Abstract
Diacylglycerol (DAG) lipase activity is required for axonal growth during development and for retrograde synaptic signaling at mature synapses. This enzyme synthesizes the endocannabinoid 2-arachidonoyl-glycerol (2-AG), and the CB1 cannabinoid receptor is also required for the above responses. We now report on the cloning and enzymatic characterization of the first specific sn-1 DAG lipases. Two closely related genes have been identified and their expression in cells correlated with 2-AG biosynthesis and release. The expression of both enzymes changes from axonal tracts in the embryo to dendritic fields in the adult, and this correlates with the developmental change in requirement for 2-AG synthesis from the pre- to the postsynaptic compartment. This switch provides a possible explanation for a fundamental change in endocannabinoid function during brain development. Identification of these enzymes may offer new therapeutic opportunities for a wide range of disorders.
Keywords: diacylglycerol lipase; CB1 receptor; anandamide; axonal growth; synaptic plasticity
Introduction
The identification of the CB1 and CB2 cannabinoid receptors, activated by the principal psychoactive component of Cannabis sativa, suggests that one or more enzymes synthesize endogenous ligands for these receptors (Pertwee, 1997; Di Marzo et al., 1998). An as yet unidentified sn-1–specific DAG lipase (DAGL) can catalyze the hydrolysis of DAG to 2-arachidonoyl-glycerol (2-AG), the most abundant endocannabinoid in tissues (Mechoulam et al., 1995; Sugiura et al., 1995). In the developing brain, pharmacological studies suggest that DAGL activity is required for axonal growth and guidance (Brittis et al., 1996), with the 2-AG being synthesized and activating CB1 receptors in the same “presynaptic” axonal growth cone (Williams et al., 2003). However, in the adult brain there is a specific postsynaptic requirement for the synthesis of one or more endocannabinoids that act as retrograde messengers on presynaptic CB1 receptors to suppress further transmitter release at both excitatory and inhibitory synapses (Wilson and Nicoll, 2002). A full elucidation of endocannabinoid signaling in the developing and adult brain clearly requires the identification and characterization of the brain DAGLs.
Results and discussion
A bioinformatic approach to identify candidate DAGLs
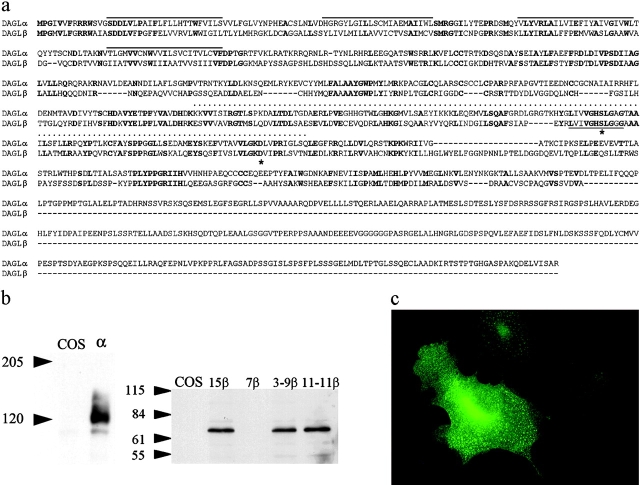
When the sequence of a Penicillium DAGL (Yamaguchi et al., 1991) is “blasted” against the human genome, two related genes (designated α and β) are identified as the only homologues (Fig. 1 a). The encoded gene products are 1,042 (α) and 672 (β) amino acids in length and show extensive homology throughout, but differ in the length of the sequence that follows the catalytic domain (Fig. 1 a). Both proteins contain a lipase-3 motif and a serine lipase motif, and they are predicted to have four transmembrane-spanning domains with the catalytic domain and amino terminus inside of the cell (Fig. 1 a). The genes are found in a wide range of species (e.g., chickens, zebrafish, and mice) with a high degree of conservation between man and mouse (97% identity for α and 79% for β; unpublished data).
Figure 1.
Characterization and expression of the novel genes. An alignment between the human α (gi|20521123) and β (gi|21040277) gene products is shown in panel a. The four putative transmembrane domains, the serine lipase motif, and the lipase 3 motif are highlighted by lines above the sequence, a dotted line, and a line below the sequence, respectively. Mutated amino acids are denoted with an asterisk. A Western blot analysis (with molecular mass markers in kD) of control and transfected COS cells expressing the α and β gene products, including the mutated clones, is shown in panel b. The localization of the α transgene in transfected COS cells is shown in panel c.
