Abstract
Transport of preproteins into the mitochondrial matrix is mediated by the presequence translocase–associated motor (PAM). Three essential subunits of the motor are known: mitochondrial Hsp70 (mtHsp70); the peripheral membrane protein Tim44; and the nucleotide exchange factor Mge1. We have identified the fourth essential subunit of the PAM, an essential inner membrane protein of 18 kD with a J-domain that stimulates the ATPase activity of mtHsp70. The novel J-protein (encoded by PAM18/YLR008c/TIM14) is required for the interaction of mtHsp70 with Tim44 and protein translocation into the matrix. We conclude that the reaction cycle of the PAM of mitochondria involves an essential J-protein.
Keywords: Hsp70; J-protein; mitochondria; protein translocation; Saccharomyces cerevisiae
Introduction
Most mitochondrial proteins are synthesized as preproteins on cytosolic ribosomes and posttranslationally transported into the organelle. The precursors of matrix proteins cross both outer and inner mitochondrial membranes by using specific channel-forming translocation machineries, the translocase of the outer membrane (TOM) complex and the presequence translocase of the inner membrane (TIM23) complex (Jensen and Johnson, 1999; Koehler, 2000; Pfanner and Geissler, 2001; Neupert and Brunner, 2002). Two main energy sources drive the translocation of preproteins. (1) The membrane potential (Δψ) across the inner membrane activates the channel, Tim23, and exerts an electrophoretic effect on the positively charged NH2-terminal targeting signals (presequences). (2) ATP hydrolysis is required for the transport of the polypeptide chain; the mitochondrial Hsp70 (mtHsp70) of the matrix forms the core of the protein import motor and directly binds to the preprotein in transit, thereby promoting the complete translocation of the preprotein into the matrix (Jensen and Johnson, 1999; Pilon and Schekman, 1999; Matouschek et al., 2000; Pfanner and Geissler, 2001; Neupert and Brunner, 2002).
Three different subunits of the presequence translocase–associated motor (PAM) of mitochondria are known, each of which is essential for cell viability: mtHsp70; the nucleotide exchange factor mitochondrial GrpE (Mge1); and the peripheral inner membrane protein Tim44, which serves as a binding partner for mtHsp70 (Jensen and Johnson, 1999; Matouschek et al., 2000; Pfanner and Geissler, 2001; Neupert and Brunner, 2002). Since Tim44 is associated with the Tim23–Tim17 complex, mtHsp70 bound to Tim44 is located close to the exit of this inner membrane protein import channel. Chaperones of the Hsp70 class, including bacterial DnaK, Hsp70s of the eukaryotic cytosol, and BiP (Kar2) of the endoplasmic reticulum, typically cooperate with cochaperones of the J-class (DnaJ homology domain) (Bukau and Horwich, 1998; Pilon and Schekman, 1999; Matlack et al., 1999; Hartl and Hayer-Hartl, 2002). Mitochondria contain three J-proteins, termed Jac1, Mdj1, and Mdj2. However, these J-proteins are neither essential for cell viability nor involved in protein translocation across the inner membrane, but rather function in protein folding and maturation in the mitochondrial matrix (Rowley et al., 1994; Westermann and Neupert, 1997; Kim et al., 2001; Lutz et al., 2001; Voisine et al., 2001). It has thus been generally assumed that the essential translocase function of mtHsp70 does not require a J-protein, but that Tim44 acts as a specialized cochaperone at the protein import site. Therefore, all current models on the function of PAM are based on the assumption that only one membrane interaction site, Tim44, exists for mtHsp70 (Glick, 1995; Voisine et al., 1999; Matouschek et al., 2000; Neupert and Brunner, 2002; Liu et al., 2003).
We found a fourth mitochondrial J-protein that is an essential inner membrane protein associated with the presequence translocase. This J-protein of 18 kD stimulates the ATPase activity of mtHsp70 and is required for protein translocation into the matrix. Thus, mtHsp70 has two functional interaction sites located close to the protein import channel, Tim44 and the novel J-protein.
