Abstract
Calcium ions, present inside all eukaryotic cells, are important second messengers in the transduction of biological signals. In mammalian cells, the release of Ca2+ from intracellular compartments is required for signaling and involves the regulated opening of ryanodine and inositol-1,4,5-trisphosphate (IP3) receptors. However, in budding yeast, no signaling pathway has been shown to involve Ca2+ release from internal stores, and no homologues of ryanodine or IP3 receptors exist in the genome. Here we show that hyperosmotic shock provokes a transient increase in cytosolic Ca2+ in vivo. Vacuolar Ca2+, which is the major intracellular Ca2+ store in yeast, is required for this response, whereas extracellular Ca2+ is not. We aimed to identify the channel responsible for this regulated vacuolar Ca2+ release. Here we report that Yvc1p, a vacuolar membrane protein with homology to transient receptor potential (TRP) channels, mediates the hyperosmolarity induced Ca2+ release. After this release, low cytosolic Ca2+ is restored and vacuolar Ca2+ is replenished through the activity of Vcx1p, a Ca2+/H+ exchanger. These studies reveal a novel mechanism of internal Ca2+ release and establish a new function for TRP channels.
Keywords: calcium signaling; ion channels; osmotic pressure; vacuoles; Saccharomyces cerevisiae
Introduction
Eukaryotic cells can sense a wide variety of environmental stresses, including changes in temperature, pH, osmolarity, and nutrient availability. They respond to these changes through a variety of signal transduction mechanisms, including activation of Ca2+-dependent signaling pathways. In mammalian cells, various stimuli are known to induce the release of Ca2+ from the endoplasmic or sarcoplasmic reticulum, the primary Ca2+ stores. In yeast, the Ca2+ concentration in the cytosol ([Ca2+]cyt)* has been shown to increase in response to the mating pheromone α factor (Iida et al., 1990), hypotonic shock (Batiza et al., 1996, Beeler et al., 1997), and addition of glucose to starving cells (Nakajima-Shimada et al., 1991). However, none of these increases in [Ca2+]cyt has been shown to depend on internal Ca2+ release, as opposed to influx from the external media. In the case of hypotonic shock, increases in [Ca2+]cyt are partially independent from external Ca2+, but there is no direct evidence of internal Ca2+ release (Batiza et al., 1996). Therefore, although yeast possesses many of the conserved elements involved in Ca2+ signaling (i.e., calmodulin, adenylate cyclase, and various protein kinases), signaling through internal Ca2+ release is still speculative in yeast.
If internal Ca2+ release exists in yeast, the vacuole is likely to be involved in this function, as it plays a major role in Ca2+ homeostasis. Indeed, free Ca2+ concentration in the yeast vacuole reaches 1.3 mM, compared with only 10 μM in the endoplasmic reticulum (Halachmi and Eilam, 1989; Strayle et al., 1999). Therefore, the yeast vacuole is the functional counterpart of the mammalian endoplasmic and sarcoplasmic reticulum for Ca2+ storage. Two transporters play complementary roles in sequestering Ca2+ into the vacuole: (a) Vcx1p, a low-affinity Ca2+/H+ exchanger that rapidly sequesters Ca2+ into the vacuole; and (b) Pmc1p, a high-affinity Ca2+ ATPase required for maintaining low [Ca2+]cyt (Cunningham and Fink, 1994, 1996; Pozos et al., 1996; Miseta et al., 1999). It has been reported that vacuolar membrane vesicles could release Ca2+ in the presence of IP3 (Belde et al., 1993); however, the mechanism and the physiological relevance of this effect have not been addressed. Although Ca2+ influx into the vacuole has been well characterized, no protein has been shown to effect vacuolar Ca2+ release.
All cells must repeatedly adapt to hypertonic shock caused by variations in water availability or solutes concentration. Yeast cells are particularly exposed to such changes, and therefore have developed multiple responses to hypertonic stress. Within minutes, cells shrink and the cytoskeleton disassembles (Morris et al., 1986; Chowdhury et al., 1992). Adaptation to these new conditions requires transcriptional induction of stress-responsive genes, as well as the accumulation of intracellular glycerol (Brown et al., 1986; Albertyn et al., 1994; Hirayama et al., 1995; Tamás et al., 1999). This transcriptional activation is mediated in part by the high-osmolarity glycerol (HOG) response pathway, which is composed of a mitogen-activated kinase cascade regulated by at least two independent osmosensors (Brewster et al., 1993; Posas et al., 1998).
