Abstract
The AP-2 adaptor complex is widely viewed as a linchpin molecule in clathrin-mediated endocytosis, simultaneously binding both clathrin and receptors. This dual interaction couples cargo capture with clathrin coat assembly, but it has now been discovered that the association with cargo is tightly regulated. Remarkably, AP-2 is not obligatory for all clathrin-mediated uptake, and several alternate adaptors appear to perform similar sorting and assembly functions at the clathrin bud site.
Keywords: endocytosis; sorting; clathrin; cargo; AP-2
Introduction
Several distinct endocytic processes provide access to the interior of eukaryotic cells, but the major and best-characterized portal is the clathrin-coated vesicle (Conner and Schmid, 2003b). Local recruitment and self-association of soluble clathrin molecules at the membrane generates a polygonal clathrin lattice, which quickly progresses into a deeply invaginated bud before detaching into the cytosol. Thus, acting as a mechanical scaffold, the clathrin coat deforms the surface membrane into vesicles containing transmembrane proteins, bound ligands and a small volume of extracellular fluid. Sites of clathrin assembly on the plasma membrane also contain the heterotetrameric AP-2 adaptor protein complex (Brodsky et al., 2001; Bonifacino and Lippincott-Schwartz, 2003). The evidence for AP-2 participating in cargo selection is now incontrovertible, yet fails to account for the full diversity of molecules clustered into endocytic clathrin coats. This review focuses on recent results suggesting that a group of cargo-selective alternate adaptors cooperate with AP-2 to ensure noncompetitive endocytosis of a variety of cargo molecules from the cell surface
AP-2 adaptor–dependent sorting
In membrane traffic, the term adaptor generally defines a class of proteins able to physically connect cargo molecules with the polymeric components of the coat (Wendland, 2002; Bonifacino and Lippincott-Schwartz, 2003). Adaptors account for the selectivity of vesicular transport as they favor enrichment of select cargo proteins within the forming vesicle. AP-2 was the first adaptor and still holds center stage in models of clathrin-dependent endocytosis. The AP-2 heterotetramer is composed of two large (α and β2, ∼100 kD), one medium (μ2, 50 kD), and one small (σ2, 17 kD) subunit (Fig. 1). Three of the subunits participate directly in clathrin coat assembly. The NH2 terminus of the α subunit binds to phosphatidylinositol 4,5-bisphosphate (PtdIns[4,5]P2), positioning AP-2 on the membrane (Gaidarov and Keen, 1999; Collins et al., 2002), whereas the globular COOH-terminal α appendage acts as a recruitment platform for a large number of so-called endocytic accessory proteins (Slepnev and De Camilli, 2000). The structurally related β2-subunit appendage shares some binding partners with the α appendage and, with the adjacent flexible hinge harboring a clathrin-binding sequence (termed the clathrin box), binds to the terminal domain of the clathrin heavy chain promoting lattice assembly (Owen et al., 2000; Brodsky et al., 2001). Transmembrane cargo proteins are bound directly by the μ2 subunit (Ohno et al., 1995), which also binds PtdIns(4,5)P2 (Collins et al., 2002; Rohde et al., 2002). The role of the σ2 subunit appears to be principally structural (Collins et al., 2002).
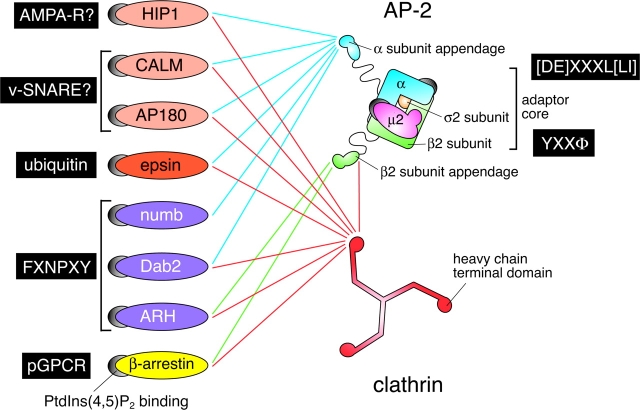
Figure 1.
The endocytic adaptor interaction web. A schematic representation of the protein–protein interactions possible between clathrin, AP-2, and alternate endocytic adaptors. The sorting signal or putative cargo types recognized by the different adaptors are boxed in black. PtdIns(4,5)P2-binding sites are indicated by the spherical gray attachments. AP-2 is modeled on the known molecular architecture of the core and appendages, but the different proteins are not to scale. pGPCR, phosphorylated G protein–coupled receptor; AMPA-R, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor.