Results and discussion
Identification of an 18-kD J-protein of the mitochondrial inner membrane associated with the presequence translocase
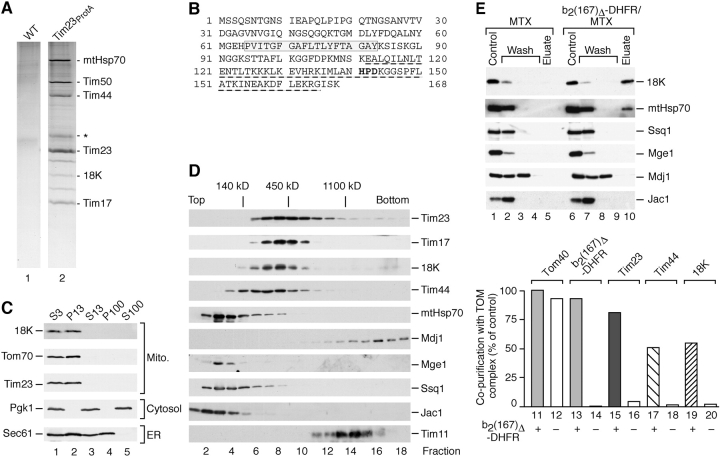
Mitochondria were isolated from a Saccharomyces cerevisiae strain expressing the channel protein Tim23 with an NH2-terminal protein A tag (Geissler et al., 2002). Upon lysis with digitonin, Tim23 was isolated by affinity chromatography, leading to the copurification of the integral translocase subunits Tim17 and Tim50 as well as the motor subunits Tim44 and mtHsp70. Additional bands observed in the purified complex included fragments of these subunits (Geissler et al., 2002). Mass spectrometric analysis led to the identification of a new protein of 18 kD (encoded by PAM18/YLR008c/TIM14) (Fig. 1 A). Deletion of the ORF was lethal to yeast cells (data not shown). The protein contains a hydrophobic segment in its middle portion and a J-domain, including the highly conserved HPD motif, at the COOH terminus (Fig. 1 B). Additional domains found in a number of J-proteins, such as a G/F region and a zinc finger motif (Kelley, 1998), are not present. Thus, the novel 18-kD protein is a mitochondrial J-protein essential for cell viability.
Figure 1.
Identification of an essential J-protein at the mitochondrial presequence translocase. (A) Purification of the presequence translocase from yeast mitochondria. Mitochondria from wild-type (WT) cells and cells expressing protein A–tagged Tim23 were lysed in digitonin and subjected to IgG chromatography. The TIM23 complex was eluted by TEV protease, separated by SDS-PAGE, and stained with colloidal Coomassie. The asterisk indicates a minor fraction of the abundant inner membrane protein ADP/ATP carrier (Geissler et al., 2002). 18K indicates the newly identified J-protein of 18 kD. (B) Deduced primary structure of the essential 18-kD J-protein of PAM. Gray box, hydrophobic segment; dashed line, J-domain. (C) Cellular fractionation. Wild-type spheroplasts were lysed and subjected to successive differential centrifugation steps. Equal volumes of the fractions were analyzed by SDS-PAGE and Western blotting. (D) Comigration with the presequence translocase. Mitochondria were solubilized in digitonin and loaded on top of a linear sucrose gradient. (E) The 18-kD J-protein is present in a functional translocase-preprotein complex. In the presence of methotrexate (MTX), purified b2(167)Δ-DHFR was arrested in mitochondria carrying Tom22His10. Mitochondria were lysed in digitonin and subjected to NiNTA–agarose chromatography. Following immunodecoration, samples were quantified using NIH-Image1.62f. The recovery of Tom40 was set to 100% (control).
Differential centrifugation of lysed yeast cells indicated that the 18-kD J-protein fractionates with mitochondria (Fig. 1 C). Upon lysis of mitochondria with digitonin and separation of protein complexes by a sucrose gradient, the protein was found to comigrate with Tim23, Tim17, and a portion of Tim44 (Fig. 1 D). Only a fraction of mtHsp70 comigrated with the presequence translocase (Dekker et al., 1997). The matrix cochaperones Mdj1, Jac1, and Mge1 did not comigrate with the presequence translocase (Fig. 1 D). To determine if the 18-kD J-protein was present in an active translocase complex, we used the model preprotein b2(167)Δ-DHFR, which consists of an NH2-terminal portion of cytochrome b 2, including the matrix targeting signal, and the COOH-terminal passenger protein dihydrofolate reductase (Dekker et al., 1997). In the presence of the ligand methotrexate, DHFR is stably folded and the preprotein accumulates in a two membrane–spanning fashion inserted both into the TOM and the TIM23 complex. When mitochondria with a tagged TOM complex are used, the presequence translocase can be copurified in the presence of arrested preprotein (Fig. 1 E, column 15 vs. 16) (Geissler et al., 2002). Although Mge1, Mdj1, Jac1, and the second Hsp70 (Ssq1) did not copurify, the 18-kD J-protein and a fraction of mtHsp70 specifically copurified with the TOM complex in the presence, but not in the absence, of preprotein (Fig. 1 E, lane 10 vs. lane 5). A quantitation revealed that the yield of copurification of the J-protein with the TOM complex was similar to that of Tim44 (Fig. 1 E, columns 17 and 19). We conclude that a J-protein is associated with the presequence translocase and is part of an active preprotein–translocase complex.
