Abstract
In this issue, Estrada et al. (2003) provide new and important insights into how the endoplasmic reticulum (ER) of budding yeast cells is inherited. Together with other studies in plant and animal cells, the results of Estrada et al. (2003) support the idea that myosin V acts as a universal motor for the transport of ER membranes.
Keywords: actin; myosin V; endoplasmic reticulum; yeast; dendritic spines
The ER is a membrane-bound organelle that functions in protein secretion, membrane protein translation, lipid synthesis, and calcium storage. Based on its localization within the cell, the nuclear ER (or nuclear envelope) can be distinguished from the peripheral ER. The latter forms a network of membrane tubules and sheet-like cisternae that is typically spread throughout the cytoplasm and is continuous with the nuclear ER (Voeltz et al., 2002).
In the first comprehensive morphological description of the ER, Keith R. Porter mentions that “…it is possible to detect a motion which might be interpreted as a migration of nodosities along the strands [of the ER]” (Porter, 1953). It is clear today that the ER indeed represents a dynamic network of membrane tubules that is continuously rearranged. Characteristic movements include the elongation and retraction of membrane tubules, the branching of tubules, the sliding of tubule junctions, and the closure of rings of ER tubules (Lee and Chen, 1988; Voeltz et al., 2002).
The motility of the ER depends on the cytoskeleton. In plants and yeast, F-actin plays a major role in mediating ER motility (Terasaki, 1990; Voeltz et al., 2002). In contrast, microtubules play the predominant role in the formation and motility of the ER in animal cells (Terasaki, 1990; Voeltz et al., 2002). Nevertheless, several observations indicate that actin is also important for ER dynamics in animal cells in vivo (Terasaki and Reese, 1994; Sturmer et al., 1995; Waterman-Storer and Salmon, 1998; Baumann and Walz, 2001).
Little is known about how the ER interacts with the actin cytoskeleton. In this issue, Estrada et al. (2003) report the exciting finding that in Saccharomyces cerevisiae the actin-based class V myosin Myo4p and its interacting partner, She3p, are part of an ER-associated machinery that is required for the inheritance of the peripheral (also called cortical) ER into daughter cells. Class V myosin motors are involved in the transport of a wide variety of cargos including membrane-bound organelles, mRNA, and proteins (Reck-Peterson et al., 2000). Myo4p was already well known for its role in the asymmetric localization of RNAs, including ASH1 mRNA, to daughter cells (Kwon and Schnapp, 2001). In this case, She3p bridges an interaction between Myo4p and the mRNA by binding to both the myosin and the RNA-binding protein, She2p (Kwon and Schnapp, 2001). Efficient accumulation of Myo4p at the tip of the growing bud is dependent on its association with the RNP cargo (Kruse et al., 2002; Gonsalvez et al., 2003).
In budding yeast, the cortical ER forms a membrane network that lies underneath the plasma membrane and is connected via membrane tubules to the nuclear ER (Voeltz et al., 2002). The motility of the cortical ER is similar to that observed for the ER network in animal cells and requires an intact actin cytoskeleton (Prinz et al., 2000). The inheritance of cortical ER takes place in parallel with bud growth. Early in the cell cycle, ER membranes are already enriched at the presumptive bud site (Fehrenbacher et al., 2002). Subsequently, ER tubules extend from the mother cell into the bud along the mother bud axis (Du et al., 2001; Fehrenbacher et al., 2002; Estrada et al., 2003). An immobilization or tethering of these tubules at the bud tip has been detected, suggesting that cortical ER can be passively drawn into the daughter cell during bud growth (Fehrenbacher et al., 2002).
In a careful microscopy study of ER inheritance in live cells, Estrada et al. (2003) are now able to show that the delivery of ER membranes into buds depends on Myo4p and She3p, as well as on F-actin. The analysis of cells expressing fluorescent protein– or HA-tagged marker proteins indicates further that Myo4p is required for the inheritance of cortical ER but not of other organelles, such as vacuoles, mitochondria, and Golgi membranes.
