Abstract
Acute transitions in cytosolic calcium ([Ca2+]i) through store-operated calcium entry channels catalyze interendothelial cell gap formation that increases permeability. However, the rise in [Ca2+]i only disrupts barrier function in the absence of a rise in cAMP. Discovery that type 6 adenylyl cyclase (AC6; EC 4.6.6.1) is inhibited by calcium entry through store-operated calcium entry pathways provided a plausible explanation for how inflammatory [Ca2+]i mediators may decrease cAMP necessary for endothelial cell gap formation. [Ca2+]i mediators only modestly decrease global cAMP concentrations and thus, to date, the physiological role of AC6 is unresolved. Present studies used an adenoviral construct that expresses the calcium-stimulated AC8 to convert normal calcium inhibition into stimulation of cAMP, within physiologically relevant concentration ranges. Thrombin stimulated a dose-dependent [Ca2+]i rise in both pulmonary artery (PAECs) and microvascular (PMVEC) endothelial cells, and promoted intercellular gap formation in both cell types. In PAECs, gap formation was progressive over 2 h, whereas in PMVECs, gap formation was rapid (within 10 min) and gaps resealed within 2 h. Expression of AC8 resulted in a modest calcium stimulation of cAMP, which virtually abolished thrombin-induced gap formation in PMVECs. Findings provide the first direct evidence that calcium inhibition of AC6 is essential for endothelial gap formation.
Keywords: adenosine 3′,5′-cyclic monophosphate; cAMP; store-operated calcium entry; thrombin; permeability
Introduction
Endothelial cells form a semipermeable barrier that separates circulating blood elements from underlying tissue (Moore et al., 1998; Michel and Curry, 1999). Cell shape and, consequently, barrier function are maintained by a balance of forces (Chicurel et al., 1998; Wang et al., 2001). Centripetally directed tension is generated by actomyosin motor function (Kolodney and Wysolmerski, 1992; Sheldon et al., 1993; Garcia et al., 1995, 1996; Garcia and Schaphorst, 1995; Goeckeler and Wysolmerski, 1995; Moy et al., 1996; Bodmer et al., 1997), and is opposed by adhesive proteins at the cell–cell border and focal adhesions at the cell–matrix border (Dejana et al., 1999; Michel and Curry, 1999; Yuan, 2000; Bazzoni and Dejana, 2001). Inflammatory mediators, which act by elevating [Ca2+]i, both increase tension and decrease adhesion important for the generation of focal intercellular gaps that form a paracellular pathway to promote fluid, solute, and protein permeability (Moore et al., 1998; Michel and Curry, 1999). Physiological transitions in cytosolic calcium ([Ca2+]i) through store-operated calcium entry pathways trigger gap formation, although [Ca2+]i-dependent targets that mediate the change in cell shape are poorly understood.
Inflammatory [Ca2+]i agonists only increase permeability in the absence of a rise in cAMP (Carson et al., 1989; Casnocha et al., 1989; Minnear et al., 1989; Morel et al., 1989; Stelzner et al., 1989; Allsup and Boarder, 1990; Oliver, 1990). cAMP-elevating agents improve constitutive barrier function and prevent the endothelial cell disruption that occurs in response to a rise in [Ca2+]i. Moreover, either inhibition of cAMP synthesis or its protein kinase (e.g., PKA) is sufficient to decrease adhesion and increase permeability (Stelzner et al., 1989; Stevens et al., 1995), suggesting that cAMP primarily regulates sites of cell–cell tethering. These findings bring into question the independent roles of [Ca2+]i and cAMP in endothelial cell barrier disruption. Results from cloning and expression of a calcium-inhibited adenylyl cyclase (type 6 adenylyl cyclase [AC6]*) in nonexcitable cells provided a plausible mechanism through which inflammatory [Ca2+]i agonists could decrease cAMP (Yoshimura and Cooper, 1992). Endothelial cells express AC6 as determined by in vivo (Chetham et al., 1997; Jourdan et al., 2001) and in vitro assays (Manolopoulos et al., 1995a,b; Stevens et al., 1995, 1997, 1999; unpublished data). Submicromolar calcium concentrations decrease cAMP accumulation by ∼30% in endothelial cell membranes (Stevens et al., 1995), and activation of store-operated calcium entry decreases cAMP and PKA activity 30–50% in intact cells (Stevens et al., 1995, 1997, 1999; unpublished data). Thus, calcium inhibition of AC6 may importantly contribute to endothelial cell gap formation.
Evidence for regulation of endothelial cell barrier function by AC6 is indirect and has been hampered by the inability to specifically control cAMP concentrations within a physiologically relevant range. A mechanism(s) accounting for calcium inhibition of AC6 is unresolved (Guillou et al., 1999; Gu and Cooper, 2000), although calcium inhibition does not require calmodulin or other calcium binding proteins (Caldwell et al., 1992). Recent studies suggest the presence of two distinct calcium binding sites on AC6, with apparent high and low affinities (Guillou et al., 1999; Gu and Cooper, 2000). Presumably the high affinity binding site accounts for enzyme calcium inhibition, because even calcium-insensitive isoforms of AC possess a low affinity binding site that inhibits enzyme activity at millimolar calcium concentrations. Structural assignment of the high affinity binding site is incomplete, making site-directed mutagenesis an untenable approach to evaluate the physiological role of AC6.
Fagan et al. (2000a) recently developed an adenovirus that expresses a calcium calmodulin–stimulated (AC8) enzyme. In their studies, adenovirus infection resulted in significant AC8 expression in the excitable GH4C1 cell type. Enzyme activity was intimately regulated by calcium entry through store-operated calcium entry pathways, relatively insensitive to regulation by calcium entry through voltage-gated calcium channels, and not stimulated by calcium release from intracellular stores. Their findings indicated that calcium-sensitive ACs functionally colocalize with store-operated calcium entry pathways (Fagan et al., 1996, 1998, 2000b), and also demonstrated that AC8 expression may represent a tenable approach to reversing the endogenous calcium inhibition of AC6. Presently, we used the adenoviral construct that expresses AC8 to examine the physiological role of endogenously expressed AC6 in nonexcitable endothelial cells. Our results indicate that thrombin-induced gap formation only proceeds when a rise in [Ca2+]i inhibits AC6 and is not observed when a rise in [Ca2+]i stimulates AC8. These results provide the first direct evidence that calcium inhibition of AC6 plays a central, dominant role in regulation of intercellular gap formation.
Results
Thrombin-induced [Ca2+]i responses in lung macro- and microvascular endothelium
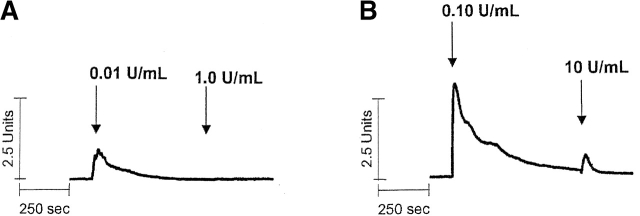
Lung macro- and microvascular endothelial cells exhibit distinct [Ca2+]i regulatory properties (Stevens et al., 1997), although the relative [Ca2+]i responses to Gq-linked agonists are poorly understood. We therefore used pulmonary artery (PAECs) and microvascular endothelial cells (PMVECs) to examine thrombin-activated [Ca2+]i signaling. In PAECs, thrombin induced a typical peak in [Ca2+]i that was due to calcium release from intracellular stores, followed by calcium entry across the cell membrane (Fig. 1 A). Thrombin-induced calcium release occurs in response to inositol 1,4,5-trisphosphate (InsP3) production that activates InsP3 receptors in the endoplasmic reticulum; the magnitude of this release phase is not influenced by acutely reducing extracellular calcium (Stevens et al., 1997). Calcium entry across the cell membrane occurs after calcium store depletion due to activation of store- and receptor-operated calcium channels (Putney, 1986).
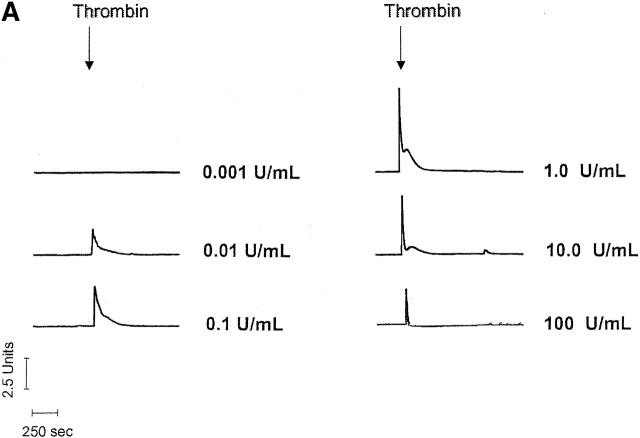
Figure 1.
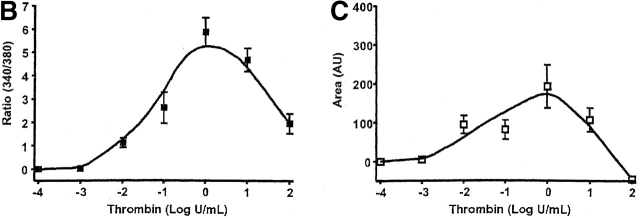
Thrombin produces a bell shape [Ca2 + ]i dose-dependent curve in PAECs. (A) Individual traces reveal that thrombin initiates [Ca2+]i signaling at concentrations below 0.01 U/ml, and reaches a maximal response at 1 U/ml. Higher thrombin concentrations reduce both calcium release (peak height) and entry (sustained plateau) phases. Indeed, in response to 100 U/ml thrombin, [Ca2+]i drops below constitutive levels during the “entry” phase, indicating pronounced inhibition of entry or stimulation of extrusion. Units on the y axis scale represent the ratio of calcium-bound (340 nm) to -unbound (380 nm) wavelengths for fura-2 fluorescence. Calcium release (B and D) and entry (C and D) phases are summarized, and are representative of n = 3–8/treatment. Area (AU, arbitrary units) on the y axis scale represents the relative area under the curve (e.g., above baseline values) generated during the Ca2+ entry phase.