Although the response to hypertonic shock has been intensively studied, whether it involves Ca2+ signaling is unknown. In addressing this question, we show that: (a) hypertonic shock induces a transient increase in cytosolic Ca2+ concentrations; (b) the Ca2+ flux comes from the vacuole; and (c) Yvc1p, a homologue of transient receptor potential (TRP) channels, is required for this release. Yvc1p has recently been cloned by Palmer et al. (2001), and has been shown to be a cation-selective channel that can conduct Ca2+, K+, or Na+. This conductance had been previously characterized by electrophysiological methods (Wada et al., 1987; Bertl and Slayman, 1990, 1992; Bertl et al., 1992). The electrophysiological properties of Yvc1p and its presence in the vacuolar fraction suggested that Yvc1p could be a vacuolar Ca2+ channel. In this study we verify this hypothesis using living cells; we demonstrate in vivo, for the first time, that Yvc1p is a vacuolar channel that mediates Ca2+ release in response to hyperosmotic stress.
Results and discussion
To analyze the effect of hypertonic shock on [Ca2+]cyt, we added media containing high NaCl, KCl, or sorbitol to cells expressing the cytosolic, luminescent Ca2+ reporter aequorin (Nakajima-Shimada et al., 1991; Batiza et al., 1996), and monitored luminescence (Fig. 1 a). All of these treatments induced an increase in [Ca2+]cyt, which peaked ∼1 min after the hypertonic shock. [Ca2+]cyt rapidly decreased and returned to its basal level by 5 min. In comparison, addition of CaCl2 to the extracellular medium induced a sudden increase in [Ca2+]cyt that peaked within the first second and then decreased rapidly (Fig. 1 a) (Miseta et al., 1999). As shown previously, this decrease is due to Ca2+ sequestration into the vacuole (Miseta et al., 1999). These experiments show that hyperosmotic shock induces a transient increase in cytosolic Ca2+, and that the timing of this response is slower than that induced by simple addition of external Ca2+.
Figure 1.
Changes in [Ca 2+ ] cyt in response to hypertonic shock. (a) Luminescence response in a wild-type strain (YPH499) after addition (Time = 0) of 0.1 M CaCl2 (gray), or after hypertonic shock treatments: 0.8 M NaCl (red); 0.9 M KCl (yellow); and 0.7 M sorbitol (blue). As measured with an osmometer, the osmolality of media with 0.8 M NaCl is the same as with 0.9 M KCl, and is ∼1.2-fold higher than with 0.7 M sorbitol. (b) Luminescence response after treatment with 0.8 M NaCl in wild-type (YPH499, black), pmc1Δ (TPYp, blue), vcx1Δ (KKY127, purple), and pmc1Δvcx1Δ (KKY124, pink) strains. All strains were transformed with PEVP11/AEQ.
To further investigate this novel Ca2+ response, we examined whether the hyperosmolarity induced Ca2+ flux comes from an external or an internal source. We repeated these experiments using media containing the Ca2+ chelators EGTA or 1,2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA), as well as using low Ca2+ SD medium (see Materials and methods), and observed no differences under these conditions (unpublished data). This strongly suggests that external Ca2+ is not required for the observed cytosolic Ca2+ peak. Next, we asked if the Ca2+ flux is released from internal stores. Because the vacuole plays an important role in Ca2+ storage and homeostasis, we investigated the hyperosmolarity induced Ca2+ flux in mutants with defects in vacuolar Ca2+ storage. In a pmc1Δvcx1Δ strain lacking both transporters for vacuolar Ca2+ storage, Ca2+ is not sequestered in the vacuole, and consequently, vacuolar [Ca2+] is dramatically reduced (Cunningham and Fink, 1996; Pozos et al., 1996). Therefore, if the hyperosmolarity induced Ca2+ flux comes from the vacuole, we expect it to be reduced in this strain. Wild-type, pmc1Δ, vcx1Δ, and pmc1Δvcx1Δ strains were subjected to high osmolarity shock (0.8 M NaCl). Strikingly, the Ca2+ increase was completely absent in the pmc1Δvcx1Δ strain (Fig. 1 b). In contrast, the single mutant pmc1Δ had a Ca2+ peak comparable to wild-type strain, and the Ca2+ response was increased in the vcx1Δ strain (Fig. 1 b). This last observation confirms that Vcx1p plays a critical role in rapidly sequestering a sudden pulse of cytosolic Ca2+ into the vacuole. Indeed, vcx1Δ cells also display a delay in restoring low [Ca2+]cyt after addition of extracellular Ca2+ (Miseta et al., 1999). Together, these results strongly suggest that the hyperosmolarity induced Ca2+ flux is generated by release of Ca2+ from the vacuole. This is the first time that Ca2+ release from the vacuole has been shown in vivo in yeast in response to a specific signal.
