichert et al., 2003). Focal complexes are dissociated by inhibitors of myosin II–dependent contractility, but not by an inhibitor of Rho-kinase (Rottner et al., 1999). Accumulation of paxillin and vinculin around large FN-coated beads is dependent on Rac but not Rho activity, and is inhibited by the myosin light chain kinase inhibitor ML-7, which indicates that forces are involved in the initiation of focal complexes (Galbraith et al., 2002). In laser tweezers experiments, reinforcement of integrin–cytoskeleton connections is correlated with the assembly of paxillin/vinculin around FNIII7–10-coated beads experiencing external forces (Galbraith et al., 2002; von Wichert et al., 2003). Because talin1 (−/−) cells are impaired in the force-dependent responses at early times, we suggest that talin1 is critical in the initiation and stabilization of focal complexes in response to forces.
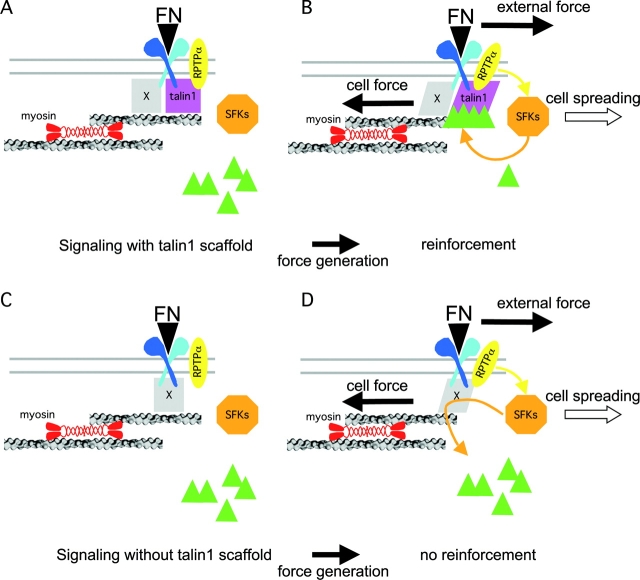
Increased diffusion of FN beads on talin1 (−/−) cells as well as the slower rate of attachment to the cytoskeleton are both consistent with a structural role for talin in the linkage between the FN–integrin complex and the cytoskeleton (Priddle et al., 1998; Brown et al., 2002). Nevertheless, the movement of FNIII7–10-coated beads out of the laser trap in talin1 (−/−) cells implies that other cytoskeletal proteins can couple integrins to the cytoskeleton (Liu et al., 2000), even though those linkages are weaker and cannot be strengthened by force application. In a separate study, we found that talin1 is required to create a discrete, but weak, mechanical linkage between the FNIII7–10 trimer–integrin complex and the cytoskeleton. The bond can slip and form new bonds repeatedly (Jiang et al., 2003; Fig. 8 A). Forces generated during reinforcement can break this linkage, suggesting that the formation of multiple discrete linkages, or the recruitment of additional proteins, is needed to strengthen integrin–cytoskeleton connections. At a molecular level, the talin1 dimer contains several binding sites for F-actin, vinculin, and integrins (Critchley, 2000; Xing et al., 2001). Therefore, talin1 may link as many as four liganded integrins to the cytoskeleton; with assistance from vinculin (Bass et al., 1999) or paxillin (Turner, 2000), talin1 may form the basis of a large complex that would be reinforced through multiple interprotein bonds. We suggest that the talin1-dependent recruitment of paxillin, vinculin, and other proteins to sites of force application enables the strengthening of linkages between integrins and the cytoskeleton (Fig. 8 B).
Figure 8.
Talin1 acts as a scaffold but does not support the signaling in the reinforcement of integrin–cytoskeleton interactions. (A) FN binding to αvβ3/integrin induces the rapid formation of a weak slipping connection between talin1 and the rearward moving cytoskeleton. (B) Sustained force applied on the FN–integrin–cytoskeleton connection induces αvβ3/integrin-dependent activation of RPTPα/SFKs, which is responsible for paxillin and vinculin (green triangles) recruitment to the talin1/cytoskeletal interface. Assembly of this complex leads to stabilization of the talin1–cytoskeleton connection and is responsible for focal complex initiation and stabilization. (C) In the absence of talin1, protein X is envisaged to support the delayed interaction of integrins with the actin cytoskeleton. (D) Protein X supports the force-induced activation of RPTPα/SFKs, but this does not result in efficient recruitment of paxillin and vinculin, and this precludes reinforcement of the initial linkage. The interaction of integrins with the actin cytoskeleton is not stabilized and focal complexes fail to form. In either case, RPTPα–SFK pathway activation is involved in stimulation of cell spreading.