Estrada et al. (2003) also present data clarifying how Myo4p acts in cortical ER inheritance. First, a point mutation in the motor domain of Myo4p has the same effect as the MYO4 deletion, indicating that the motor activity of this myosin is required for cortical ER inheritance. Second, fractionation studies show that Myo4p and She3p are associated with the ER via an interaction that is independent of She2p and RNA. And finally, Estrada et al. (2003) are able to demonstrate that the inheritance of cortical ER is independent of Myo4p's function in mRNA transport. In conclusion, the results establish ER membranes as a new cargo for the class V myosin Myo4p.
Interestingly, in the absence of Myo4p approximately one quarter of daughter cells still receive cortical ER (Estrada et al., 2003), indicating that cortical ER can also be inherited via a Myo4p-independent mechanism. Such an alternative mechanism might involve the passive tethering of ER membranes to the cell cortex of the growing bud as described by Fehrenbacher et al. (2002) (Fig. 1). Relevant to this, an interaction between the β subunit of the Sec61p–ER translocation complex (Seb1p–Sbh1p) and the exocyst complex has been detected (Toikkanen et al., 2003). This connection is noteworthy given that the exocyst complex localizes to the tip of growing buds and Sec3p, one of its components, is required for ER inheritance (Wiederkehr et al., 2003).
Figure 1.
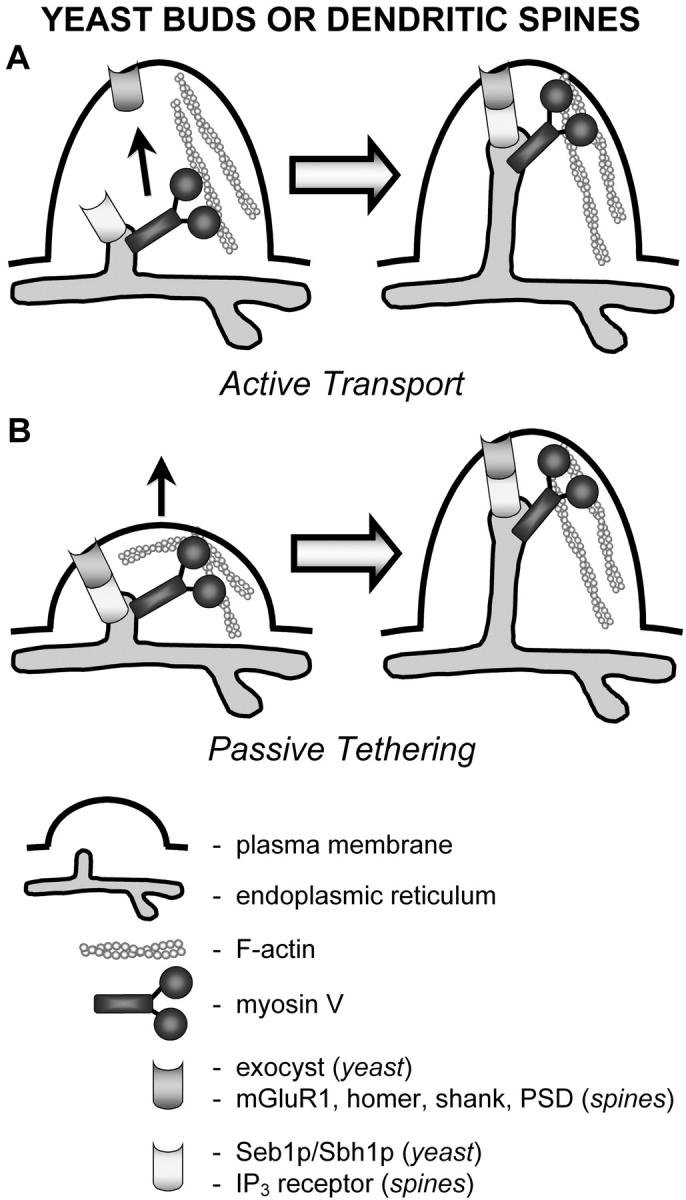
Two models of how ER membranes can be delivered to yeast buds and dendritic spines, whose dimensions are similar. Myosin V could actively transport ER tubules (A) or play a role in tethering ER to the cell surface (B). Interactions between ER membrane proteins and plasma membrane proteins have been detected in both the bud and dendritic spines. These interactions could contribute to the tethering of ER membranes after their myosin V–dependent delivery, or they could allow passive inheritance or localization of ER during bud or spine formation. Other possibilities exist. For example, myosin V could transport a protein or mRNA into the spine that in turn is required for anchoring the ER. PSD, postsynaptic density.
