Abstract
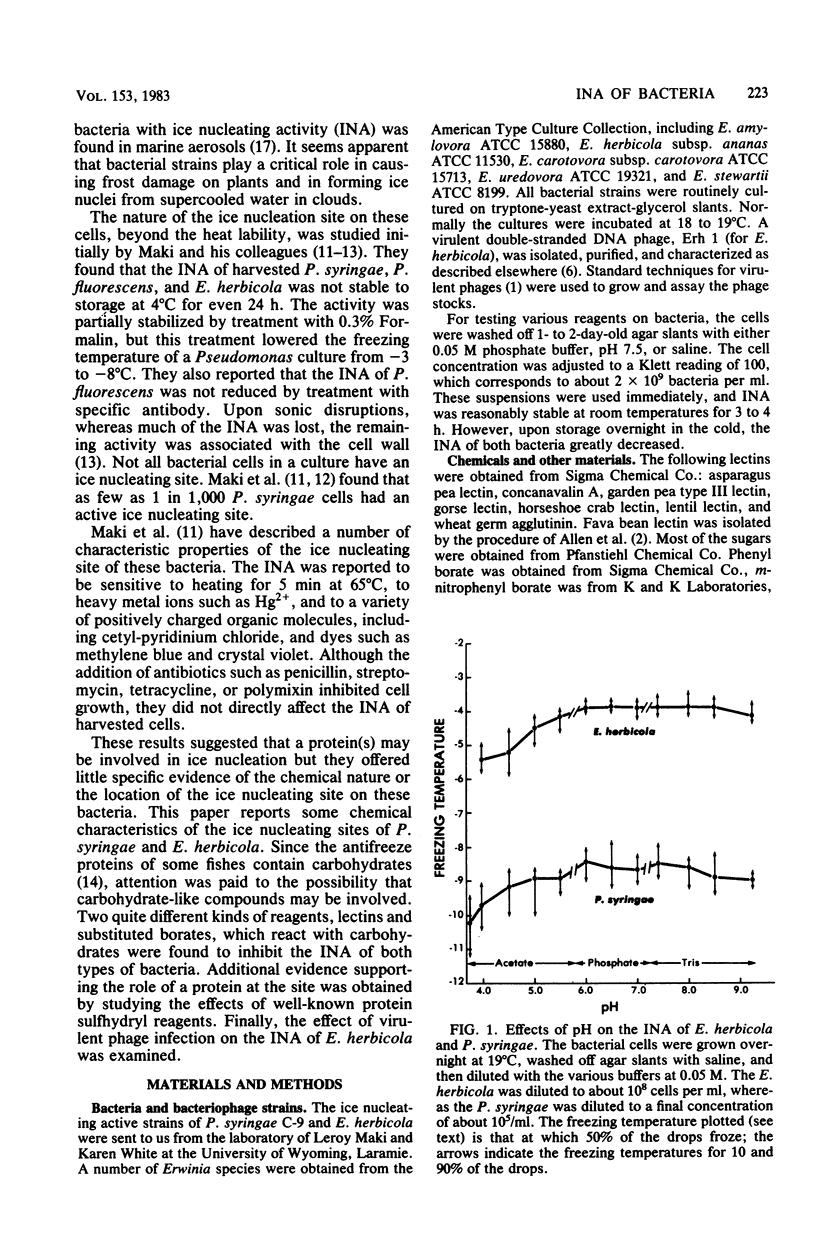
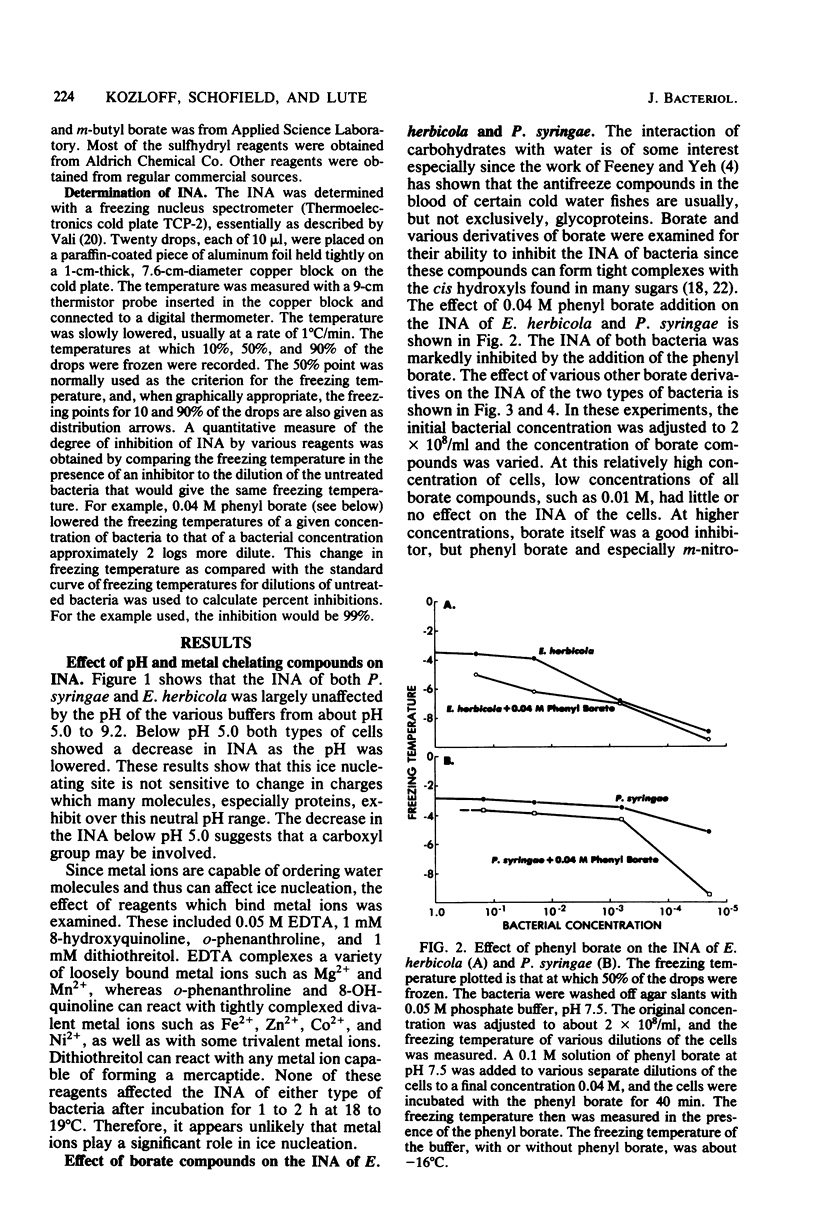
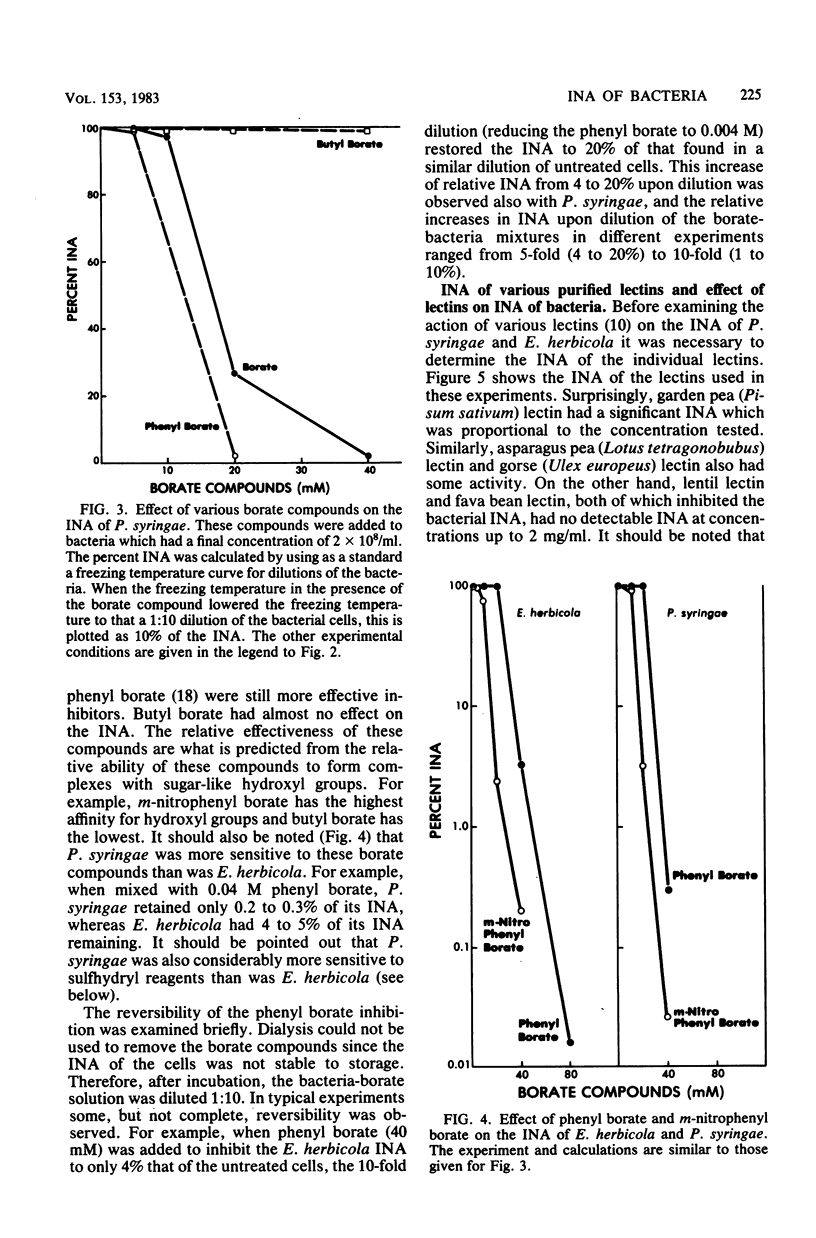
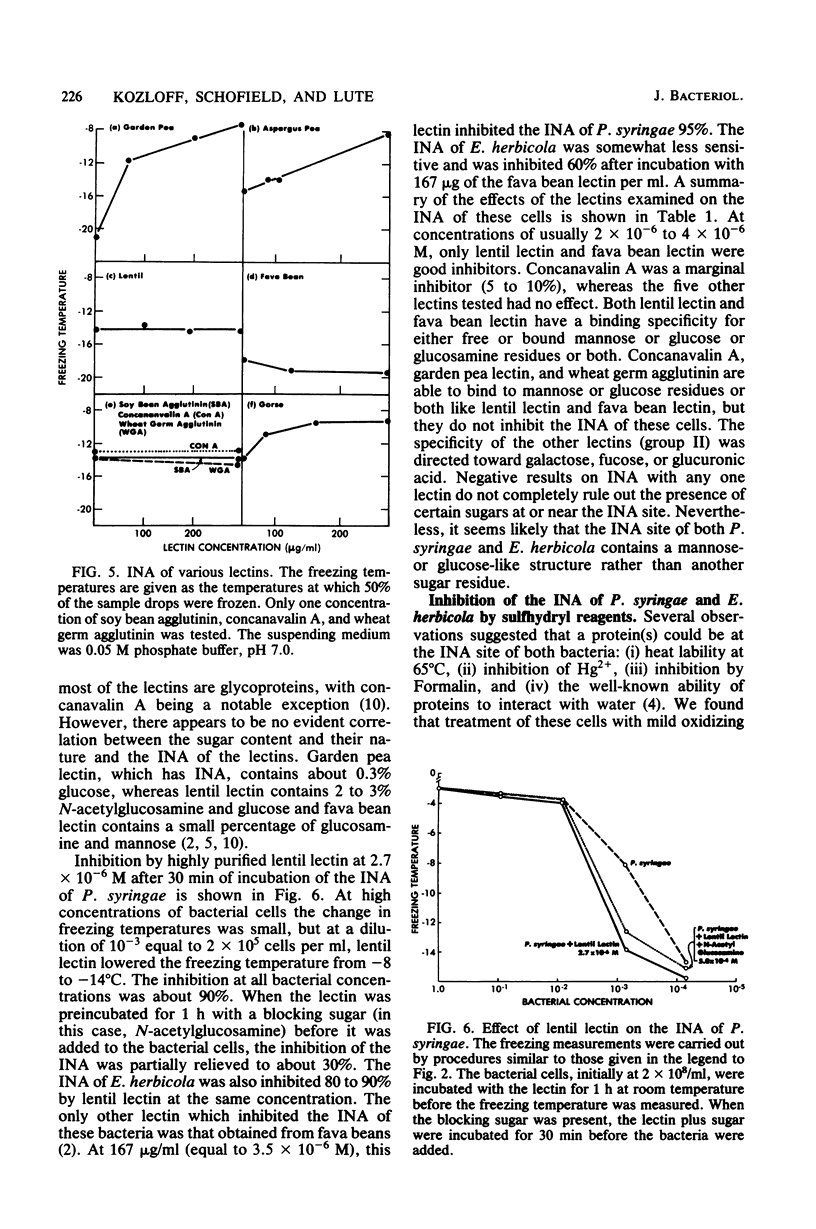
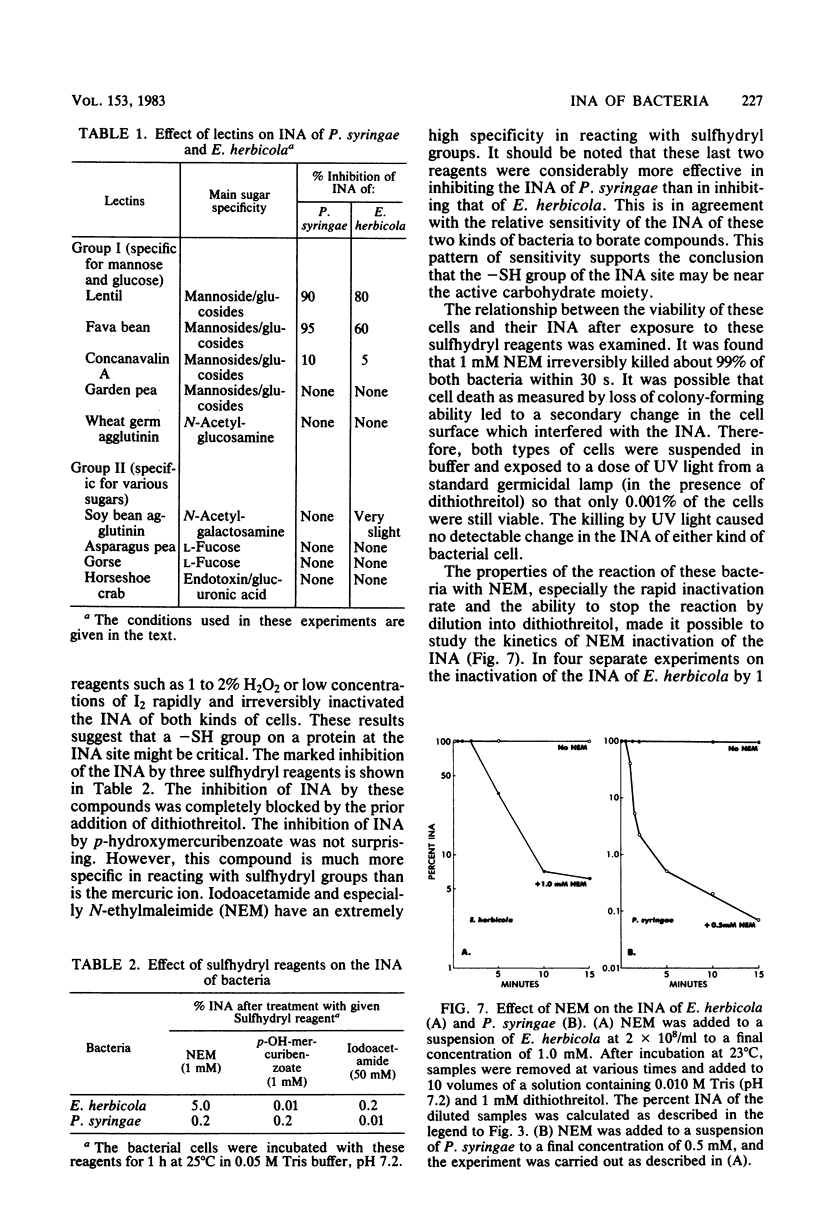
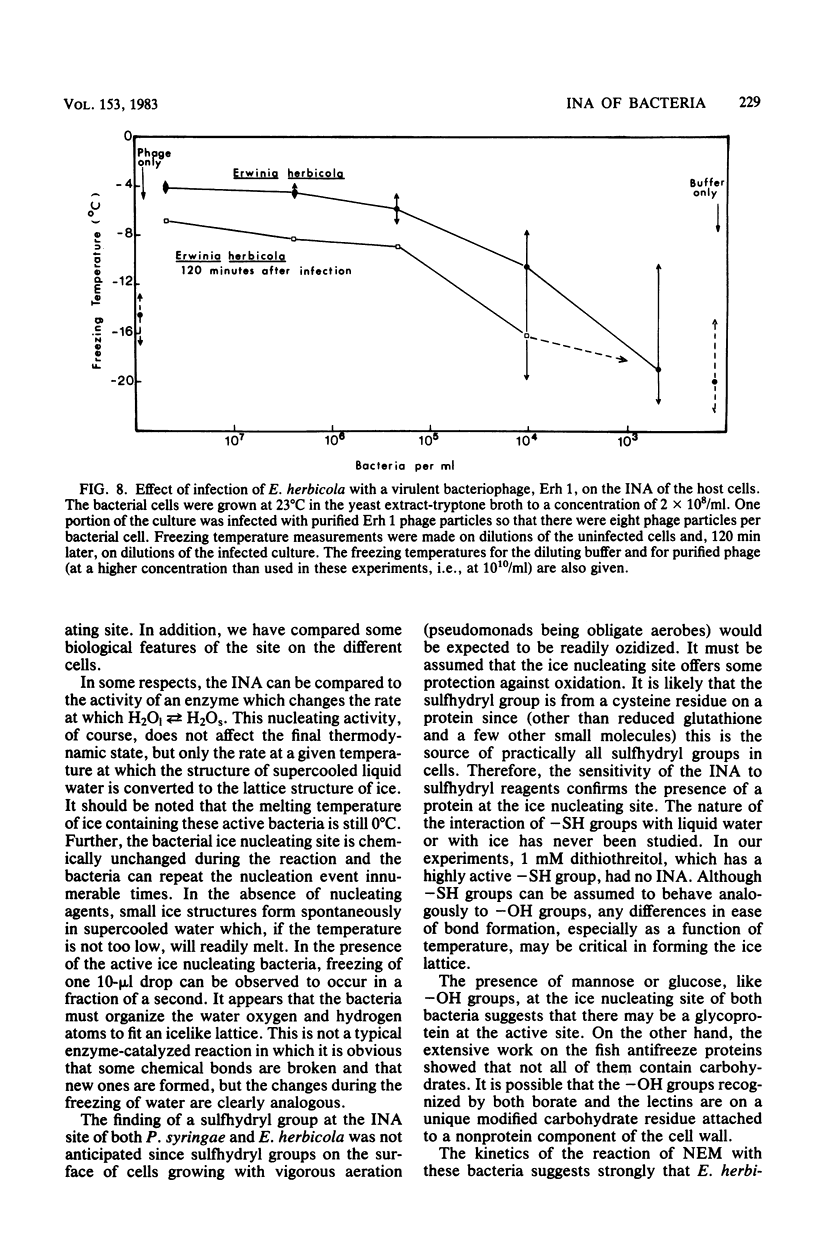
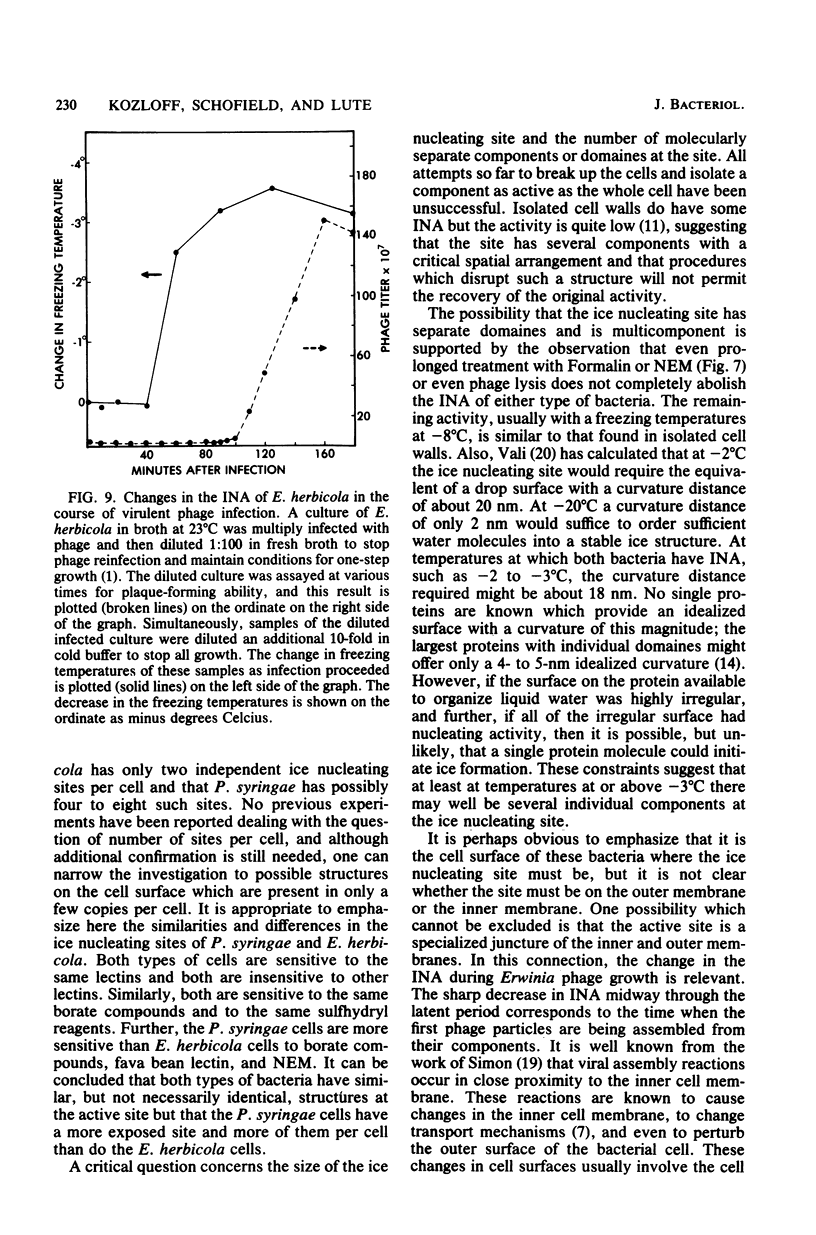
Chemical and biological properties of the ice nucleating sites of Pseudomonas syringae, strain C-9, and Erwinia herbicola have been characterized. The ice nucleating activity (INA) for both bacteria was unchanged in buffers ranging from pH 5.0 to 9.2, suggesting that there were no essential groups