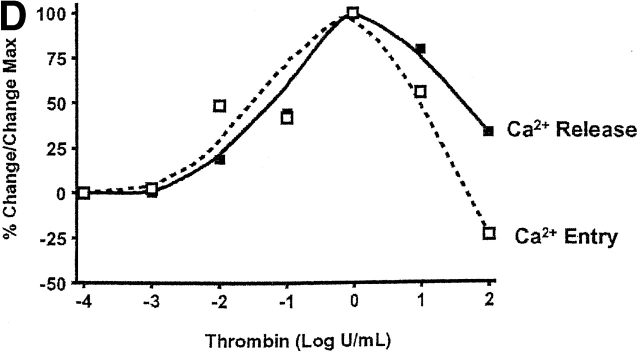
Dose response curves revealed that 1 U/ml thrombin maximally activated both calcium release and entry in PAECs, indicating direct coupling between calcium store depletion and calcium entry through store-operated calcium entry pathways (Fig. 1, B–D). The correlation between calcium release and entry was exact until 1 U/ml thrombin. At higher thrombin concentrations (100 U/ml), however, calcium release was reduced to 30% of its maximal response and calcium entry was 25% below basal levels. The thrombin [Ca2+]i dose response was bell shaped, suggesting that higher thrombin concentrations, in particular, activated interrelated regulatory pathways. These findings are generally compatible with thrombin's simultaneous activation of multiple signaling pathways (Molino et al., 1997). Because the correlation between calcium release and entry was not apparent at high thrombin concentrations, these data suggest that thrombin activated feedback inhibition of calcium entry channels.
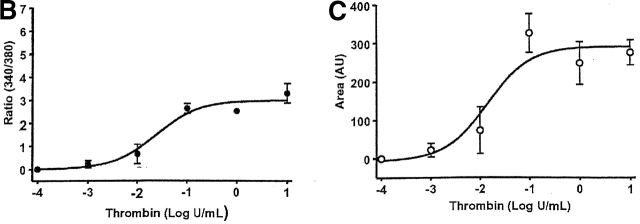
The thrombin-induced [Ca2+]i responses were different in PMVECs than in PAECs. Thrombin induced a sigmoidal [Ca2+]i dose response curve in PMVECs, both in calcium release and entry (Fig. 2). There was good association between calcium release and entry over the entire dose range (Fig. 2 D), generally compatible with the mechanism of store-operated calcium entry. PMVECs were more sensitive to thrombin than were PAECs. Even though thrombin did not initiate a [Ca2+]i rise in PMVECs until 0.1 U/ml (versus 0.01 U/ml in PAECs), the microvascular cell dose response curve was left shifted, indicating greater thrombin sensitivity; in PMVECs the EC50 = 0.05 U/ml and in PAECs the EC50 = 0.1 U/ml.
Figure 2.
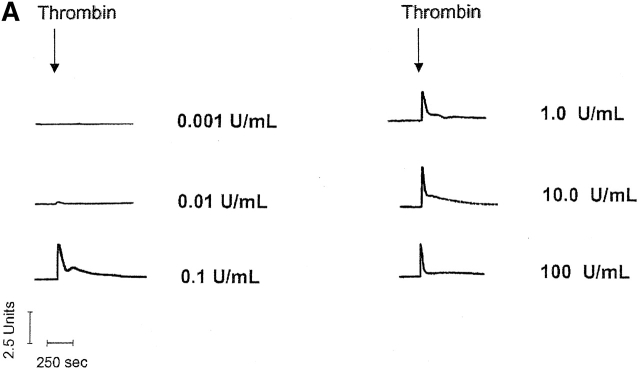
Thrombin produces a sigmoidal [Ca2 + ]i dose-dependent curve in PMVECs. (A) Individual traces reveal that thrombin initiates [Ca2+]i signaling at concentrations near 0.01 U/ml, and reaches a maximal response near 0.1 U/ml. This maximal [Ca2+]i response is sustained after application of higher thrombin concentrations. Indeed, the [Ca2+]i response to 0.1 and 10 U/ml thrombin are nearly identical. Units on the y axis scale represent the ratio of calcium-bound (340 nm) to -unbound (380 nm) wavelengths for fura-2 fluorescence. Calcium release (B and D) and entry (C and D) phases are summarized, and are representative of n = 3–4/treatment. Area (AU, arbitrary units) on the y axis scale represents the relative area under the curve (e.g., above baseline values) generated during the Ca2+ entry phase.
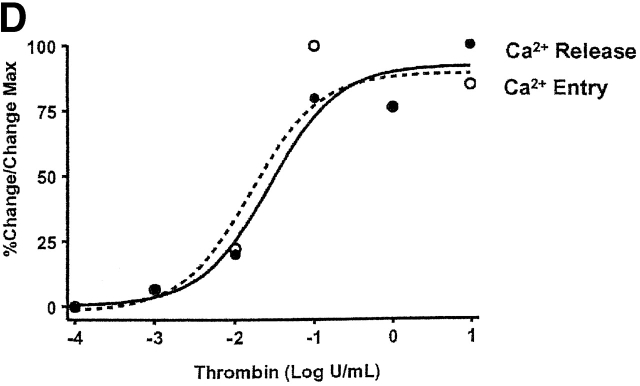
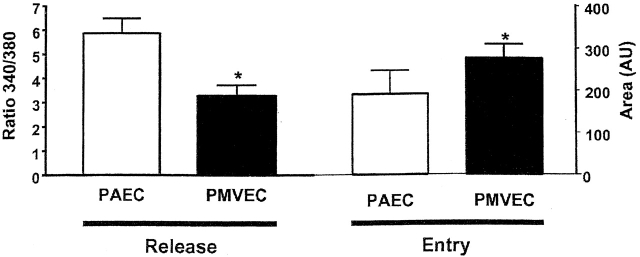
Calcium release and entry coupling was not similar between PMVECs and PAECs. Even though good association between calcium release and entry was observed in PMVECs, these findings are incompatible with the notion that store-operated calcium entry pathways represent the predominant mode of calcium entry in microvascular cells. Peak calcium release was ∼40% lower in PMVECs than in PAECs, suggesting that either thrombin does not equivalently stimulate InsP3 production in both cell types or that InsP3 does not equivalently stimulate calcium release in PMVECs. Prior work supports the latter possibility; InsP3 is rapidly hydrolyzed in cytosol (Ribeiro et al., 1997) and we have recently demonstrated that the endoplasmic reticulum is twofold farther from the plasmalemma in PMVECs (∼250 nm) than in PAECs (∼100 nm) (unpublished data). Although not specifically examined in the present study, a decrease in thrombin-induced calcium release may result from hydrolysis of InsP3 before receptor binding. Although thrombin-induced calcium release was lower in PMVECs than in PAECs, the calcium entry response was ∼30% higher (Fig. 3). Thus, although calcium store depletion and calcium entry correlate within a cell type, the magnitude of calcium release and entry are not associated between cell types.
Figure 3.
Coupling between Ca2 + store depletion and activation of Ca2 + entry is dissimilar between PAECs and PMVECs. Whereas maximal thrombin-induced Ca2+ release is greater in PAECs, maximal Ca2+ entry is elevated in PMVECs (n = 3/treatment). An asterisk denotes significant difference from corresponding PAEC value.
Thrombin produces homologous desensitization of its receptor(s), resulting in decreased [Ca2+]i signaling, inhibition of protein phosphorylation, and preservation of endothelial cell barrier function (Brass et al., 1991; Levin and Santell, 1991; Weintraub et al., 1992; Kruse et al., 1995; Yan et al., 1998). Maximal or near-maximal thrombin doses are typically used for desensitization studies. We examined whether lower thrombin concentrations desensitize subsequent [Ca2+]i responses to EC100 thrombin concentrations. Fig. 4, A, C, and D, illustrates that an EC20 concentration of thrombin effectively desensitizes PAECs to subsequent activation by maximal doses. In PMVECs, similar low concentrations of thrombin only reduced the maximal response by 40%, indicating that 0.01 U/ml was insufficient to fully desensitize thrombin-induced [Ca2+]i signaling in microvascular cells. However, 0.1 U/ml thrombin induced a large [Ca2+]i rise and abolished the subsequent [Ca2+]i response to maximal thrombin concentrations in PMVECs (Fig. 4, B–D). Thus, thrombin-induced calcium entry is abolished by receptor desensitization, as it is in the presence of nominal extracellular calcium.
Figure 4.
Low thrombin concentrations acutely desensitize [Ca2 + ]i signaling. (A) Application of an EC20 thrombin dose acutely abolishes the subsequent [Ca2+]i response to an EC100 thrombin concentration in PAECs. Similar inhibition was not observed in PMVECs (unpublished data). Units on the y axis scale represent the ratio of calcium-bound (340 nm) to -unbound (380 nm) wavelengths for fura-2 fluorescence. (B) Application of an EC100 thrombin concentration acutely reduces the subsequent [Ca2+]i response to a higher thrombin dose in PMVECs. Summary data are shown for Ca2+ release (C) and entry (D) phases, and are representative of n = 3/treatment. Area (AU, arbitrary units) on the y axis scale represents the relative area under the curve (e.g., above baseline values) generated during the Ca2+ entry phase.