Transmembrane proteins require an internalization signal for rapid endocytosis. Several signals are known, and AP-2 engages the YXXØ (where X is any amino acid and Ø is a bulky hydrophobic residue) and [DE]XXXL[LI] dileucine signals (Bonifacino and Traub, 2003). The 20YTRF sequence in the cytosolic domain of the transferrin receptor (TfR) is a typical YXXØ motif, and protein chimeras show this motif is both autonomous and transplantable. Consequently, the TfR is widely used as a marker for this type of sorting signal. YXXØ signals bind physically to the μ2 subunit (Ohno et al., 1995), and cocrystals of the μ2 β-sandwich domain complexed with several YXXØ peptides explain the critical role of the anchor Tyr and the ability of μ2 to accommodate various hydrophobic residues at the Ø position (Owen and Evans, 1998). Yet, until recently, it was not clear why AP-2 does not bind cytosolic YXXØ signals in proteins located elsewhere in the cell. AP-2 cycles onto and off the membrane and, in the cytosolic state, the YXXØ binding surface is inaccessible; the structure of the adaptor core shows the μ2 β-sandwich domain is packed too closely to the adjacent adaptor β2 subunit to allow unhindered access (Collins et al., 2002). Phosphorylation of 156Thr, a residue located within a disordered loop adjacent to the β-sandwich region that engages the YXXØ motif, drives a conformational change in μ2 that increases the apparent affinity of AP-2 for YXXØ sequences ∼25-fold (Ricotta et al., 2002). Accordingly, a T156A mutation inhibits TfR uptake but the mutant AP-2 adaptor is still correctly recruited to the plasma membrane (Olusanya et al., 2001). Likewise, a μ2 D176A/W421A mutant that can be phosphorylated but cannot engage the YXXØ motif still localizes to the cell surface (Nesterov et al., 1999). This shows that AP-2 recruitment to the cell surface is not governed by cargo interactions.
156Thr is phosphorylated by adaptor-associated kinase 1 (AAK1) (Conner and Schmid, 2002), a member of the Ark family of protein kinases. 156Thr lies within a sequence tract 150ITSQVTG, in good agreement with the LXX[TQ]XTG consensus recognized by the S. cerevisiae Ark family kinases (Henry et al., 2003). AAK1 appears to recognize AP-2 by first binding to the α appendage that projects from the heterotetrameric adaptor core (Fig. 1) (Conner and Schmid, 2002). How then is 156Thr phosphorylation confined to AP-2 at the membrane? In answer, it has just been found that clathrin maximizes μ2 kinase activity (Conner et al., 2003; Jackson et al., 2003, in this issue). AAK1 activation by assembled clathrin apparently involves multiple contacts between the kinase and the clathrin heavy and light chains (Conner et al., 2003), and this engagement may displace a putative pseudosubstrate 846LVNQSLG sequence at the COOH terminus of AAK1 to promote 156Thr phosphorylation (Jackson et al., 2003). The experimental confirmation that 156Thr-phosphorylated AP-2 is largely confined to the plasma membrane and the potentiating effect of clathrin on kinase activity provides an elegant affinity-modulation model to explain precise YXXØ cargo capture only at clathrin bud sites (Jackson et al., 2003).
AP-2–independent sorting
One surprising conclusion from the crystallographic studies on the μ2 subunit is that the first internalization sequence identified, 802FDNPVY within the cytosolic domain of the low density lipoprotein receptor (LDLR), is structurally incompatible with the YXXØ interaction surface (Owen and Evans, 1998). The FXNPXY motif differs from the YXXØ type in that it adopts a type-I β-turn conformation and the terminal Tyr residue can be substituted with Phe with no loss of activity. Overexpression studies show clearly that saturating YXXØ-driven endocytosis has no effect on the kinetics of uptake of either FXNPXY- or [DE]XXXL[LI]-harboring proteins (Marks et al., 1996; Warren et al., 1998). To explain these surprising results, discrete but unidentified intermediate connector proteins were postulated to oversee the recognition and internalization of the LDLR (and EGF receptor [EGFR]) (Warren et al., 1998). There is also good genetic evidence for the existence of connector proteins. Autosomal recessive hypercholesterolemia (ARH) patients have a clinical phenotype almost indistinguishable from familial hypercholesterolemia but have normal LDLR alleles (Norman et al., 1999). Linkage analysis fails to reveal defects in clathrin heavy and light chain or AP-2 subunit genes in these individuals (Eden et al., 2001), and sequence analysis of patient μ2 cDNAs shows no abnormalities (Norman et al., 1999). In these patients then, a normal LDLR fails to internalize properly from the sinusoidal surface of the hepatocyte, despite apparently normal AP-2. Again, alternate sorting adaptors were invoked (Norman et al., 1999; Eden et al., 2001).
