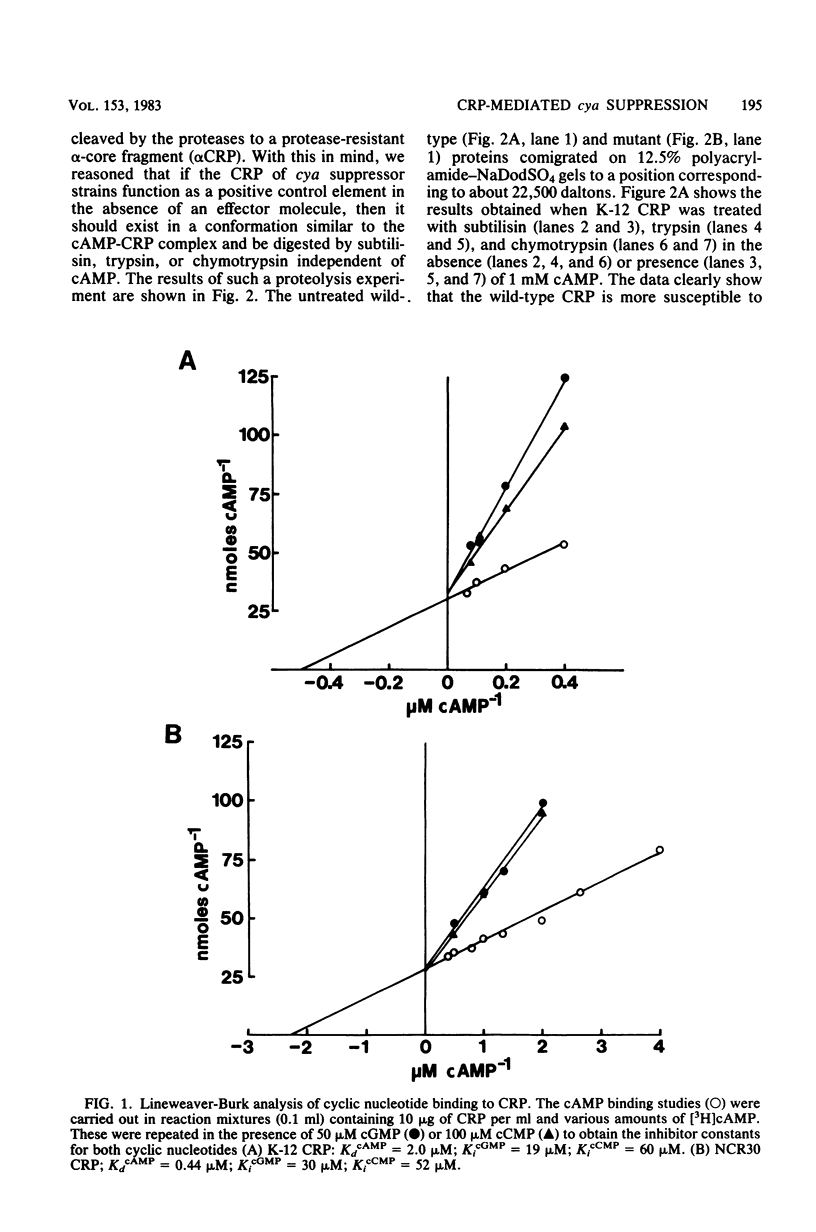
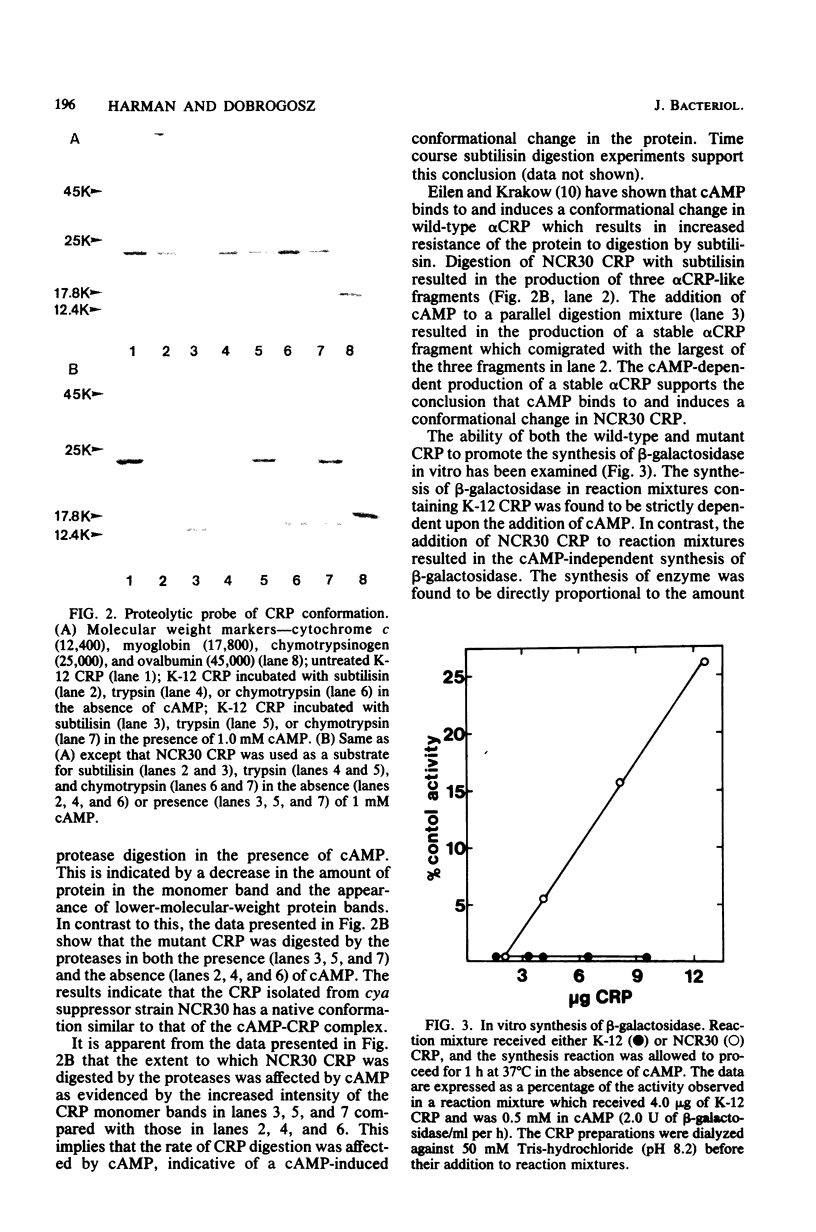
Abstract
Escherichia coli strain NCR30 contains a cya lesion and a second-site cya suppressor mutation that lies in the crp gene. NCR30 shows a pleiotropic phenotypic reversion to the wild-type state in expressing many operons that require the cyclic AMP (cAMP)-cAMP receptor protein (CRP) complex for positive control. In vivo beta-galactosidase synthesis in NCR30 was sensitive to glucose-mediated repression, which was relieved not only by cAMP but also by cyclic GMP and cyclic CMP. The CRP isolated from NCR30 differed from the protein isolated from wild-type E. coli in many respects. The mutant protein bound cAMP with four to five times greater affinity than wild-type CRP. Protease digestion studies indicated that native NCR30 CRP exists in the cAMP-CRP complex-like conformation. The protein conferred a degree of cAMP independence on the in vitro synthesis of beta-galactosidase. In addition, the inherent positive control activity of the mutant protein in vitro was enhanced by those nucleotides that stimulate in vivo beta-galactosidase synthesis in NCR30. The results of this study supported the conclusion that the crp allele of NCR30 codes for a protein having altered effector specificity yet capable of promoting positive control over catabolite-sensitive operons in the absence of an effector molecule.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. K. Suppression of defects in cyclic adenosine 3',5'-monophosphate metabolism in Escherichia coli. J Bacteriol. 1980 Oct;144(1):205–209. doi: 10.1128/jb.144.1.205-209.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. B., Perlman R. L., Pastan I. Effect of adenosine 3',5'-monophosphate analogues on the activity of the cyclic adenosine 3',5'-monophosphate receptor in Escherichia coli. J Biol Chem. 1972 May 10;247(9):2717–2722. [PubMed] [Google Scholar]
- Boone T., Wilcox G. A rapid high-yield purification procedure for the cyclic adenosine 3',5'-monophosphate receptor protein from Escherichia coli. Biochim Biophys Acta. 1978 Jul 17;541(4):528–534. doi: 10.1016/0304-4165(78)90162-9. [DOI] [PubMed] [Google Scholar]
- Botsford J. L., Drexler M. The cyclic 3',5'-adenosine monophosphate receptor protein and regulation of cyclic 3',5'-adenosine monophosphate synthesis in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):47–56. doi: 10.1007/BF00270375. [DOI] [PubMed] [Google Scholar]
