Abstract
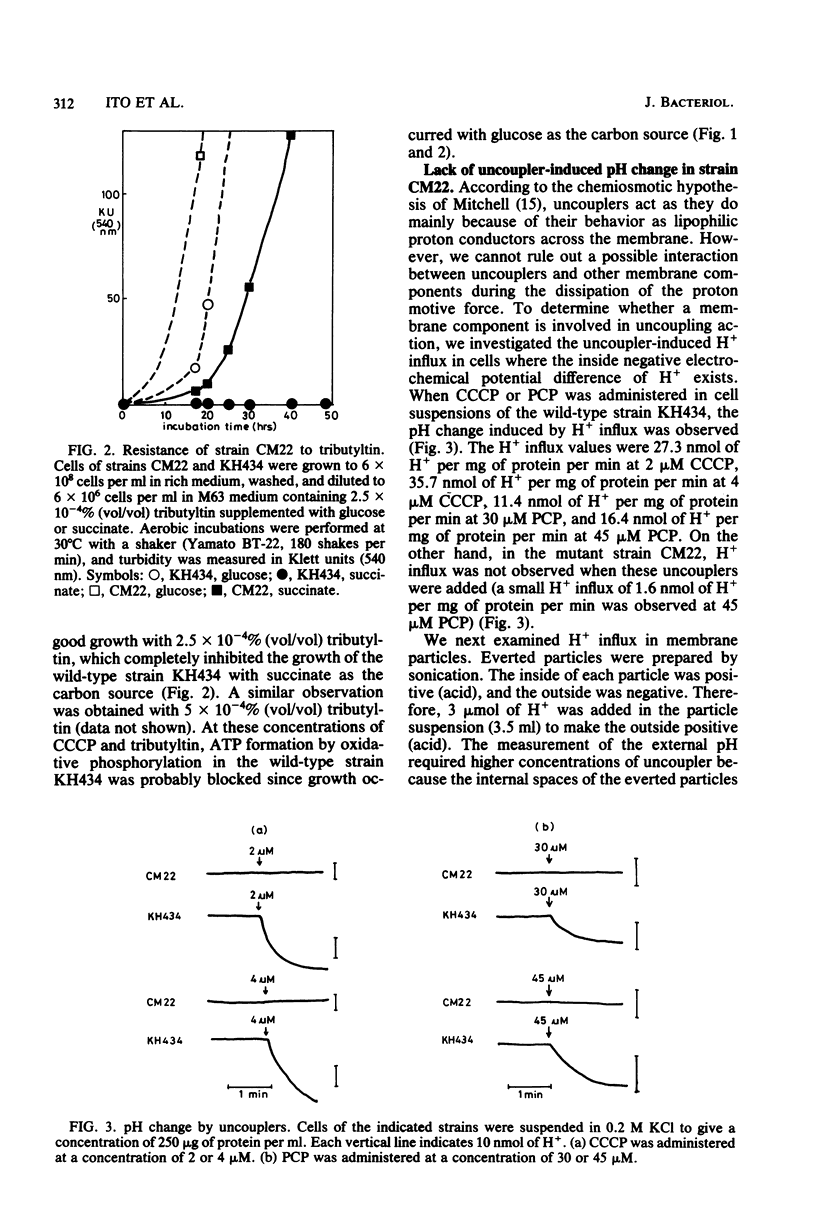
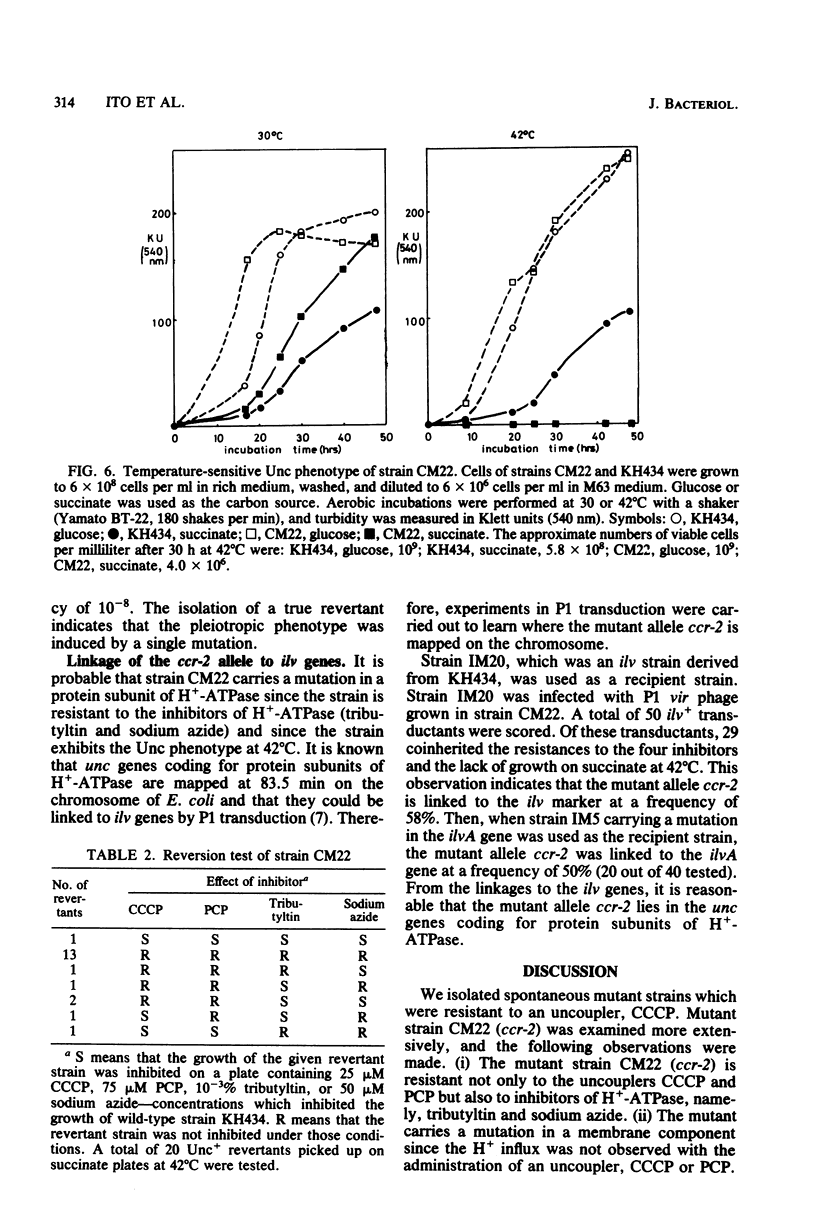
Two spontaneous Escherichia coli mutant strains which are resistant to an oxidative phosphorylation uncoupler, carbonyl cyanide-m-chlorophenyl hydrazone, were isolated. Strain CM22 (ccr-2) was resistant to another uncoupler, pentachlorophenol, and to the inhibitors of proton-translocating ATPase, namely tributyltin and sodium azide. Carbonyl cyanide-m-chlorophenyl hydrazone or pentachlorophenol administered to cell suspensions of strain CM22 did not cause a pH change induced by H+ influx, and a similar result was obtained with everted particles. The respiratory rate of strain CM22 with succinate was twice that of wild-type strain KH434. When carbonyl cyanide-m-chlorophenyl hydrazone was administered, a stimulation of O2 uptake was observed in wild-type strain KH434 but not in the mutant strain CM22. Strain CM22 did not grow on succinate at 42 degrees C. Isolation of a true revertant at a frequency of 10(-8) demonstrated that the pleiotropic phenotype was induced by a single mutation. P1 transduction indicated that the mutant allele, ccr-2, was cotransduced with the ilv genes at a frequency of about 55%.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein C., Tiankova L., Kepes A. Respiratory control in Escherichia coli K 12. Eur J Biochem. 1979 Mar;94(2):387–392. doi: 10.1111/j.1432-1033.1979.tb12905.x. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. Studies on electron transport and energy-linked reactions using mutants of Escherichia coli. Biochim Biophys Acta. 1974 Apr 30;346(1):1–25. doi: 10.1016/0304-4173(74)90010-x. [DOI] [PubMed] [Google Scholar]