for which a change in charge in this range was critical. The INA of both bacteria was also unaffected by the addition of metal chelating compounds. Borate compounds and certain lectins markedly inhibited the INA of both types of bacterial cells. Butyl borate was not an inhibitor, but borate, phenyl borate, and m-nitrophenyl borate were, in order, increasingly potent inhibitors. These compounds have a similar order of affinity for cis hydroxyls, particularly for those found on sugars. Lentil lectin and fava bean lectin, which have binding sites for mannose or glucose, inhibited the INA of both bacteria. All other lectins examined had no effect. The inhibition of INA by these two types of reagents indicate that sugar-like groups are at or near the ice nucleating site. Sulfhydryl reagents were potent inhibitors of the INA of both bacteria. When treated with N-ethylmaleimide, p-hydroxymercuribenzoate, or iodoacetamide, the INA was irreversibly inhibited by 99%. The kinetics of inactivation with N-ethylmaleimide suggested that E. herbicola cells have at least two separate ice nucleating sites, whereas P. syringae cells have possibly four or more separate sites. The effect of infection with a virulent phage (Erh 1) on the INA of E. herbicola was examined. After multiple infection of a bacterial culture the INA was unchanged until 40 to 45 min, which was midway through the 95-min latent period. At that time, the INA activity began falling and 99% of the INA was lost by 55 min after infection, well before any cells had lysed. This decrease in INA before lysis is attributed to phage-induced changes in the cell wall.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Desai N. N., Neuberger A. Purification of the glycoprotein lectin from the broad bean (Vicia faba) and