As a next step, we aimed to identify the channel responsible for this Ca2+ release. We examined the yeast genome for putative Ca2+ channels and found a candidate ORF, recently characterized as YVC1, that shows significant homology to the TRP family of ion channels (Palmer et al., 2001). The first TRP channel was discovered in Drosophila melanogaster and is required for phototransduction (Montell and Rubin, 1989). Multiple homologues have since been identified in mammals, Xenopus, squid, and worms, and are involved in such diverse sensory functions as pain, heat, olfaction, and osmolarity signaling; they may also be involved in replenishing intracellular Ca2+ stores (Putney and McKay, 1999; Harteneck et al., 2000; Clapham et al., 2001). TRP channels have been the subject of intense investigation recently, yet their gating mechanisms and biological role are not fully understood (Harteneck et al., 2000; Clapham et al., 2001; Montell, 2001). The discovery of a TRP homologue in Saccharomyces cerevisiae prompted us to search other fungal genomes for YVC1 homologues. We found a single homologue in Candida albicans, Neurospora crassa, and in 5 of the 14 hemiascomycetous yeast genomes that have been partially sequenced (Souciet et al., 2000). Next, we analyzed the phylogenetic relationship between these new fungal TRP channels and animal TRPs from worm and mammals (Fig. 2). The resulting tree shows that the newly defined cluster of fungal TRPs forms a distinct subfamily (Fig. 2), in addition to the previously described Short, Osm-like, and Long subfamilies (Harteneck et al., 2000; Clapham et al., 2001), also defined, respectively, as TRPC, TRPV, and TRPM subfamilies (Montell, 2001).
Figure 2.
Phylogenetic tree of TRP family of ion channels displaying the new fungal subfamily. Ca, C. albicans; Ce, C. elegans; d, D. melanogaster; h, human; m, mouse; Nc, N. crassa; r, rat; Sc, S. cerevisiae; and TRP homologues cluster into four subfamilies.
As a first step toward characterizing yeast Yvc1p, we determined its localization in vivo using a COOH-terminal green fluorescent protein (GFP) fusion. Interestingly, Yvc1–GFP was specifically localized to the vacuolar membrane (Fig. 3 a). This localization of the yeast TRP homologue is in contrast to other TRP channels studied thus far, which localize to the plasma membrane (Pollock et al., 1995; McKay et al., 2000; Xu and Beech, 2001). Next, we characterized the effect of Yvc1p levels on yeast cell growth. Although yvc1Δ had no apparent growth defects, cells expressing high levels of Yvc1p were extremely sensitive to the presence of CaCl2 in the medium (Fig. 3 b). Furthermore, this sensitivity was Ca2+-specific, as MgCl2 at the same concentration did not affect growth (Fig. 3 b), and cells overexpressing YVC1 did not show increased sensitivity to NaCl or KCl (0.6 to 1.2 M) (unpublished data). This Ca2+ sensitivity strongly suggests that Yvc1p, like some other TRP channels, participates in Ca2+ homeostasis and acts to increase cytosolic [Ca2+]. Based on this finding, as well as its localization to the vacuolar membrane, Yvc1p is a good candidate for a Ca2+ channel that mediates vacuolar Ca2+ release. This hypothesis is also consistent with the electrophysiological properties of YVC1, which has been shown to be permeable to Ca2+, among other cations (Bertl and Slayman, 1990, 1992; Bertl et al., 1992; Palmer et al., 2001).
