Abstract
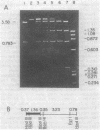
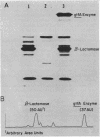
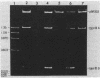
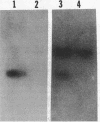
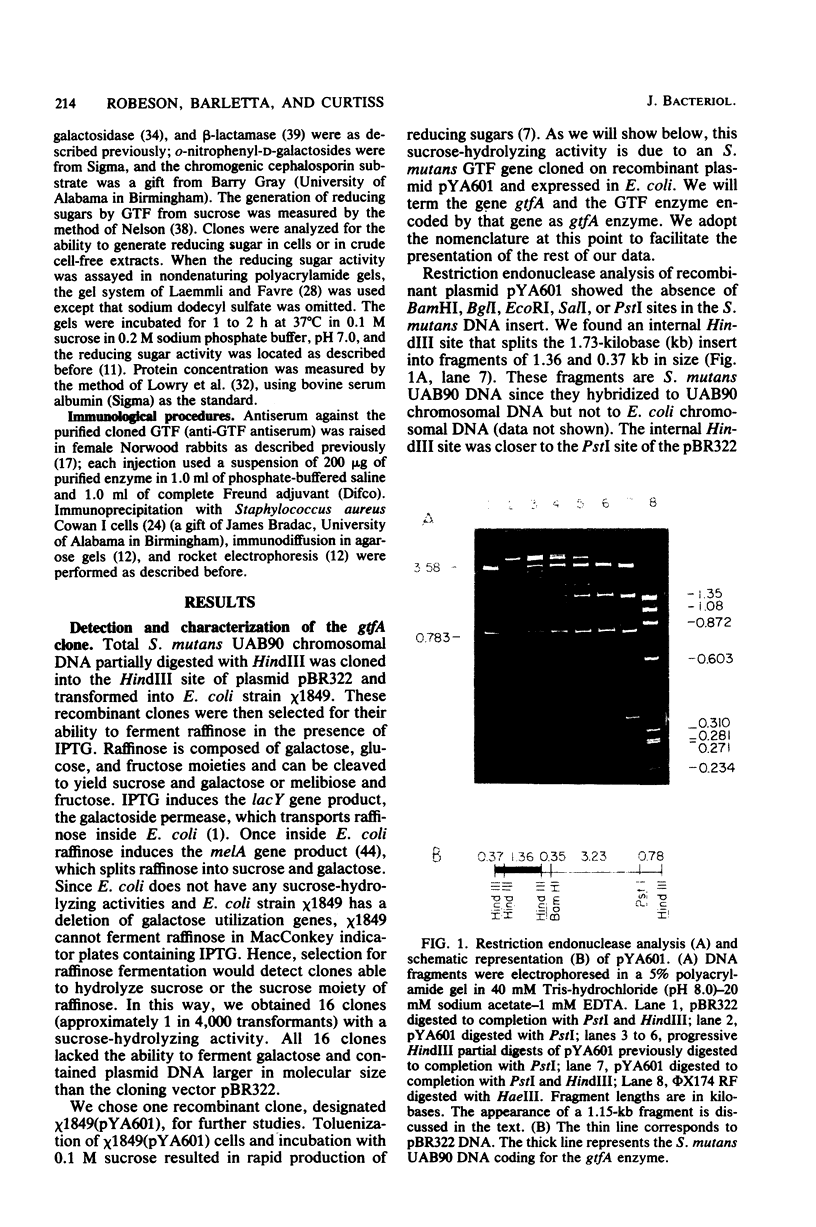
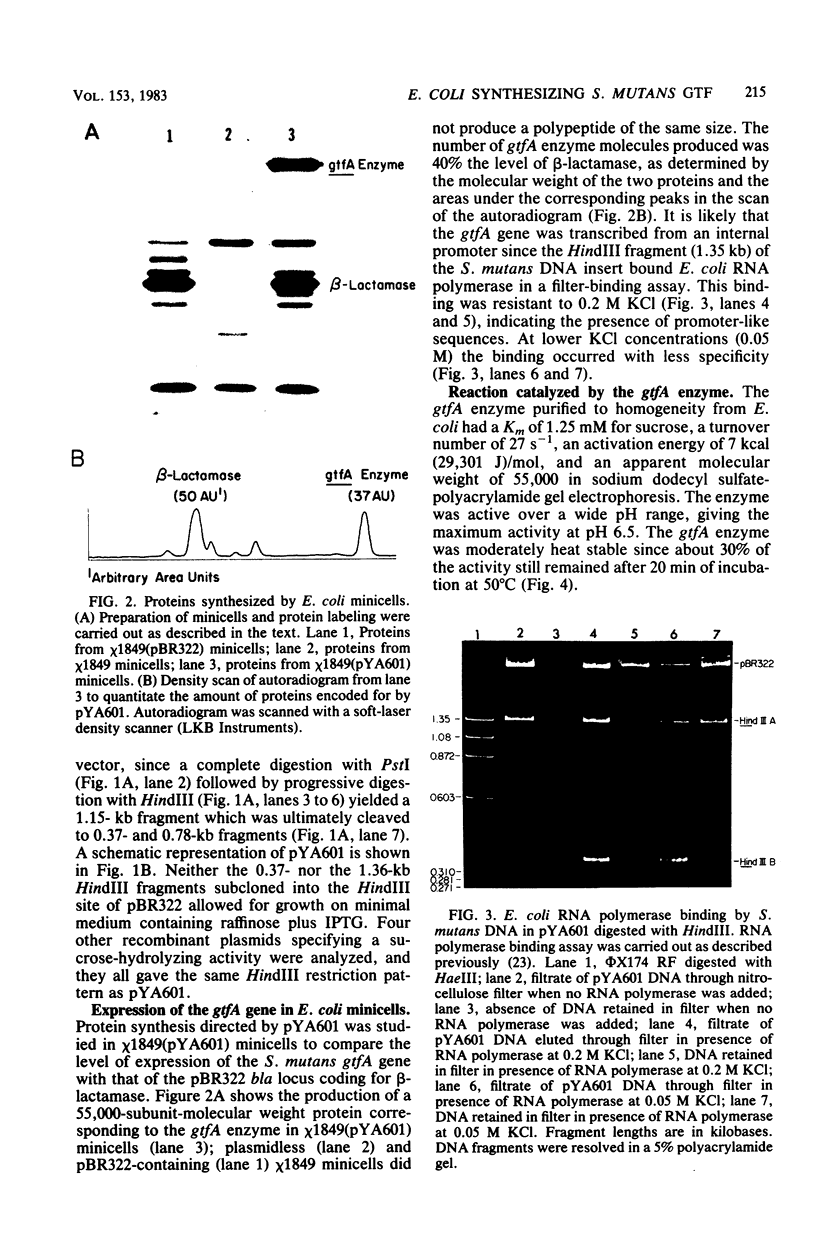
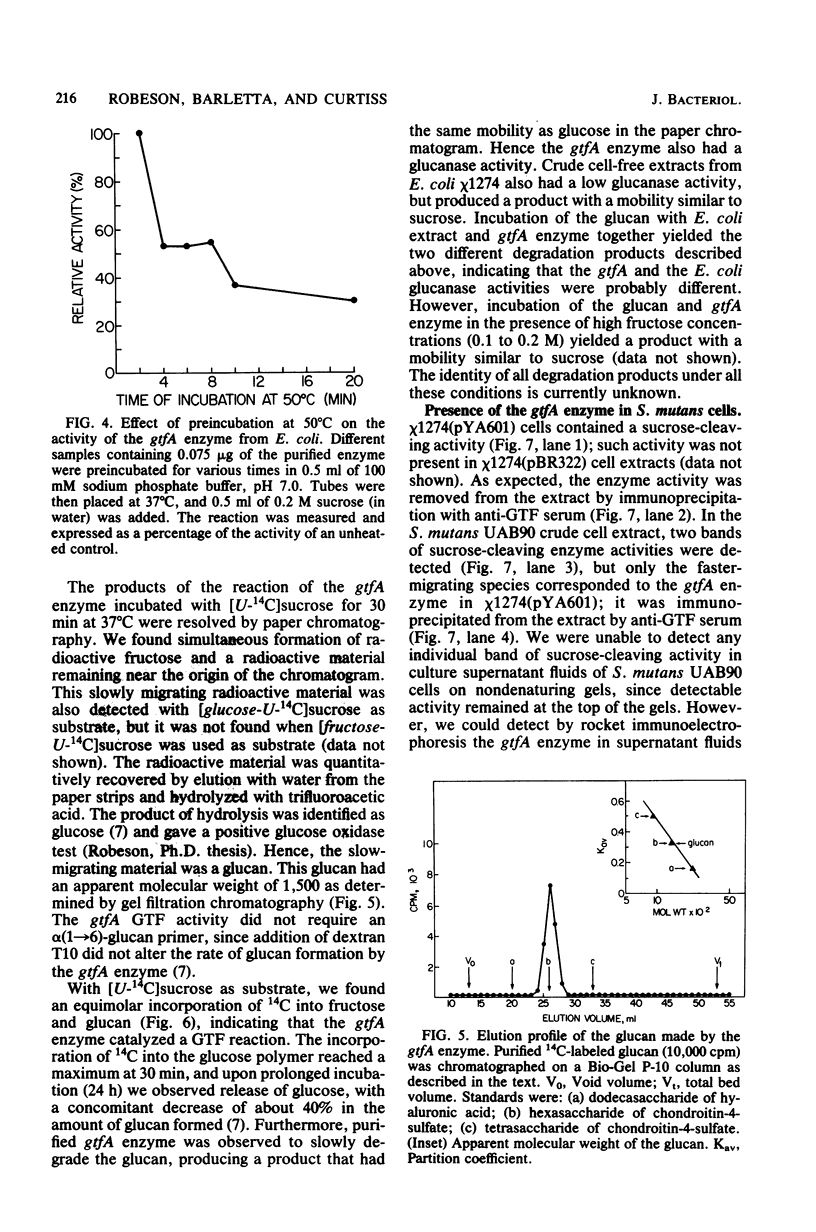
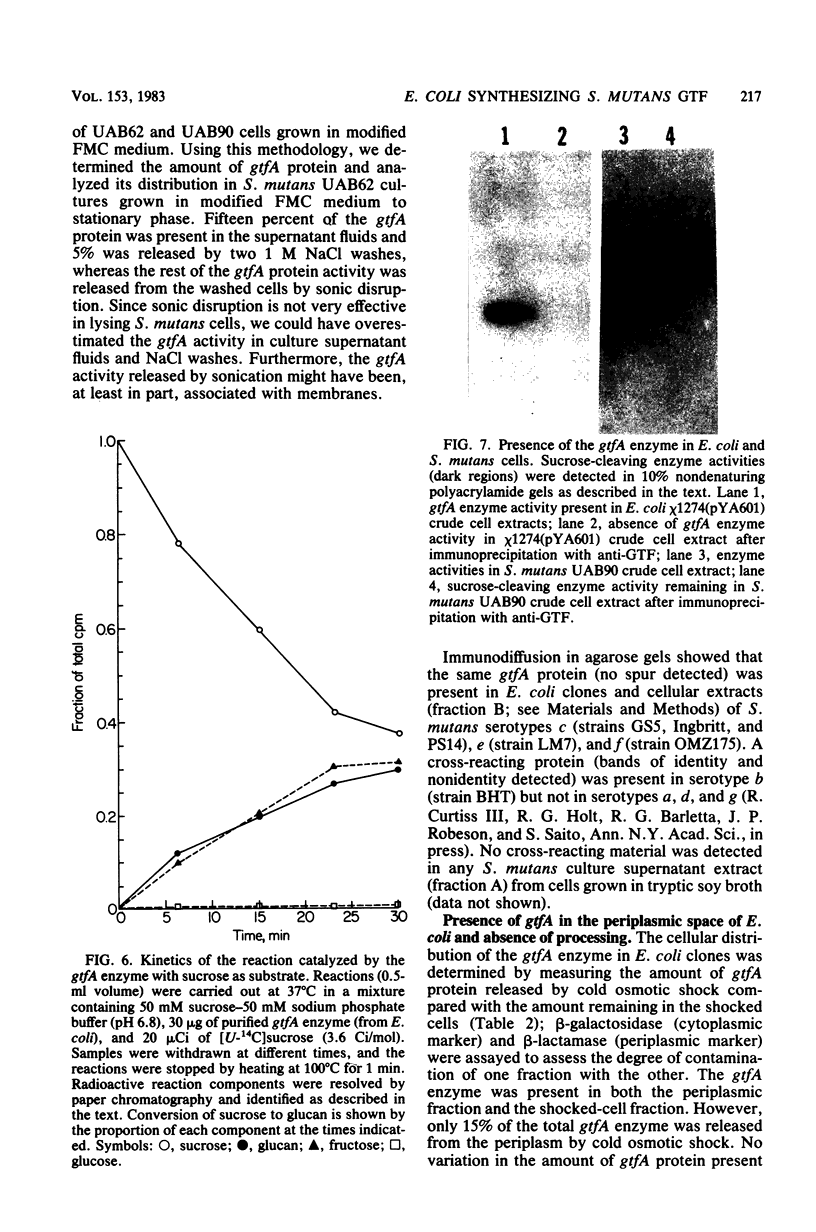
Chromosomal DNA from Streptococcus mutans strain UAB90 (serotype c) was cloned into Escherichia coli K-12. The clone bank was screened for any sucrose-hydrolyzing activity by selection for growth on raffinose in the presence of isopropyl-beta-D-thiogalactoside. A clone expressing an S. mutans glucosyltransferase was identified. The S. mutans DNA encoding this enzyme is a 1.73-kilobase fragment cloned into the HindIII site of plasmid pBR322. We designated the gene gtfA. The plasmid-encoded gtfA enzyme, a 55,000-molecular-weight protein, is synthesized at 40% the level of pBR322-encoded beta-lactamase in E. coli minicells. Using sucrose as substrate, the gtfA enzyme catalyzes the formation of fructose and a glucan with an apparent molecular weight of 1,500. We detected the gtfA protein in S. mutans cells with antibody raised against the cloned gtfA enzyme. Immunologically identical gtfA protein appears to be present in S. mutans cells of serotypes c, e, and f, and a cross-reacting protein was made by serotype b cells. Proteins from serotype a, g, and d S. mutans cells did not react with antibody to gtfA enzyme. The gtfA activity was present in the periplasmic space of E. coli clones, since 15% of the total gtfA activity was released by cold osmotic shock and the clones were able to grow on sucrose as sole carbon source.