Now, elegant work using siRNA to ablate AP-2 in HeLa cells shows that the LDLR and EGFR can still use clathrin-mediated endocytosis to enter the cell when AP-2 activity is compromised. Knock down of the clathrin heavy chain effectively eliminates uptake of TfR, EGFR, and a LDLR FDNPVY-sequence reporter, but silencing of either the μ2 or α subunit of AP-2 selectively depresses only TfR endocytosis (Motley et al., 2003). AP-2 disruption leads to ∼10-fold fewer clathrin coats at the surface but those that are present are morphologically normal. Thus, other proteins must be able to recruit clathrin, select cargo, and promote proper vesicle assembly. A concurrent siRNA study also finds that TfR uptake is suppressed by both clathrin heavy chain and AP-2 α subunit knock down (Hinrichsen et al., 2003). But, although clathrin depletion did not perturb the AP-2 distribution grossly, AP-2 knock down abolished clathrin localization to the plasma membrane. Still, EGFR uptake proceeds normally (Hinrichsen et al., 2003). The discordance may be due to the different clathrin antibodies used in the two studies, as both groups find clathrin-coated structures at the cell surface after AP-2 silencing, albeit at low frequency. The EGFR also rapidly saturates the clathrin-dependent internalization pathway (Warren et al., 1998; Jiang et al., 2003) and switches to macropinocytic internalization from dynamic membrane ruffles upon activation with higher EGF concentrations (Yamazaki et al., 2002). The macropinocytic route is spatially distinct from the TfR-positive clathrin-dependent pathway (Yamazaki et al., 2002), and varies between different cultured cells (Jiang et al., 2003), so macropinocytosis could still facilitate EGFR uptake in the face of complete clathrin incapacitation at the cell surface.
An alternative approach, using viral-mediated overexpression of AAK1 to interrupt AP-2 function, also shows that EGFR uptake is clearly independent of AP-2 (Conner and Schmid, 2003a). In cells containing excessive levels of AAK1, AP-2 becomes mislocalized, no longer clustering in characteristic random spots at the cell surface, despite proper clathrin placement in puncta throughout the cell. The AAK1 overexpressors, which do not internalize Tf, endocytose EGF efficiently (Conner and Schmid, 2003a). In fact, the seminal observation that heterotetrameric adaptors are not necessary to sustain clathrin coat assembly or sorting was actually made in yeast several years ago (Huang et al., 1999; Yeung et al., 1999). The congruence of all these independent investigations leaves little doubt that alternate adaptors participate in garnering cargo into the clathrin bud site.
Alternate clathrin adaptors
The arrestins.
There is already a well-accepted precedent for alternate sorting adaptors in mammalian cells: β-arrestin 1 and 2 (Claing et al., 2002; Marchese et al., 2003). Within seconds of agonist application, diffuse cytosolic β-arrestin 2–GFP concentrates at preexisting sites of clathrin assembly, guiding activated G protein–coupled receptors (GPCRs) into the cell (Santini et al., 2002). The capability of the β-arrestins to mesh stimulated GPCRs with the clathrin machinery depends on four functional attributes; the ability to engage the phosphorylated cargo receptor, a capacity to bind to PtdIns(4,5)P2 (Gaidarov et al., 1999), and the ability to bind physically to both clathrin and AP-2 (Claing et al., 2002; Marchese et al., 2003) (Fig. 1). The concerted effect of these interactions promotes rapid endocytosis, and impairing any one leads to defects in GPCR internalization, as does either β-arrestin gene disruption or RNAi silencing (Claing et al., 2002; Marchese et al., 2003). A clathrin box and an AP-2 appendage-binding determinant are tandemly arrayed at the COOH terminus of β-arrestin, an ordered region that becomes unstructured upon binding activated GPCRs (Milano et al., 2002). Remarkably, several known endocytic components, previously termed accessory factors (Slepnev and De Camilli, 2000), display these same four functional attributes and now represent candidate monomeric adaptors.