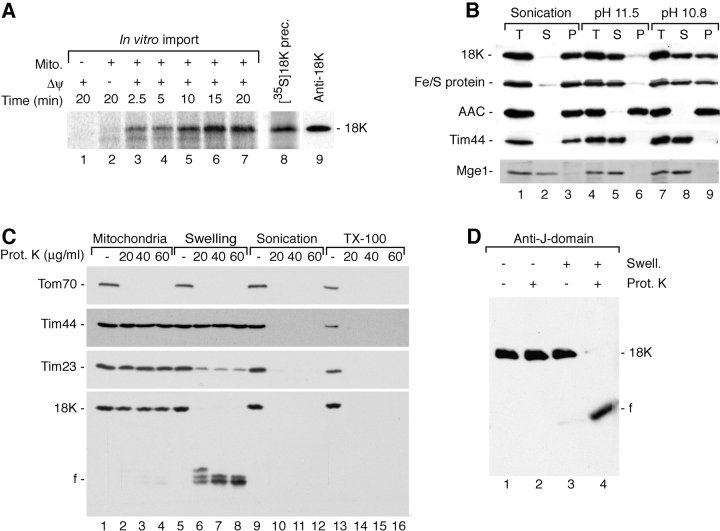
The in vitro synthesized 35S-labeled precursor of the 18-kD J-protein was incubated with isolated yeast mitochondria and transported to a protease-protected location only in the presence of a Δψ (Fig. 2 A). The imported protein had the same mobility on SDS-PAGE as the precursor (Fig. 2 A), indicating that the precursor is not proteolytically processed during import, consistent with the lack of an identifiable presequence in the primary structure (Fig. 1 B). After sonication of mitochondria, the J-protein remained associated with the membrane fraction (Fig. 2 B, lane 3). Since mitochondrial outer membrane proteins are imported independent of a Δψ, these findings indicate that the protein is associated with the mitochondrial inner membrane. Treatment of membranes at pH 11.5 leads to an extraction of peripheral membrane proteins, whereas integral membrane proteins that are embedded in the lipid phase remain in the membrane sheets. The 18-kD J-protein was largely extracted from the membranes at pH 11.5, although it contains one predicted transmembrane segment, whereas the ADP/ATP carrier, which contains six transmembrane segments, remained in the pellet fraction (Fig. 2 B, lane 6). However, the Rieske Fe/S-protein of the bc 1 complex, which spans the inner membrane with one transmembrane segment, showed the same behavior as the J-protein. A small fraction of both of these two proteins remained in the pellet fraction at pH 11.5 (Fig. 2 B, lane 6). At pH 10.8, nearly half of these proteins remained inextractable, whereas Tim44 was still completely extracted (Fig. 2 B, lane 9). The single transmembrane segment of the Fe/S-protein is not entirely embedded in the lipid phase of the inner membrane, but spans the membrane in association with other proteins (Lange and Hunte, 2002), suggesting that the single hydrophobic segment of the 18-kD J-protein similarly spans the membrane in association with other proteins.
Figure 2.
The J-domain is exposed to the mitochondrial matrix. (A) The 35S-labeled precursor of the 18-kD J-protein of PAM was imported into isolated wild-type mitochondria for the indicated times. After proteinase K treatment, samples were analyzed by SDS-PAGE and digital autoradiography. Radiolabeled precursor (lane 8) and Western blot of the authentic, mature 18-kD protein (lane 9) are shown for comparison. (B) Mitochondria were sonicated in the presence of 500 mM NaCl or subjected to treatment at alkaline pH. Samples were left untreated (T) or subjected to centrifugation at 100,000 g. S, supernatant; P, pellet. (C) Part of the J-protein is exposed to the intermembrane space. Mitochondria were either directly treated with proteinase K or subjected to hypotonic swelling, sonication, or Triton X-100 lysis before proteinase K treatment. Samples were separated by SDS-PAGE and subjected to Western blotting. (D) The COOH-terminal portion with the J-domain is exposed to the matrix. Mitochondria were left untreated or subjected to hypotonic swelling before proteinase K treatment. Samples were subjected to SDS-PAGE and Western blotting with affinity-purified anti–18-kD J-domain antibodies.