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arditti R. R., Scaife J. G., Beckwith J. R. The nature of mutants in the lac promoter region. J Mol Biol. 1968 Dec;38(3):421–426. doi: 10.1016/0022-2836(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi N. Y., Ehrlich S. D., Lederberg J. Functional expression of two Bacillus subtilis chromosomal genes in Escherichia coli. J Bacteriol. 1978 Feb;133(2):816–821. doi: 10.1128/jb.133.2.816-821.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M., Birked D., Granath K. Analyses of glucans from cariogenic and mutant Streptococcus mutans. Infect Immun. 1978 Jul;21(1):17–27. doi: 10.1128/iai.21.1.17-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Moriyama T., Miyake Y., Mizutani K., Tanaka O. Purification and properties of glucosyltransferase responsible for water-insoluble glucan synthesis from Streptococcus mutans. Infect Immun. 1982 Jul;37(1):1–9. doi: 10.1128/iai.37.1.1-9.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel O., Wang S. F. Determination of enzymatic activity in polyacrylamide gels. I. Enzymes catalyzing the conversion of nonreducing substrates to reducing products. Anal Biochem. 1969 Mar;27(3):545–554. doi: 10.1016/0003-2697(69)90068-2. [DOI] [PubMed] [Google Scholar]
- Gray O., Chang S. Molecular cloning and expression of Bacillus licheniformis beta-lactamase gene in Escherichia coli and Bacillus subtilis. J Bacteriol. 1981 Jan;145(1):422–428. doi: 10.1128/jb.145.1.422-428.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Abiko Y., Curtiss R., 3rd Characterization of the Streptococcus mutans plasmid pva318 cloned into Escherichia coli. Infect Immun. 1981 Mar;31(3):1034–1043. doi: 10.1128/iai.31.3.1034-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J., Hunter E., Vogt P. K. Inhibition of avian sarcoma virus replication by glucosamine: a specific effect on the synthesis and processing of viral proteins. Virology. 1976 Jun;71(2):402–411. doi: 10.1016/0042-6822(76)90368-8. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Mutants in transmission of chemotactic signals from two independent receptors of E. coli. Cell. 1979 Mar;16(3):617–625. doi: 10.1016/0092-8674(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Heller K. B., Wilson T. H. Sucrose transport by the Escherichia coli lactose carrier. J Bacteriol. 1979 Nov;140(2):395–399. doi: 10.1128/jb.140.2.395-399.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. G., Abiko Y., Saito S., Smorawinska M., Hansen J. B., Curtiss R., 3rd Streptococcus mutans genes that code for extracellular proteins in Escherichia coli K-12. Infect Immun. 1982 Oct;38(1):147–156. doi: 10.1128/iai.38.1.147-156.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagusztyn-Krynicka E. K., Smorawinska M., Curtiss R., 3rd Expression of Streptococcus mutans aspartate-semialdehyde dehydrogenase gene cloned into plasmid pBR322. J Gen Microbiol. 1982 May;128(5):1135–1145. doi: 10.1099/00221287-128-5-1135. [DOI] [PubMed] [Google Scholar]