Figure 3.
Functional characterisation of Yvc1p. (a) Yvc1–GFP localization to the vacuolar membrane, as visualized by fluorescence microscopy using an FITC filter. (b) YVC1 overexpression causes Ca2+ sensitivity, as shown by serial fivefold dilutions of wild-type (YPH499) strain transformed with a control (CTL) or the pYVC1-L-HA plasmid allowing high expression levels. Because YVC1-expressing cells grew slightly more slowly than control cells on regular media (unpublished data), low Ca2+ SD medium (see Materials and methods) was used to perform this experiment in order to have identical growth on the control plate. (c) YVC1 is required for vacuolar Ca2+ release in response to hypertonic shock. Luminescence response of the wild-type strain (YPH499), carrying pYVC1-U for YVC1 overexpression (WT+YVC1o.p.) or a control plasmid (WT), and of the yvc1Δ strain (VDY23) carrying a control plasmid (yvc1Δ). (d) Same experiment as c in pmc1Δvcx1Δ (KKY124) and pmc1Δvcx1Δyvc1Δ (VDY40) strains. (e) Same experiment as c in vcx1Δ (KKY127) and vcx1Δyvc1Δ (VDY31) strains.
We tested whether YVC1 was involved in the hyperosmolarity induced Ca2+ increase by examining [Ca2+]cyt in cells lacking or overexpressing YVC1. The yvc1Δ strain displayed no significant increase in [Ca2+]cyt after hypertonic treatment (Fig. 3 c). In contrast, YVC1 overexpression greatly enhanced the magnitude of the Ca2+ peak induced by high osmolarity (Fig. 3 c). These results indicate that Yvc1p mediates increased [Ca2+]cyt in response to hypertonic shock. To confirm that this YVC1-mediated Ca2+ release is dependent on vacuolar Ca2+, we examined [Ca2+]cyt in pmc1Δ, vcx1Δ and pmc1Δvcx1Δ strains carrying a yvc1Δ allele or overexpressing YVC1. The pmc1Δvcx1Δ strain showed no vacuolar Ca2+ release even when YVC1 was overexpressed (Fig. 3 d). This is likely due to low vacuolar [Ca2+], and shows that YVC1-dependent Ca2+ release comes from the vacuole. As expected, changes in [Ca2+]cyt observed in the Δpmc1 background lacking or overexpressing YVC1 were equivalent to those seen in the wild-type strain (unpublished data). In a vcx1Δ background, deletion of YVC1 completely eliminated the Ca2+ increase induced by hypertonic shock (Fig. 3 e). In contrast, overexpression of YVC1 in the vcx1Δ strain caused a dramatic increase in this Ca2+ response (Fig. 3 e). Thus, overexpression of YVC1 and mutational inactivation of VCX1 both increase the amplitude of the hyperosmolarity induced Ca2+ peak, and these two effects are additive. These observations underscore the importance of Vcx1p in antagonizing and potentially modulating YVC1-dependent Ca2+ release. Together, these results show that following hypertonic shock, Yvc1p effects Ca2+ release from the vacuole into the cytosol, and that this release is followed by rapid Ca2+ sequestration into the vacuole by Vcx1p (Fig. 4).
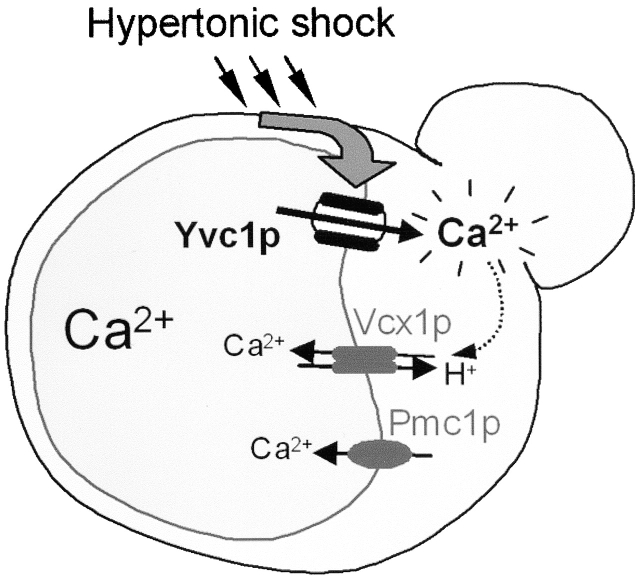
Figure 4.