Enzymatic characterization of the novel genes
Transfection of the two novel genes in COS cells led to the expression of products with appropriate molecular mass (∼70 kD for the β gene and ∼120 kD for the α gene) with protein expression localized to the plasma membrane (Fig. 1, b and c). COS cells expressing the highest levels of the gene products (clones 12α and 15β) were selected for enzymatic characterization with a clone that expressed no detectable transgene taken as a control (clone 7β; Fig. 1 b). When using sn-1-stearoyl-2-[14C]arachidonoyl-glycerol as a substrate, and measuring the 2-[14C]AG released, we confirmed that both enzymes are mostly expressed in the 10,000 g membrane fraction (Fig. 2 a), exhibit optimal activity at pH 7 (not depicted), and follow Michaelis–Menten kinetics, with Km values in the range of the possible concentrations of DAGs in animal tissues (154.7 ± 19.1 and 74.1 ± 4.9 μM for the α and β form, respectively; Fig. 2 b). Both enzymes exhibit very little, if any, monoacylglycerol lipase, phospholipase A1/A2, triacylglycerol lipase, and anandamide amidase activity (unpublished data). To investigate their substrate selectivity, three types of radiolabeled DAG substrates were synthesized. A three- to eightfold selectivity for the sn-1 over the sn-2 position of DAGs (Fig. 2 c) was demonstrated by comparing the rate of the formation of [14C]oleic acid and sn-1-[14C]oleoyl-glycerol from sn-1-[14C]oleoyl-2-oleoyl-glycerol; or by comparing the rate of the formation of mono[14C]oleoyl-glycerol from either sn-1-[14C]oleoyl-2-oleoyl-glycerol or sn-1-oleoyl-2-[14C]oleoyl-glycerol. The similar activities of the enzymes observed when using as substrates either sn-1-[14C]oleoyl-2-arachidonyl glyceryl ether, which cannot be hydrolyzed on the 2 position, or sn-1-[14C]oleoyl-2-arachidonoyl glycerol, further demonstrates that the two lipases are selective for the sn-1 position of DAGs. Of the two enzymes, the β form appears to prefer sn-1-oleoyl-2 acyl-glycerols with linoleic ≥ oleic > arachidonic > stearic acid on the 2 position, whereas the α form appears to work equally well with all fatty acids (Fig. 2 d and not depicted). Both enzymes are equally sensitive to Ser/Cys-hydrolase inhibitors such as p-hydroxy-mercuri-benzoate and HgCl2, but not to PMSF. Importantly, both enzymes are inhibited by RHC80267, a drug that blocks 2-AG formation from intact cells (Bisogno et al., 1997; Stella et al., 1997). Glutathione and Ca2+ stimulate both enzymes (Fig. 2 e). Based on homology with other serine lipases, two of the three amino acids that likely constitute the “catalytic triad” can readily be identified as serine 443 and aspartic acid 495 in the β form of the enzyme (Fig. 1 a). Accordingly, substitution of the serine (clone 11-11β) or aspartic acid (clone 3-9β) with alanines abolished enzymatic activity (Fig. 2 a). Based on these results, we can conclude that the products of the genes are specific sn-1-DAGLs; therefore, we designate them as DAGLα and DAGLβ.
