Abstract
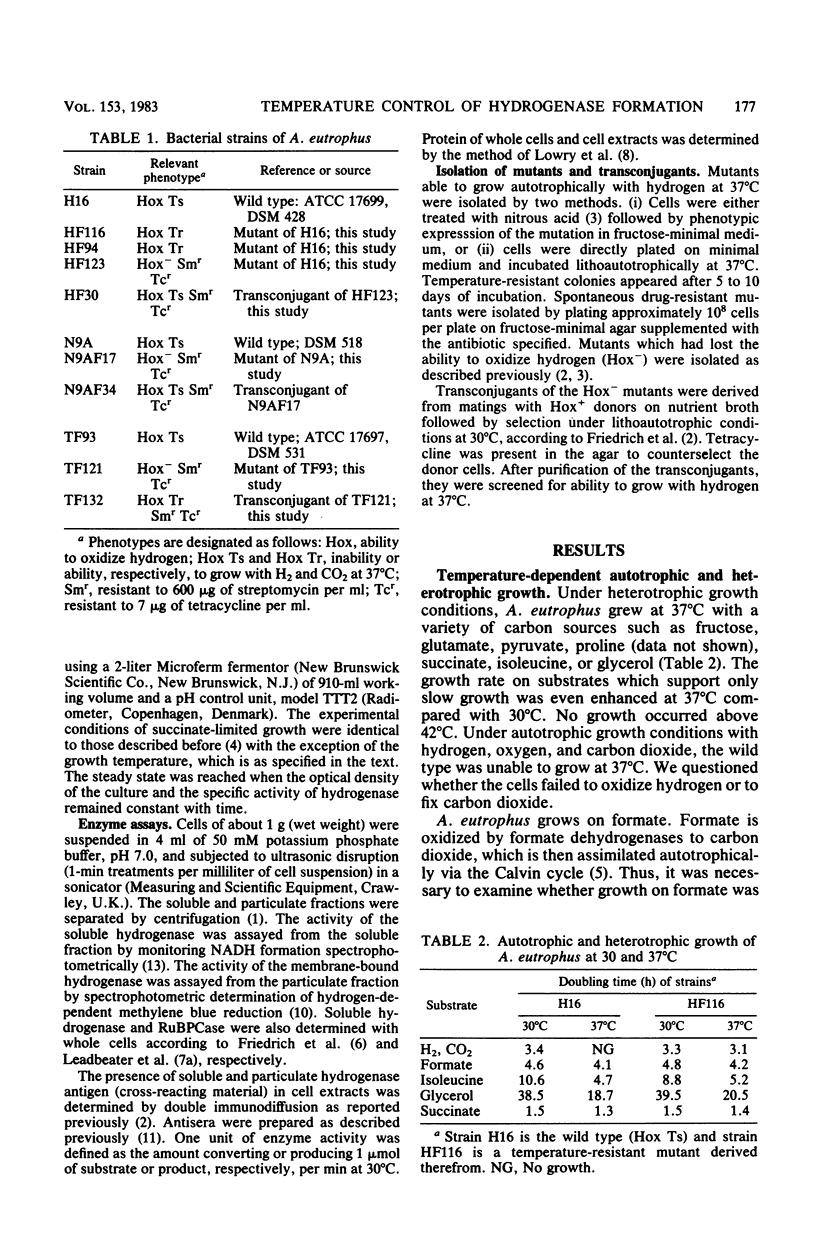
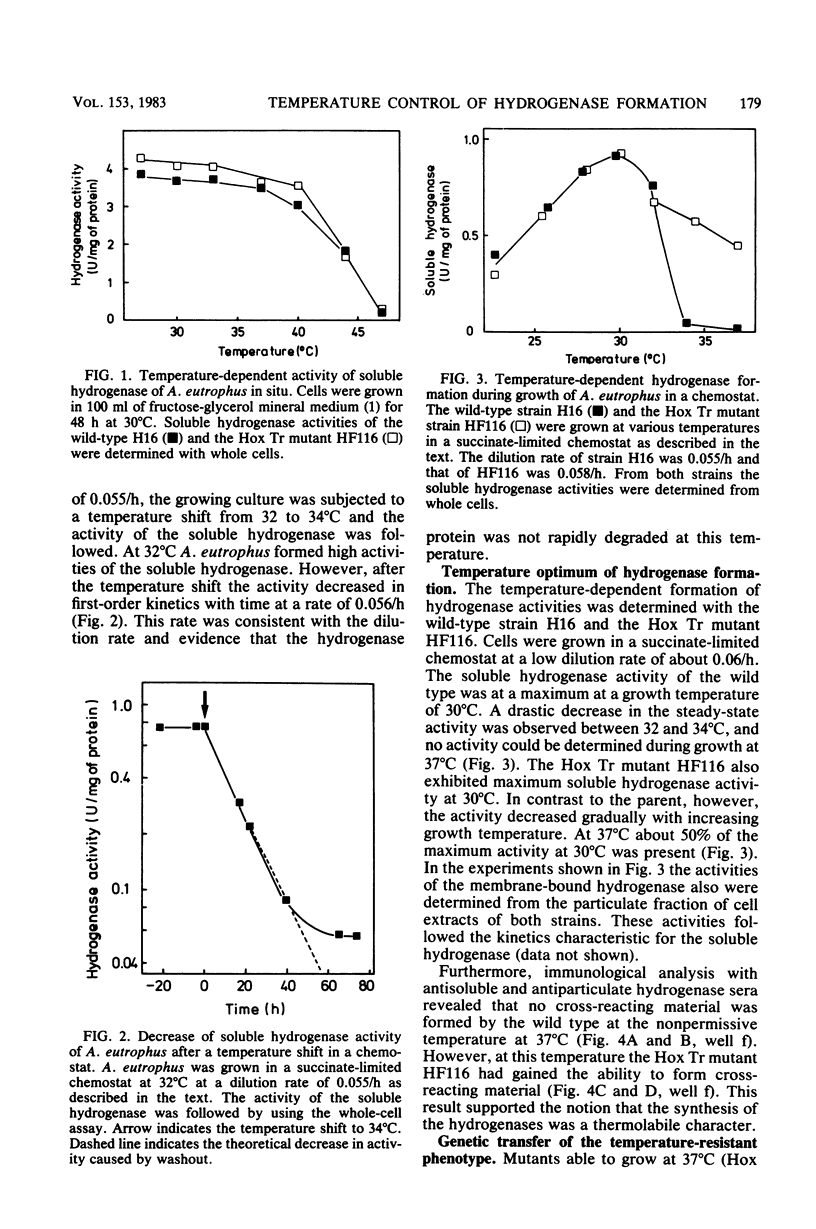
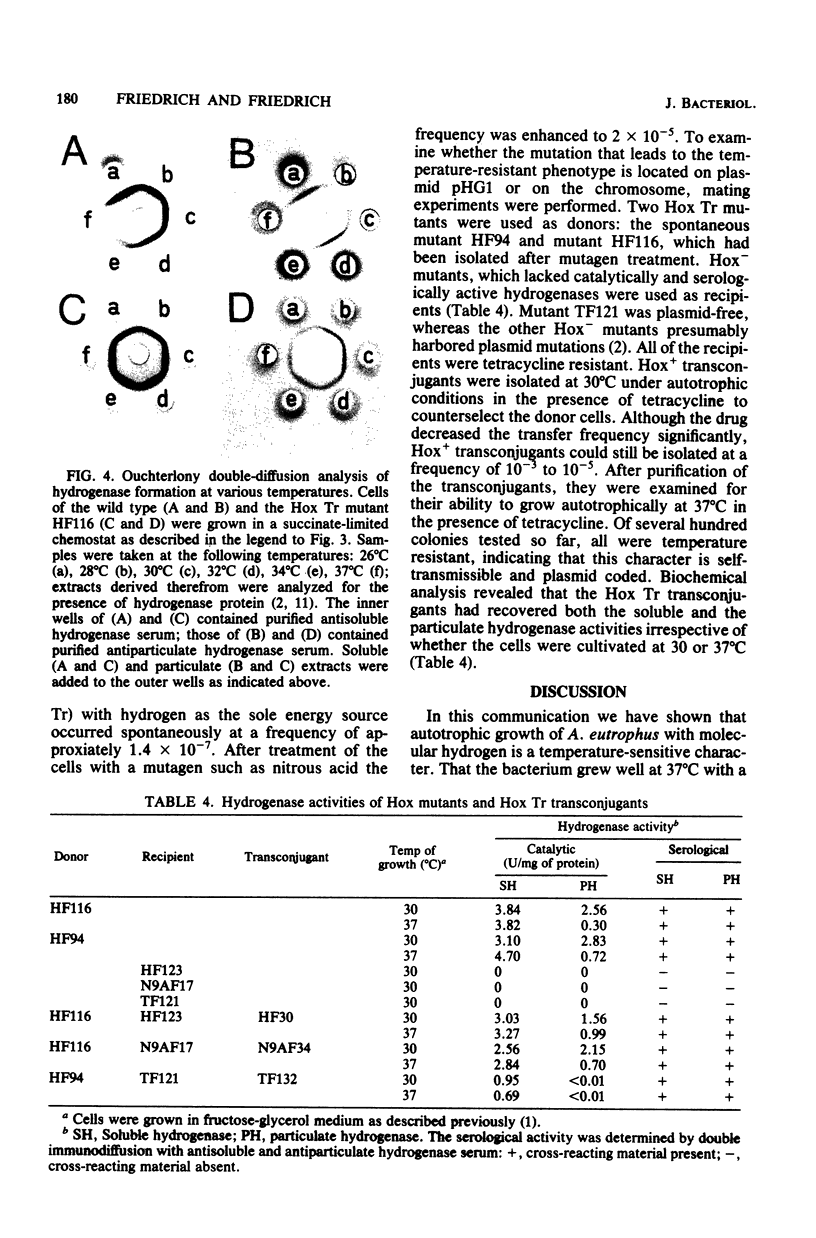
Alcaligenes eutrophus grew well autotrophically with molecular hydrogen at 30 degrees C, but failed to grow at 37 degrees C (Hox Ts). At this temperature the strain grew well heterotrophically with a variety of organic compounds and with formate as an autotrophic substrate, restricting the thermolabile character to hydrogen metabolism. The soluble hydrogenase activity was stable at 37 degrees C. The catalytic properties of the wild-type enzyme were identical to those of a mutant able to grow lithoautotrophically at 37 degrees C (Hox Tr). Soluble hydrogenase was not rapidly degraded at elevated temperatures since the preformed enzyme remained stable for at least 5 h in resting cells or was diluted by growth, as shown in temperature shift experiments. Immunochemical studies revealed that the formation of the hydrogenase proteins was temperature sensitive. No cross-reactivity was detected above temperatures of 34 degrees C. The genetic