To address the topology of the novel J-protein, we tested its accessibility to proteinase K. The protein was protected from the protease in mitochondria, but became accessible upon opening of the outer membrane, thus generating a fragment of ∼11 kD (Fig. 2 C). This fragment was degraded when the matrix was opened by sonication or the membranes were dissolved by detergent (Fig. 2 C). Thus, the J-protein exposes a domain to the intermembrane space, whereas another portion is accessible from the matrix side. To determine which terminus was exposed to the intermembrane space, we affinity purified antibodies against the expressed COOH-terminal J-domain. Upon swelling and proteinase K treatment, the generated fragment was efficiently recognized by these antibodies (Fig. 2 D). Thus, the J-domain is protected against protease treatment and exposed to the matrix space.
The essential J-protein is involved in protein import into mitochondria
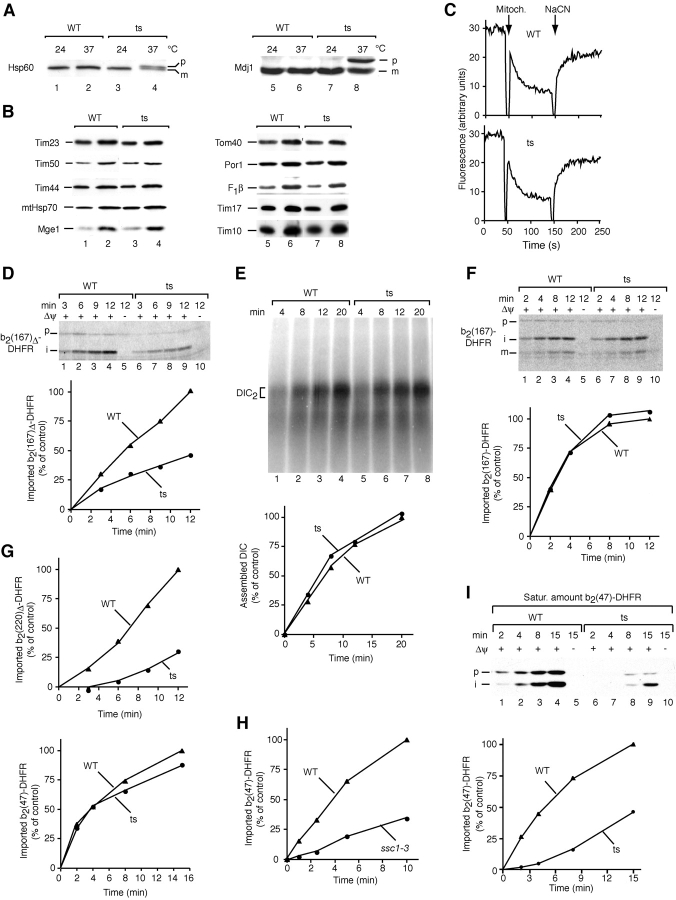
To assess the role of the 18-kD J-protein in protein transport, we generated conditional alleles by error-prone PCR and selected a mutant strain with a temperature-sensitive lethal growth phenotype at 37°C. When the mutant cells were shifted to the nonpermissive temperature, mitochondrial precursor proteins accumulated in vivo (Fig. 3 A). Mutant cells grown at 24°C contained wild-type amounts of all mitochondrial marker proteins analyzed (Fig. 3 B). To minimize indirect effects on mitochondrial functions, the mutant cells were grown at the permissive temperature of 24°C, and isolated mitochondria were preincubated at 37°C before import reactions. J mutant mitochondria were as competent in the generation of a Δψ as wild-type mitochondria (Fig. 3 C). The matrix-targeted preprotein b2(167)Δ-DHFR was synthesized and radiolabeled in rabbit reticulocyte lysate and incubated with isolated mitochondria. Its import, determined by proteolytic processing and transport to a protease-protected location, was up to threefold reduced in the mutant mitochondria compared with wild-type (Fig. 3 D). Import of a carrier protein that uses the second inner membrane translocase (TIM22 complex) was not affected (Fig. 3 E), indicating a specific impairment of the presequence pathway in the mutant mitochondria. When the inner membrane sorting signal of cytochrome b 2 is included in the preprotein, the resulting protein b2(167)-DHFR becomes arrested in the inner membrane by this hydrophobic sorting signal and can be imported in the absence of functional mtHsp70 (Voos et al., 1993). Indeed, the import of b2(167)-DHFR into the mutant mitochondria was similar to that in wild-type mitochondria (Fig. 3 F), raising the possibility that the import defect is related to a functional impairment of mtHsp70.