Figure 2.
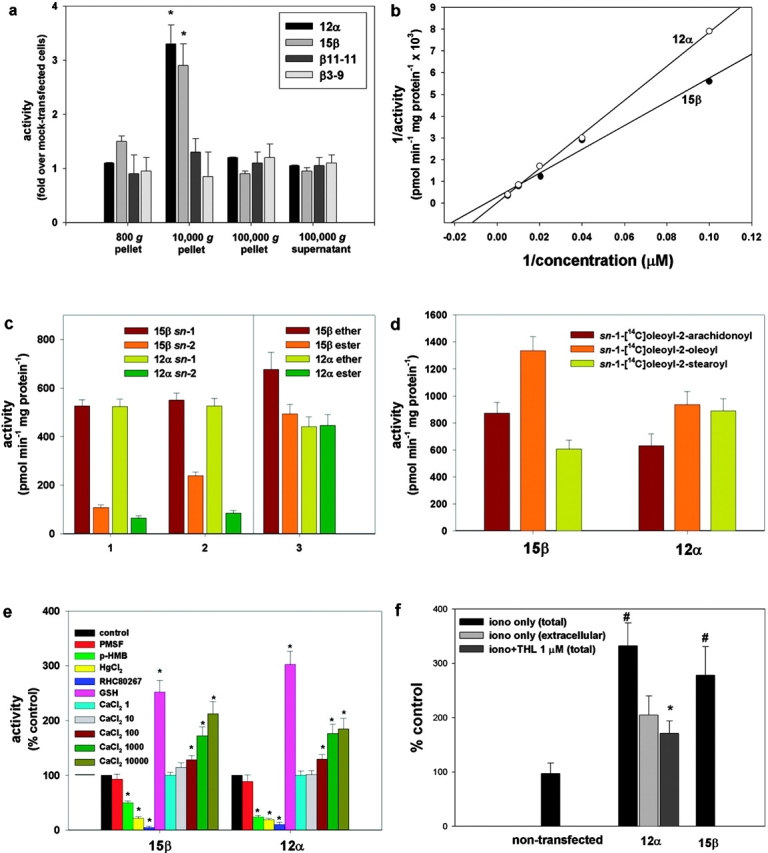
Enzymatic characterization of the new lipase gene products expressed in COS cells (clones 12α and 15β). (a) Subcellular fractionation of the enzymes using sn-1-stearoyl-2-[14C]arachidonoyl-glycerol as a substrate. Controls included two clones expressing the highest levels of the β gene product with single point mutations in Asp 494 to Ala (clone 3-9β) and Ser 443 to Ala (clone 11-11β). Activity is expressed as fold over the activity in fractions from a clone transfected with the β construct that expressed no detectable transgene (clone 7β, control cells). (b) Lineweaver–Burk profiles of the activities in the 10,000 g pellets from clones 12α and 15β. Data points were subtracted from the activity found in control cells (clone 7β). Vmax values were 33.3 ± 4.5 and 3.45 ± 0.16 nmol min−1 mg protein−1 for the α and β form, respectively. (c) Selectivity of the two DAGLs for the sn-1 position of DAGs. The rate of formation is shown for: (1) [14C]oleic acid (sn-1 bars) and sn-1-[14C]oleoyl-glycerol (sn-2 bars) from sn-1-[14C]oleoyl-2-oleoyl-glycerol, this indicates sn-1 and sn-2 selectivity, respectively; (2) mono[14C]oleoyl-glycerol from either sn-1-[14C]oleoyl-2-oleoyl-glycerol (sn-2 bars) or sn-1-oleoyl-2-[14C]oleoyl-glycerol (sn-1 bars), this indicates sn-2 and sn-1 selectivity, respectively; and (3) [14C]oleic acid from either sn-1-[14C]oleoyl-2-arachidonyl glyceryl ether (ether bars), or sn-1-[14C]oleoyl-2-arachidonoyl glycerol (ester bars), this indicates sn-1 selectivity, without and with possible interference from sn-2 selectivity, respectively. (d) Rate of formation of [14C]oleic acid using DAG substrates with different fatty acids on the 2-position. (e) Effect of inhibitors, glutathione (GSH), and calcium on enzyme activity assessed using sn-1-[14C]oleoyl-2-arachidonoyl-glycerol as substrate. 1 mM PMSF; 1 mM p-hydroxy-mercuri-benzoate (p-HMB); 5 mM HgCl2; and 100 μM RHC80267. The concentrations of CaCl2 in the assay buffer were 1, 10, 100, 1,000, and 10,000 μM. (f) Formation of 2-AG from intact COS cells (nontransfected or clones 12α and 15β), stimulated for 20 min with vehicle (DMSO, 0.1%), 4 μM ionomycin, or ionomycin + 1 μM THL after a 5-min preincubation with THL. 2-AG levels were measured in cells plus medium (total) by liquid chromatography-mass spectrometry. Results are expressed as a percentage of 2-AG formation from vehicle-stimulated cells (12.1 ± 4.3 pmol/mg extracted lipids). In one set of experiments, the amounts of 2-AG produced after ionomycin stimulation were measured separately in cells and medium, and the percentage of the amount of 2-AG found in the medium (extracellular) is shown. Data are means ± SEM of n ≥ 4. *, P < 0.05, control cells; #, P < 0.05, iono only (total), t test.
Both enzymes contribute to the Ca2+-dependent biosynthesis/release of endocannabinoid 2-AG from intact cells
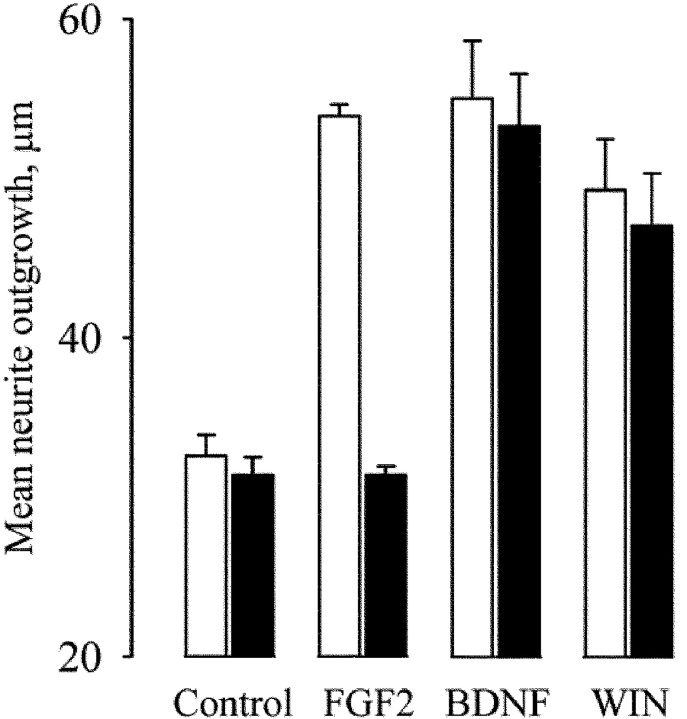