ARH, Disabled-2, and numb.
Three lines of evidence point to the phosphotyrosine-binding (PTB) domain playing a vital role in FXNPXY signal recognition. First, the PTB domain specifically recognizes an FXNPXpY sequence, but PTB is actually a misnomer as many PTB domains have a higher selectivity for the nonphosphorylated FXNPXY sequence (Howell et al., 1999; Morris and Cooper, 2001). Second, characterization of the genetic lesion in ARH patients reveals that a novel PTB domain adaptor, termed ARH, is necessary for LDLR endocytosis in hepatocytes, lymphocytes, and macrophages (Garcia et al., 2001; Eden et al., 2002). The pivotal role of ARH in facilitating LDL uptake is plainly demonstrated by phenotypic rescue of ARH-patient lymphoblasts expressing retrovirally introduced ARH (Eden et al., 2002). In line with this, hepatocytes of ARH−/− mice accumulate the LDLR at the sinusoidal surface (Jones et al., 2003). Third, Disabled-1 (Dab1), a protein that regulates cortical lamination in the brain, uses a PTB domain to bind to the FXNPXY motifs in two LDLR family members, the VLDL and apoER2 receptors (Herz, 2001). The Dab1 PTB domain is 65% identical to Dab2, a related protein that, at steady-state, colocalizes extremely well with AP-2 and clathrin (Mishra et al., 2002a; Morris and Cooper, 2001). A vexing but consistent finding is that fibroblasts derived from ARH patients have normal LDLR activity (Garcia et al., 2001; Eden et al., 2002). This may reflect functional redundancy with another PTB domain protein(s), possibly Dab2. In mice, Dab2 gene disruption is lethal, but conditional knock out in the embryo leads to viable animals that excrete proteins normally recovered in the kidney by the scavenger receptor megalin, another LDLR family member (Morris et al., 2002). The Dab2−/− proteinuria is reminiscent of but milder than that seen in megalin−/− mice. This suggests that Dab2 plays an important role in the endocytic retrieval of filtered protein in the nephron.
The Dab1/2 and ARH PTB modules represent a distinct subset of PTB domains that also includes the endocytic protein numb (Santolini et al., 2000; Berdnik et al., 2002). Crystal structures of the Dab1/2 PTB domains confirm that the FXNPXY motif binds in a β-turn conformation and explains why a Phe but not pTyr can replace the terminal Tyr residue (Stolt et al., 2003; Yun et al., 2003). Furthermore, the PTB module is structurally related to the pleckstrin homology domain and, importantly, the Dab1/2, ARH, and numb PTB domains all bind PtdIns(4,5)P2 (Dho et al., 1999; Howell et al., 1999; Mishra et al., 2002a,b). This allows these PTB domains to bind to the plasma membrane and an internalization sequence simultaneously. Indeed, overexpression of a tandem Dab2 PTB fusion selectively abolishes LDLR internalization without affecting TfR uptake (Mishra et al., 2002b).
Outside of the PTB domain, the COOH-terminal segments of ARH, Dab2, and numb are predicted to be disordered and, like β-arrestin, contain adjacent clathrin box and/or AP-2 appendage-binding sequences. (Santolini et al., 2000; Morris and Cooper, 2001; He et al., 2002; Mishra et al., 2002a,b) (Fig. 1). The Xenopus ARH orthologue requires these determinants to drive the internalization of the vitellogenin receptor, an FXNPXY-containing member of the LDLR family (Zhou et al., 2003). And in Drosophila there is strong genetic evidence for an endocytic role for Numb and clear binary Numb–AP-2 interactions (Berdnik et al., 2002). So, like β-arrestins, the PTB adaptors can bind cargo, PtdIns(4,5)P2, the clathrin terminal domain, and AP-2 (Fig. 1).
The epsin superfamily.