- Cook W. R., Kalb V. F., Jr, Peace A. A., Bernlohr R. W. Is cyclic guanosine 3',5'-monophosphate a cell cycle regulator? J Bacteriol. 1980 Mar;141(3):1450–1453. doi: 10.1128/jb.141.3.1450-1453.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessein A., Schwartz M., Ullmann A. Catabolite repression in Escherichia coli mutants lacking cyclic AMP. Mol Gen Genet. 1978 Jun 1;162(1):83–87. doi: 10.1007/BF00333853. [DOI] [PubMed] [Google Scholar]
- Dobrogosz W. J. Altered end-product patterns and catabolite repression in Escherichia coli. J Bacteriol. 1966 Jun;91(6):2263–2269. doi: 10.1128/jb.91.6.2263-2269.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebright R. H., Wong J. R. Mechanism for transcriptional action of cyclic AMP in Escherichia coli: entry into DNA to disrupt DNA secondary structure. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4011–4015. doi: 10.1073/pnas.78.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilen E., Krakow J. S. Cyclic AMP-mediated intersubunit disulfide crosslinking of the cyclic AMP receptor protein of Escherichia coli. J Mol Biol. 1977 Jul;114(1):47–60. doi: 10.1016/0022-2836(77)90282-0. [DOI] [PubMed] [Google Scholar]
- Eilen E., Krakow J. S. Effects of cyclic nucleotides on the conformational states of the alpha core of the cyclic AMP receptor protein. Biochim Biophys Acta. 1977 Jul 22;493(1):115–121. doi: 10.1016/0005-2795(77)90264-1. [DOI] [PubMed] [Google Scholar]
- Eilen E., Pampeno C., Krakow J. S. Production and properties of the alpha core derived from the cyclic adenosine monophosphate receptor protein of Escherichia coli. Biochemistry. 1978 Jun 27;17(13):2469–2473. doi: 10.1021/bi00606a001. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenborn A. M., Clore G. M., Blazy B., Baudras A. Conformational selection of syn-cAMP upon binding to the cAMP: receptor protein. FEBS Lett. 1981 Dec 21;136(1):160–164. doi: 10.1016/0014-5793(81)81237-9. [DOI] [PubMed] [Google Scholar]
- Guidi-Rontani C., Danchin A., Ullmann A. Catabolite repression in Escherichia coli mutants lacking cyclic AMP receptor protein. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5799–5801. doi: 10.1073/pnas.77.10.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow J. S. Cyclic adenosine monophosphate receptor: effect of cyclic AMP analogues on DNA binding and proteolytic inactivation. Biochim Biophys Acta. 1975 Apr 2;383(4):345–350. doi: 10.1016/0005-2787(75)90303-2. [DOI] [PubMed] [Google Scholar]