In agreement with the Ca2+ requirement of the novel enzymes, and with the Ca2+ dependence of 2-AG biosynthesis in neurons (Bisogno et al., 1997; Stella et al., 1997), ionomycin stimulation of clone 12α or 15β cells led to the production, and release into the media, of significantly higher amounts of 2-AG than control COS cells (Fig. 2 f). A strong correlation between the expression of the novel genes and endogenous DAGL activity was found in a wide range of cell types (e.g., N18TG2 neuroblastoma, C6 glioma, RBL-2H3 basophilic leukemia, human Caco-2, and embryonic kidney HEK-293 cells; unpublished data). More importantly, tetrahydrolipstatin (THL), a second DAGL inhibitor (Lee et al., 1995), potently inhibited both DAGLα and DAGLβ from clone 12α and 15β cell homogenates (IC50 = 60 and 100 nM, respectively). THL (5-min preincubation at 1 μM) also decreased the ionomycin-induced release of 2-AG from intact N18TG2, C6, and RBL-2H3 cells (66.7 ± 5.9, 93.5 ± 7.3, and 99.2 ± 10.1% inhibition, respectively; means ± SEM, n = 3, P < 0.01), as well as in clone 12α (Fig. 2 f). FGF2 stimulates neurite outgrowth from cerebellar neurons via a pathway that requires DAGL activity to generate 2-AG and the consequent activation of the CB1 receptor (Williams et al., 2003). THL inhibited the neurite outgrowth stimulated by FGF2 with no effect on the response stimulated by a CB1 agonist or brain-derived neuronotrophic factor (BDNF; Fig. 3), demonstrating that it is acting specifically, and upstream of the CB1 receptor, in the FGF signaling pathway. Thus, the novel enzymes make and release 2-AG as endocannabinoid, and THL is a useful tool to investigate the cellular function of the two enzymes.
Figure 3.
THL inhibits the neurite outgrowth response stimulated by FGF2. Rat cerebellar neurons were isolated at postnatal day 2 and cultured over monolayers of 3T3 cells in control media or media supplemented with 5 ng/ml FGF2, 5 ng/ml BDNF, or 0.2 μM WIN55,2122-2 as indicated. The experiments were done in the absence (white bars) and presence (black bars) of 10 μM THL. After 18 h, the cultures were fixed and stained for GAP-43, and the mean length of the longest neurite was determined from ∼140 neurons under each culture condition. For the control and FGF2 data, results were pooled from three independent experiments. For the BDNF and CB1 agonist data, the results were pooled from two independent experiments. Bars show SEM. Dose–response experiments (not depicted) indicated an IC50 = 2 μM for the inhibition by THL of FGF2 response.
A switch in enzyme expression from axons to dendrites during development
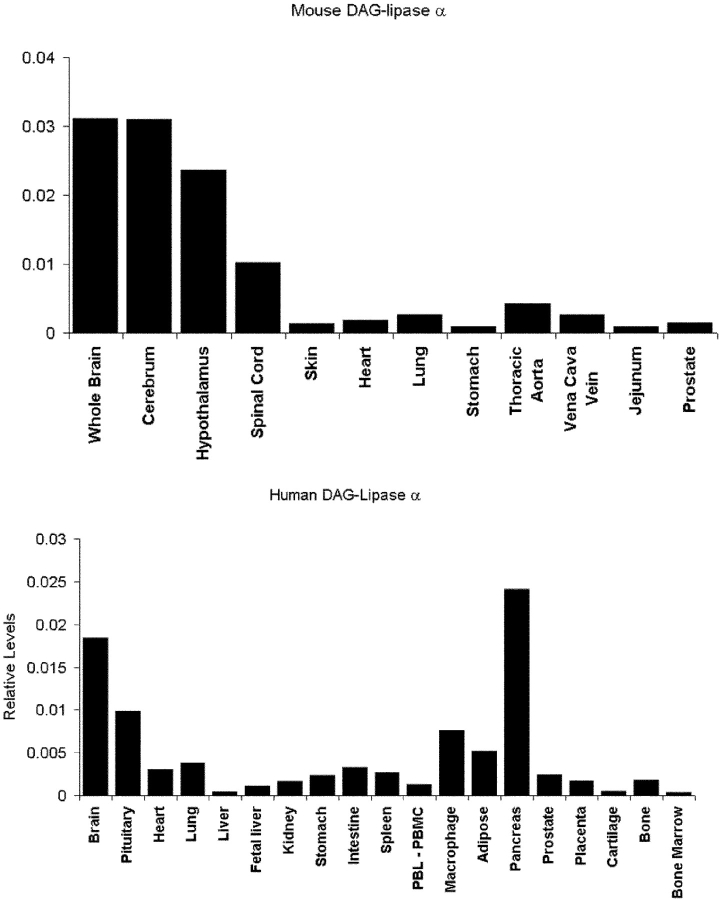