- Janda W. M., Kuramitsu H. K. Regulation and extracellular glucosyltransferase production and the relationship between extracellular and cell-associated activities in Streptococcus mutans. Infect Immun. 1976 Jul;14(1):191–202. doi: 10.1128/iai.14.1.191-202.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. B., Reznikoff W. S. Tryptophan-transducing bacteriophages: in vitro studies with restriction endonucleases HindII + III and Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1977 Oct;132(1):270–281. doi: 10.1128/jb.132.1.270-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H., Ingersoll L. Immunological relationships between glucosyltransferases from Streptococcus mutans serotypes. Infect Immun. 1976 Sep;14(3):636–644. doi: 10.1128/iai.14.3.636-644.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Kanazawa H., Ozols J., Wu H. C. An Escherichia coli mutant with an amino acid alteration within the signal sequence of outer membrane prolipoprotein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4891–4895. doi: 10.1073/pnas.75.10.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Shiota T., Devenyns D. Low sucrose levels promote extensive Streptococcus mutans-induced dental caries. Infect Immun. 1977 May;16(2):712–714. doi: 10.1128/iai.16.2.712-714.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison H., Larrimore S., Curtiss R., 3rd Isolation and characterization of Streptococcus mutans mutants defective in adherence and aggregation. Infect Immun. 