A second group of putative alternate adaptors includes epsin 1, AP180/CALM, and HIP1/Hip1R (Wendland, 2002). These proteins contain a structurally related NH2-terminal PtdIns(4,5)P2-binding domain (the ENTH/ANTH domain) (Ford et al., 2001) and all bind to and colocalize with AP-2/clathrin, and, in each case, plausible cargo molecules can be assigned (Fig. 1). The case for epsin has recently been reviewed (Wendland, 2002), where ubiquitin interaction motifs (UIMs) allow ubiquitin recognition. Ubiquitination is the principal internalization signal in S. cerevisiae and the yeast epsins, Ent1p and Ent2p, use embedded UIMs to promote rapid endocytosis (Shih et al., 2002). Mutant alleles of Liquid facets, the Drosophila epsin, prevent internalization of the transmembrane Notch ligand Delta in compound eye progenitors and, consequently, severely malformed eyes develop (Overstreet et al., 2003). The ubiquitin connection comes from the fact that ubiquitination of Delta by the RING E3 ubiquitin ligase Neuralized is necessary for Delta endocytosis (Kramer, 2001). In mammals, epsin UIMs may operate similarly where, for example, the EGFR is multiply monoubiquitinated upon ligand binding. However, the wide array of components ubiquitinated upon EGFR activation complicates the interpretation of many studies, as does the fact that the EGFR has a YXXØ internalization sequence. Both AP180 and HIP1 lack UIMs but, intriguingly, UNC-11, the C. elegans AP180 orthologue, may participate in the sorting of synaptobrevin. Genetic disruption of the UNC-11 gene leads to selective missorting of this v-SNARE at the presynaptic plasma membrane (Nonet et al., 1999). Finally, GluR1-containing AMPA receptor endocytosis is defective in neurons from HIP1−/− mice (Metzler et al., 2003). For these proteins the molecular basis for cargo recognition is unknown but the similarity between the ANTH domain and another cargo recognition module, the VHS domain, is highly suggestive. The ability of the epsin ENTH domain alone to rescue epsin deletions in both yeast and flies also hints at additional roles for this domain (Overstreet et al., 2003).
Perspective
Recent progress makes it clear that alternate endocytic adaptors display grossly similar properties that enable them to perform the fundamental tasks required of an adaptor: cargo recognition and coat assembly (Wendland, 2002; Bonifacino and Lippincott-Schwartz, 2003). Despite little overall sequence identity, all have clathrin box and/or AP-2 interaction sequences that govern associations with the clathrin heavy chain and the AP-2 adaptor appendages (Fig. 1). Another unifying architectural theme is an NH2-terminal lipid-binding module in an otherwise largely unfolded polypeptide ideally suited to presentation of short protein–protein interaction motifs. All are able to engage PtdIns(4,5)P2, favoring plasma membrane localization although, in each case, the precise mode of PtdIns(4,5)P2 binding is different. Nonetheless, on a PtdIns(4,5)P2-containing membrane in vitro, AP180, epsin, Dab2, and HIP1 each can collaborate with AP-2 to promote optimal clathrin recruitment and assembly (Ford et al., 2001; Mishra et al., 2001, 2002a). This is significant because the surface density of clathrin coats in AP-2–deficient cells is <10% of normal (Motley et al., 2003). So, although the alternate monomeric adaptors can apparently sustain endocytic uptake of certain receptors in the absence of AP-2, optimal coat assembly and trafficking evidently requires AP-2. This is readily apparent in Drosophila, where some α-subunit mutations are lethal and α-appendage mutant alleles phenocopy certain numb mutants, but AP-2 clearly acts downstream of Numb (Berdnik et al., 2002).
Dab2, epsin 1, and CALM populate common clathrin structures at the cell surface, indicating that these adaptors expand the sorting repertoire of the coat rather than generating separate classes of transport vesicle. The biologic utility of a diverse cargo recognition machinery is highlighted by elegant Drosophila studies. Endocytosis is not simply about nutrition and cellular homeostasis but is also fundamental to whole developmental programs. There are times during ontogeny when cells require decisive clearance of certain receptors from the surface, or inappropriate specification of cell fate ensues (Berdnik et al., 2002; Overstreet et al., 2003). Multiple adaptors can provide the plasticity to allow precise temporal control even in the face of high traffic volumes. Next, it will be vital to learn whether cargo engagement by alternate adaptors is as strictly regulated as in AP-2. Nevertheless, further characterization of the alternate adaptors promises to provide a complete molecular explanation for the capture of the whole repertoire of cargo at the clathrin bud, and functionally similar proteins appear to act at clathrin bud sites on the trans-Golgi network as well (Duncan and Payne, 2003).
Acknowledgments
I am indebted to Gerry Apodaca, Peter Keyel, and Matthew Hawryluk for their valuable comments and suggestions.