- Krakow J. S., Pastan I. Cyclic adenosine monophosphate receptor: loss of cAMP-dependent DNA binding activity after proteolysis in the presence of cyclic adenosine monophosphate. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2529–2533. doi: 10.1073/pnas.70.9.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. A., Murthy N. S., Krakow J. S. Ligand-induced change in the radius of gyration of cAMP receptor protein from Escherichia coli. FEBS Lett. 1980 Jan 1;109(1):121–124. doi: 10.1016/0014-5793(80)81324-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Majors J. Specific binding of CAP factor to lac promoter DNA. Nature. 1975 Aug 21;256(5519):672–674. doi: 10.1038/256672a0. [DOI] [PubMed] [Google Scholar]
- Melton T., Snow L. L., Freitag C. S., Dobrogosz W. J. Isolation and characterization of cAMP suppressor mutants of Escherichia coli K12. Mol Gen Genet. 1981;182(3):480–489. doi: 10.1007/BF00293939. [DOI] [PubMed] [Google Scholar]
- Nissley P., Anderson W. B., Gallo M., Pastan I., Perlman R. L. The binding of cyclic adenosine monophosphate receptor to deoxyribonucleic acid. J Biol Chem. 1972 Jul 10;247(13):4264–4269. [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Takebe Y., Kaziro Y. A possible involvement of cya gene in the synthesis of cyclic guanosine 3':5'-monophosphate in E. coli. Cell. 1977 Oct;12(2):521–528. doi: 10.1016/0092-8674(77)90128-3. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Shibuya M., Kaziro Y. A new extragenic suppressor of cya mutation. Mutant cyclic AMP receptor protein with an increased affinity for cyclic AMP. J Biochem. 1978 Jun;83(6):1615–1623. doi: 10.1093/oxfordjournals.jbchem.a132073. [DOI] [PubMed] [Google Scholar]
- Ullmann A. Are cyclic AMP effects related to real physiological phenomena? Biochem Biophys Res Commun. 1974 Mar 25;57(2):348–352. doi: 10.1016/0006-291x(74)90936-x. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Tillier F., Monod J. Catabolite modulator factor: a possible mediator of catabolite repression in bacteria. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3476–3479. doi: 10.1073/pnas.73.10.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Regulation of lac operon expression: reappraisal of the theory of catabolite repression. J Bacteriol. 1978 Dec;136(3):947–954. doi: 10.1128/jb.136.3.947-954.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J. 1968 Feb;106(4):847–858. doi: 10.1042/bj1060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox G., Meuris P., Bass R., Englesberg E. Regulation of the L-arabinose operon BAD in vitro. J Biol Chem. 1974 May 10;249(9):2946–2952. [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]