The expression of the novel sn-1-DAGLs will determine when and where they can make and release 2-AG in the developing and adult brain. In general, antibodies to DAGLα and β showed the same qualitative staining pattern, demonstrating that they are normally coexpressed; and similar staining patterns were seen with three independent antisera to DAGLα (unpublished data). We found that all developing axonal tracts examined coexpress the enzymes. For example, in the mouse at embryonic day 10, DAGLα and DAGLβ are expressed in axons crossing the floor plate of the spinal cord (Fig. 4, a–c). At day 14, DAGLβ can be seen to specifically label the retinal ganglion fiber tract and also the optic nerve (Fig. 4 d). A similar, but less pronounced, staining was seen for DAGLα (unpublished data). Remarkably, both enzymes are absent from axonal tracts in the adult mouse brain, with this shown for DAGLα in the optic and anterior commissures in Fig. 4, e and f. This contrasted with the very strong staining of both regions with antibodies to the CB1 receptor (Fig. 4, g and h). In the cerebellum, the highest levels of DAGLα and DAGLβ are seen in the dendritic field with staining also apparent in the deep cerebellar nuclei (Fig. 4, i and j); however, both enzymes are again absent in the axonal tracts. A high power image of DAGLα expression within the Purkinje cell dendritic field of the cerebellum clearly shows that the enzyme is specifically expressed in the tubular-like structures that characterize the dendritic tree of the Purkinje cell (Fig. 4 k). The staining for DAGLβ was qualitatively similar but considerably less pronounced than that for DAGLα (Fig. 4, i and j), suggesting a substantial down-regulation of the β form of the enzyme during development. Given the maintained expression of high levels of DAGLα in the adult nervous system, we assessed the relative level of transcripts for the gene in various adult tissues in the mouse and human by TaqMan RT-PCR. In the mouse, the highest levels of transcripts were found in the nervous system, with barely detectable levels found in the skin, heart, lung, and various other tissues (Fig. 5), which is in agreement with the highest relative abundance of 2-AG and other 2-acylglycerols in the rodent brain (Kondo et al., 1998). In the human, high levels of expression were again found in the brain, relative to most tissues, with high levels also noted in the pancreas (Fig. 5). The pancreatic expression is of interest given the established role for DAGL activity in amylase secretion in this tissue (Hou et al., 1997).
Figure 4.
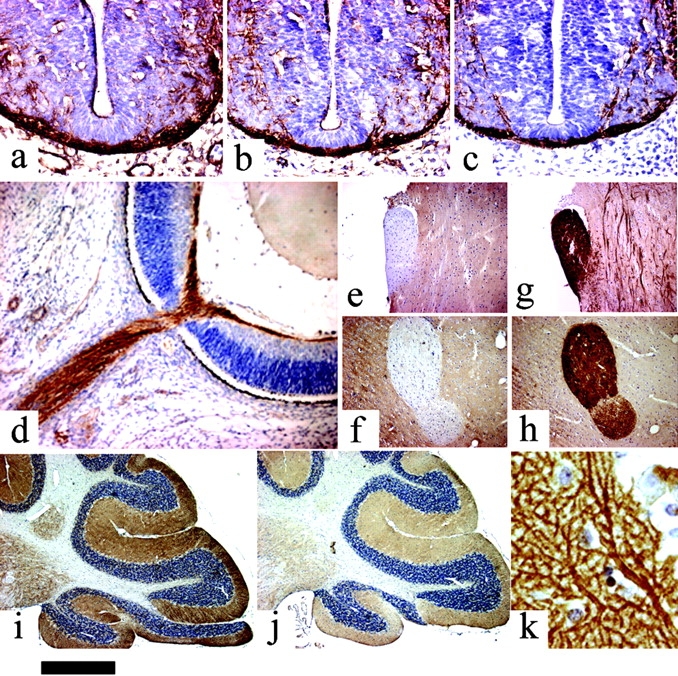
Expression of the novel lipases in the mouse nervous system. DAGLα (a) and DAGβ (b) are expressed in neurofilament-positive axonal tracts (c) in the embryonic spinal cord (day 10). DAGLβ is expressed in the embryonic retinal ganglion fiber layer and optic nerve at day 14 (d). Note the lack of expression of DAGLα in the optic (e) and anterior commissures (f) in the adult. This contrasts with strong staining for the CB1 receptor in these tracts (g and h). DAGLα (i) and DAGLβ (j) expression in the adult cerebellum is restricted to the deep cerebellar nuclei and synaptic fields, including the Purkinje cell dendrites (k). Bar: (a–c) ∼130 μm; (d) ∼220 μm; (e–j) ∼500 μm; (k) ∼20 μm.
Figure 5.