Thrombin-induced endothelial cell gap formation
Thrombin disrupts the endothelial cell barrier and increases macromolecular, solute, and water permeability (Casnocha et al., 1989; Minnear et al., 1989; Lum et al., 1993; Garcia et al., 1995, 1996; Garcia and Schaphorst, 1995; Bartha et al., 2000; Moore et al., 2000; Norwood et al., 2000). Barrier disruption is induced by an increase in centripetally directed tension that is mediated through the activation of an actomyosin-based motor, and by a decrease in cell adhesion that is mediated through the loss of cadherin-dependent cell–cell tethering. Signaling events that promote increased tension and decreased adhesion are incompletely understood, though calcium entry across the cell membrane is recognized as an important catalyst (Moore et al., 1998). Despite the implied role of increased [Ca2+]i in the loss of endothelial cell barrier function, prior studies have not distinguished between [Ca2+]i-dependent signaling events and simultaneous activation of other signaling pathways, particularly in macro- and microvascular endothelia. We therefore examined the specific role of thrombin-induced calcium signaling in PAECs and PMVECs.
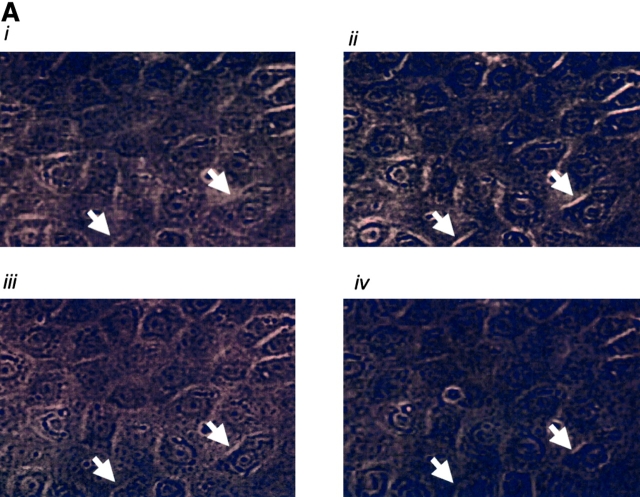
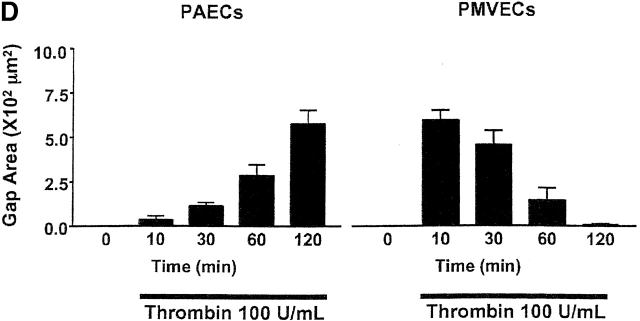
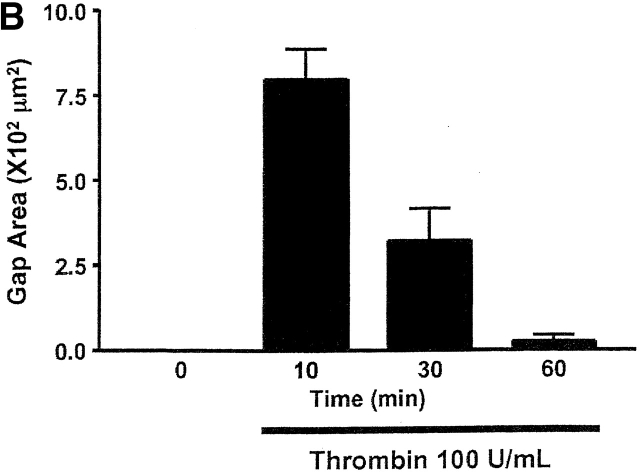
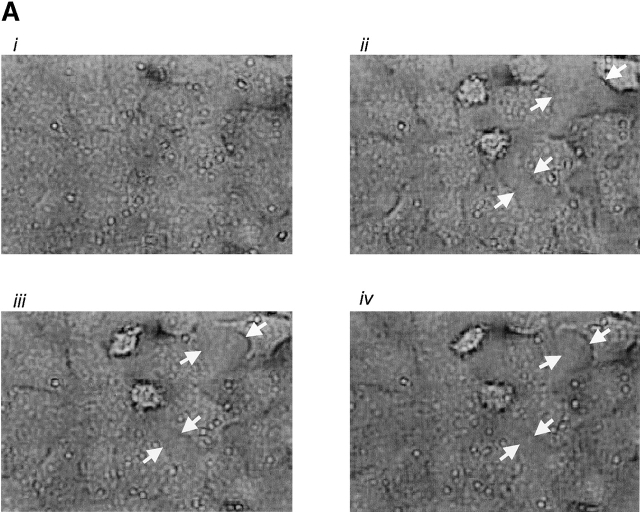
Fig. 5 illustrates thrombin-induced gap formation in PAECs and PMVECs. Acute application of 1 U/ml (EC100) thrombin to PAEC monolayers produced small gaps between cells that resealed over a 1-h time course (Fig. 5 A). Gaps were difficult to clearly resolve by light microscopy, though they were evident by the appearance of translucent cell–cell borders. These findings are compatible with prior studies indicating that increased macromolecular permeability occurs through only minor ultrastructural disturbances in adherens junctions (McDonald et al., 1999; Moy et al., 2000). Higher thrombin concentrations (100 U/ml) produced larger, more visible intercellular gaps that persisted and, indeed, increased over a 2-h time course (Fig. 5, B and D). Because [Ca2+]i responses are lower in response to 100 U/ml than to 1 U/ml thrombin (Fig. 1 D), these data indicate that the large gaps generated in response to higher thrombin concentrations occur after activation of calcium-independent signaling pathways.
Figure 5.
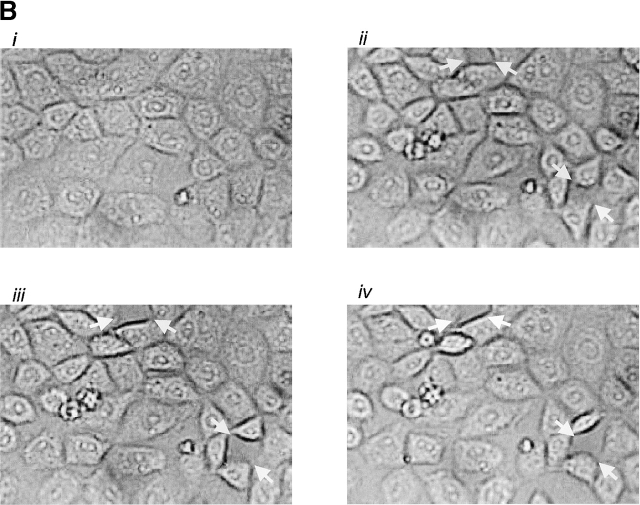
Thrombin induces interendothelial cell gap formation in PAECs and PMVECs, although only gaps in PMVECs reseal within 2 h. (A) In basal PAEC monolayers (i), application of 1 U/ml thrombin induced intercellular gaps within 10 min (ii) that persisted over 60 (iii) and 120 (iv) min, though gaps were difficult to clearly resolve by light microscopy. Gaps are seen as translucent borders indicated by arrows in ii–iv. (B) To more clearly resolve gaps, PAECs were grown to confluence (i) and 100 U/ml thrombin was applied. Gaps apparent at 10 min (ii) were sustained over 30 (iii) and 60 (iv) min. (C) Application of 100 U/ml thrombin to PMVECs (i) resulted in the formation of large gaps at 10 min (ii) that were easily resolved. In contrast to PAECs, gaps in PMVECs resealed over 30 (iii) and 60 (iv) min. See Video 1, available at http://www.jcb.org/cgi/content/full/jcb.200204022/DC1 . (D) Direct comparison of gaps between cell types reveals that whereas gap size increases temporally in PAECs, gap size decreases temporally in PMVECs. (E) The capacity of PMVECs to reseal their barrier is evident even when large gaps have formed. Arrows indicate intercellular gaps. n = 6–8/treatment.
Our prior studies indicated that direct activation of store-operated calcium entry did not increase PMVEC permeability (Kelly et al., 1998). Similarly, in the intact pulmonary circulation, thapsigargin did not induce intercellular gaps in small arteries, veins, or capillaries, but gap formation was prominent in large arteries and veins (Chetham et al., 1999). In our present study, thrombin (EC100) induced gap formation in cultured PMVECs, though unlike conduit cells, the response was not sustained (Fig. 5, C–E). Indeed, gaps that formed in PMVECs rapidly resealed; these findings are illustrated in the supplementary video (Video 1, available online at http://www.jcb.org/cgi/content/full/jcb.200204022/DC1) that accompanies Fig. 5 C. A 1-h time course is compressed to ∼45 s in the video. Gaps were fully formed 5–10 min after thrombin application and subsequently resealed within 1–2 h.
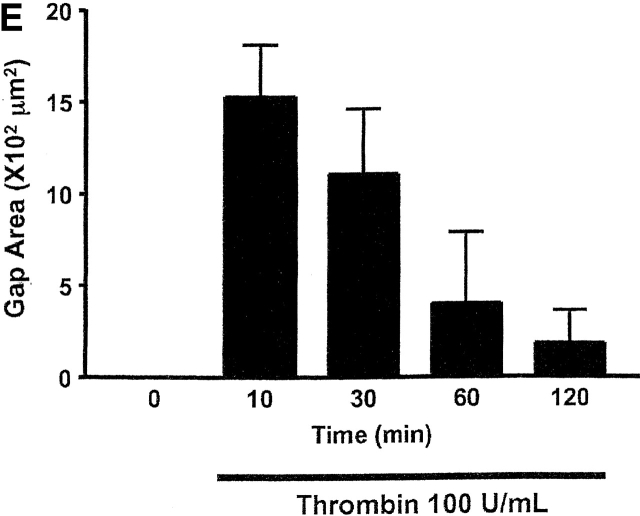
Thrombin induced PAEC and PMVEC gaps that varied in size, though clearly the largest gaps were observed in PMVECs. A direct comparison between cell types is shown in Fig. 5 D, where equivalent gap sizes are plotted. Whereas gap size progressively increased over 2 h in PAECs, gap size progressively decreased after 10 min in PMVECs. As shown in Fig. 5 E, even the largest PMVEC gaps were nearly completely resealed by 2 h. These findings indicate that PMVECs possess a unique capacity to repair or reseal intercellular gaps, even in the continued presence of thrombin.