This work was supported by National Institutes of Health grant DK53249.
Abbreviations used in this paper: AAK1, adaptor-associated kinase 1; ARH, autosomal recessive hypercholesterolemia; Dab1, Disabled-1; EGFR, EGF receptor; GPCR, G protein–coupled receptor; LDLR, low density lipoprotein receptor; PTB, phosphotyrosine-binding; PtdIns(4,5)P2, phosphatidylinositol 4,5-bisphosphate; TfR, transferrin receptor; UIM, ubiquitin interaction motif.
References
- Berdnik, D., T. Torok, M. Gonzalez-Gaitan, and J. Knoblich. 2002. The endocytic protein α-Adaptin is required for Numb-mediated asymmetric cell division in Drosophila. Dev. Cell. 3:221–231. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., and J. Lippincott-Schwartz. 2003. Coat proteins: shaping membrane transport. Nat. Rev. Mol. Cell Biol. 4:409–414. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., and L.M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395–447. [DOI] [PubMed] [Google Scholar]
- Brodsky, F.M., C.Y. Chen, C. Knuehl, M.C. Towler, and D.E. Wakeham. 2001. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17:517–568. [DOI] [PubMed] [Google Scholar]
- Claing, A., S.A. Laporte, M.G. Caron, and R.J. Lefkowitz. 2002. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and β-arrestin proteins. Prog. Neurobiol. 66:61–79. [DOI] [PubMed] [Google Scholar]
- Collins, B.M., A.J. McCoy, H.M. Kent, P.R. Evans, and D.J. Owen. 2002. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 109:523–535. [DOI] [PubMed] [Google Scholar]
- Conner, S.D., and S.L. Schmid. 2002. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J. Cell Biol. 156:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner, S.D., and S.L. Schmid. 2003. a. Differential requirements for AP-2 in clathrin-mediated endocytosis. J. Cell Biol. 162:773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner, S.D., and S.L. Schmid. 2003. b. Regulated portals of entry into the cell. Nature. 422:37–44. [DOI] [PubMed] [Google Scholar]
- Conner, S.D., T. Schroter, and S.L. Schmid. 2003. AAK1 mediated μ2 phosphorylation is stimulated by assembled clathrin. Traffic. In press. [DOI] [PubMed] [Google Scholar]
- Dho, S.E., M.B. French, S.A. Woods, and C.J. McGlade. 1999. Characterization of four mammalian numb protein isoforms. Identification of cytoplasmic and membrane-associated variants of the phosphotyrosine binding domain. J. Biol. Chem. 274:33097–33104. [DOI] [PubMed] [Google Scholar]
- Duncan, M.C., and G.S. Payne. 2003. ENTH/ANTH domains expand to the Golgi. Trends Cell Biol. 13:211–215. [DOI] [PubMed] [Google Scholar]
- Eden, E.R., R.P. Naoumova, J.J. Burden, M.I. McCarthy, and A.K. Soutar. 2001. Use of homozygosity mapping to identify a region on chromosome 1 bearing a defective gene that causes autosomal recessive homozygous hypercholesterolemia in two unrelated families. Am. J. Hum. Genet. 68:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden, E.R., D.D. Patel, X. Sun, J.J. Burden, M. Themis, M. Edwards, P. Lee, C. Neuwirth, R.P. Naoumova, and A.K. Soutar. 2002. Restoration of LDL-receptor function in cells from patients with autosomal recessive hypercholesterolemia by retroviral expression of ARH1. J. Clin. Invest. 110:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, M.G., B.M. Pearse, M.K. Higgins, Y. Vallis, D.J. Owen, A. Gibson, C.R. Hopkins, P.R. Evans, and H.T. McMahon. 2001. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 291:1051–1055. [DOI] [PubMed] [Google Scholar]
- Gaidarov, I., and J.H. Keen. 1999. Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J. Cell Biol. 146:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov, I., J.G. Krupnick, J.R. Falck, J.L. Benovic, and J.H. Keen. 1999. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J. 18:871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, C.K., K. Wilund, M. Arca, G. Zuliani, R. Fellin, M. Maioli, S. Calandra, S. Bertolini, F. Cossu, N. Grishin, et al. 2001. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science. 292:1394–1398. [DOI] [PubMed] [Google Scholar]
- He, G., S. Gupta, P. Michaely, H.H. Hobbs, and J.C. Cohen. 2002. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin and AP-2. J. Biol. Chem. 277:44044–44049. [DOI] [PubMed] [Google Scholar]