The most dramatic example to date of actin-dependent ER motility is the process of cytoplasmic streaming in plant cells, where the ER network and, by virtue of cytoplasmic viscosity, most of the remaining organelles and constituents are pulled through the cell by an actin-based motor along massive, polarized actin cables (Kachar and Reese, 1988). The motor responsible for this ER movement has recently been identified as myosin V (also called plant myosin XI; Kashiyama et al., 2000; Morimatsu et al., 2000; Kimura et al., 2003).
Myosin V has also been implicated in ER localization and motility in animal cells. The first evidence for this came from studies of cerebellar Purkinje neurons from dilute mice and rats (Dekker-Ohno et al., 1996; Takagishi et al., 1996). These animals carry null mutations in the myosin Va heavy chain gene and are characterized by a lightened coat color and neurological abnormalities (Mercer et al., 1991). Smooth ER is normally present in the dendritic spines of Purkinje cells, where it plays a critical role in synaptic transmission through its ability to regulate local calcium levels. In dilute mice and rats, these spines are devoid of smooth ER membranes (Dekker-Ohno et al., 1996; Takagishi et al., 1996). This deficiency in ER localization or “inheritance” is thought to be responsible for the defect in long-term depression at the parallel fiber–Purkinje cell synapse, and for at least some of the neurological abnormalities exhibited by dilute animals (Miyata et al., 2000). It is currently unclear exactly how myosin V localizes smooth ER in spines. There is considerable evidence that, like in yeast, vertebrate myosin V is an ER-associated motor (see next paragraph), so myosin V might actively transport smooth ER into spines. As in yeast, however, a protein link connecting the ER to the plasma membrane appears to exist in dendritic spines (Sheng and Kim, 2002), so passive tethering may also play a role (Fig. 1).
Support for the idea that vertebrate myosin V is an ER-associated motor has come from immunoelectron microscopy, which shows myosin Va on smooth ER in the dendritic spines of Purkinje cells (Petralia et al., 2001) and in the axons of mouse optic nerves (Rao et al., 2002). Moreover, biochemical studies have shown that myosin Va cofractionates with the ER (Ohashi et al., 2002). Direct evidence of a role for myosin V in ER motility has come from studies using extruded squid axoplasm and Xenopus oocyte extracts (Tabb et al., 1998; Wollert et al., 2002). Tabb et al. (1998) found that ER vesicles isolated from squid axoplasm move along actin filaments in vitro, that myosin V is associated with these vesicles, and that antibodies directed against myosin V block the motility of these ER vesicles. More recently, Wollert et al. (2002) showed in Xenopus egg extracts that myosin V colocalizes extensively with ER tubules and that the actin-dependent motility and network forming capabilities of these tubules are dependent on the myosin.