Calcium-sensitive ACs and endothelial cell gap formation
Because thrombin activates multiple intracellular signaling cascades, we sought to examine the specific role of [Ca2+]i in gap formation. In PAECs, low thrombin concentrations (0.01 U/ml; EC20) desensitized the subsequent [Ca2+]i response to maximal doses (1.0 U/ml). In PMVECs, higher thrombin doses (0.10 U/ml) were required to desensitize subsequent [Ca2+]i responses. However, the use of higher concentrations elicited a large [Ca2+]i response and therefore the desensitization approach could not resolve between [Ca2+]i-dependent and -independent signal transduction pathways (Fig. 6, A and B), indicating that more direct approaches were required to specifically divulge the requirement of [Ca2+]i in thrombin-induced gap formation.
Figure 6.
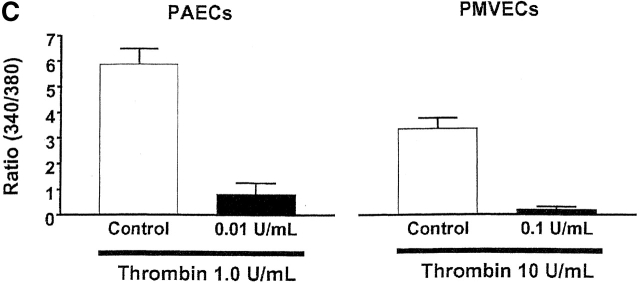
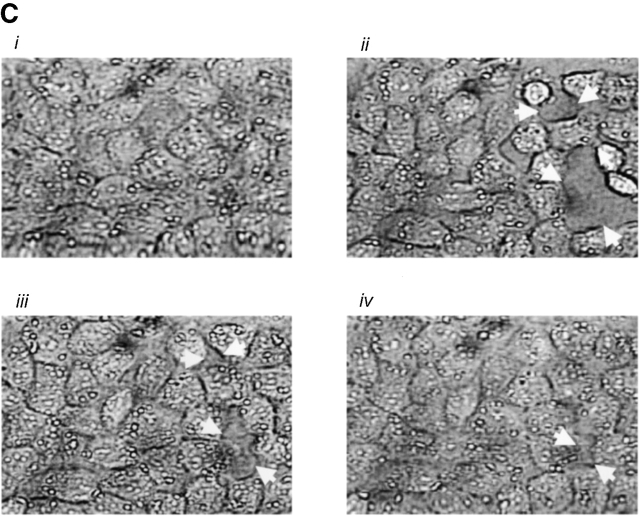
A desensitizing thrombin concentration induces gap formation in PMVECs. Application of 0.1 U/ml thrombin increased [Ca2+]i and desensitized the subsequent response to 10 U/ml thrombin (Fig. 4 B). (A) Similar treatment in confluent PMVECs (i) revealed that the desensitizing [Ca2+]i response to 0.1 U/ml thrombin induced gap formation within 10 min (ii), which resolved over 30 (iii) and 60 (iv) min. (B) Summary of gaps formed by the desensitizing dose of thrombin shows resolution of gaps over a 60-min time course. Arrows indicate intercellular gaps. n = 3/treatment.
Though Ca2+ entry is a well-recognized stimulus for endothelial cell barrier disruption, the Ca2+-sensitive targets mediating this response are poorly understood. We have previously reported that Ca2+ entry reduces cAMP in PAECs, and that the decrease in cAMP contributes to endothelial cell gap formation (Stevens et al., 1995). Indeed, the inverse relationship between Ca2+ and cAMP is mediated through AC6, which is specifically inhibited by Ca2+ entry through store-operated Ca2+ entry pathways (Fagan et al., 1996, 1998, 2000b). Despite studies implicating AC6 in endothelial barrier disruption, conclusive proof for this idea has been limited by our inability to specifically control cAMP levels within the physiological range. The Ca2+-sensitive region of AC6 is unknown, and thus studies seeking to specifically eliminate enzyme Ca2+ inhibition are implausible (Guillou et al., 1999; Gu and Cooper, 2000). Our present approach therefore exploited an adenovirus vector constructed to express the Ca2+-stimulated AC8. Prior work from Fagan et al. (2000a) established that the AC8/adenovirus construct expresses functional enzyme that converts Ca2+ inhibition into Ca2+ stimulation of cAMP.
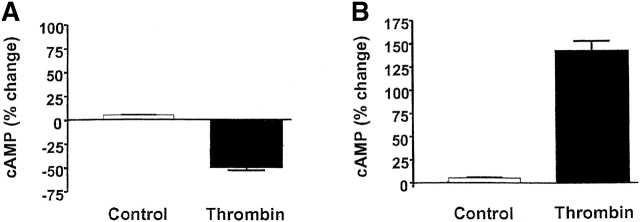
Expression of the AC8/adenovirus construct did not change basal PMVEC cAMP concentrations (control = 124 ± 9 fmol cAMP vs. AC8 = 120 ± 11 fmol cAMP). Forskolin and rolipram synergistically increase cAMP in PMVECs, and allow easy resolution of AC6 Ca2+ inhibition (Stevens et al., 1999; unpublished data). Similar to our previous reports (Stevens et al., 1995, 1997, 1999; unpublished data), thrombin inhibited cAMP in PMVECS that endogenously expressed AC6 (Fig. 7 A). However, in cells expressing the AC8/adenovirus construct, the thrombin-induced Ca2+ inhibition was converted into Ca2+ stimulation of cAMP (Fig. 7 B). Thus, cells infected with the AC8/adenoviral construct provided the first available model to specifically examine the physiological role of AC6 in regulation of endothelial cell barrier apposition.
Figure 7.
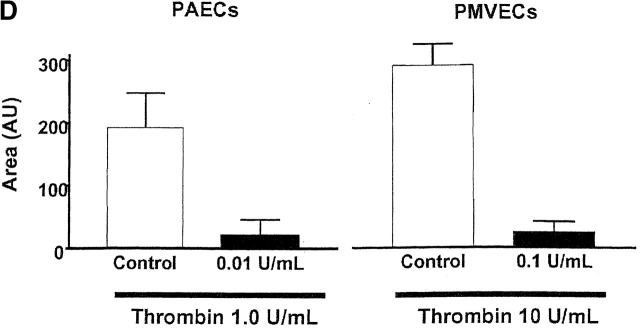
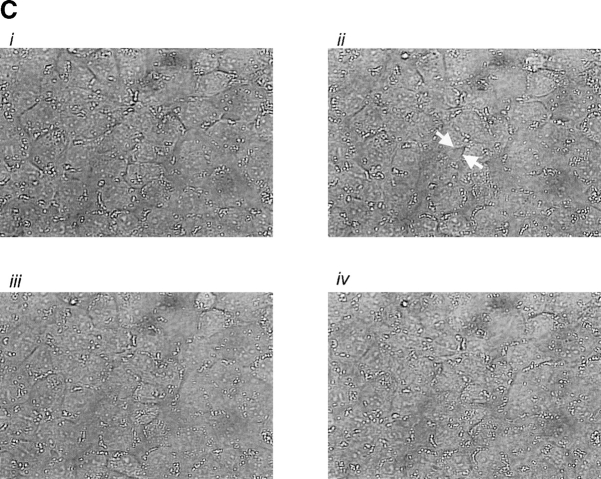
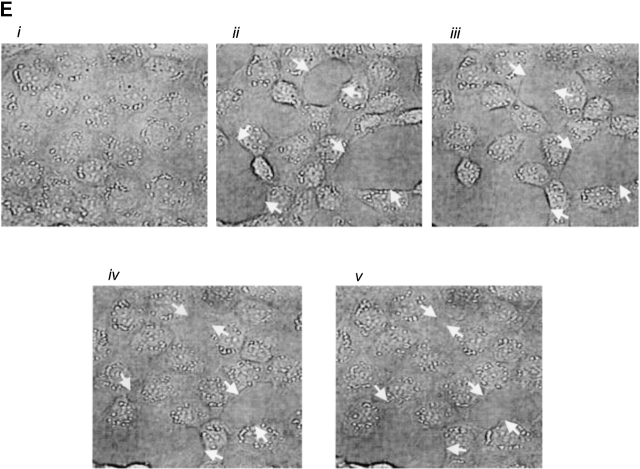
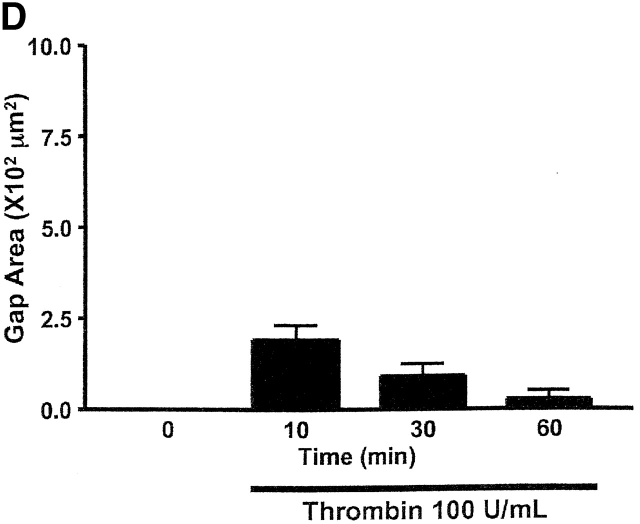
Expression of AC8 prevents thrombin from inducing gaps in PMVECs. (A) Confluent PMVECs were incubated with the type 4 phosphodiesterase inhibitor rolipram (10 μM) for 10 min before thrombin (100 U/ml) treatment. 5 min after addition of thrombin, PMVECs were collected for cAMP determination. Thrombin decreased cAMP from 1293 ± 123 to 648 ± 37 fmol/well, consistent with calcium inhibition of endogenous AC6. (B) Expression of AC8 did not change basal cAMP concentrations. However, in cells infected with the AC8/adenovirus construct, rolipram (10 μM) and thrombin (100 U/ml) treatment resulted in an increase in cAMP, indicating that endogenous calcium inhibition had been converted to stimulation of AC. (C) Cells expressing the AC8/adenovirus construct (i) did not respond to thrombin with significant gap formation at 10 (ii), 30 (iii), or 60 (iv) min. Arrows indicate intercellular gaps. See Video 2, available at http://www.jcb.org/cgi/content/full/jcb.200204022/DC1 . (D) Summary data reveals that thrombin induced few gaps in cells expressing the AC8/adenovirus construct. Gaps that did form were small in size and resealed rapidly (n = 7/treatment). (E) Cells expressing an alkaline phosphatase/adenovirus construct were used to confirm that adenoviral infection and expression, per se, did not provide nonspecific protection against thrombin-induced gap formation. Cells are shown before (i) and 10 (ii), 30 (iii), 60 (iv), and 90 (v) min after thrombin stimulation. Arrows indicate intercellular gaps. Video 3 demonstrates that expression of alkaline phosphatase does not inhibit thrombin-induced gap formation.