Relative expression of mouse and human DAGLα in various adult tissues. TaqMan RT-RCR was used to measure the relative level of mouse (top) and human (bottom) DAGLα transcripts in various tissues as indicated. In both instances, the number of mRNA copies detected for DAGLα was divided by the number of mRNA copies of GAPDH and β-actin detected in each sample. The average of these two figures are plotted. For the human, data were obtained for two males and two females; there were no obvious gender differences, and the results from all four individuals were pooled.
In summary, this study is the first to report the identification of specific sn-1 DAGLs playing a major role in biosynthesizing the endocannabinoid 2-AG. Expression studies have revealed that although both enzymes are expressed in axonal tracts during development, expression in the adult becomes restricted to synaptic fields. It is also clear that in the adult cerebellum, expression within a synaptic field can be restricted to the dendritic compartment. Therefore, the ability of these enzymes to make and release 2-AG is temporally and spatially regulated in the brain in a manner that correlates well with two of the key functions of the endocannabinoid signaling system. In fact, in the developing embryo, the enzymes are available within a growth cone to make 2-AG able to act in an autocrine way on CB1 receptors to promote axonal growth and guidance (Williams et al., 2003). In contrast, in the adult, the enzymes are lost from axonal tracts but remain expressed in synaptic fields. In the case of the cerebellar Purkinje cell, this expression is localized to the postsynaptic dendrite, and this correlates with the postsynaptic requirement for the synthesis of an endocannabinoid as a retrograde messenger for depolarization-induced suppression of excitatory neurotransmission at this synapse (Kreitzer and Regehr, 2001; Diana et al., 2002). With the identification of the first DAGLs, it will now be possible to test their importance in physiologically relevant endocannabinoid signaling pathways and to evaluate their potential as therapeutic targets. In the latter context, there is a growing interest in 2-AG/CB1 signaling in terms of therapies for a range of conditions including neurodegenerative diseases (Di Marzo et al., 2000; Panikashvili et al., 2001), obesity (Di Marzo et al., 2001), and the extinction of aversive memories (Marsicano et al., 2002).
Materials and methods
Cloning and expression of DAGLs
A full-length clone of the human α gene (gi|20521122) was obtained from the Kazusa DNA Research Institute (KIAA0659), and the mouse β gene (gi|16359288) was obtained from I.M.A.G.E. Consortium (4921222). The coding sequence for the α gene was amplified by PCR and inserted into pcDNA3.1D/V5-His-TOPO (Invitrogen) to generate an expression construct with an in-frame 3′ V5 epitope tag. The coding sequence for the β gene was amplified by PCR and subcloned into pCMV-Tag4A (Stratagene) using the NotI and XhoI restriction sites, in-frame with a 3′ FLAG epitope tag. Single point mutations in the β gene were generated using the QuikChange Site-Directed Mutagenesis kit (Stratagene). Plasmids were transfected into COS-7 cells using lipofectamine plus (Invitrogen), and stable transfected clones were selected using G418. For Western blotting, equal amounts of protein lysate were separated on SDS–polyacrylamide gels and transferred to nitrocellulose Hybond ECL (Amersham Biosciences). Primary antibodies used were mouse anti-V5 (Invitrogen) at 1:5,000 and mouse anti-Flag (Stratagene) at 1:1,000. The secondary antibody was anti–mouse HRP (Vector Laboratories) used at 1:3,000.
Synthesis of substrates
In brief, the compounds were obtained from the R (−) solketal esterified with either unlabeled or 14C-labeled oleic acid using N'-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride/4-dimethylaminopyridin, and deprotecting the acetonide with hydrochloride/methanol. The primary alcoholic group was protected selectively with triisopropylsilyl chloride, whereas the free secondary alcohol was esterified with various fatty acids, either unlabeled or 14C-labeled. Finally, the sn-1,2-diacyl-glycerol with two different acyl groups in position sn-1 and 2 was obtained by removing selectively the sylyl group with tetrabutylammonium fluoride/acetic acid. To prepare sn-1-[14C]oleoyl-2-arachidonyl glyceryl ether, the previously prepared 2-AG ether (noladin) was esterified with [14C]oleic acid using N'-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride /4-dimethylaminopyridin. 2-[3H]arachidonoyl-glycerol and arachidonoyl-[14C]ethanol-amide were synthesized as described previously (Bisogno et al., 1997) with minor modifications.