- Cyboron G. W., Dryer R. L. Uncoupling of hamster brown adipose and liver mitochondria by 2-azido-4-nitrophenol and binding properties of the reagent. Arch Biochem Biophys. 1977 Feb;179(1):141–146. doi: 10.1016/0003-9861(77)90097-2. [DOI] [PubMed] [Google Scholar]
- Decker S. J., Lang D. R. Membrane bioenergetic parameters in uncoupler-resistant mutants of Bacillus megaterium. J Biol Chem. 1978 Oct 10;253(19):6738–6743. [PubMed] [Google Scholar]
- Decker S. J., Lang D. R. Mutants of Bacillus megaterium resistant to uncouplers of oxidative phosphorylation. J Biol Chem. 1977 Sep 10;252(17):5936–5938. [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Hanstein W. G., Hatefi Y. Characterization and localization of mitochondrial uncoupler binding sites with an uncoupler capable of photoaffinity labeling. J Biol Chem. 1974 Mar 10;249(5):1356–1362. [PubMed] [Google Scholar]
- Hanstein W. G. Uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 1976 Sep 27;456(2):129–148. doi: 10.1016/0304-4173(76)90010-0. [DOI] [PubMed] [Google Scholar]
- Ito M., Ohnishi Y. Isolation of Escherichia coli mutants which are resistant to an inhibitor of H+-ATPase, tributyltin and also to uncouplers of oxidative phosphorylation. FEBS Lett. 1981 Dec 28;136(2):225–230. doi: 10.1016/0014-5793(81)80623-0. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Sone N., Hirata H., Yoshida M. Structure and function of H+-ATPase. J Bioenerg Biomembr. 1979 Aug;11(3-4):39–78. doi: 10.1007/BF00743196. [DOI] [PubMed] [Google Scholar]
- Kovác L. Biochemical mutants: an approach to mitochondrial energy coupling. Biochim Biophys Acta. 1974 Oct 31;346(2):101–135. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Nelson N. Structure and function of chloroplast ATPase. Biochim Biophys Acta. 1976 Nov 30;456(3-4):314–338. doi: 10.1016/0304-4173(76)90003-3. [DOI] [PubMed] [Google Scholar]