Figure 3.
The essential mitochondrial J-protein is selectively required for import of matrix proteins. (A) Accumulation of the precursors of mitochondrial proteins in J mutant cells (pam18–1; ts) shifted to 37°C for 16 h. (B) Protein levels. Isolated mitochondria (15-μg protein, odd-numbered lanes; 30-μg protein, even-numbered lanes) were analyzed by Western blotting. Nonrelevant gel lanes were excised digitally. (C) J mutant mitochondria (ts) generate a Δψ. The Δψ was assessed by fluorescence quenching using the dye DiSC3(5). (D) Impaired import of a matrix protein. 35S-labeled precursor was imported into mitochondria. Samples were treated with proteinase K and analyzed by digital autoradiography. (E) Import of a carrier protein is not affected. Dicarboxylate carrier (DIC) import and assembly into its dimeric form were analyzed by blue native PAGE and digital autoradiography. (F) Sorting of cytochrome b 2 at the inner membrane is not inhibited in J mutant mitochondria. Import of radiolabeled precursor was performed as described for D. (G) A relation of the J dependence on preprotein length. Radiolabeled precursors were imported as described above. (H) Import of b2(47)-DHFR is inhibited in ssc1–3 mitochondria. (I) J mutant mitochondria are impaired in import of saturating amounts of b2(47)-DHFR. Urea denaturated purified precursor was imported for the indicated times. Samples were treated with proteinase K and analyzed by Western blotting. The quantification shown is the average of at least three independent experiments. Import into wild-type mitochondria after the longest incubation time was set to 100% (control). p, precursor; i, intermediate; m, mature.
When testing radiochemical amounts of matrix-targeted preproteins of different lengths, we observed that import of a long preprotein, b2(220)Δ-DHFR, was about fourfold reduced in the mutant mitochondria, whereas a short preprotein, b2(47)-DHFR, was imported with an efficiency close to that of wild-type mitochondria (Fig. 3 G). The import of radiolabeled b2(47)-DHFR depends on the function of mtHsp70 as shown with the mutant mitochondria ssc1–3 (Fig. 3 H). We thus used saturating amounts of b2(47)-DHFR such that the import motor had to undergo several rounds of translocation (Lim et al., 2001). Under these conditions, import of b2(47)-DHFR was indeed significantly impaired in the J mutant mitochondria (Fig. 3 I). We conclude that the essential mitochondrial J-protein is required for the import of matrix proteins.
The essential J-protein stimulates mtHsp70
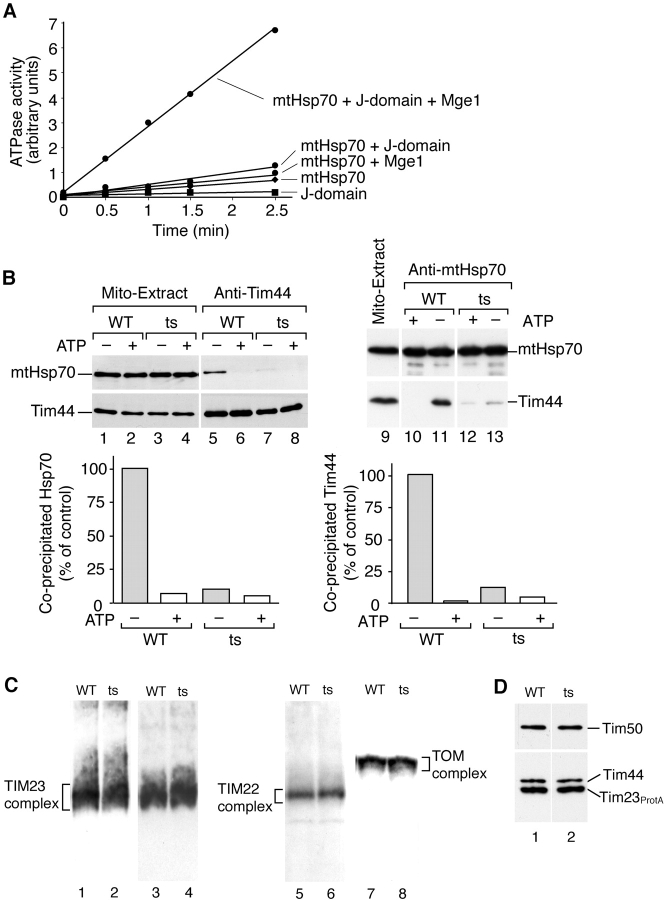
We expressed and purified the J-domain and analyzed its effect on the ATPase activity of purified mtHsp70 under steady-state conditions. An incubation of the J-domain with mtHsp70 only slightly stimulated the ATP turnover, similar to an incubation of purified Mge1 with mtHsp70 (Fig. 4 A) (Weiss et al., 2002). However, addition of the J-domain to mtHsp70 in the presence of Mge1 led to an ∼10-fold stimulation of the ATPase activity (Fig. 4 A). Tim44 did not stimulate the ATPase activity of mtHsp70 (data not shown). We conclude that the J-domain stimulates the activity of mtHsp70 and thus the novel 18-kD J-protein is a subunit of PAM.