Model for vacuolar Ca 2+ release and sequestration. Hypertonic shock induces vacuolar Ca2+ release by YVC1, which requires the presence of at least one of the Ca2+ transporters Pmc1p or Vcx1p. After Ca2+ release, Vcx1p rapidly sequesters Ca2+ into the vacuole and decreases cytosolic [Ca2+].
Yeast actively sequester Ca2+ in their vacuole. In these studies we establish that, as in other eukaryotic cells, this Ca2+ can be released into the cytosol in response to external stimuli. We also show that this release is followed by refilling of the internal store. However, two key questions remain: (a) What leads to Yvc1p channel opening?; and (b) What are the physiological consequences of Ca2+ release? We investigated whether other environmental changes besides hypertonic shock induced Ca2+ release by Yvc1p. First, we found that the Ca2+ peak induced by injection of extracellular Ca2+ (Fig. 1 a) was not affected by YVC1 deletion or overexpression (unpublished data). Thus, in vivo, a brief increase in [Ca2+]cyt is apparently not sufficient to trigger Yvc1p opening, although the YVC1 cation conductance observed in isolated vacuoles is activated by Ca2+ (Wada et al., 1987; Bertl and Slayman, 1990, 1992; Bertl et al., 1992; Palmer et al., 2001). Other conditions, such as hypotonic shock or the addition of 0.03% SDS or 7% ethanol, also induced a transient increase in cytosolic Ca2+ (Batiza et al., 1996; unpublished data); however, YVC1 was similarly not required for these Ca2+ peaks (unpublished data). Therefore, the response of Yvc1p to hypertonic shock appears to be specific. We are currently investigating the role of YVC1-mediated Ca2+ release in hypertonic stress signaling. The signaling pathway activated by hypertonic shock has been well characterized in yeast, and is composed of the HOG mitogen-activated kinase cascade (Posas et al., 1998). Further studies will examine the relationship between components of the HOG pathway and the Ca2+ increase mediated by YVC1.
In conclusion, we show that internal Ca2+ release in yeast is mediated by a novel class of Ca2+ release channel, which is unrelated to IP3 or ryanodine receptors. Instead, this release requires a homologue of the TRP family of ion channels, Yvc1p. Like TRP channels in multicellular organisms, YVC1 acts in sensory transduction. However, YVC1 is the first TRP channel homologue shown to mediate Ca2+ release from an intracellular store.
Materials and methods
Yeast strains and media
Strains were isogenic to YPH499 (MATa ura3–52 lys2–801 ade2–101 trp1-Δ63 his3-Δ200 leu2-Δ1) (Sikorski and Hieter, 1989). TPYp is Matα pmc1Δ::TRP1; KKY127 is Mata vcx1Δ; KKY124 is Matα pmc1Δ::TRP1 vcx1Δ; VDY23 is MATa yvc1Δ::Kan R; VDY25 is Matα pmc1Δ::TRP1 yvc1Δ::Kan R; VDY31 is Mat a vcx1Δ yvc1Δ::Kan R; and VDY40 is Matα pmc1Δ::TRP1 vcx1Δ yvc1Δ::Kan R. SD medium (Sherman et al., 1986) contained twice the recommended levels of supplements with 3.5 g of ammonium chloride per liter substituted for ammonium sulfate. Low Ca2+ SD medium ([Ca2+] ∼ 0.24 μM) was the SD medium in which CaCl2 was omitted and calcium pantothenate was replaced by sodium pantothenate as described previously (Iida et al., 1994).