- Henry, K.R., K. D'Hondt, J.S. Chang, D.A. Nix, M.J. Cope, C.S. Chan, D.G. Drubin, and S.K. Lemmon. 2003. The actin-regulating kinase Prk1p negatively regulates Scd5p, a suppressor of clathrin deficiency, in actin organization and endocytosis. Curr. Biol. 13:1564–1569. [DOI] [PubMed] [Google Scholar]
- Herz, J. 2001. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 29:571–581. [DOI] [PubMed] [Google Scholar]
- Hinrichsen, L., J. Harborth, L. Andrees, K. Weber, and E.J. Ungewickell. 2003. Effect of clathrin heavy chain- and α-adaptin specific small interfering RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 278. [DOI] [PubMed] [Google Scholar]
- Howell, B.W., L.M. Lanier, R. Frank, F.B. Gertler, and J.A. Cooper. 1999. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol. Cell. Biol. 19:5179–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, K.M., K. D'Hondt, H. Riezman, and S.K. Lemmon. 1999. Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 18:3897–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, A.P., A. Flett, C. Smythe, L. Hufton, F.R. Wettey, and E. Smythe. 2003. Clathrin promotes incorpotration of cargo into coated pits by activation of the AP2 adaptor μ2 kinase. J. Cell Biol. 163:231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X., F. Huang, A. Marusyk, and A. Sorkin. 2003. Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol. Biol. Cell. 14:858–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C., R.E. Hammer, W.P. Li, J.C. Cohen, H.H. Hobbs, and J. Herz. 2003. Normal sorting, but defective endocytosis of the LDL receptor in mice with autosomal recessive hypercholesterolemia. J. Biol. Chem. 278:29024–29030. [DOI] [PubMed] [Google Scholar]
- Kramer, H. 2001. Neuralized: regulating notch by putting away delta. Dev. Cell. 1:725–726. [DOI] [PubMed] [Google Scholar]
- Marchese, A., C. Chen, Y.M. Kim, and J.L. Benovic. 2003. The ins and outs of G protein-coupled receptor trafficking. Trends Biochem. Sci. 28:369–376. [DOI] [PubMed] [Google Scholar]
- Marks, M.S., W.L.H. Ohno, and J.S. Bonifacino. 1996. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J. Cell Biol. 135:341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler, M., B. Li, L. Gan, J. Georgiou, C.A. Gutekunst, Y. Wang, E. Torre, R.S. Devon, R. Oh, V. Legendre-Guillemin, et al. 2003. Disruption of the endocytic protein HIP1 results in neurological deficits and decreased AMPA receptor trafficking. EMBO J. 22:3254–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano, S.K., H.C. Pace, Y.M. Kim, C. Brenner, and J.L. Benovic. 2002. Scaffolding functions of arrestin-2 revealed by crystal structure and mutagenesis. Biochemistry. 41:3321–3328. [DOI] [PubMed] [Google Scholar]
- Mishra, S.K., N.R. Agostinelli, T.J. Brett, I. Mizukami, T.S. Ross, and L.M. Traub. 2001. Clathrin- and AP-2-binding sites in HIP1 uncover a general assembly role for endocytic accessory proteins. J. Biol. Chem. 276:46230–46236. [DOI] [PubMed] [Google Scholar]
- Mishra, S.K., P.A. Keyel, M.J. Hawryluk, N.R. Agostinelli, S.C. Watkins, and L.M. Traub. 2002. a. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 21:4915–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, S.K., S.C. Watkins, and L.M. Traub. 2002. b. The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc. Natl. Acad. Sci. USA. 99:16099–16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, S.M., and J.A. Cooper. 2001. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic. 2:111–123. [DOI] [PubMed] [Google Scholar]