Recent studies show that the deletion of RPTPα from cells inhibits αvβ3/integrin-dependent SFK activation and reinforcement very effectively (von Wichert et al., 2003). In talin1 (−/−)WT cells, inhibition of αvβ3/integrin signaling by GPen treatment also inhibits the reinforcement process but does not totally suppress linkages to the cytoskeleton. Likewise, the initial talin1-dependent connections between integrin and the cytoskeleton form normally in RPTPα (−/−) cells (Jiang et al., 2003). In the case of talin1 (−/−) cells, the activation of SFKs is normal, with no difference in the amount of SFK and FAK activation in response to forces at early times (Fig. 8 D). This suggests that force acts at two levels in reinforcement of integrin–cytoskeleton connections. At early times, force activates parallel enzymatic and structural changes and talin1 is involved in structural sensing of force on integrin–cytoskeleton linkages. Consistent with our suggestion, a recent work has shown that talin is not required for integrin-mediated signaling to regulate gene expression during D. melanogaster embryogenesis (Brown et al., 2002). We propose that FN–integrin–talin1–actin connections and αvβ3/integrin-dependent activation of RPTPα/SFKs comprise two major elements in a minimum reinforcing module, with RPTPα/SFKs being the regulatory component and talin1-actin being the scaffolding modified in response to force (Fig. 8).
Functional analysis, using microinjection of talin antibodies (Nuckolls et al., 1992; Bolton et al., 1997), or recombinant domains of talin (Hemmings et al., 1996) show that talin is involved in stabilization of adhesion sites. At later times, talin1 (−/−) cells are able to form adhesion sites (Priddle et al., 1998; Fig. 1) and are responsive to matrix stretching (Fig. 7), which indicates that other proteins can substitute for talin1 in building later integrin–cytoskeleton connections. Indeed, when talin localization to adhesion sites is altered by antibody injection (Nuckolls et al., 1992) or sequestration of phosphoinositides (Martel et al., 2001) there is no simultaneous disruption of mature adhesion sites. However, antibody injection disrupts newly formed adhesion sites or prevents their formation, which further emphasizes the critical role of talin during early formation of adhesion sites, rather than maturation or stabilization of older adhesion sites. Tensin, which colocalizes with paxillin, seems a better candidate to functionally replace talin1 than filaminA or α-actinin (unpublished data).
Talin has been described as an early component of adhesion site precursors and has been localized at the distal margins of lamellipodia (Izzard, 1988) as high affinity integrin (Kiosses et al., 2001; Nishizaka et al., 2000), and RPTPα (von Wichert et al., 2003). Therefore, components of the early force-sensing apparatus are concentrated at the leading edge of the cell and respond rapidly to localized changes in the stiffness of ECM proteins (Choquet et al., 1997). In terms of a general model, we suggest that talin1 is a critical part of the minimum specific complex linking integrins to the cytoskeleton (Jiang et al., 2003). Cells build on this complex in response to force, as emphasized by the dramatic effect of talin1 disruption on cell migration at gastrulation (Monkley et al., 2000). Although the activation of signaling pathways by force exerted on integrins is important for cellular processes such as motility, proliferation, apoptosis, and morphogenesis, the structural scaffold provided by talin also appears to be a critical factor.
Materials and methods
Cell culture
The human melanoma lines M2 and A7 were cultured as described previously (Glogauer et al., 1998). The SV40 T-antigen immortalized talin1-deficient fibroblast-like cell line dj26.28 was derived from embryoid bodies produced from talin (−/−) ES cells and maintained in DMEM/F-12 medium supplemented with 15% (vol/vol) FBS. Transfection of plasmids encoding EGFP (CLONTECH Laboratories, Inc.), paxillin-GFP, HA-tagged full-length mouse talin1 (provided by E. Fukumoto and K. Yamada, National Institutes of Health, Bethesda, MD), or mouse talin1 (residues 1–2299) lacking the COOH-terminal actin-binding domain was performed with Fugene 6 (Roche).
Spreading assays
Spreading assays were performed as described previously with VN- and FN-coated surfaces (von Wichert et al., 2003). For paxillin distribution assays, cells were transiently transfected using Fugene 6 with paxillin-GFP, plated as described in the previous paragraph, and subsequently analyzed by confocal microscopy (100×; model Fluoview 300; Olympus). All the cells displaying at least one distinct adhesion site were scored positive. The criteria used to classify an adhesion site were its intensity compared with the surrounding region.
