Abstract
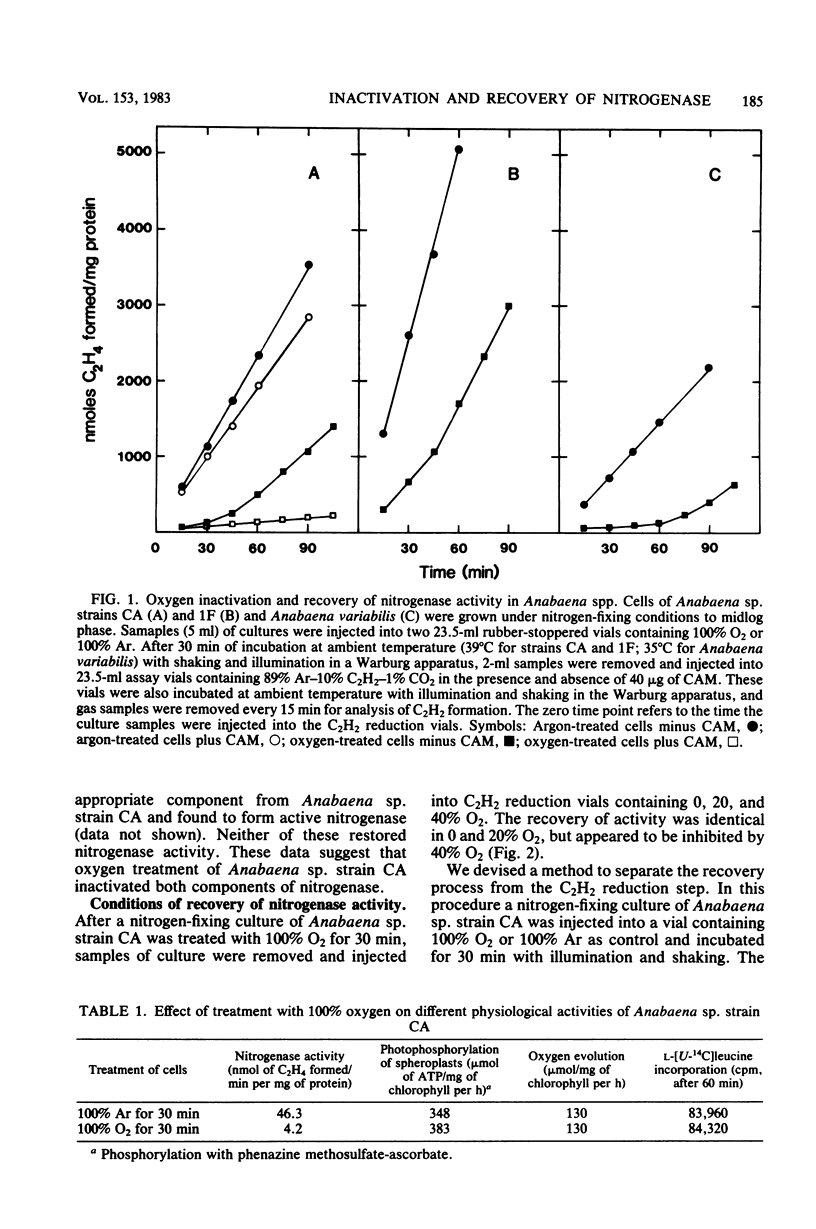
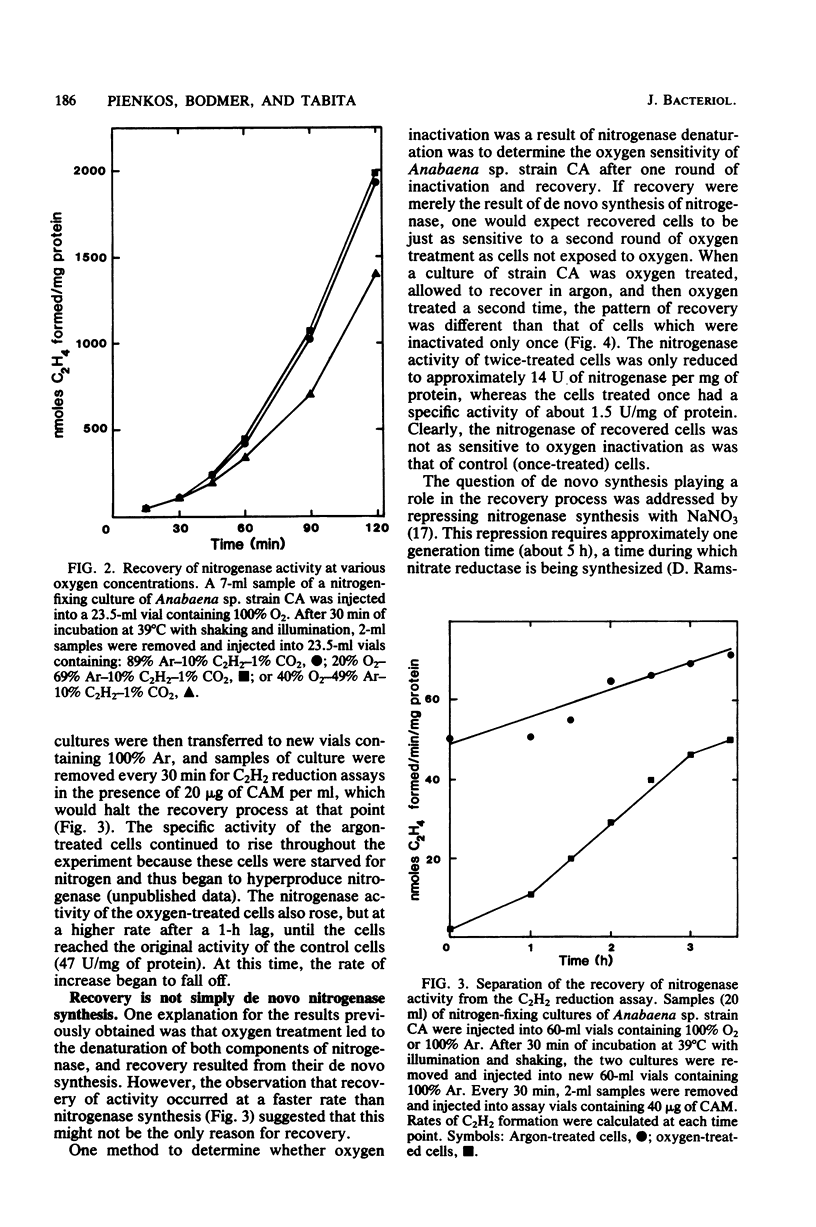
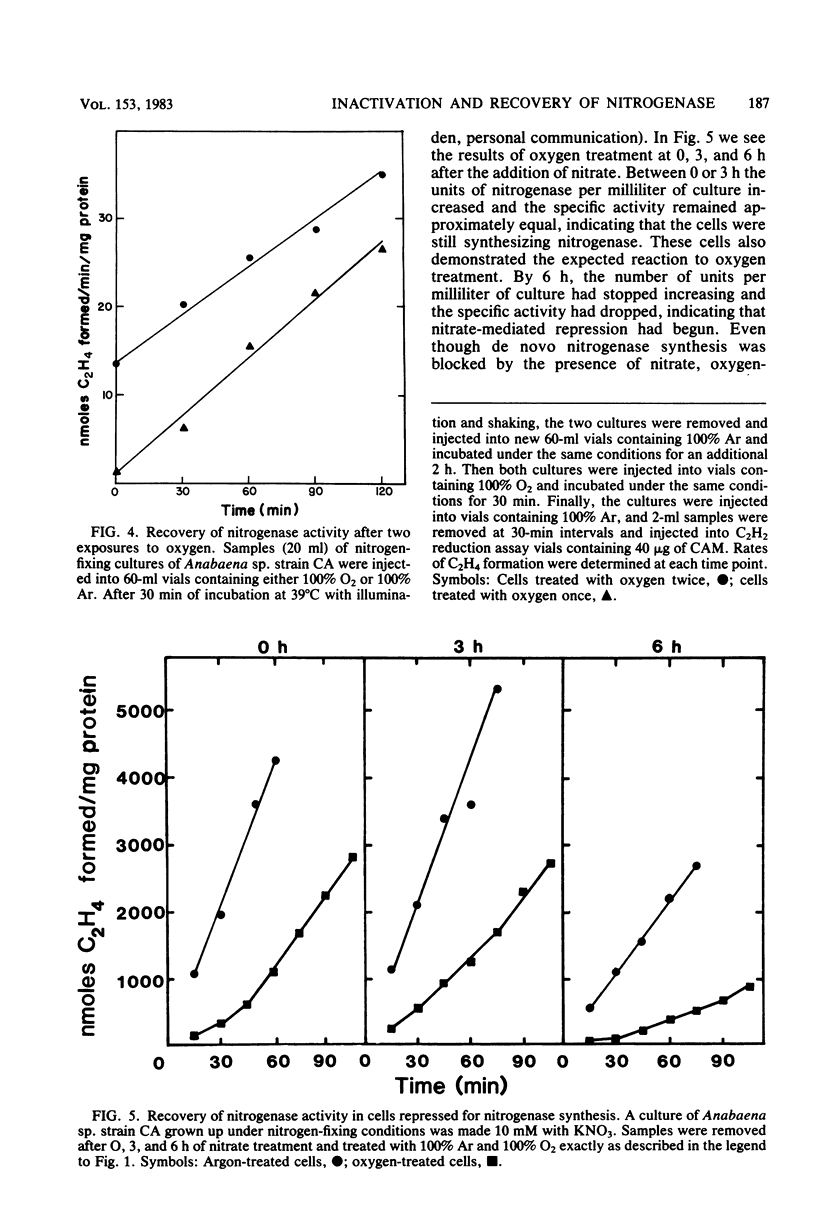
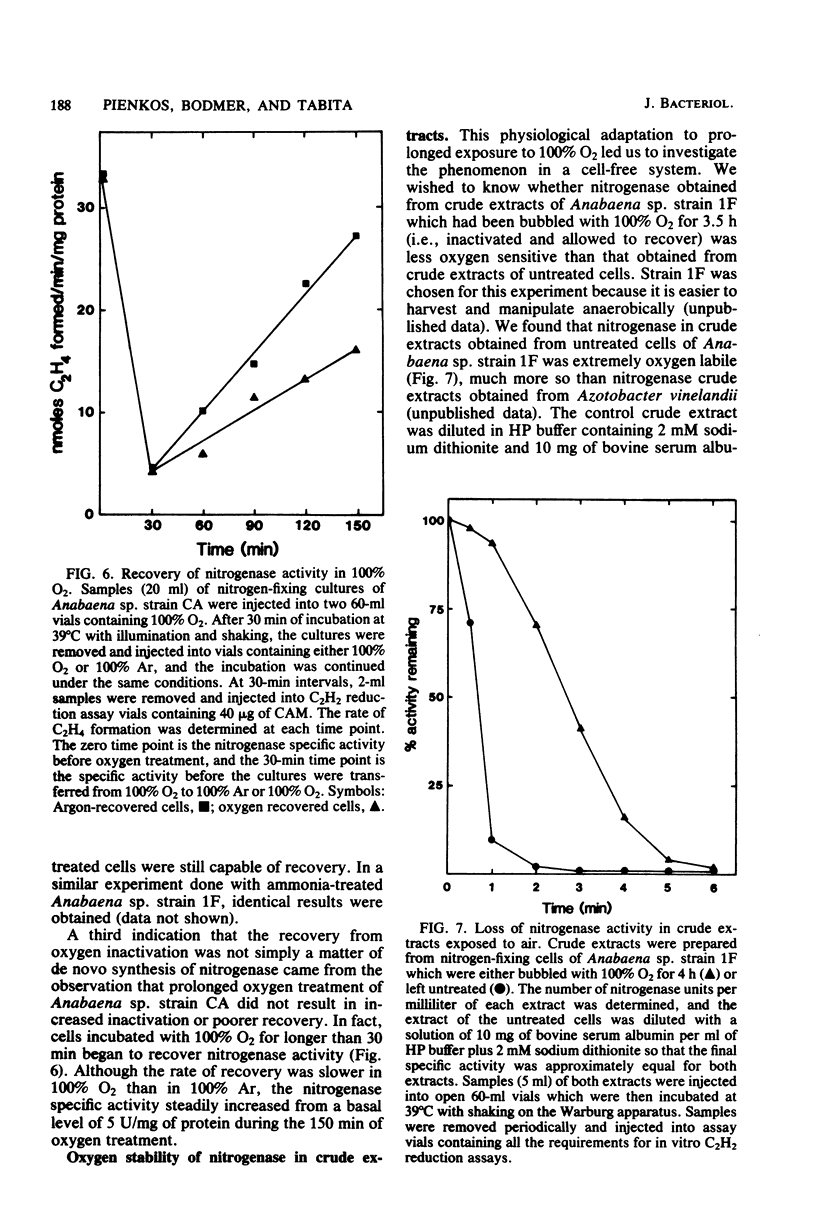
Exposure of nitrogen-fixing cultures of Anabaena spp. to 100% oxygen resulted in the rapid decline of nitrogenase activity. When oxygen-treated cells were transferred to 100% argon, nitrogenase activity was quickly restored in a process that required protein synthesis. Anaerobiosis was not essential for the recovery process; in fact, cells of Anabaena sp. strains CA and 1F will recover nitrogenase activity after prolonged incubation in 100% oxygen. Oxygen treatment acted directly on the intracellular nitrogenase and did not affect other metabolic processes. Examination of crude extracts of oxygen-treated Anabaena sp. strain CA indicated that both components of nitrogenase are inactivated. However, several lines of evidence suggest that oxygen treatment does not result in irreversible denaturation of nitrogenase, but rather results in