Vector construction and gene deletion
YVC1–GFP plasmid (VDp88) was constructed by cloning a PCR fragment containing YVC1 and 737 pb upstream sequence into the SacI/NheI sites of pGRU2, provided by Bertrand Daignan-Fornier (Institut de Biochemie et Génétique Cellulaires, Bordeaux, France). Hemagglutinin (HA)-tagged YVC1 overexpression plasmids were constructed by a two-step PCR: PCR-amplified 3× (HA) was used as a downstream primer to amplify YVC1 (YOR087/088W). This fragment was cut by Xho/BglII and cloned into Xho/Bam sites in pVT100L or pVT100U (Vernet et al., 1987), leading to pYVC1-HA-L for high expression of YVC1, and pYVC1-HA-U, for moderate expression, respectively. We verified that full-length Yvc1-HA protein (∼78 kD) was expressed in yeast by Western blotting. pYVC1-U, used for aequorin experiments, is a nontagged version of pYVC1-HA-U: the HA tag was removed by a NotI digest and self-religation. To delete YVC1, a fragment comprising ORF YOR087/088 and 736 bp of upstream sequence was cloned between the XhoI and BamHI sites of pcDNA3.1 (Invitrogen). This plasmid was then cut by EcoRI, which removed the sequence from position −41 to +1780 from the ATG, and a cassette containing the kan r gene was inserted into this site. The resulting plasmid was used to amplify a deletion cassette that was used to transform yeast. Kanamycin-resistant colonies were selected and Δyvc1 knockout was checked by PCR as described previously (Güldener et al., 1996).
Aequorin experiments
Yeast carrying the PEVP11/AEQ plasmid, provided by Patrick H. Masson (University of Wisconsin-Madison, Madison, WI) (Batiza et al., 1996) were inoculated from a saturated overnight culture to OD600 = 0.5 in SD media with 2 μM coelenterazine, and were grown overnight at room temperature to reconstitute aequorin from apoaequorin. For each experiment, an aliquot of 250 μl (OD600 = 2–3) was harvested. Cells were resuspended in 100 μl SD media and transferred to luminometer tubes. The baseline luminescence was recorded every second for 30 s (1-s integration) using a Berthold LB9507 luminometer, and was reported in relative luminescence units/s. Hypertonic shock was performed by adding 100 μl SD containing twice the desired final concentration of sorbitol, KCl, or NaCl. To ensure that total reconstituted aequorin was not limiting in our assays, we measured the maximal luminescence after addition of 0.1% digitonin. The maximal luminescence was 4,000,000 relative luminescence units or more, which is 10× higher than the highest signal observed in our assays. Because light units cannot be accurately converted into intracellular Ca2+ concentrations, our results are presented as relative quantities.
Phylogenetic tree
Evolutionary distances between peptide sequences aligned with ClustalW were calculated with the PHILIP protdist software (Felsenstein, 1993), and the tree was subsequently plotted by the neighbor-joining method (Saitou and Nei, 1987). The GenBank accession numbers for the proteins used are the following (available at GenBank/EMBL/DDBJ accession no.): ScYVC1 (S. cerevisiae, YOR087/088w) (Palmer et al., 2001); CaTRP (sequence 11894–9852 from Candida albicans genome contig 1.802); NcTRP (sequence 4727–6751 from N. crassa genome contig 6–2259); rVR1 (T09054); mOTRPC4 (AAG17543); rVRL-1 (NP_035836); mCaT1 (BAA99538); rCaT2 (BAA99541); CeOTRPC2 (CAA96644); CeOTRPC1 (T37241); CeLTRPC2 (CAA92726); CeLTRPC1 (CAB02303); mLTRPC7 (AAK57433); hChaK2 (AAK31202); hLTRPC2 (NP_003298); hLTRPC4 (NP_060106); mLTRPC5 (AAF98120);CeSTRPC1 (AAA28168); CeSTRPC2 (AAK21447); dTRPL (P48994); dTRP (P19334); mTRPC1 (AAB50622); mTRPC4 (AAC05179); mTRPC5 (AAC13550); mTRPC6 (AAC06146); mTRPC3 (NP_062383); mTRPC7 (AAD42069); and mTRPC2 (AAG29950).
Acknowledgments
We thank Patrick Masson for providing the PEVP11/AEQ plasmid, Bertrand Daignan-Fornier for pGRU2, Kim Williams for her advice on aequorin assays, Richard Lewis and Clifford Slayman for helpful discussion, and Vicky Heath, Kim Kafadar, and Sidney Shaw for critical reading of the manuscript.
This work was supported by National Institutes of Health research grant GM48728.