Preparation of FN-coated silica beads
0.64-μm silica beads (Bang Laboratories, Inc.) were coated with the trimer of FN as described previously (Jiang et al., 2003).
Laser trap experiments
For bead binding assays, beads were held for 3 s on the cell surface 0.2–0.5 μm from the leading edge using a 100-mW (20 pN/μm) optical gradient laser trap setup (model Axiovert 100TV; Carl Zeiss MicroImaging, Inc.) (Felsenfeld et al., 1996; Choquet et al., 1997). Beads were scored as attached if they remained in focus in the plane of the membrane for 10 s after inactivating the trap.
For MSD assays, ligand-coated beads were held in the 100-mW laser trap on the cell surface until the bead had moved >500 nm from the trap center. x and y coordinates were determined from video micrographs using single particle tracking routines performed with Isee software (Invision Corporation) running on a Silicon Graphics O2 workstation. The MSD was calculated using an algorithm modified from Qian et al. (1991).
For reinforcement assays, ligand-coated beads were held in a 100-mW laser trap on the cell surface for up to 30 s or until the bead had moved >600 nm from the trap center. Beads still in the trap after 30 s were scored as “no escape.” Beads were tested with a second pulse of the trap (100 mW) positioned <0.5 μm behind the bead (toward the leading edge). Beads were scored as “reinforced” if they could not be rapidly (within <100 ms) displaced by >100 nm after the 100-mW test pulse.
Large bead assays
Large bead assays were performed as described previously (von Wichert et al., 2003).
Living cells stretching experiments
Talin1 (−/−) or talin1 (−/−)WT cells transiently transfected with paxillin-GFP were plated on the pronectin-coated silicone membrane for 10 min (Flexcell International) and stretched biaxially (10% in each dimension) for 2 min. For living cell experiments, the GFP fluorescence was observed by fluorescence microscopy (model BX50; Olympus; using a 60×, 0.9 NA water immersion objective); 5 min after stretch, the silicone substrate was relaxed to its original size, and the fluorescence was recorded. Otherwise, for determination of the percentage of responsive cells, talin1 (−/−), talin1 (−/−)WT, M2, and A7 cells (transfected as indicated in the figure legends), cultured on silicone membranes, were stretched for 2 min and fixed with 3.7% formaldehyde/PBS. After fixation, the cells were permeabilized with 0.1% Triton X-100/PBS and subjected to immunostaining as indicated.
TIRF microscopy
FN-coated coverglass was placed on the TIRF microscope at 37°C with a cell suspension in 0.5% serum media. A cooled CCD camera (model CoolSnap fx; Roper Scientific) recorded digital grayscale images from the microscope to a computer running custom image capture software operating as a plugin from within the free ImageJ (http://rsb.info.nih.gov/ij) software package. TIRF images were captured every 10 s. The TIRF laser was synchronously shuttered with the CCD camera to mitigate phototoxicity and photobleaching.
Western blot
Spreading cells were lysed (1% NP-40 , 2 mM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 1 mM PMSF) and lysates were diluted in 3× SDS-PAGE sample buffer. Equal amounts of proteins were analyzed by SDS-PAGE, followed by Western blotting using a polyclonal phosphospecific anti-SFK, monoclonal anti–c-Src, polyclonal anti–FAK-Y397, polyclonal anti–αv integrin, polyclonal anti–α5 integrin, polyclonal anti–β1 integrin, polyclonal anti–β3 integrin, monoclonal anti-filamin, monoclonal antitalin antibodies, with immunoreactive bands being visualized by ECL detection.
Materials
The monoclonal antitalin antibodies were obtained from Biogenesis (TD77) and Sigma-Aldrich (8d4). The anti–β1 and anti–α5 integrin were obtained from Santa Cruz Biotechnology, Inc. The polyclonal anti–αv and anti–β3 integrin antibodies were obtained from Chemicon. Monoclonal antipaxillin antibody was obtained from Transduction Laboratories. The anti-Src antibody (Ab327) was obtained from Oncogene Research Products. The phosphospecific (Y416) anti-SFK antibody was obtained from Cell Signaling. The phosphospecific (Y397) anti-FAK antibody was obtained from Biosource International. ECL reagent and peroxidase coupled anti–rabbit and anti–mouse IgG antibodies were obtained from Amersham Biosciences.
Acknowledgments
We are grateful to Emiko Fukumoto and Kenneth Yamada for providing us with the HA-talin1 construct. We thank J. Sable for expert technical assistance and support. We also thank Anthony Baer, Yasuhiro Sawada, Masako Tamada, and Goetz von Wichert for comments on the manuscript.