- Morris, S.M., M.D. Tallquist, C.O. Rock, and J.A. Cooper. 2002. Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J. 21:1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley, A., N.A. Bright, M.N. Seaman, and M.S. Robinson. 2003. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162:909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterov, A., R.E. Carter, T. Sorkina, G.N. Gill, and A. Sorkin. 1999. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant μ2 subunit and its effects on endocytosis. EMBO J. 18:2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet, M.L., A.M. Holgado, F. Brewer, C.J. Serpe, B.A. Norbeck, J. Holleran, L. Wei, E. Hartwieg, E.M. Jorgensen, and A. Alfonso. 1999. UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol. Biol. Cell. 10:2343–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, D., X.M. Sun, M. Bourbon, B.L. Knight, R.P. Naoumova, and A.K. Soutar. 1999. Characterization of a novel cellular defect in patients with phenotypic homozygous familial hypercholesterolemia. J. Clin. Invest. 104:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno, H., M.-C. Fournier, H. Bosshart, I. Rhee, S. Miyatake, T. Saito, A. Galluser, T. Kirchhausen, and J.S. Bonifacino. 1995. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 269:1872–1875. [DOI] [PubMed] [Google Scholar]
- Olusanya, O., P.D. Andrews, J.R. Swedlow, and E. Smythe. 2001. Phosphorylation of threonine 156 of the μ2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr. Biol. 11:896–900. [DOI] [PubMed] [Google Scholar]
- Overstreet, E., X. Chen, B. Wendland, and J.A. Fischer. 2003. Either part of a Drosophila epsin protein, divided after the ENTH domain, functions in endocytosis of Delta in the developing eye. Curr. Biol. 13:854–860. [DOI] [PubMed] [Google Scholar]
- Owen, D.J., and P.R. Evans. 1998. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 282:1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, D.J., Y. Vallis, B.M. Pearse, H.T. McMahon, and P.R. Evans. 2000. The structure and function of the β2-adaptin appendage domain. EMBO J. 19:4216–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotta, D., S.D. Conner, S.L. Schmid, K. von Figura, and S. Honing. 2002. Phosphorylation of the AP2 μ subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J. Cell Biol. 156:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde, G., D. Wenzel, and V. Haucke. 2002. A phosphatidylinositol (4,5)-bisphosphate binding site within μ2-adaptin regulates clathrin-mediated endocytosis. J. Cell Biol. 158:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini, F., I. Gaidarov, and J.H. Keen. 2002. G protein-coupled receptor/arrestin3 modulation of the endocytic machinery. J. Cell Biol. 156:665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolini, E., C. Puri, A.E. Salcini, M.C. Gagliani, P.G. Pelicci, C. Tacchetti, and P.P. Di Fiore. 2000. Numb is an endocytic protein. J. Cell Biol. 151:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, S.C., D.J. Katzmann, J.D. Schnell, M. Sutanto, S.D. Emr, and L. Hicke. 2002. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 4:389–393. [DOI] [PubMed] [Google Scholar]
- Slepnev, V.I., and P. De Camilli. 2000. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat. Rev. Neurosci. 1:161–172. [DOI] [PubMed] [Google Scholar]
- Stolt, P.C., H. Jeon, H.K. Song, J. Herz, M.J. Eck, and S.C. Blacklow. 2003. Origins of peptide selectivity and phosphoinositide binding revealed by structures of Disabled-1 PTB domain complexes. Structure. 11:569–579. [DOI] [PubMed] [Google Scholar]
- Warren, R.A., F.A. Green, P.E. Stenberg, and C.A. Enns. 1998. Distinct saturable pathways for the endocytosis of different tyrosine motifs. J. Biol. Chem. 273:17056–17063. [DOI] [PubMed] [Google Scholar]
- Wendland, B. 2002. Epsins: adaptors in endocytosis? Nat. Rev. Mol. Cell Biol. 3:971–977. [DOI] [PubMed] [Google Scholar]
- Yamazaki, T., K. Zaal, D. Hailey, J. Presley, J. Lippincott-Schwartz, and L.E. Samelson. 2002. Role of Grb2 in EGF-stimulated EGFR internalization. J. Cell Sci. 115:1791–1802. [DOI] [PubMed] [Google Scholar]
- Yeung, B.G., H.L. Phan, and G.S. Payne. 1999. Adaptor complex-independent clathrin function in yeast. Mol. Biol. Cell. 10:3643–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, M., L. Keshvara, C.G. Park, Y.M. Zhang, J.B. Dickerson, J. Zheng, C.O. Rock, T. Curran, and H.W. Park. 2003. Crystal structures of the dab homology domains of mouse disabled 1 and 2. J. Biol. Chem. 278:36572–36581. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., J. Zhang, and M.L. King. 2003. Xenopus ARH couples lipoprotein receptors with the AP-2 complex in oocytes and embryos and is required for vitellogenesis. J. Biol. Chem. In press. [DOI] [PubMed] [Google Scholar]