Together, the findings summarized above suggest strongly that myosin V functions in ER transport in animals, plants, and yeast. Nevertheless, many questions remain unanswered. First, live cell imaging coupled with the use of cells expressing mutant myosin V molecules that move faster or slower (used to great effect to verify the role of Myo2p, the other yeast myosin V, in secretory vesicle transport; Schott et al., 2002) are needed to solidify Myo4p's role in ER movement and inheritance. Second, we need to determine how myosin V interacts with ER membranes. In melanocytes Rab27a and melanophilin recruit myosin Va to melanosomes (Fukuda et al., 2002; Hume et al., 2002; Nagashima et al., 2002; Wu et al., 2002) and in budding yeast Rab GTPases appear to play roles in linking Myo2p to secretory vesicles and mitochondria (Pruyne and Bretscher, 2000; Itoh et al., 2002; Wagner et al., 2002). In contrast, an apparently unrelated Myo2p receptor comprising the proteins Vac8p and Vac17p exists on the yeast vacuole (Ishikawa et al., 2003, Tang et al., 2003). The results of Estrada et al. (2003) suggest that the Myo4p-interacting protein She3p, which is also associated with ER membranes and required for cortical ER inheritance, might be part of the link between myosin V and the ER. Finally, the motility of the ER depends not only on myosin V but on microtubules as well. This is especially the case in animal cells, although in yeast microtubules play a role in the movement of nuclear ER late in the cell cycle (Fehrenbacher et al., 2002). Therefore, it is important to determine how the actin- and microtubule-based movements of the ER are coordinated. Answers to these questions should broaden our knowledge of how motors recognize their cargos and how their activity in the transport of different cargos is regulated.
References
- Baumann, O., and B. Walz. 2001. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int. Rev. Cytol. 205:149–214. [DOI] [PubMed] [Google Scholar]
- Dekker-Ohno, K., S. Hayasaka, Y. Takagishi, S. Oda, N. Wakasugi, K. Mikoshiba, M. Inouye, and H. Yamamura. 1996. Endoplasmic reticulum is missing in dendritic spines of Purkinje cells of the ataxic mutant rat. Brain Res. 714:226–230. [DOI] [PubMed] [Google Scholar]
- Du, Y., M. Pypaert, P. Novick, and S. Ferro-Novick. 2001. Aux1p/Swa2p is required for cortical endoplasmic reticulum inheritance in Saccharomyces cerevisiae. Mol. Biol. Cell. 12:2614–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada, P., J. Kim, J. Coleman, L. Walker, B. Dunn, P. Takizawa, P. Novick, and S. Ferro-Novick. 2003. Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J. Cell Biol. 163:1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher, K.L., D. Davis, M. Wu, I. Boldogh, and L.A. Pon. 2002. Endoplasmic reticulum dynamics, inheritance, and cytoskeletal interactions in budding yeast. Mol. Biol. Cell. 13:854–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, M., T.S. Kuroda, and K. Mikoshiba. 2002. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J. Biol. Chem. 277:12432–12436. [DOI] [PubMed] [Google Scholar]
- Gonsalvez, G.B., K.A. Lehmann, D.K. Ho, E.S. Stanitsa, J.R. Williamson, and R.M. Long. 2003. RNA-protein interactions promote asymmetric sorting of the ASH1 mRNA ribonucleoprotein complex. RNA. 9:1383–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume, A.N., L.M. Collinson, C.R. Hopkins, M. Strom, D.C. Barral, G. Bossi, G.M. Griffiths, and M.C. Seabra. 2002. The leaden gene product is required with Rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic. 3:193–202. [DOI] [PubMed] [Google Scholar]