Figure 4.
The J-domain stimulates the ATPase activity of mtHsp70 and influences the Tim44–mtHsp70 interaction. (A) ATPase activity of mtHsp70 under steady-state conditions using [α32P]ATP, purified mtHsp70, Mge1, and the J-domain of the 18-kD protein of PAM. Purified Tim44 did not stimulate the ATPase activity. (B) Disturbance of the Tim44–mtHsp70 interaction in J mutant (ts) mitochondria. Mitochondria were lysed under nondenaturing conditions and subjected to coprecipitation with antibodies directed against Tim44 or mtHsp70 (Voisine et al., 1999). Precipitates were analyzed by SDS-PAGE and immunodecoration (5% of the mitochondrial extracts are shown). Quantification: the amount of coprecipitated protein from wild type under ATP-depleted conditions was set to 100% (control). (C) Blue native PAGE of mitochondria lysed with digitonin, followed by immunodecoration with antibodies directed against Tim23, Tim22, and Tom40. (D) Protein A tagged Tim23 was expressed in wild-type and J mutant cells. Mitochondria were isolated, lysed with digitonin, and subjected to IgG chromatography.
To address if the J-protein influences the interaction of mtHsp70 with Tim44, we performed a coprecipitation of the Tim44–mtHsp70 complex upon lysis of mitochondria under nondenaturing conditions. From wild-type mitochondria, the Tim44–mtHsp70 complex was coprecipitated under ATP-depleted conditions (Voisine et al., 1999), whereas with J mutant mitochondria, the yield of Tim44–mtHsp70 complex was strongly decreased, independent if antibodies against Tim44 or mtHsp70 were used (Fig. 4 B). One concern was the possibility that the inhibitory effect of the J mutation on the Tim44–mtHsp70 interaction and protein import may be indirectly caused by a disturbed assembly of the Tim23–Tim17 complex. We thus analyzed the 90-kD Tim23–Tim17 complex by blue native electrophoresis under nonpermissive conditions. The TIM23 complex of the mutant mitochondria was indistinguishable from that of wild-type mitochondria (Fig. 4 C, lanes 1–4, two different gels were used). Moreover, the association of Tim44 with the TIM23 complex was not disturbed (Fig. 4 D). Together with the finding that the insertion of a protein into the inner membrane by the TIM23 complex was not affected in the J mutant (Fig. 3 F), we conclude that the TIM23 complex was correctly assembled and functional.
In summary, we report that an 18-kD integral inner membrane protein with a J-domain constitutes an essential component of the mitochondrial import motor PAM. The J-protein is associated with the presequence translocase. Its inactivation impairs the interaction of mtHsp70 with Tim44 and inhibits the translocation of preproteins into the mitochondrial matrix. The identification of this J-protein indicates that the reaction cycle of mtHsp70 at the protein import site of the mitochondrial inner membrane requires a functional interaction with two essential membrane proteins, the 18-kD J-protein and Tim44. The J-protein promotes the reaction cycle of mtHsp70 by stimulating its ATPase activity.
Materials and methods
Yeast strains and plasmids
Deletion of the ORF YLR008c was found to be lethal to S. cerevisiae cells by two methods, sporulation/tetrad analysis and plasmid shuffling, in the BMA64 and YPH499 backgrounds. Temperature conditional alleles were generated by error prone PCR and subsequently introduced into a gene deletion strain (covered by a plasmid containing the wild-type gene) by gap repair and plasmid shuffle. The mutant YPH-BG-Mdj3–66 (pam18–1) was selected. The mutations led to the amino acid changes S2G, Q4R, V30A, Q46L, N91S, T97S, M108V, K111E, T125A, E131G, N155S. The entire ORF and a fragment encoding amino acids 84–168 were amplified by PCR and cloned into pET10N or pGEX4T1, respectively, for expression in E. coli. For in vitro transcription, the ORF was cloned into pGEM-4Z.
