Abstract
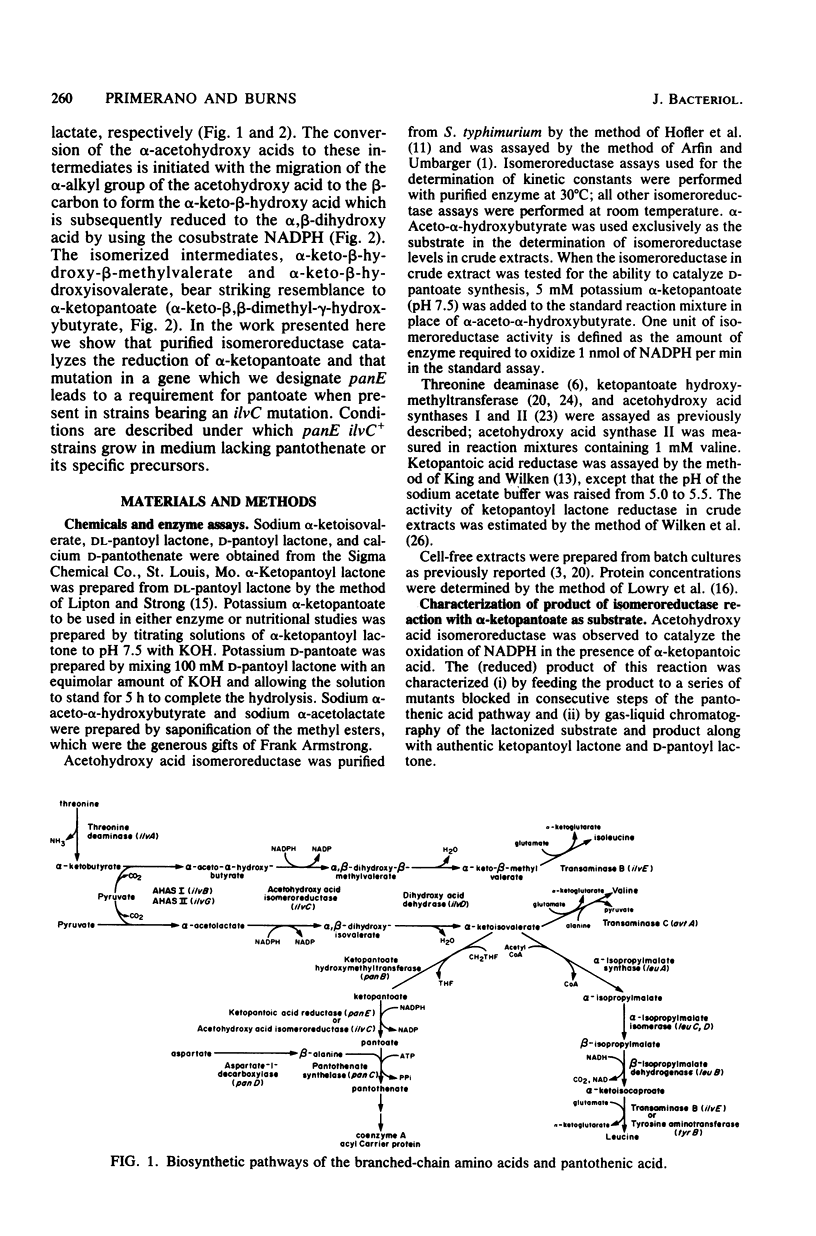
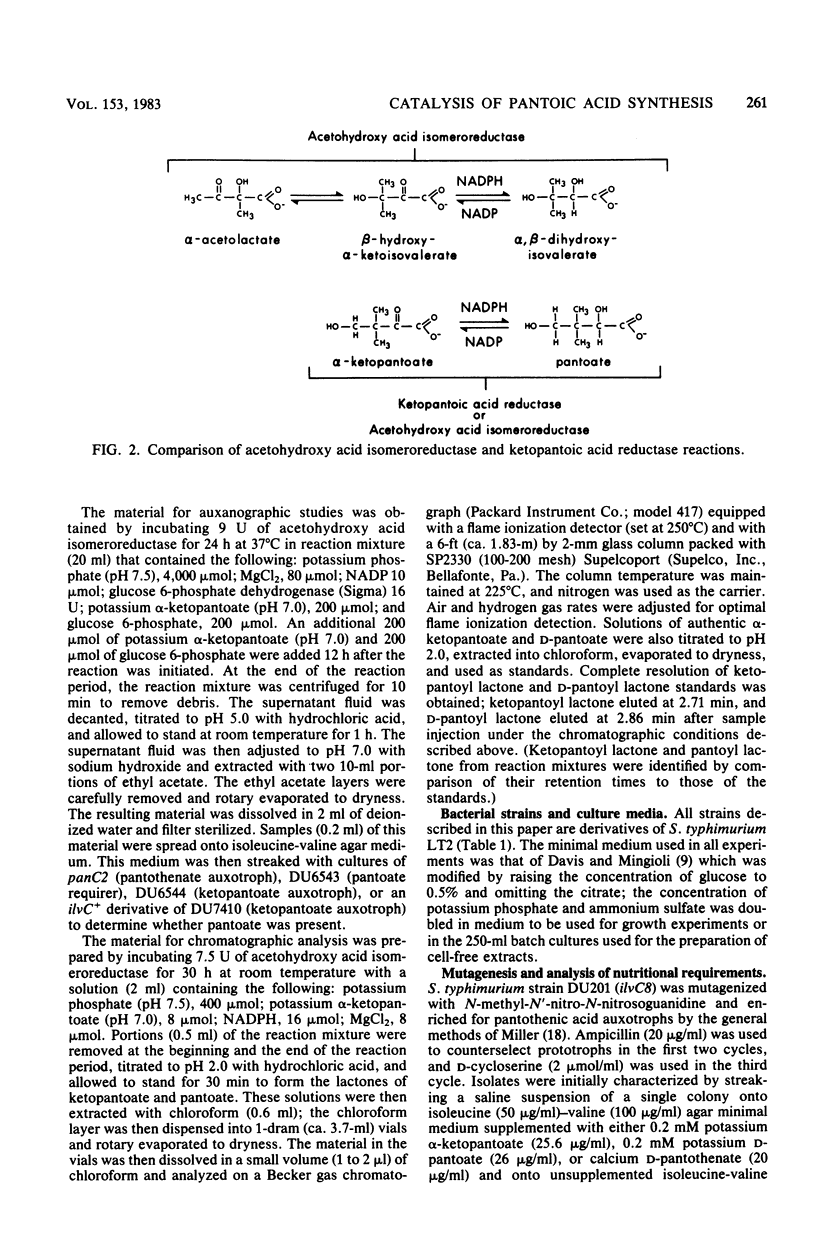
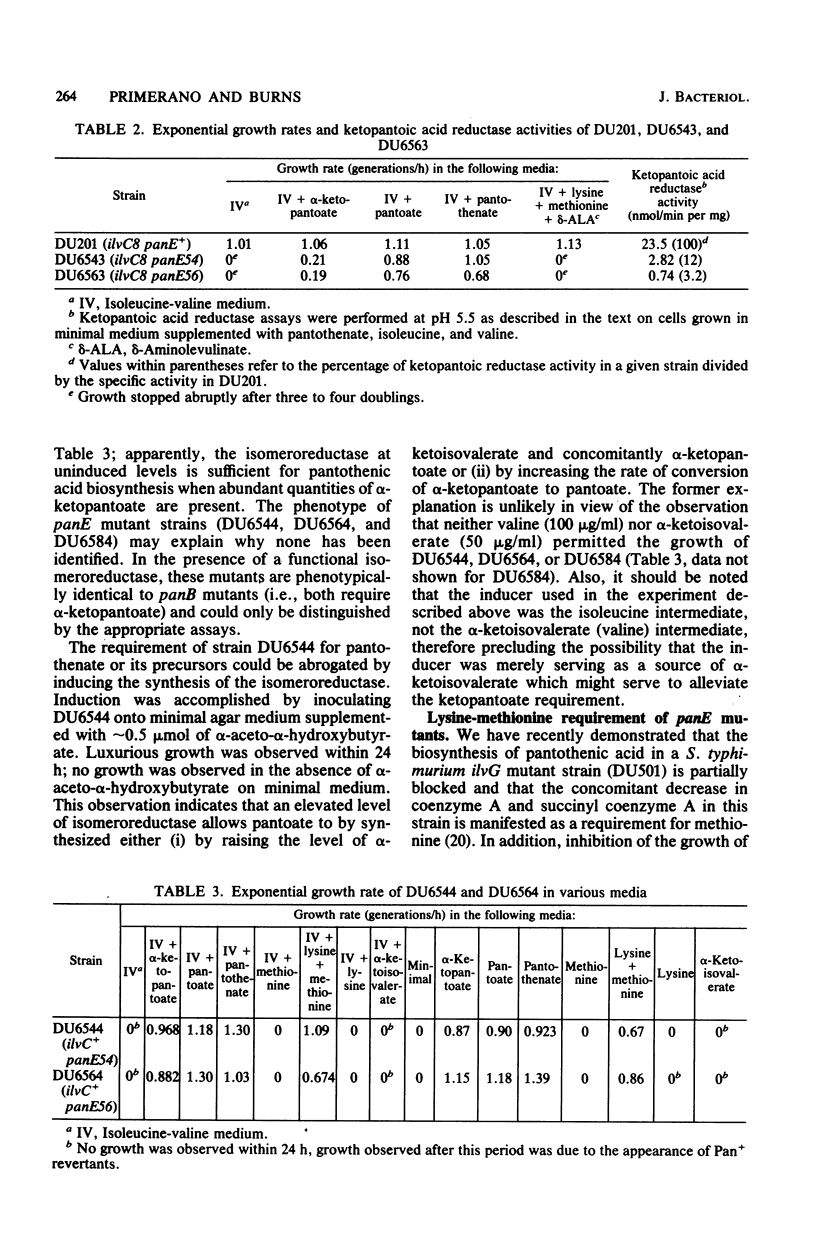
Structural genes have been identified for all of the enzymes involved in the biosynthesis of pantothenic acid in Salmonella typhimurium and Escherichia coli K-12, with the exception of ketopantoic acid reductase, which catalyzes the conversion of α-ketopantoate to pantoate. The acetohydroxy acid isomeroreductase from S. typhimurium efficiently bound α-ketopantoate (Km = 0.25 mM) and catalyzed its reduction at 1/20 the rate at which α-acetolactate was reduced. Since two enzymes could apparently participate in the synthesis of pantoate, a S. typhimurium ilvC8 strain was mutagenized to derive strains completely blocked in the conversion of α-ketopantoate to pantoate. Several isolates were obtained that grew in isoleucine-valine medium supplemented with either pantoate or pantothenate, but not in the same medium supplemented with α-ketopantoate or β-alanine. The mutations that conferred pantoate auxotrophy (designated panE) to these isolates appeared to be clustered, but were not linked to panB or panC. All panE strains tested had greatly reduced levels of ketopantoic acid reductase (3 to 12% of the activity present in DU201). The capacity of the isomeroreductase to synthesize pantoate in vivo was assessed by determining the growth requirements of ilvC+ derivatives