- Ishikawa, K., N.L. Catlett, J.L. Novak, F. Tang, J.J. Nau, and L.S. Weisman. 2003. Identification of an organelle-specific myosin V receptor. J. Cell Biol. 160:887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, T., A. Watabe, E. Toh, and Y. Matsui. 2002. Complex formation with Ypt11p, a rab-type small GTPase, is essential to facilitate the function of Myo2p, a class V myosin, in mitochondrial distribution in Saccharomyces cerevisiae. Mol. Cell. Biol. 22:7744–7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar, B., and T.S. Reese. 1988. The mechanism of cytoplasmic streaming in characean algal cells: sliding of endoplasmic reticulum along actin filaments. J. Cell Biol. 106:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiyama, T., N. Kimura, T. Mimura, and K. Yamamoto. 2000. Cloning and characterization of a myosin from characean alga, the fastest motor protein in the world. J. Biochem. (Tokyo). 127:1065–1070. [DOI] [PubMed] [Google Scholar]
- Kimura, Y., N. Toyoshima, N. Hirakawa, K. Okamoto, and A. Ishijima. 2003. A kinetic mechanism for the fast movement of Chara myosin. J. Mol. Biol. 328:939–950. [DOI] [PubMed] [Google Scholar]
- Kruse, C., A. Jaedicke, J. Beaudouin, F. Bohl, D. Ferring, T. Guttler, J. Ellenberg, and R.P. Jansen. 2002. Ribonucleoprotein-dependent localization of the yeast class V myosin Myo4p. J. Cell Biol. 159:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, S., and B.J. Schnapp. 2001. RNA localization: SHEdding light on the RNA-motor linkage. Curr. Biol. 11:R166–R168. [DOI] [PubMed] [Google Scholar]
- Lee, C., and L.B. Chen. 1988. Dynamic behavior of endoplasmic reticulum in living cells. Cell. 54:37–46. [DOI] [PubMed] [Google Scholar]
- Mercer, J.A., P.K. Seperack, M.C. Strobel, N.G. Copeland, and N.A. Jenkins. 1991. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature. 349:709–713. [DOI] [PubMed] [Google Scholar]
- Miyata, M., E.A. Finch, L. Khiroug, K. Hashimoto, S. Hayasaka, S.I. Oda, M. Inouye, Y. Takagishi, G.J. Augustine, and M. Kano. 2000. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron. 28:233–244. [DOI] [PubMed] [Google Scholar]
- Morimatsu, M., A. Nakamura, H. Sumiyoshi, N. Sakaba, H. Taniguchi, K. Kohama, and S. Higashi-Fujime. 2000. The molecular structure of the fastest myosin from green algae, Chara. Biochem. Biophys. Res. Commun. 270:147–152. [DOI] [PubMed] [Google Scholar]
- Nagashima, K., S. Torii, Z. Yi, M. Igarashi, K. Okamoto, T. Takeuchi, and T. Izumi. 2002. Melanophilin directly links Rab27a and myosin Va through its distinct coiled-coil regions. FEBS Lett. 517:233–238. [DOI] [PubMed] [Google Scholar]
- Ohashi, S., K. Koike, A. Omori, S. Ichinose, S. Ohara, S. Kobayashi, T.A. Sato, and K. Anzai. 2002. Identification of mRNA/protein (mRNP) complexes containing Puralpha, mStaufen, fragile X protein, and myosin Va and their association with rough endoplasmic reticulum equipped with a kinesin motor. J. Biol. Chem. 277:37804–37810. [DOI] [PubMed] [Google Scholar]
- Petralia, R.S., Y.X. Wang, N. Sans, P.F. Worley, J.A. Hammer III, and R.J. Wenthold. 2001. Glutamate receptor targeting in the postsynaptic spine involves mechanisms that are independent of myosin Va. Eur. J. Neurosci. 13:1722–1732. [DOI] [PubMed] [Google Scholar]
- Porter, K.R. 1953. Observations on a submicroscopic basophilic component of the cytoplasm. J. Exp. Med. 97:727–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz, W.A., L. Grzyb, M. Veenhuis, J.A. Kahana, P.A. Silver, and T.A. Rapoport. 2000. Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J. Cell Biol. 150:461–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. J. Cell Sci. 113:571–585. [DOI] [PubMed] [Google Scholar]