In vitro import into yeast mitochondria
Growth of yeast cells and isolation of mitochondria were performed according to Ryan et al. (2001). For isolation of mutant and wild-type mitochondria, yeast cells were grown at 24°C. Synthesis and transport of 35S-labeled proteins into isolated mitochondria was performed as described (Ryan et al., 2001). For import into mutant mitochondria, mitochondria were preincubated at 37°C for 15 min, and import reactions were performed at 25°C. Import of saturating amounts of urea-denaturated preprotein was performed using 280 pmol of purified b2(47)-DHFR preprotein/mg mitochondrial protein (Dekker et al., 1997). Samples were subsequently separated by SDS-PAGE and either subjected to digital autoradiography (radiolabeled preproteins) or probed with anti-DHFR IgGs (saturating amounts of preprotein).
Fractionation and coimmunoprecipitation
Hypotonic swelling of mitochondria, subsequent treatment with proteinase K and carbonate extraction were performed as described (Ryan et al., 2001). Mitochondria were sonicated on ice (3 × 30 s with 40% duty cycle in a Branson Sonifier 250) in the presence of 5 μg/ml proteinase K in 10 mM Tris, pH 7.4, 500 mM NaCl. Coimmunoprecipitations were performed as described (Voisine et al., 1999). Purification of recombinant proteins and immunization of rabbits was performed as described in Geissler et al. (2002) or by the manufacturer (Amersham Biosciences).
Miscellaneous
Purification of the TIM23 complex was performed according to Geissler et al. (2002) with the exception that elution of the IgG–Sepharose bound complex was achieved by TEV–protease cleavage overnight at 4°C. Isolation of a TOM–TIM translocation intermediate, complex analysis on sucrose gradients, Δψ measurements, digest of proteins in gel, preparation for nano-HPLC and ESI mass spectrometry were performed as described in Geissler et al. (2002). Blue native PAGE was performed as described by Dekker et al. (1997). For ATPase assays, all proteins were purified to homogeneity. MtHsp70 (Ssc1) was purified from mitochondria by NiNTA chromatography. Mge1 was isolated according to Dekker and Pfanner (1997) and the J-domain (GST-Pam1884–168) was purified as described above. ATPase activity was determined according to Dekker and Pfanner (1997).
Acknowledgments
We thank Drs. T. Sommer, T.H. Stevens, and B. Trumpower for antibodies and I. Perschil and N. Zufall for expert technical assistance.
This work was supported by the Sonderforschungsbereich 388, Max Planck Research Award, Nationales Genomforschungsnetz, BMBF, and the Fonds der Chemischen Industrie. M. Lind is a recipient of a postdoctoral fellowship from the Wenner-Gren foundations.
K.N. Truscott and W. Voos contributed equally to this work.
Abbreviations used in this paper: Δψ, membrane potential; mtHsp70, mitochondrial Hsp70; PAM, presequence translocase–associated motor.
References
- Bukau, B., and A.L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell. 92:351–366. [DOI] [PubMed] [Google Scholar]
- Dekker, P.J., and N. Pfanner. 1997. Role of mitochondrial GrpE and phosphate in the ATPase cycle of matrix Hsp70. J. Mol. Biol. 270:321–327. [DOI] [PubMed] [Google Scholar]
- Dekker, P.J., F. Martin, A.C. Maarse, U. Bömer, H. Müller, B. Guiard, M. Meijer, J. Rassow, and N. Pfanner. 1997. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 16:5408–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler, A., A. Chacinska, K.N. Truscott, N. Wiedemann, K. Brandner, A. Sickmann, H.E. Meyer, C. Meisinger, N. Pfanner, and P. Rehling. 2002. The mitochondrial presequence translocase: an essential role of Tim50 in directing preproteins to the import channel. Cell. 111:507–518. [DOI] [PubMed] [Google Scholar]
- Glick, B.S. 1995. Can Hsp70 proteins act as force-generating motors? Cell. 80:11–14. [DOI] [PubMed] [Google Scholar]
- Hartl, F.U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 295:1852–1858. [DOI] [PubMed] [Google Scholar]