information of Hox resides on a self-transmissible plasmid in A. eutrophus. Using Hox Tr mutants as donors of hydrogen-oxidizing ability resulted in Hox+ transconjugants which not only had recovered plasmid pHG1 and both hydrogenase activities but also were temperature resistant. This is evidence that the Hox Tr phenotype is coded by plasmid pHG1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Friedrich B., Heine E., Finck A., Friedrich C. G. Nickel requirement for active hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981 Mar;145(3):1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Hogrefe C., Schlegel H. G. Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol. 1981 Jul;147(1):198–205. doi: 10.1128/jb.147.1.198-205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Schlegel H. G. Aromatic amino acid biosynthesis in Alcaligenes eutrophus H16. II. The isolation and characterization of mutants auxotrophic for phenylalanine and tyrosine. Arch Microbiol. 1975 Apr 7;103(2):141–149. doi: 10.1007/BF00436341. [DOI] [PubMed] [Google Scholar]
- Friedrich C. G. Depression of hydrogenase during limitation of electron donors and derepression of ribulosebisphosphate carboxylase during carbon limitation of Alcaligenes eutrophus. J Bacteriol. 1982 Jan;149(1):203–210. doi: 10.1128/jb.149.1.203-210.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H., Shanmugam K. T. Temperature control of nitrogen fixation in Klebsiella pneumoniae. Arch Microbiol. 1979;123(3):259–265. doi: 10.1007/BF00406659. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus. I. Solubilization, purification, and biochemical properties. Biochim Biophys Acta. 1979 Apr 12;567(2):315–324. doi: 10.1016/0005-2744(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus: II. Localization and immunological comparison with other hydrogenase systems. Antonie Van Leeuwenhoek. 1980;46(1):1–14. doi: 10.1007/BF00422224. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Purification and properties of soluble hydrogenase from Alcaligenes eutrophus H 16. Biochim Biophys Acta. 1976 Nov 8;452(1):66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]