Enzyme assays
Confluent cells were harvested in Tris-HCl buffer, pH 7, and homogenized in a homogenizer (Dounce). The homogenates were centrifuged at 4°C sequentially at 800 g (5 min), 10,000 g (25 min), and 100,000 g (70 min). Each fraction or, for most of the experiments, the 10,000-g fraction was incubated at pH 7.0 (or in citrate 50 mM or Tris-HCl 50 mM buffers at different pH values) at 37°C for 15 min, with different radiolabeled substrates (i.e., for DAGL activity, with various synthetic 14C-labeled DAGs [1.0 mCi/mmol, 50 μM] or with sn-1-stearoyl-2-[14C]arachidonoyl-glycerol from Amersham Biosciences, 56.0 mCi/mmol, at different concentrations; for monoacylglycerol lipase activity, with synthetic 2-[3H]arachidonoyl-glycerol, 1.0 mCi/mmol, 50 μM; for triacylglycerol lipase activity, with 1,2,3-tri-[14C]oleoyl-glycerol from NEN, 100.0 mCi/mmol, 50 μM; for phospholipase A1/A2 activity, or sn-1-[14C]oleoyl-2-[14C]oleoyl-phosphatidylcholine from NEN, 104.0 mCi/mmol, 20 μM; and for fatty acid amide hydrolase activity, with synthetic arachidonoyl-[14C]ethanolamide, 5.0 mCi/mmol, 25 μM). After the incubation, lipids were extracted three times with 2 vol chloroform/methanol 2:1 (by vol), and the extracts were lyophilized under vacuum. Extracts were fractionated by TLC on silica on polypropylene plates using chloroform/methanol/NH4OH (85:15:0.1, by vol) as the eluting system. Under these conditions, the migration index of free fatty acids, monoacylglycerols, and diacylglycerols was 0.25, 0.65, and 0.9, respectively; the migration index of phospholipids and triacylglycerols was 0.05 and 1.0, respectively. Bands corresponding to each class of lipids were cut, and their radioactivity was counted with a β-counter. Using sn-1-stearoyl-2-[14C]arachidonoyl-glycerol as the substrate, the DAGL reaction rate was linear up to 20 min and reached a plateau after 30 min, with both DAGLα and β. Saturation of DAGLα and β activity was reached with 500 and 400 μM, respectively, when using sn-1-stearoyl-2-[14C]arachidonoyl-glycerol as the substrate.
Intact cell stimulation and 2-AG analyses
Confluent cells were stimulated for 20 min at 37°C with either vehicle or 4 μM ionomycin or 1 μM ionomycin + THL, after a 5-min preincubation, with THL in DME medium without serum. Immediately after the stimulation, cells, medium, or cells plus medium were extracted three times with 2 vol chloroform/methanol 2:1 (by vol), and the extracts were lyophilized under vacuum. Each extract was purified by open bed chromatography over silica columns, followed by 2-AG quantification by means of isotope dilution atmospheric pressure chemical ionization–liquid chromatography–mass spectrometry (Marsicano et al., 2002).
Immunohistochemistry
Rabbit antibodies, raised and affinity purified against the GASPTKQDDLVISAR epitope in DAGLα, and the SSDSPLDSPTKYPTL epitope in DAGLβ, were used at 1.5–3.0 μg/ml for immunostaining. An affinity-purified antibody against the CB1 receptor (PA1-745; Affinity BioReagents, Inc.) was used at 20 μg/ml IgG. An antineurofilament monoclonal antibody (N5264; Sigma-Aldrich) was used at a dilution of 1:1,000. 6-μm sections of formalin-fixed, paraffin wax–embedded tissues were subjected to heat-mediated antigen retrieval to disclose antigenic sites before being incubated in primary antibody solutions overnight at 4°C. After washing, the sections were incubated with biotinylated secondary antibodies (E0432 and E0433; Dakopatts) followed by detection with an HRP-streptavidin-biotin kit (model K377; Dakopatts). To visualize the V5 epitope tag, cells were fixed with 4% PFA and processed for indirect immunofluorescence. Cells were permeabilized with 0.2% Triton X-100/PBS and stained with mouse anti-V5 primary antibody at 1:200, followed by Alexa Fluor 488 goat anti–mouse IgG secondary (Molecular Probes) at 1:2,000. Slides were viewed on a microscope (model Axiovert 135; Carl Zeiss MicroImaging, Inc.), using 10×/0.25 Achrostigmat or 63×/1.4 Oil Pan-Apochromat lens. Images were captured with an AxioCam using KS300 software (Carl Zeiss MicroImaging, Inc.). Images were processed in Adobe Photoshop, with brightness and contrast being the only adjustments made.
Neurite outgrowth studies
Neurite outgrowth studies and reagents used therein were as described previously (Williams et al., 2003).
Real-time RT-PCR analysis of DAGLα expression in various tissues
TaqMan RT-PCR for DAGLα was performed essentially as described previously (Bond et al., 2002). Primers were based on sequences encoded by exon 19; for the mouse, the forward and reverse primers were ttcgccgagttcattgacag and tctcaggcaccatcatgca. For the human, the forward and reverse primers were cctcttcaacctggacagcaa and gggccctcagcgtagtca. To normalize data and to correct for variations in RNA and/or cDNA quality and quantity, parallel TaqMan assays were run for two housekeeping genes, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin.
Acknowledgments
TaqMan analysis was performed by K. Philpott and R. Davies (GlaxoSmithKline, Harlow, UK).