Remarkably, infection with the AC8/adenoviral construct nearly abolished thrombin-induced PMVEC gap formation, shown in Fig. 7, C and D, and the accompanying time-compressed video (Video 2, available online at http://www.jcb.org/cgi/content/full/jcb.200204022/DC1). Fig. 7 D summarizes all gaps formed in AC8-expressing cells treated with thrombin; this figure differs from control experiments (e.g., Fig. 5 D) that standardized gap size between PAECs and PMVECs. Indeed, Fig. 5 E illustrates PMVEC gaps that exceeded 15 × 102 μm2 in some cases. Cells infected with the AC8/adenoviral construct possessed very few gaps that were of low magnitude (e.g., ≤2.4 × 102 μm2). Although even small gaps rarely formed in cells expressing AC8, the video clearly illustrates that thrombin initiated cell movement. Thrombin induces myosin light chain phosphorylation and increases actomyosin motor function. The relative contributions of increased motor function and decreased cell adhesion to gap formation is still controversial. However, our data suggest cAMP primarily regulates adhesion as opposed to “contraction,” and suggests that increased motor function does not form gaps if cell–cell adhesion is maintained.
It is not clear in AC8-expressing cells how small rises in global cAMP could, with such strength, oppose thrombin-induced gap formation. Recent studies (Rich et al., 2000, 2001) implicate discrete cAMP pools, including its site of synthesis (e.g., plasmalemma) and the bulk cytosol, in location-specific function. Thus, AC localization is an important determinant of enzyme (and cAMP) function independent of global cAMP concentrations. We examined localization of AC8 by expressing it as a fusion with yellow fluorescent protein (YFP). Fig. 8 illustrates nearly uniform expression of every cell infected with the YFP–AC8/adenoviral construct. Confocal imaging from abluminal-to-luminal cell aspects reveals punctate staining at abluminal and luminal cell aspects, with staining enrichment at cell–cell borders, suggesting that the enzyme localizes to sites of cell contact. Indeed, the video (Video 6) accompanying Fig. 8 E demonstrates dynamic movement of YFP–AC8 at sites of cell–cell contact. As seen in this video, YFP–AC8 localization to cell–cell borders is retained in accordance with cell movement in response to thrombin, including membrane association during small, transient gap formation. Taken together, these data support the idea that AC localization to sites of cell–cell contact is an important determinant of how localized changes in calcium-dependent cAMP concentrations regulate intercellular gap formation.
Figure 8.
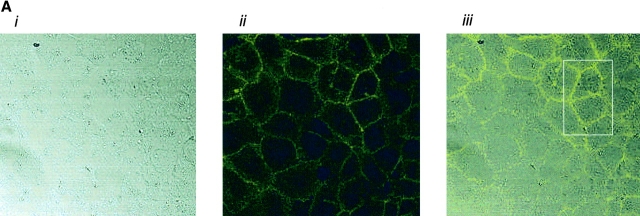
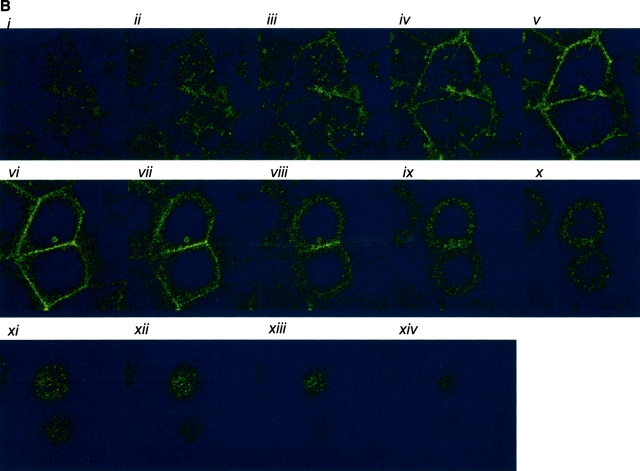
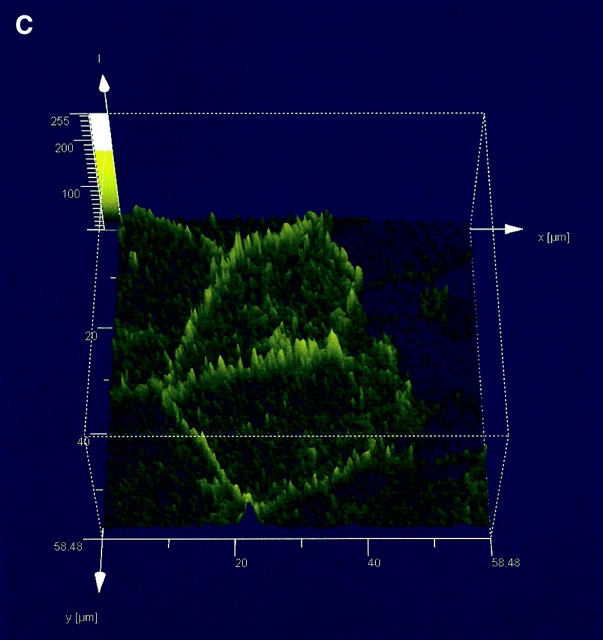
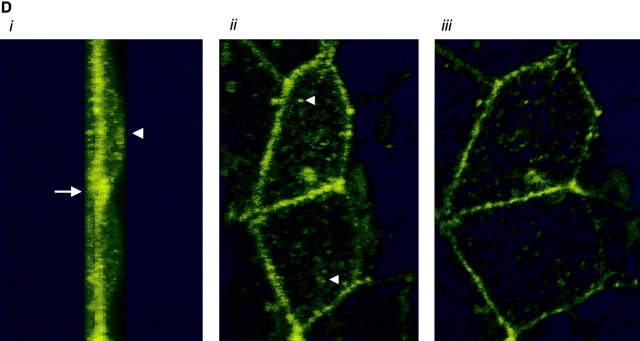
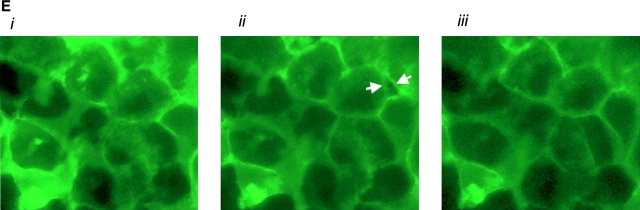
AC 8 localizes to sites of cell–cell contact. (A) Subconfluent PMVECs (i) were infected with the YFP–AC8 adenovirus construct (ii). Overlay of phase contrast and fluorescent images (iii) reveals that virtually every cell expressed YFP–AC8. The box indicates cells analyzed by confocal microscopy in B–D. (B) Abluminal-to-luminal cell slices were analyzed by confocal microscopy at 0.5-μm resolution (i–xiv, abluminal to luminal). Punctate staining is observed on abluminal and luminal cell aspects, whereas staining enrichment was observed at cell–cell borders. Video 4 illustrates the abluminal-to-luminal expression pattern of YFP–AC8 . (C) Cell coordinates are shown on the x and y axes. Fluorescent intensity is shown on the z axis. Fluorescence intensity is greatest at sites of cell–cell contact. (D) 3-D reconstruction of confocal images taken in B demonstrates high fluorescent intensity at sites of cell–cell contact from side (i, arrow denotes AC8 expression at cell–cell borders and arrowhead denotes the cell's luminal aspect), angled (ii, arrowheads denote punctate staining), and horizontal (iii) views. Video 5 shows the lateral rotation of the 3-D image. (E) Response of YFP–AC8 was observed after application with thrombin (20 U/ml). Staining is most apparent at sites of cell–cell contact (i), and remains most intense at sites of cell–cell contact even after thrombin application for 10 (ii) and 30 (iii) min. Video 6 represents a compression of the 30-min thrombin response.
Discussion
Inflammatory [Ca2+]i agonists induce interendothelial cell gaps. However, a rise in [Ca2+]i only promotes gap formation if cAMP levels are not also increased. We have previously demonstrated that endothelial cells express AC6, and physiological [Ca2+]i transitions through store-operated calcium entry channels reduce enzyme activity (Stevens et al., 1995, 1997, 1999; unpublished data). In prior studies, activation of store-operated calcium entry decreased global cAMP by ∼30%. We have not firmly established whether a 30% decrease in global cAMP contributes to intercellular gap formation; indeed, because of difficulties regulating cAMP within a narrow concentration range, we have heretofore been unable to clearly establish the independent effects of [Ca2+]i and cAMP. Consequently, the physiological significance of AC6 was unclear. Use of an adenoviral construct expressing AC8 has allowed precise control over calcium-regulated cAMP production. For the first time, our findings directly implicate AC6 in thrombin-induced endothelial cell gap formation.