Footnotes
Abbreviations used in this paper: [Ca2+]cyt, concentration of Ca2+ in the cytosol; GFP, green fluorescent protein; HA, hemagglutinin; HOG, high-osmolarity glycerol; IP3, inositol-1,4,5-trisphosphate; TRP, transient receptor potential.
References
- Albertyn, J., S. Hohmann, J.M. Thevelein, and B.A. Prior. 1994. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 14:4135–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiza, A.F., T. Schulz, and P.H. Masson. 1996. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 271:23357–23362. [DOI] [PubMed] [Google Scholar]
- Beeler, T., K. Gable, and T. Dunn. 1997. Activation of divalent cation influx into S. cerevisiae cells by hypotonic downshift. J. Membr. Biol. 160:77–83. [DOI] [PubMed] [Google Scholar]
- Belde, P.J.M., J.H. Vossen, G.W.F.H. Borst-Pauwels, and A.P.R. Theuvenet. 1993. Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of Saccharomyces cerevisiae. FEBS Lett. 323:113–118. [DOI] [PubMed] [Google Scholar]
- Bertl, A., and C.L. Slayman. 1990. Cation-selective channels in the vacuolar membrane of Saccharomyces: dependence on calcium, redox state, and voltage. Proc. Natl. Acad. Sci. USA. 87:7824–7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl, A., and C.L. Slayman. 1992. Complex modulation of cation channels in the tonoplast and plasma membrane of Saccharomyces cerevisiae: single-channel studies. J. Exp. Biol. 172:271–287. [DOI] [PubMed] [Google Scholar]
- Bertl, A., D. Gradmann, and C.L. Slayman. 1992. Calcium- and voltage-dependent ion channels in Saccharomyces cerevisiae. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 338:63–72. [DOI] [PubMed] [Google Scholar]
- Brewster, J.L., T. de Valoir, N.D. Dwyer, E. Winter, and M. Gustino. 1993. An osmosensin signal transduction pathway in yeast. Science. 259:1760–1763. [DOI] [PubMed] [Google Scholar]
- Brown, A.D., K.F. Mackenzie, and K.K. Singh. 1986. Selected aspects of microbial osmoregulation. FEMS Microbiol. Rev. 39:31–36. [Google Scholar]
- Chowdhury, S., K.W. Smith, and M.C. Gustin. 1992. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J. Cell Biol. 118:561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham, D.E., L.W. Runnels, and C. Strübing. 2001. The TRP ion channel family. Nat. Rev. Neurosci. 6:387–396. [DOI] [PubMed] [Google Scholar]
- Cunningham, K.W., and G.R. Fink. 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homologue of plasma membrane Ca2+ ATPases. J. Cell Biol. 124:351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, K.W., and G.R. Fink. 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein, J. 1993. PHYLIP (Phylogeny Inference Package) version 3.5c. Department of Genetics, University of Washington, Seattle.
- Güldener, U., S. Heck, T. Fiedler, J. Beinhauer, and J.H. Hegemann. 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halachmi, D., and Y. Eilam. 1989. Cytosolic and vacuolar Ca2+ concentrations in yeast cells measured with the Ca2+-sensitive fluorescence dye indo-1. FEBS Lett. 25655-61. [DOI] [PubMed]
- Harteneck, C., T.D. Plant, and G. Schultz. 2000. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 23:159–166. [DOI] [PubMed] [Google Scholar]
- Hirayama, T., T. Maeda, H. Saito, and K. Shinozaki. 1995. Cloning and characterization of seven cDNAs for hyper-osmolarity-responsive (HOR) genes of Saccharomyces cerevisiae. Mol. Gen. Genet. 249:127–138. [DOI] [PubMed] [Google Scholar]
- Iida, H., Y. Yagawa, and Y. Anraku. 1990. Cell cycle control by Ca2+ in Saccharomyces cerevisiae. J. Biol. Chem. 265:13391–13399. [PubMed] [Google Scholar]
- Iida, H., H. Nakamura, T. Ono, M.S. Okumura, and Y. Anraku. 1994. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell Biol. 14:8259–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay, R.R., C.L. Szymeczek-Seay, J.P. Lievremont, G.S. Bird, C. Zitt, E. Jungling, A. Luckhoff, and J.W. Putney, Jr. 2000. Cloning and expression of the human transient receptor potential 4 (TRP4) gene: localization and functional expression of human TRP4 and TRP3. Biochem. J. 351:735–746. [PMC free article] [PubMed] [Google Scholar]