This work was supported by the Wellcome Trust, Volkswagen Stiftung, Italian Ministry of Education, University and Research (Fondo per gli Investimenti della Ricerca di Base), and the UK Medical Research Council. A Human Frontier in Science Program Organization fellowship partly supported T. Bisogno.
T. Bisogno, F. Howell, and G. Williams contributed equally to this work.
Abbreviations used in this paper: 2-AG, 2-arachidonoyl-glycerol; BDNF, brain-derived neuronotrophic factor; DAGL, DAG lipase; THL, tetrahydrolipstatin.
References
- Bisogno, T., N. Sepe, D. Melck, S. Maurelli, L. De Petrocellis, and V. Di Marzo. 1997. Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem. J. 322:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond, B.C., D.J. Virley, N.J. Cairns, A.J. Hunter, G.B. Moore, S.J. Moss, A.W. Mudge, F.S. Walsh, E. Jazin, and P. Preece. 2002. The quantification of gene expression in an animal model of brain ischaemia using TaqMan real-time RT-PCR. Brain Res. Mol. Brain Res. 106:101–116. [DOI] [PubMed] [Google Scholar]
- Brittis, P.A., J. Silver, F.S. Walsh, and P. Doherty. 1996. Fibroblast growth factor receptor function is required for the orderly projection of ganglion cell axons in the developing mammalian retina. Mol. Cell. Neurosci. 8:120–128. [DOI] [PubMed] [Google Scholar]
- Diana, M.A., C. Levenes, K. Mackie, and A. Marty. 2002. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J. Neurosci. 22:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo, V., D. Melck, T. Bisogno, and L. De Petrocellis. 1998. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 21:521–528. [DOI] [PubMed] [Google Scholar]
- Di Marzo, V., M.P. Hill, T. Bisogno, A.R. Crossman, and J.M. Brotchie. 2000. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson's disease. FASEB J. 14:1432–1438. [DOI] [PubMed] [Google Scholar]
- Di Marzo, V., S.K. Goparaju, L. Wang, J. Liu, S. Batkai, Z. Jarai, F. Fezza, G.I. Miura, R.D. Palmiter, T. Sugiura, and G. Kunos. 2001. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 410:822–825. [DOI] [PubMed] [Google Scholar]
- Hou, W., Y. Arita, and J. Morisset. 1997. Endogenous arachidonic acid release and pancreatic amylase secretion. Pancreas. 14:301–308. [DOI] [PubMed] [Google Scholar]
- Kondo, S., H. Kondo, S. Nakane, T. Kodaka, A. Tokumura, K. Waku, and T. Sugiura. 1998. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through Ca2+-dependent and -independent mechanisms. FEBS Lett. 429:152–156. [DOI] [PubMed] [Google Scholar]
- Kreitzer, A.C., and W.G. Regehr. 2001. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 29:717–727. [DOI] [PubMed] [Google Scholar]
- Lee, M.W., F.B. Kraemer, and D.L. Severson. 1995. Characterization of a partially purified diacylglycerol lipase from bovine aorta. Biochim. Biophys. Acta. 1254:311–318. [DOI] [PubMed] [Google Scholar]
- Marsicano, G., C.T. Wotjak, S.C. Azad, T. Bisogno, G. Rammes, M.G. Cascio, H. Hermann, J. Tang, C. Hofmann, W. Zieglgansberger, et al. 2002. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 418:530–534. [DOI] [PubMed] [Google Scholar]
- Mechoulam, R., S. Ben-Shabat, L. Hanus, M. Ligumsky, N.E. Kaminski, A.R. Schatz, A. Gopher, S. Almog, B.R. Martin, D.R. Compton, et al. 1995. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50:83–90. [DOI] [PubMed] [Google Scholar]
- Panikashvili, D., C. Simeonidou, S. Ben-Shabat, L. Hanus, A. Breuer, R. Mechoulam, and E. Shohami. 2001. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 413:527–531. [DOI] [PubMed] [Google Scholar]
- Pertwee, R.G. 1997. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 74:129–180. [DOI] [PubMed] [Google Scholar]
- Stella, N., P. Schweitzer, and D. Piomelli. 1997. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 388:773–778. [DOI] [PubMed] [Google Scholar]
- Sugiura, T., S. Kondo, A. Sukagawa, S. Nakane, A. Shinoda, K. Itoh, A. Yamashita, and K. Waku. 1995. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215:89–97. [DOI] [PubMed] [Google Scholar]
- Williams, E.J., F.S. Walsh, and P. Doherty. 2003. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J. Cell Biol. 160:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R.I., and R.A. Nicoll. 2002. Endocannabinoid signaling in the brain. Science. 296:678–682. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, S., T. Mase, and K. Takeuchi. 1991. Cloning and structure of the mono- and diacylglycerol lipase-encoding gene from Penicillium camembertii U-150. Gene. 103:61–67. [DOI] [PubMed] [Google Scholar]