Emerging evidence suggests that PAECs and PMVECs arise from distinct embryological origins and retain phenotypic differences even when their environments are the same (deMello et al., 1997; deMello and Reid, 2000; Stevens et al., 2001; Maeda et al., 2002). Previous studies using these cells have demonstrated that their calcium and cyclic nucleotide regulatory properties significantly differ (Stevens et al., 1997, 1999), although they both possess store-operated calcium entry pathways and they both express AC6. PMVECs form a more restrictive barrier in vivo and in vitro to protect the alveolar-capillary network from fluid accumulation and to optimize gas exchange (Kelly et al., 1998; Chetham et al., 1999). Direct activation of store-operated calcium entry using thapsigargin increases PAEC, but does not increase PMVEC, macromolecular permeability (Kelly et al., 1998; Chetham et al., 1999). Our present studies therefore used the inflammatory [Ca2+]i agonist thrombin, which, like thapsigargin, activates store-operated calcium entry. In addition, however, thrombin activates Gi and G12/13 signaling pathways that inhibit AC (Brass et al., 1991) and activate Rho signaling cascades (Vouret-Craviari et al., 1998; Carbajal and Schaeffer, 1999; Carbajal et al., 2000; Sah et al., 2000; van Nieuw Amerongen et al., 2000a,b; Wojciak-Stothard et al., 2001), respectively. Activation of Rho and its kinase promotes the cytoskeletal reorganization that is important for induction of intercellular gap formation.
We found that thrombin induces gap formation in both PAECs and PMVECs. High thrombin concentrations were required to break cell–cell adhesions in microvascular cells. Indeed, our dose response curves were conducted over thrombin concentrations that exceeded its normal range. High thrombin concentrations revealed unique responses in PAECs and PMVECs. Calcium regulatory mechanisms are different between these cell types at high thrombin concentrations; PAECs posses a feedback inhibitory mechanism not present in PMVECs. In addition, gap formation in PAECs was slow and progressive, whereas gap formation in PMVECs was rapid and quickly resolved, suggesting that microvascular cells possess a motility/repair mechanism not present in their macrovascular counterparts. The gaps that formed in PMVECs were substantially larger than those present in PAECs. While speculative, PMVECs may possess a higher resting tension so that the disruption of cell–cell adhesion produces large gaps that form rapidly.
In PAECs, gaps formed in response to the high thrombin concentrations that also inhibited calcium entry. Thus, intercellular gap formation can be induced by [Ca2+]i-independent as well as [Ca2+]i-dependent signaling cascades. AC6 is a calcium entry–sensitive target that coordinates [Ca2+]i and cAMP signaling pathways. AC6 functionally colocalizes with store-operated calcium entry channels (Fagan et al., 1996, 1998, 2000b), indicating that thrombin may inhibit AC6 activity by stimulating calcium entry and activating Gi.
Our present studies sought to specifically determine the relevance of AC6 in thrombin-induced gap formation. Despite the activation of multiple signaling pathways, we found that converting calcium inhibition to modest stimulation of cAMP nearly abolished thrombin-induced gap formation in PMVECs. In AC8-expressing cells, AC8 localized to sites of cell–cell contact. Digitally time-compressed videos revealed that although AC8-expressing cells did not form gaps, they still exhibited significant movement that was generally consistent with activation of motor function. It therefore appears that AC6 decreases the cAMP necessary to break cell–cell adhesions without significantly altering actomyosin function per se. cAMP activation of its protein kinase reduces endothelial cell myosin light chain kinase activity (Garcia et al., 1997), indicating that cAMP is capable of reducing endothelial cell motor function. However, our findings suggest that the principal role of calcium-sensitive cAMP synthesis is not to regulate actomyosin interaction, but rather to control cell–cell adhesion. In future studies, it will be important to determine relevant A-kinase targets that may control development of intercellular gap formation.
In summary, our studies have used two distinct endothelial cell phenotypes, PAECs and PMVECs, to evaluate the role of AC6 in thrombin-induced gap formation. Thrombin activates store-operated calcium entry in both cell types, along with other reported signaling cascades. Nonetheless, calcium entry across the cell membrane is an important determinant of gap formation. We have established for the first time that calcium entry is required to inhibit AC6 activity, which decreases cAMP necessary for generation of intercellular gaps. These findings firmly establish a physiological role for AC6 in nonexcitable endothelial cells: AC6 critically regulates gap formation.
Materials and methods
Reagents
All reagents were obtained from Sigma-Aldrich, unless otherwise specified. Thrombin used in calcium and videomicroscopy experiments was from Sigma-Aldrich, catalog no. T-6634, lot 19H7612 (from bovine plasma) or catalog no. T-5772, lot 71K7612 (from rat plasma). Fura-2/AM and Pluronic® F-127 were purchased from Molecular Probes.
Isolation and culture of pulmonary endothelial cells
PAECs and PMVECs were isolated and cultured using a method described in detail by Stevens et al. (1999). Cells were routinely passaged by scraping. Cultures were characterized using SEM, uptake of 1,1'-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine–labeled low-density lipoprotein (DiI-acetylated LDL), and a lectin binding panel.
Construction of the virus expressing AC8
A full-length description of the adenovirus expressing AC8 has been reported in detail elsewhere (Fagan et al., 2000a). However, for construction of an adenovirus-transducing vector encoding the YFP–AC8 fusion protein, the YFP–AC8 fusion protein gene was excised from the plasmid pEYFP-C1 by digesting with NheI, filling in with T4 DNA polymerase in the presence of dNTPs, and digesting with XbaI. The plasmid pShuttleCMV (He et al., 1998) was digested with XhoI, blunt ended with T4 DNA polymerase in the presence of dNTPs, and digested with XbaI. The YFP–AC8 gene and pShuttleCMV fragments were resolved by low melting point agarose gel electrophoresis, excised, and the DNA fragments were recovered by melting the agarose followed by phenol extraction and ethanol precipitation. The DNA fragments were ligated to generate the plasmid pShuttleCMV-YFP–AC8. The YFP–AC8 gene was removed from pShuttleCMV-YFP–AC8 by digestion with NotI and XbaI and ligated with pShuttleE1A DNA (Schaack et al., 2001) that had been digested with the same restriction enzymes. The shuttle vectors encoding YFP–AC8 were then linearized by digestion with PmeI, and 20 ng of each was used to electroporate Escherichia coli strain BJ5183 carrying the plasmid pAdEasy-1(He et al., 1998), a modification that we previously found helpful for plasmid construction (Orlicky and Schaack, 2001), to introduce the YFP–AC8 genes into the adenovirus chromosome by homologous recombination (Chartier et al., 1996; Crouzet et al., 1997; He et al., 1998). Resulting colonies were screened by agarose gel electrophoresis of undigested plasmid DNAs. Supercoiled plasmids that migrated slower than linear 12-kb marker DNA were then used to transform E. coli DH5, and the plasmids tested by restriction digestion, and grown in large scale. The resulting plasmids were named pAdEasyCMV-YFP–AC8 and pAdEasyE1A-YFP–AC8. The plasmid DNAs were digested with PacI to liberate the adenovirus chromosomes and used to transfect (Jordan et al., 1996) 293 cells (Graham et al., 1977) to make viruses. Despite repeated attempts, no plaques were isolated from cells transfected with pAdEasyCMV-YFP–AC8. However, fluorescent plaques were readily obtained from cells transfected with pAdEasyE1A-YFP–AC8, suggesting that the level of overexpression of the YFP–AC8 gene that results from the use of the CMV major immediate early promoter inhibited virus production (Schaack et al., 2001). AdEasyE1A-YFP–AC8 virus was plaque purified and grown in large scale using 293 cells, purified by successive banding on CsCl step and isopycnic gradients, and dialyzed using three changes of 10 mM Tris-HCl, pH 8.0, 135 mM NaCl, 1 mM MgCl2, 50% vol/vol glycerol at 4°C. The particle concentration of the virus was quantitated by determination of the absorbance at 260 nm, where one A260 unit was considered equal to 1012 virus particles. The particle/plaque forming unit ratio for viruses prepared in this manner is reproducibly close to 100:1. The virus was stored at −20°C.
Infection with adenovirus/AC8 construct
PMVECs were seeded onto 25-mm glass coverslips and grown to ∼60% confluence, at which point they were infected with the adenoviral vector constructed to express Ca2+-stimulated AC8 at a titer of 100 plaque forming units/cell. Initial studies using PAECs (unpublished data) and GH4C1 cells established a dose-dependent increase in calcium-dependent cAMP synthesis over a multiplicity of infection range from 0 to 200. A multiplicity of infection of 100 provided reproducible calcium-stimulated cAMP responses and was therefore selected as the dose used in our present studies. Cells were used 48 h after infection for cAMP measurement and videomicroscopy studies.
Calcium measurement
Calcium measurements and endothelial cell calibrations have been described elsewhere (Stevens et al., 1994).
cAMP measurements
cAMP content was determined using a Biotrack cAMP competitive enzyme-immunoassay system (Amersham Pharmacia Biotech).
Confocal fluorescence microscopy
Fluorescent images were acquired on a Leica TCS SP2 confocal laser scanning microscope at a 514-nm excitation. Slices were taken through cells at 0.5-μm sections.
Time-lapsed fluorescence microscopy
40–48 h after infection with the YFP–AC8 construct, media was removed from cells and replaced with Krebs/2 mM Ca2+ buffer. Fluorescent images were acquired on an Olympus IX70 inverted microscope at 488-nm excitation and processed using Spot Software (Diagnostic Instruments). Time-lapse experiments were performed using the MetaMorph® (Universal Imaging Corp.) system. Images were collected at 40-s intervals over 30 min.