of panE ilvC8 strains. These strains required either α-ketopantoate, pantoate, or pantothenate when the isomeroreductase was present at low levels; when the synthesis of isomeroreductase was induced, panE ilvC+ strains grew in unsupplemented medium. These phenotypes indicate that a high level of isomeroreductase is sufficient for the synthesis of pantoate. panE ilvC+ strains also grew in medium supplemented with lysine and methionine. This phenotype resembles that of some S. typhimurium ilvG mutants (e.g., DU501) which are partially blocked in the biosynthesis of coenzyme A and are limited for succinyl coenzyme A. panE ilvC+ strains which lack the acetohydroxy acid synthases required only methionine for growth (in the presence of leucine, isoleucine, and valine). This and other evidence suggested that the synthesis of pantoic acid by isomeroreductase was blocked by the α-acetohydroxy acids and that pantoic acid synthesis was enhanced in the absence of these intermediates, even when the isomeroreductase was at low levels. panE ilvC+ strains reverted to pantothenate independence. Several of these revertants were shown to have elevated isomeroreductase levels under noninduced and induced conditions; the suppressing mutation in each revertant was shown to be closely linked to ilvC by P22 transduction. This procedure presents a means for obtaining mutants with altered regulation of isomeroreductase.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Umbarger H. E. Purification and properties of the acetohydroxy acid isomeroreductase of Salmonella typhimurium. J Biol Chem. 1969 Mar 10;244(5):1118–1127. [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazey D. L., Burns R. O. Gene ilvY of Salmonella typhimurium. J Bacteriol. 1980 Jun;142(3):1015–1018. doi: 10.1128/jb.142.3.1015-1018.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazey D. L., Burns R. O. Genetic organization of the Salmonella typhimurium ilv gene cluster. Mol Gen Genet. 1979;177(1):1–11. doi: 10.1007/BF00267247. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Beta-alanine synthesis in Escherichia coli. J Bacteriol. 1980 Mar;141(3):1291–1297. doi: 10.1128/jb.141.3.1291-1297.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Littel K. J., Jackowski S. Genetic and biochemical analyses of pantothenate biosynthesis in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1982 Mar;149(3):916–922. doi: 10.1128/jb.149.3.916-922.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King H. L., Jr, Dyar R. E., Wilken D. R. Ketopantoyl lactone and ketopantoic acid reductases. Characterization of the reactions and purification of two forms of ketopantoyl lactone reductase. J Biol Chem. 1974 Aug 10;249(15):4689–4695. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langley D., Guest J. R. Biochemical and genetic characterics of deletion and other mutant strains of Salmonella typhimurium LT2 lacking alpha-keto acid dehydrogenase complex activities,. J Gen Microbiol. 1974 Jun;82(2):319–335. doi: 10.1099/00221287-82-2-319. [DOI] [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega M. V., Cárdenas A., Ubiera D. panD, a new chromosomal locus of Salmonella typhimurium for the biosynthesis of beta-alanine. Mol Gen Genet. 1975 Sep 29;140(2):159–164. doi: 10.1007/BF00329783. [DOI] [PubMed] [Google Scholar]
- Primerano D. A., Burns R. O. Metabolic basis for the isoleucine, pantothenate or methionine requirement of ilvG strains of Salmonella typhimurium. J Bacteriol. 1982 Jun;150(3):1202–1211. doi: 10.1128/jb.150.3.1202-1211.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Teller J. H., Powers S. G., Snell E. E. Ketopantoate hydroxymethyltransferase. I. Purification and role in pantothenate biosynthesis. J Biol Chem. 1976 Jun 25;251(12):3780–3785. [PubMed] [Google Scholar]
- Wilken D. R., King H. L., Jr, Dyar R. E. Ketopantoic acid and ketopantoyl lactone reductases. Stereospecificity of transfer of hydrogen from reduced nicotinamide adenine dinucleotide phosphate. J Biol Chem. 1975 Mar 25;250(6):2311–2314. [PubMed] [Google Scholar]
- Wilken D. R., King H. L., Jr, Dyar R. E. Ketopantoyl lactone reductases. Methods Enzymol. 1979;62:209–215. doi: 10.1016/0076-6879(79)62220-6. [DOI] [PubMed] [Google Scholar]