a reversible inactivation which may serve as a protection mechanism. Nitrogenase present in crude extracts from cells of Anabaena sp. strain 1F which had been incubated for a prolonged period in 100% oxygen was less sensitive to oxygen in vitro than was nitrogenase of a crude extract of untreated cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Binder A., Tel-or E., Avron M. Photosynthetic activities of membrane preparations of the blue-green alga Phormidium luridum. Eur J Biochem. 1976 Aug 1;67(1):187–196. doi: 10.1111/j.1432-1033.1976.tb10648.x. [DOI] [PubMed] [Google Scholar]
- Bothe H., Tennigkeit J., Eisbrenner G. The utilization of molecular hydrogen by the blue-green alga Anabaena cylindrica. Arch Microbiol. 1977 Jul 26;114(1):43–49. doi: 10.1007/BF00429628. [DOI] [PubMed] [Google Scholar]
- Dervartanian D. V., Shethna Y. I., Beinert H. Purification and properties of two iron-sulfur proteins from Azotobacter vinelandii. Biochim Biophys Acta. 1969 Dec 23;194(2):548–563. doi: 10.1016/0005-2795(69)90117-2. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Postgate J. R. Nitrogenase. Nature. 1974 Jun 28;249(460):805–810. doi: 10.1038/249805a0. [DOI] [PubMed] [Google Scholar]
- Gotto J. W., Tabita F. R., Van Baalen C. Mutants of Anabaena strain CA altered in their ability to grow under nitrogen-fixing conditions. J Bacteriol. 1979 Nov;140(2):327–332. doi: 10.1128/jb.140.2.327-332.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker H., Veeger C. Involvement of the cytoplasmic membrane in nitrogen fixation by Azotobacter vinelandii. Eur J Biochem. 1977 Jul 1;77(1):1–10. doi: 10.1111/j.1432-1033.1977.tb11634.x. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Kostel P. J., Benemann Purification and properties of nitrogenase from the cyanobacterium, Anabaena cylindrica. Eur J Biochem. 1979 Jul;98(1):275–284. doi: 10.1111/j.1432-1033.1979.tb13186.x. [DOI] [PubMed] [Google Scholar]
- Haury J. F., Wolk C. P. Classes of Anabaena variabilis mutants with oxygen-sensitive nitrogenase activity. J Bacteriol. 1978 Nov;136(2):688–692. doi: 10.1128/jb.136.2.688-692.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G. R., Smith G. D. Hydrogen metabolism by filamentous cyanobacteria. Arch Biochem Biophys. 1980 Nov;205(1):36–50. doi: 10.1016/0003-9861(80)90081-8. [DOI] [PubMed] [Google Scholar]
- Paerl H. W., Kellar P. E. Nitrogen-fixing anabaena: physiological adaptations instrumental in maintaining surface blooms. Science. 1979 May 11;204(4393):620–622. doi: 10.1126/science.204.4393.620. [DOI] [PubMed] [Google Scholar]
- Pienkos P. T., Klevickis S., Brill W. J. In vitro activation of inactive nitrogenase component I with molybdate. J Bacteriol. 1981 Jan;145(1):248–256. doi: 10.1128/jb.145.1.248-256.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Stacey G., Bottomley P. J., Van Baalen C., Tabita F. R. Control of heterocyst and nitrogenase synthesis in cyanobacteria. J Bacteriol. 1979 Jan;137(1):321–326. doi: 10.1128/jb.137.1.321-326.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]