Online supplemental material
Supplemental videos are available at http://www.jcb.org/cgi/content/full/jcb.200204022/DC1. Videos 1–3 are time-compressed movies of PMVECs, demonstrating that thrombin-induced gap formation occurs only when arise in [Ca2+] reduces cAMP. Videos 4–6 illustrate localization of AC (YFP–AC8) primarily at sites of cell–cell adhesion.
Supplemental Material
Acknowledgments
The authors wish to thank Tray Weathington for assistance with cell culture experiments and Michael Carmichael, Ray Butler (in memory), and Joanne Brookfield for assistance in producing digitally time-compressed videos. We also thank Atsushi Tanaka for assistance in plasmid construction and Alexander Sorkin for assistance in examination of the fluorescence spectrum of YFP–AC8 synthesized in 293 cells infected with the plaque-purified stocks of the virus.
This work was supported by PO1 HL66299 and RO1 HL60024 (to T. Stevens) and RO1 GM32483 (to D.M.F. Cooper).
The online version of this article includes supplemental material.
Footnotes
Abbreviations used in this paper: AC, adenylyl cyclase; InsP3, inositol 1,4,5-triphosphate; PAEC, pulmonary artery endothelial cell; PMVEC, pulmonary microvascular endothelial cell; YFP, yellow fluorescent protein.
References
- Allsup, D.J., and M.R. Boarder. 1990. Comparison of P2 purinergic receptors of aortic endothelial cells with those of adrenal medulla: evidence for heterogeneity of receptor subtype and of inositol phosphate response. Mol. Pharmacol. 38:84–91. [PubMed] [Google Scholar]
- Bartha, K., E. Domotor, F. Lanza, V. Adam-Vizi, and R. Machovich. 2000. Identification of thrombin receptors in rat brain capillary endothelial cells. J. Cereb. Blood Flow Metab. 20:175–182. [DOI] [PubMed] [Google Scholar]
- Bazzoni, G., and E. Dejana. 2001. Pores in the sieve and channels in the wall: control of paracellular permeability by junctional proteins in endothelial cells. Microcirculation. 8:143–152. [DOI] [PubMed] [Google Scholar]
- Bodmer, J.E., J. Van Engelenhoven, G. Reyes, K. Blackwell, A. Kamath, D.M. Shasby, and A.B. Moy. 1997. Isometric tension of cultured endothelial cells: new technical aspects. Microvasc. Res. 53:261–271. [DOI] [PubMed] [Google Scholar]
- Brass, L.F., D.R. Manning, A.G. Williams, M.J. Woolkalis, and M. Poncz. 1991. Receptor and G protein-mediated responses to thrombin in HEL cells. J. Biol. Chem. 266:958–965. [PubMed] [Google Scholar]
- Caldwell, K.K., C.L. Boyajian, and D.M.F. Cooper. 1992. The effects of Ca2+ and calmodulin on adenylyl cyclase activity in plasma membranes derived from neural and non-neural cells. Cell Calcium. 13:107–121. [DOI] [PubMed] [Google Scholar]
- Carbajal, J.M., and R.C. Schaeffer, Jr. 1999. RhoA inactivation enhances endothelial barrier function. Am. J. Physiol. 277:C955–C964. [DOI] [PubMed] [Google Scholar]
- Carbajal, J.M., M.L. Gratrix, C.H. Yu, and R.C. Schaeffer, Jr. 2000. ROCK mediates thrombin's endothelial barrier dysfunction. Am. J. Physiol. Cell Physiol. 279:C195–C204. [DOI] [PubMed] [Google Scholar]
- Carson, M.R., S.S. Shasby, and D.M. Shasby. 1989. Histamine and inositol phosphate accumulation in endothelium: cAMP and a G protein. Am. J. Physiol. 257:L259–L264. [DOI] [PubMed] [Google Scholar]
- Casnocha, S.A., S.G. Eskin, E.R. Hall, and L.V. McIntire. 1989. Permeability of human endothelial monolayers: effect of vasoactive agonists and cAMP. J. Appl. Physiol. 67:1997–2005. [DOI] [PubMed] [Google Scholar]
- Chartier, C., E. Degryse, M. Gantzer, A. Dieterle, A. Pavirani, and M. Mehtali. 1996. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J. Virol. 70:4805–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetham, P.M., H.A. Guldemeester, N. Mons, G.H. Brough, J.P. Bridges, W.J. Thompson, and T. Stevens. 1997. Ca(2+)-inhibitable adenylyl cyclase and pulmonary microvascular permeability. Am. J. Physiol. 273:L22–L30. [DOI] [PubMed] [Google Scholar]
- Chetham, P.M., P. Babal, J.P. Bridges, T.M. Moore, and T. Stevens. 1999. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. Am. J. Physiol. 276:L41–L50. [DOI] [PubMed] [Google Scholar]
- Chicurel, M.E., C.S. Chen, and D.E. Ingber. 1998. Cellular control lies in the balance of forces. Curr. Opin. Cell Biol. 10:232–239. [DOI] [PubMed] [Google Scholar]
- Crouzet, J., L. Naudin, C. Orsini, E. Vigne, L. Ferrero, A. Le Roux, P. Benoit, M. Latta, C. Torrent, D. Branellec, et al. 1997. Recombinational construction in Escherichia coli of infectious adenoviral genomes. Proc. Natl. Acad. Sci. USA. 94:1414–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana, E., G. Bazzoni, and M.G. Lampugnani. 1999. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp. Cell Res. 252:13–19. [DOI] [PubMed] [Google Scholar]
- deMello, D.E., and L.M. Reid. 2000. Embryonic and early fetal development of human lung vasculature and its functional implications. Pediatr. Dev. Pathol. 3:439–449. [DOI] [PubMed] [Google Scholar]
- deMello, D.E., D. Sawyer, N. Galvin, and L.M. Reid. 1997. Early fetal development of lung vasculature. Am. J. Respir. Cell Mol. Biol. 16:568–581. [DOI] [PubMed] [Google Scholar]
- Fagan, K.A., R. Mahey, and D.M.F. Cooper. 1996. Functional co-localization of transfected Ca(2+)-stimulable adenylyl cyclases with capacitative Ca2+ entry sites. J. Biol. Chem. 271:12438–12444. [DOI] [PubMed] [Google Scholar]
- Fagan, K.A., N. Mons, and D.M.F. Cooper. 1998. Dependence of the Ca2+-inhibitable adenylyl cyclase of C6-2B glioma cells on capacitative Ca2+ entry. J. Biol. Chem. 273:9297–9305. [DOI] [PubMed] [Google Scholar]
- Fagan, K.A., R.A. Graf, S. Tolman, J. Schaack, and D.M.F. Cooper. 2000. a. Regulation of a Ca2+-sensitive adenylyl cyclase in an excitable cell. Role of voltage-gated versus capacitative Ca2+ entry. J. Biol. Chem. 275:40187–40194. [DOI] [PubMed] [Google Scholar]
- Fagan, K.A., K.E. Smith, and D.M.F. Cooper. 2000. b. Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J. Biol. Chem. 275:26530–26537. [DOI] [PubMed] [Google Scholar]
- Garcia, J.G., and K.L. Schaphorst. 1995. Regulation of endothelial cell gap formation and paracellular permeability. J. Investig. Med. 43:117–126. [PubMed] [Google Scholar]
- Garcia, J.G., F.M. Pavalko, and C.E. Patterson. 1995. Vascular endothelial cell activation and permeability responses to thrombin. Blood Coagul. Fibrinolysis. 6:609–626. [DOI] [PubMed] [Google Scholar]
- Garcia, J.G., A.D. Verin, and K.L. Schaphorst. 1996. Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin. Thromb. Hemost. 22:309–315. [DOI] [PubMed] [Google Scholar]
- Garcia, J.G., V. Lazar, L.I. Gilbert-McClain, P.J. Gallagher, and A.D. Verin. 1997. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am. J. Respir. Cell Mol. Biol. 16:489–494. [DOI] [PubMed] [Google Scholar]
- Goeckeler, Z.M., and R.B. Wysolmerski. 1995. Myosin light chain kinase–regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J. Cell Biol. 130:613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, F.L., J. Smiley, W.C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59–74. [DOI] [PubMed] [Google Scholar]
- Gu, C., and D.M.F. Cooper. 2000. Ca(2+), Sr(2+), and Ba(2+) identify distinct regulatory sites on adenylyl cyclase (AC) types VI and VIII and consolidate the apposition of capacitative cation entry channels and Ca(2+)-sensitive ACs. J. Biol. Chem. 275:6980–6986. [DOI] [PubMed] [Google Scholar]
- Guillou, J.L., H. Nakata, and D.M.F. Cooper. 1999. Inhibition by calcium of mammalian adenylyl cyclases. J. Biol. Chem. 274:35539–35545. [DOI] [PubMed] [Google Scholar]
- He, T.C., S. Zhou, L.T. da Costa, J. Yu, K.W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA. 95:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, M., A. Schallhorn, and F.M. Wurm. 1996. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 24:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan, K.B., N.A. Mason, L. Long, P.G. Philips, M.R. Wilkins, and N.W. Morrell. 2001. Characterization of adenylyl cyclase isoforms in rat peripheral pulmonary arteries. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L1359–L1369. [DOI] [PubMed] [Google Scholar]
- Kelly, J.J., T.M. Moore, P. Babal, A.H. Diwan, T. Stevens, and W.J. Thompson. 1998. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am. J. Physiol. 274:L810–L819. [DOI] [PubMed] [Google Scholar]
- Kolodney, M.S., and R.B. Wysolmerski. 1992. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J. Cell Biol. 117:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse, H.J., C. Mayerhofer, W. Siess, and P.C. Weber. 1995. Thrombin receptor-activating peptide sensitizes the human endothelial thrombin receptor. Am. J. Physiol. 268:C36–C44. [DOI] [PubMed] [Google Scholar]