The work in D.R. Critchley's laboratory was funded by the Wellcome Trust, and D.H. Sutton was supported by a Biotechnology and Biological Sciences Research Council committee studentship. This work was supported by National Institutes of Health grant GM36277 to M.P. Sheetz.
Abbreviations used in this paper: ABS, actin-binding site; ES, embryonic stem; FN, fibronectin; MSD, mean square displacement; RPTPα, receptor-like protein phosphatase α; SFK, Src family kinase; TIRF, total internal reflection fluorescence; VN, vitronectin.
References
- Balaban, N.Q., U.S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472. [DOI] [PubMed] [Google Scholar]
- Bass, M.D., B.J. Smith, S.A. Prigent, and D.R. Critchley. 1999. Talin contains three similar vinculin-binding sites predicted to form an amphipathic helix. Biochem. J. 341:257–263. [PMC free article] [PubMed] [Google Scholar]
- Boettiger, D., L. Lynch, S. Blystone, and F. Huber. 2001. Distinct ligand-binding modes for integrin alpha(v)beta(3)-mediated adhesion to fibronectin versus vitronectin. J. Biol. Chem. 276:31684–31690. [DOI] [PubMed] [Google Scholar]
- Bolton, S.J., S.T. Barry, H. Mosley, B. Patel, B.M. Jockusch, J.M. Wilkinson, and D.R. Critchley. 1997. Monoclonal antibodies recognizing the N- and C-terminal regions of talin disrupt actin stress fibers when microinjected into human fibroblasts. Cell Motil. Cytoskeleton. 36:363–376. [DOI] [PubMed] [Google Scholar]
- Brown, N.H., S.L. Gregory, W.L. Rickoll, L.I. Fessler, M. Prout, R.A. White, and J.W. Fristrom. 2002. Talin is essential for integrin function in Drosophila. Dev. Cell. 3:569–579. [DOI] [PubMed] [Google Scholar]
- Calderwood, D.A., R. Zent, R. Grant, D.J. Rees, R.O. Hynes, and M.H. Ginsberg. 1999. The talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 274:28071–28074. [DOI] [PubMed] [Google Scholar]
- Cary, L.A., R.A. Klinghoffer, C. Sachsenmaier, and J.A. Cooper. 2002. SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading. Mol. Cell. Biol. 22:2427–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet, D., D.P. Felsenfeld, and M.P. Sheetz. 1997. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 88:39–48. [DOI] [PubMed] [Google Scholar]
- Critchley, D.R. 2000. Focal adhesions - the cytoskeletal connection. Curr. Opin. Cell Biol. 12:133–139. [DOI] [PubMed] [Google Scholar]
- Felsenfeld, D.P., D. Choquet, and M.P. Sheetz. 1996. Ligand binding regulates the directed movement of beta1 integrins on fibroblasts. Nature. 383:438–440. [DOI] [PubMed] [Google Scholar]
- Felsenfeld, D.P., P.L. Schwartzberg, A. Venegas, R. Tse, and M.P. Sheetz. 1999. Selective regulation of integrin–cytoskeleton interactions by the tyrosine kinase Src. Nat. Cell Biol. 1:200–206. [DOI] [PubMed] [Google Scholar]
- Galbraith, C.G., K.M. Yamada, and M.P. Sheetz. 2002. The relationship between force and focal complex development. J. Cell Biol. 159:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger, B., and A. Bershadsky. 2002. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell. 110:139–142. [DOI] [PubMed] [Google Scholar]
- Glogauer, M., P. Arora, D. Chou, P.A. Janmey, G.P. Downey, and C.A. McCulloch. 1998. The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J. Biol. Chem. 273:1689–1698. [DOI] [PubMed] [Google Scholar]
- Hemmings, L., D.J. Rees, V. Ohanian, S.J. Bolton, A.P. Gilmore, B. Patel, H. Priddle, J.E. Trevithick, R.O. Hynes, and D.R. Critchley. 1996. Talin contains three actin-binding sites each of which is adjacent to a vinculin-binding site. J. Cell Sci. 109:2715–2726. [DOI] [PubMed] [Google Scholar]
- Huang, S., and D.E. Ingber. 2000. Shape-dependent control of cell growth, differentiation, and apoptosis: switching between attractors in cell regulatory networks. Exp. Cell Res. 261:91–103. [DOI] [PubMed] [Google Scholar]
- Izzard, C.S. 1988. A precursor of the focal contact in cultured fibroblasts. Cell Motil. Cytoskeleton. 10:137–142. [DOI] [PubMed] [Google Scholar]
- Jiang, G., G. Giannone, D.R. Critchley, E. Fukumoto, and M.P. Sheetz. 2003. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 424:334–337. [DOI] [PubMed] [Google Scholar]