- Jensen, R.E., and A.E. Johnson. 1999. Protein translocation: is Hsp70 pulling my chain? Curr. Biol. 9:R779–R782. [DOI] [PubMed] [Google Scholar]
- Kelley, W.L. 1998. The J-domain family and the recruitment of chaperone power. Trends Biochem. Sci. 23:222–227. [DOI] [PubMed] [Google Scholar]
- Kim, R., S. Saxena, D.M. Gordon, D. Pain, and A. Dancis. 2001. J-domain protein, Jac1p, of yeast mitochondria required for iron homeostasis and activity of Fe-S cluster proteins. J. Biol. Chem. 276:17524–17532. [DOI] [PubMed] [Google Scholar]
- Koehler, C.M. 2000. Protein translocation pathways of the mitochondrion. FEBS Lett. 476:27–31. [DOI] [PubMed] [Google Scholar]
- Lange, C., and C. Hunte. 2002. Crystal structure of the yeast cytochrome bc 1 complex with its bound substrate cytochrome c. Proc. Natl. Acad. Sci. USA. 99:2800–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, J.H., F. Martin, B. Guiard, N. Pfanner, and W. Voos. 2001. The mitochondrial Hsp70-dependent import system actively unfolds preproteins and shortens the lag phase of translocation. EMBO J. 20:941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q., P. D'Silva, W. Walter, J. Mszalek, and E.A. Craig. 2003. Regulated cycling of mitochondrial Hsp70 at the protein import channel. Science. 300:139–141. [DOI] [PubMed] [Google Scholar]
- Lutz, T., B. Westermann, W. Neupert, and J.M. Herrmann. 2001. The mitochondrial proteins Ssq1 and Jac1 are required for the assembly of iron sulfur clusters in mitochondria. J. Mol. Biol. 307:815–825. [DOI] [PubMed] [Google Scholar]
- Matlack, K.E., B. Misselwitz, K. Plath, and T.A. Rapoport. 1999. BiP acts as a molecular ratchet during posttranslational transport of prepro-alpha factor across the ER membrane. Cell. 97:553–564. [DOI] [PubMed] [Google Scholar]
- Matouschek, A., N. Pfanner, and W. Voos. 2000. Protein unfolding by mitochondria. The Hsp70 import motor. EMBO Rep. 1:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert, W., and M. Brunner. 2002. The protein import motor of mitochondria. Nat. Rev. Mol. Cell Biol. 3:555–565. [DOI] [PubMed] [Google Scholar]
- Pfanner, N., and A. Geissler. 2001. Versatility of the mitochondrial protein import machinery. Nat. Rev. Mol. Cell Biol. 2:339–349. [DOI] [PubMed] [Google Scholar]
- Pilon, M., and R. Schekman. 1999. Protein translocation: how Hsp70 pulls it off. Cell. 97:679–682. [DOI] [PubMed] [Google Scholar]
- Rowley, N., C. Prip-Buus, B. Westermann, C. Brown, E. Schwarz, B. Barrell, and W. Neupert. 1994. Mdj1p, a novel chaperone of the DnaJ family, is involved in mitochondrial biogenesis and protein folding. Cell. 77:249–259. [DOI] [PubMed] [Google Scholar]
- Ryan, M.T., W. Voos, and N. Pfanner. 2001. Assaying protein import into mitochondria. Methods Cell Biol. 65:189–215. [DOI] [PubMed] [Google Scholar]
- Voisine, C., E.A. Craig, N. Zufall, O. von Ahsen, N. Pfanner, and W. Voos. 1999. The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell. 97:565–574. [DOI] [PubMed] [Google Scholar]
- Voisine, C., Y.C. Cheng, M. Ohlson, B. Schilke, K. Hoff, H. Beinert, J. Marszalek, and E.A. Craig. 2001. Jac1, a mitochondrial J-type chaperone, is involved in the biogenesis of Fe/S clusters in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 98:1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos, W., B.D. Gambill, B. Guiard, N. Pfanner, and E.A. Craig. 1993. Presequence and mature part of preproteins strongly influence the dependence of mitochondrial protein import on heat shock protein 70 in the matrix. J. Cell Biol. 123:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, C., A. Niv, and A. Azem. 2002. Two-step purification of mitochondrial Hsp70, Ssc1p, using Mge1(His)6 immobilized on Ni-agarose. Protein Expr. Purif. 24:268–273. [DOI] [PubMed] [Google Scholar]
- Westermann, B., and W. Neupert. 1997. Mdj2p, a novel DnaJ homolog in the mitochondrial inner membrane of the yeast Saccharomyces cerevisiae. J. Mol. Biol. 272:477–483. [DOI] [PubMed] [Google Scholar]