- Levin, E.G., and L. Santell. 1991. Thrombin- and histamine-induced signal transduction in human endothelial cells. Stimulation and agonist-dependent desensitization of protein phosphorylation. J. Biol. Chem. 266:174–181. [PubMed] [Google Scholar]
- Lum, H., T.T. Andersen, A. Siflinger-Birnboim, C. Tiruppathi, M.S. Goligorsky, J.W. Fenton II, and A.B. Malik. 1993. Thrombin receptor peptide inhibits thrombin-induced increase in endothelial permeability by receptor desensitization. J. Cell Biol. 120:1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, S., S. Suzuki, T. Suzuki, M. Endo, T. Moriya, M. Chida, T. Kondo, and H. Sasano. 2002. Analysis of intrapulmonary vessels and epithelial-endothelial interactions in the human developing lung. Lab. Invest. 82:293–301. [DOI] [PubMed] [Google Scholar]
- Manolopoulos, V.G., J. Liu, B.R. Unsworth, and P.I. Lelkes. 1995. a. Adenylyl cyclase isoforms are differentially expressed in primary cultures of endothelial cells and whole tissue homogenates from various rat tissues. Biochem. Biophys. Res. Commun. 208:323–331. [DOI] [PubMed] [Google Scholar]
- Manolopoulos, V.G., M.M. Samet, and P.I. Lelkes. 1995. b. Regulation of the adenylyl cyclase signaling system in various types of cultured endothelial cells. J. Cell. Biochem. 57:590–598. [DOI] [PubMed] [Google Scholar]
- McDonald, D.M., G. Thurston, and P. Baluk. 1999. Endothelial gaps as sites for plasma leakage in inflammation. Microcirculation. 6:7–22. [PubMed] [Google Scholar]
- Michel, C.C., and F.E. Curry. 1999. Microvascular permeability. Physiol. Rev. 79:703–761. [DOI] [PubMed] [Google Scholar]
- Minnear, F.L., M.A. DeMichele, D.G. Moon, C.L. Rieder, and J.W. Fenton II. 1989. Isoproterenol reduces thrombin-induced pulmonary endothelial permeability in vitro. Am. J. Physiol. 257:H1613–H1623. [DOI] [PubMed] [Google Scholar]
- Molino, M., M.J. Woolkalis, J. Reavey-Cantwell, D. Pratico, P. Andrade-Gordon, E.S. Barnathan, and L.F. Brass. 1997. Endothelial cell thrombin receptors and PAR-2. Two protease-activated receptors located in a single cellular environment. J. Biol. Chem. 272:11133–11141. [DOI] [PubMed] [Google Scholar]
- Moore, T.M., P.M. Chetham, J.J. Kelly, and T. Stevens. 1998. Signal transduction and regulation of lung endothelial cell permeability. Interaction between calcium and cAMP. Am. J. Physiol. 275:L203–L222. [DOI] [PubMed] [Google Scholar]
- Moore, T.M., N.R. Norwood, J.R. Creighton, P. Babal, G.H. Brough, D.M. Shasby, and T. Stevens. 2000. Receptor-dependent activation of store-operated calcium entry increases endothelial cell permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L691–L698. [DOI] [PubMed] [Google Scholar]
- Morel, N.M., A.B. Dodge, W.F. Patton, I.M. Herman, H.B. Hechtman, and D. Shepro. 1989. Pulmonary microvascular endothelial cell contractility on silicone rubber substrate. J. Cell. Physiol. 141:653–659. [DOI] [PubMed] [Google Scholar]
- Moy, A.B., J. Van Engelenhoven, J. Bodmer, J. Kamath, C. Keese, I. Giaever, S. Shasby, and D.M. Shasby. 1996. Histamine and thrombin modulate endothelial focal adhesion through centripetal and centrifugal forces. J. Clin. Invest. 97:1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy, A.B., M. Winter, A. Kamath, K. Blackwell, G. Reyes, I. Giaever, C. Keese, and D.M. Shasby. 2000. Histamine alters endothelial barrier function at cell-cell and cell-matrix sites. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L888–L898. [DOI] [PubMed] [Google Scholar]
- Norwood, N., T.M. Moore, D.A. Dean, R. Bhattacharjee, M. Li, and T. Stevens. 2000. Store-operated calcium entry and increased endothelial cell permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L815–L824. [DOI] [PubMed] [Google Scholar]
- Oliver, J.A. 1990. Adenylate cyclase and protein kinase C mediate opposite actions on endothelial junctions. J. Cell. Physiol. 145:536–542. [DOI] [PubMed] [Google Scholar]
- Orlicky, D.J., and J. Schaack. 2001. Adenovirus transduction of 3T3-L1 cells. J. Lipid Res. 42:460–466. [PubMed] [Google Scholar]
- Putney, J.W., Jr. 1986. A model for receptor-regulated calcium entry. Cell Calcium. 7:1–12. [DOI] [PubMed] [Google Scholar]
- Ribeiro, C.M., J. Reece, and J.W. Putney, Jr. 1997. Role of the cytoskeleton in calcium signaling in NIH 3T3 cells. An intact cytoskeleton is required for agonist-induced [Ca2+]i signaling, but not for capacitative calcium entry. J. Biol. Chem. 272:26555–26561. [DOI] [PubMed] [Google Scholar]
- Rich, T.C., K.A. Fagan, H. Nakata, J. Schaack, D.M.F. Cooper, and J.W. Karpen. 2000. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J. Gen. Physiol. 116:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich, T.C., K.A. Fagan, T.E. Tse, J. Schaack, D.M.F. Cooper, and J.W. Karpen. 2001. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc. Natl. Acad. Sci. USA. 98:13049–13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah, V.P., T.M. Seasholtz, S.A. Sagi, and J.H. Brown. 2000. The role of Rho in G protein-coupled receptor signal transduction. Annu. Rev. Pharmacol. Toxicol. 40:459–489. [DOI] [PubMed] [Google Scholar]
- Schaack, J., B. Allen, D.J. Orlicky, M.L. Bennett, I.H. Maxwell, and R.L. Smith. 2001. Promoter strength in adenovirus transducing vectors: down-regulation of the adenovirus E1A promoter in 293 cells facilitates vector construction. Virology. 291:101–109. [DOI] [PubMed] [Google Scholar]
- Sheldon, R., A. Moy, K. Lindsley, S. Shasby, and D.M. Shasby. 1993. Role of myosin light-chain phosphorylation in endothelial cell retraction. Am. J. Physiol. 265:L606–L612. [DOI] [PubMed] [Google Scholar]
- Stelzner, T.J., J.V. Weil, and R.F. O'Brien. 1989. Role of cyclic adenosine monophosphate in the induction of endothelial barrier properties. J. Cell. Physiol. 139:157–166. [DOI] [PubMed] [Google Scholar]
- Stevens, T., B. Fouty, D. Cornfield, and D.M. Rodman. 1994. Reduced PO2 alters the behavior of Fura-2 and Indo-1 in bovine pulmonary artery endothelial cells. Cell Calcium. 16:404–412. [DOI] [PubMed] [Google Scholar]
- Stevens, T., Y. Nakahashi, D.N. Cornfield, I.F. McMurtry, D.M.F. Cooper, and D.M. Rodman. 1995. Ca(2+)-inhibitable adenylyl cyclase modulates pulmonary artery endothelial cell cAMP content and barrier function. Proc. Natl. Acad. Sci. USA. 92:2696–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, T., B. Fouty, L. Hepler, D. Richardson, G. Brough, I.F. McMurtry, and D.M. Rodman. 1997. Cytosolic Ca2+ and adenylyl cyclase responses in phenotypically distinct pulmonary endothelial cells. Am. J. Physiol. 272:L51–L59. [DOI] [PubMed] [Google Scholar]
- Stevens, T., J. Creighton, and W.J. Thompson. 1999. Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am. J. Physiol. 277:L119–L126. [DOI] [PubMed] [Google Scholar]
- Stevens, T., R. Rosenberg, W. Aird, T. Quertermous, F.L. Johnson, J.G. Garcia, R. Hebbel, R.M. Tuder, and S. Garfinkel. 2001. Endothelial cell phenotypes in heart, lung and blood diseases. Am. J. Physiol. 281:C1422–C1433. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen, G.P., S. van Delft, M.A. Vermeer, J.G. Collard, and V.W. van Hinsbergh. 2000. a. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ. Res. 87:335–340. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen, G.P., M.A. Vermeer, and V.W. van Hinsbergh. 2000. b. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler. Thromb. Vasc. Biol. 20:E127–E133. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari, V., P. Boquet, J. Pouyssegur, and E. Van Obberghen-Schilling. 1998. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol. Biol. Cell. 9:2639–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, N., K. Naruse, D. Stamenovic, J.J. Fredberg, S.M. Mijailovich, I.M. Tolic-Norrelykke, T. Polte, R. Mannix, and D.E. Ingber. 2001. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. USA. 98:7765–7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub, W.H., P.A. Negulescu, and T.E. Machen. 1992. Calcium signaling in endothelia: cellular heterogeneity and receptor internalization. Am. J. Physiol. 263:C1029–C1039. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard, B., S. Potempa, T. Eichholtz, and A.J. Ridley. 2001. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J. Cell Sci. 114:1343–1355. [DOI] [PubMed] [Google Scholar]
- Yan, W., C. Tiruppathi, H. Lum, R. Qiao, and A.B. Malik. 1998. Protein kinase C beta regulates heterologous desensitization of thrombin receptor (PAR-1) in endothelial cells. Am. J. Physiol. 274:C387–C395. [DOI] [PubMed] [Google Scholar]
- Yoshimura, M., and D.M.F. Cooper. 1992. Cloning and expression of a Ca(2+)-inhibitable adenylyl cyclase from NCB-20 cells. Proc. Natl. Acad. Sci. USA. 89:6716–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, S.Y. 2000. Signal transduction pathways in enhanced microvascular permeability. Microcirculation. 7:395–403. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.