a comparison of its properties with lectins of similar specificity. Biochem J. 1976 Apr 1;155(1):127–135. doi: 10.1042/bj1550127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney R. E., Yeh Y. Antifreeze proteins from fish bloods. Adv Protein Chem. 1978;32:191–282. doi: 10.1016/s0065-3233(08)60576-8. [DOI] [PubMed] [Google Scholar]
- Fliegerová O., Salvetová A., Tichá M., Kocourek J. Studies on phytohemagglutinins. XIX. Subunit structure of the lentil isophytohemagglutinins. Biochim Biophys Acta. 1974 Jun 7;351(2):416–426. doi: 10.1016/0005-2795(74)90206-2. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Chapman V., DeLong S. Defective packing of an unusual DNA in a virulent Erwinia phage, Erh 1. Prog Clin Biol Res. 1981;64:253–269. [PubMed] [Google Scholar]
- Kozloff L. M., Zorzopulos J. Zinc uptake and incorporation into proteins in T4D bacteriophage-infected Escherichia coli. J Biol Chem. 1978 Aug 10;253(15):5548–5550. [PubMed] [Google Scholar]
- Lindow S. E., Arny D. C., Upper C. D. Distribution of ice nucleation-active bacteria on plants in nature. Appl Environ Microbiol. 1978 Dec;36(6):831–838. doi: 10.1128/aem.36.6.831-838.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki L. R., Galyan E. L., Chang-Chien M. M., Caldwell D. R. Ice nucleation induced by pseudomonas syringae. Appl Microbiol. 1974 Sep;28(3):456–459. doi: 10.1128/am.28.3.456-459.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. 3. Membrane-associated intracellular bacteriophages. Virology. 1969 Jun;38(2):285–296. doi: 10.1016/0042-6822(69)90370-5. [DOI] [PubMed] [Google Scholar]
- ZITTLE C. A. Reaction of borate with substances of biological interest. Adv Enzymol Relat Subj Biochem. 1951;12:493–527. doi: 10.1002/9780470122570.ch9. [DOI] [PubMed] [Google Scholar]