- Rao, M.V., L.J. Engle, P.S. Mohan, A. Yuan, D. Qiu, A. Cataldo, L. Hassinger, S. Jacobsen, V.M. Lee, A. Andreadis, et al. 2002. Myosin Va binding to neurofilaments is essential for correct myosin Va distribution and transport and neurofilament density. J. Cell Biol. 159:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson, S.L., D.W. Provance, Jr., M.S. Mooseker, and J.A. Mercer. 2000. Class V myosins. Biochim. Biophys. Acta. 1496:36–51. [DOI] [PubMed] [Google Scholar]
- Schott, D.H., R.N. Collins, and A. Bretscher. 2002. Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J. Cell Biol. 156:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, M., and M.J. Kim. 2002. Postsynaptic signaling and plasticity mechanisms. Science. 298:776–780. [DOI] [PubMed] [Google Scholar]
- Sturmer, K., O. Baumann, and B. Walz. 1995. Actin-dependent light-induced translocation of mitochondria and ER cisternae in the photoreceptor cells of the locust Schistocerca gregaria. J. Cell Sci. 108:2273–2283. [DOI] [PubMed] [Google Scholar]
- Tabb, J.S., B.J. Molyneaux, D.L. Cohen, S.A. Kuznetsov, and G.M. Langford. 1998. Transport of ER vesicles on actin filaments in neurons by myosin V. J. Cell Sci. 111:3221–3234. [DOI] [PubMed] [Google Scholar]
- Takagishi, Y., S. Oda, S. Hayasaka, K. Dekker-Ohno, T. Shikata, M. Inouye, and H. Yamamura. 1996. The dilute-lethal (dl) gene attacks a Ca2+ store in the dendritic spine of Purkinje cells in mice. Neurosci. Lett. 215:169–172. [DOI] [PubMed] [Google Scholar]
- Tang, F., E.J. Kauffman, J.L. Novak, J.J. Nau, N.L. Catlett, and L.S. Weisman. 2003. Regulated degradation of a class V myosin receptor directs movement of the yeast vacuole. Nature. 422:87–92. [DOI] [PubMed] [Google Scholar]
- Terasaki, M. 1990. Recent progress on structural interactions of the endoplasmic reticulum. Cell Motil. Cytoskeleton. 15:71–75. [DOI] [PubMed] [Google Scholar]
- Terasaki, M., and T.S. Reese. 1994. Interactions among endoplasmic reticulum, microtubules, and retrograde movements of the cell surface. Cell Motil. Cytoskeleton. 29:291–300. [DOI] [PubMed] [Google Scholar]
- Toikkanen, J.H., K.J. Miller, H. Soderlund, J. Jantti, and S. Keranen. 2003. The beta subunit of the Sec61p endoplasmic reticulum translocon interacts with the exocyst complex in Saccharomyces cerevisiae. J. Biol. Chem. 278:20946–20953. [DOI] [PubMed] [Google Scholar]
- Voeltz, G.K., M.M. Rolls, and T.A. Rapoport. 2002. Structural organization of the endoplasmic reticulum. EMBO Rep. 3:944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, W., P. Bielli, S. Wacha, and A. Ragnini-Wilson. 2002. Mlc1p promotes septum closure during cytokinesis via the IQ motifs of the vesicle motor Myo2p. EMBO J. 21:6397–6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer, C.M., and E.D. Salmon. 1998. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr. Biol. 8:798–806. [DOI] [PubMed] [Google Scholar]
- Wiederkehr, A., Y. Du, M. Pypaert, S. Ferro-Novick, and P. Novick. 2003. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol. Biol. Cell. 14:4770–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert, T., D.G. Weiss, H.H. Gerdes, and S.A. Kuznetsov. 2002. Activation of myosin V–based motility and F-actin–dependent network formation of endoplasmic reticulum during mitosis. J. Cell Biol. 159:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X.S., K. Rao, H. Zhang, F. Wang, J.R. Sellers, L.E. Matesic, N.G. Copeland, N.A. Jenkins, and J.A. Hammer III. 2002. Identification of an organelle receptor for myosin-Va. Nat. Cell Biol. 4:271–278. [DOI] [PubMed] [Google Scholar]