- Miseta, A., R. Kellermayer, D.P. Aiello, L. Fu, and D.M. Bedwell. 1999. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 451:132–136. [DOI] [PubMed] [Google Scholar]
- Montell, C. 2001. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Sciences's STKE (http://stke.sciencemag.org/cgi/content/full/OC_sigtrans;2001/90/re1). [DOI] [PubMed]
- Montell, C., and G.M. Rubin. 1989. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 2:1313–1323. [DOI] [PubMed] [Google Scholar]
- Morris, G.J., L. Winters, G.E. Coulson, and K.J. Clarke. 1986. Effect of osmotic stress on the ultrastructure and viability of the yeast Saccharomyces cerevisiae J. Gen. Microbiol. 2023–2034. [DOI] [PubMed] [Google Scholar]
- Nakajima-Shimada, J., H. Iida, F.I. Tsuji, and Y. Anraku. 1991. Monitoring of intracellular calcium in Saccharomyces cerevisiae with an apoaequorin cDNA expression system. Proc. Natl. Acad. Sci. USA. 88:6878–6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, C.P., X.L. Zhou, J. Lin, S.H. Loukin, C. Kung, and Y. Saimi. 2001. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. USA. 98:7801–7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock, J.A., A. Assaf, A. Peretz, C.D. Nichols, M.H. Mojet, R.C. Hardie, and B. Minke. 1995. TRP, a protein essential for inositide-mediated Ca2+ influx is localized adjacent to the calcium stores in Drosophila photoreceptors. J. Neurosci. 15:3747–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas, F., M. Takekawa, and H. Saito. 1998. Signal transduction by MAP kinase cascades in budding yeast. Curr. Opin. Microbiol. 1:175–182. [DOI] [PubMed] [Google Scholar]
- Pozos, T.C., I. Sekler, and M.S. Cyert. 1996. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol. Cell. Biol. 16:3730–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney, J.W., and R.R. McKay. 1999. Capacitative calcium entry channels. Bioessays. 21:38–46. [DOI] [PubMed] [Google Scholar]
- Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425. [DOI] [PubMed] [Google Scholar]
- Sherman, F., G.R. Fink, and J.B. Hicks. 1986. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 186 pp.
- Sikorski, R.S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in S. cerevisiae. Genetics. 122:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souciet, J., M. Aigle, F. Artguenare, G. Blandin, M. Bolton-Fukuhara, E. Bon, P. Brottier, S. Casararegola, J. de Montigny, B. Dujon, et al. 2000. Genomic exploration of the hemiascomycetous yeasts: a set of species for molecular evolution studies. FEBS Lett. 487:3–12. [DOI] [PubMed] [Google Scholar]
- Strayle, J., T. Pozzan, and H.K. Rudolph. 1999. Steady-state free Ca(2+) in the yeast endoplasmic reticulum reaches only 10 microM and is mainly controlled by the secretory pathway pump pmr1. EMBO J. 18:4733–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamás, M.J., K. Luyten, F.C. Sutherland, A. Hernandez, J. Albertyn, H. Valadi, H. Li, B.A. Prior, S.G. Kilian, J. Ramos, et al. 1999. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 31:1087–1104. [DOI] [PubMed] [Google Scholar]
- Vernet, T., D. Dignard, and D.Y. Thomas. 1987. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 52:225–233. [DOI] [PubMed] [Google Scholar]
- Wada, Y., Y. Ohsumi, M. Tanifuji, M. Kasai, and Y. Anraku. 1987. Vacuolar ion channel of the yeast S. cerevisiae. J. Biol. Chem. 262:17260–17263. [PubMed] [Google Scholar]
- Xu, S.Z., and D.J. Beech. 2001. TrpC1 is a membrane-spanning subunit of store-operated Ca(2+) channels in native vascular smooth muscle cells. Circ. Res. 88:84–87. [DOI] [PubMed] [Google Scholar]