- Kiosses, W.B., S.J. Shattil, N. Pampori, and M.A. Schwartz. 2001. Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat. Cell Biol. 3:316–320. [DOI] [PubMed] [Google Scholar]
- Lauffenburger, D.A., and A.F. Horwitz. 1996. Cell migration: a physically integrated molecular process. Cell. 84:359–369. [DOI] [PubMed] [Google Scholar]
- Liu, S., D.A. Calderwood, and M.H. Ginsberg. 2000. Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 113:3563–3571. [DOI] [PubMed] [Google Scholar]
- Martel, V., C. Racaud-Sultan, S. Dupe, C. Marie, F. Paulhe, A. Galmiche, M.R. Block, and C. Albiges-Rizo. 2001. Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J. Biol. Chem. 276:21217–21227. [DOI] [PubMed] [Google Scholar]
- Miyamoto, S., S.K. Akiyama, and K.M. Yamada. 1995. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 267:883–885. [DOI] [PubMed] [Google Scholar]
- Monkley, S.J., X.H. Zhou, S.J. Kinston, S.M. Giblett, L. Hemmings, H. Priddle, J.E. Brown, C.A. Pritchard, D.R. Critchley, and R. Fassler. 2000. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev. Dyn. 219:560–574. [DOI] [PubMed] [Google Scholar]
- Monkley, S.J., C.A. Pritchard, and D.R. Critchley. 2001. Analysis of the mammalian talin2 gene TLN2. Biochem. Biophys. Res. Commun. 286:880–885. [DOI] [PubMed] [Google Scholar]
- Nishizaka, T., Q. Shi, and M.P. Sheetz. 2000. Position-dependent linkages of fibronectin-integrin-cytoskeleton. Proc. Natl. Acad. Sci. USA. 97:692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuckolls, G.H., L.H. Romer, and K. Burridge. 1992. Microinjection of antibodies against talin inhibits the spreading and migration of fibroblasts. J. Cell Sci. 102:753–762. [DOI] [PubMed] [Google Scholar]
- Pelham, R.J., Jr., and Y. Wang. 1997. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 94:13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher, M.D., and E. Ruoslahti. 1987. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J. Biol. Chem. 262:17294–17298. [PubMed] [Google Scholar]
- Priddle, H., L. Hemmings, S. Monkley, A. Woods, B. Patel, D. Sutton, G.A. Dunn, D. Zicha, and D.R. Critchley. 1998. Disruption of the talin gene compromises focal adhesion assembly in undifferentiated but not differentiated embryonic stem cells. J. Cell Biol. 142:1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, H., M.P. Sheetz, and E.L. Elson. 1991. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys. J. 60:910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveline, D., E. Zamir, N.Q. Balaban, U.S. Schwarz, T. Ishizaki, S. Narumiya, Z. Kam, B. Geiger, and A.D. Bershadsky. 2001. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner, K., A. Hall, and J.V. Small. 1999. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 9:640–648. [DOI] [PubMed] [Google Scholar]
- Sawada, Y., and M.P. Sheetz. 2002. Force transduction by Triton cytoskeletons. J. Cell Biol. 156:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwaelder, S.M., and K. Burridge. 1999. Bidirectional signaling between the cytoskeleton and integrins. Curr. Opin. Cell Biol. 11:274–286. [DOI] [PubMed] [Google Scholar]
- Stossel, T.P., J. Condeelis, L. Cooley, J.H. Hartwig, A. Noegel, M. Schleicher, and S.S. Shapiro. 2001. Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell Biol. 2:138–145. [DOI] [PubMed] [Google Scholar]
- Turner, C.E. 2000. Paxillin interactions. J. Cell Sci. 113:4139–4140. [DOI] [PubMed] [Google Scholar]
- von Wichert, G., G. Jiang, A. Kostic, K. De Vos, J. Sap, and M.P. Sheetz. 2003. RPTP-α acts as a transducer of mechanical force on αv/β3-integrin–cytoskeleton linkages. J. Cell Biol. 161:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H.B., M. Dembo, S.K. Hanks, and Y. Wang. 2001. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl. Acad. Sci. USA. 98:11295–11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, B., A. Jedsadayanmata, and S.C. Lam. 2001. Localization of an integrin binding site to the C terminus of talin. J. Biol. Chem. 276:44373–44378. [DOI] [PubMed] [Google Scholar]