1981 Dec;34(3):1044–1055. doi: 10.1128/iai.34.3.1044-1055.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison H., Larrimore S., Hull S., Curtiss R., 3rd Isolation and characterization of Streptococcus mutans mutants with altered cellular morphology or chain length. Infect Immun. 1982 Oct;38(1):282–291. doi: 10.1128/iai.38.1.282-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Harlander S. K., Germaine G. R. Streptococcus mutans dextransucrase: availability of disaggregated enzyme after growth in a chemically defined medium. Infect Immun. 1976 May;13(5):1522–1524. doi: 10.1128/iai.13.5.1522-1524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherp H. W. Dental caries: prospects for prevention. Science. 1971 Sep 24;173(4003):1199–1205. doi: 10.1126/science.173.4003.1199. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A. Antigenic relatedness of glucosyltransferase enzymes from streptococcus mutans. Infect Immun. 1977 Jan;15(1):91–103. doi: 10.1128/iai.15.1.91-103.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Sarthy A., Beckwith J. Escherichia coli pleiotropic mutant that reduces amounts of several periplasmic and outer membrane proteins. J Bacteriol. 1979 Oct;140(1):229–239. doi: 10.1128/jb.140.1.229-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa K., Kawata S., Nishimura S., Ikeda Y., Yoshimura Y. Mutanolysin, bacteriolytic agent for cariogenic Streptococci: partial purification and properties. Antimicrob Agents Chemother. 1974 Aug;6(2):156–165. doi: 10.1128/aac.6.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]