Abstract
The β1 family of integrins has been primarily studied as a set of receptors for the extracellular matrix. In this paper, we define a novel role for α3β1 integrin in association with the tetraspanin CD151 as a component of a cell–cell adhesion complex in epithelial cells that directly stimulates cadherin-mediated adhesion. The integrin–tetraspanin complex affects epithelial cell–cell adhesion at the level of gene expression both by regulating expression of PTPμ and by organizing a multimolecular complex containing PKCβII, RACK1, PTPμ, β-catenin, and E-cadherin. These findings demonstrate how integrin-based signaling can regulate complex biological responses at multiple levels to determine cell morphology and behavior.
Keywords: cell contact; tetraspanin; phosphatase; tyrosine phosphorylation; PKCβII
Introduction
Most, if not all, cellular morphogenetic events result from modulation in cell–cell or cell–substratum adhesion. Traditionally, integrins are thought to mediate cell–matrix adhesion and cadherins cell–cell adhesion. There are well-known exceptions to this rule, such as the leukocyte–endothelium cell–cell interactions mediated by the β2 family of integrins (Carter et al., 1990; Larjava et al., 1990). Nevertheless, integrins of the β1 family have primarily been studied as receptors for the ECM. However, certain members of the β1 family of integrins, when complexed with another family of transmembrane proteins, known as tetraspanins, have been hypothesized to be involved in cell–cell adhesion (Fitter et al., 1999).
Tetraspanins are a group of cell surface molecules that have four transmembrane domains. Tetraspanins are thought to play an important role in a variety of normal and pathological processes, such as cell differentiation, cell motility, egg–sperm fusion, and tumor cell metastasis (Berditchevski, 2001; Boucheix and Rubinstein, 2001). In vivo, α3β1 integrin and CD151 are coexpressed in a variety of epithelial cells, including basal keratinocytes of the skin and glomerular epithelial cells of the kidney (Sterk et al., 2000, 2002). It has been found that the α3β1–CD151 complex is very stable and can withstand conditions that disrupt all other integrin–tetraspanin and tetraspanin–tetraspanin interactions (Yauch et al., 1998; Serru et al., 1999). Notably, the interaction of CD151 and α3β1 integrin has been found to affect cell motility and signaling (Zhang et al., 2001; Yang et al., 2002). Yauch et al. (1998)(2000) reported that the stalk region of the α3 extracellular domain (between 570 and 705 aa) and a region of large extracellular domain of CD151 (between 186 and 217 aa) is important for stable association of these two molecules (Berditchevski et al., 2001). Tetraspanins are not found in focal contacts, nor do they appear to have any effect on ECM adhesion mediated by integrins (Berditchevski et al., 1996; Berditchevski and Odintsova, 1999). However, numerous reports have described the localization of tetraspanins at sites of cell–cell contact, suggesting a possible role in cell–cell adhesion tetraspanins in promoting cell–cell interactions (Fitter et al., 1999; Yanez-Mo et al., 2001).
Several studies have demonstrated that disruption of integrin function in epithelial cells leads to a loss of the cortical cytoskeleton and the assembly of actin stress fibers (Carter et al., 1990; Hodivala-Dilke et al., 1998; Wang et al., 1999). In Wang et al. (1999), it was shown that α3β1 integrin–deficient collecting duct epithelial cells assembled actin stress fibers instead of a subcortical cytoskeleton, and that there was reduced association of the cadherin–catenin complex with α-actinin in α3β1-deficient cells. Here, we show that association of α3β1 integrin with CD151 does indeed promote association of the cadherin–catenin complex with the actin cytoskeleton and cadherin-mediated cell–cell adhesion. Two levels of regulation were evident. First, the integrin–tetraspanin complex regulates the gene expression of PTPμ, a transmembrane protein tyrosine phosphatase previously shown to be involved in cadherin-mediated adhesion (Brady-Kalnay et al., 1995, 1998; Hellberg et al., 2002). Second, we observed a unique complex involving PKCβII, RACK1, and PTPμ used by integrins to regulate cadherin-mediated cell–cell adhesion, possibly by modulating tyrosine phosphorylation of β-catenin. It was possible to identify a large multimolecular complex containing α3β1 integrin–CD151–PKCβII–RACK1–PTPμ–E-cadherin–β-catenin, whose presence in epithelial cells was dependent on the integrin–tetraspanin interaction. The α3β1 integrin receptors associated with the cadherin–catenin complex could be distinguished from those involved in binding laminin. Therefore, two distinct populations of integrins are present in epithelial cells, one involved in cell–matrix adhesion and another involved in cell–cell adhesion.
Results
Expression of chimeric integrin receptors on the surface of α3β1-deficient cells and their association with CD151
Yauch et al. (2000) previously characterized a set of chimeric integrin α subunits, used here, containing the α6 extracellular domain and the α3 cytoplasmic domain (Fig. 1 A). Under their conditions, and confirmed in our studies (Fig. 1 B), CD151 was only able to interact with α subunits containing the α3-stalk region. α3β1-Deficient cells were stably transfected with α6/α3 chimeric integrins, and heterodimeric expression of chimeric α subunits with β1 integrin was confirmed (Fig. 1 B); henceforth, transfected cell lines are designated as α3-stalk and α6-stalk. As in previously published studies (Yauch et al., 2000), only wild-type α3 or α3-stalk subunits interacted with CD151 (Fig. 1 C), the amount of CD151 being equal in all cell lines (Fig. 1 C).
Figure 1.
Association of integrins and CD151. In all panels, the cell lines are designated below the panel showing the first blot; when shown, reblots are below the cell line designations. In all figures: WT, wild-type cells; KO, α3β1 integrin-deficient cells; α3-stalk, cells expressing α6/α3 chimeric integrin that has α3-stalk region; α6-stalk, cells expressing α6/α3 chimeric integrin that has α6-stalk region. (A) A schematic representation of the chimeric α integrin subunits, showing the inclusion of either the α3- or α6-stalk region. (B) Immunoprecipitation of surface-labeled cells using a polyclonal antibody to the α3 integrin cytoplasmic domain or an α6 mAb. Cells were labeled with biotin, lysed using 1% Brij 96 buffer, and lysates were subjected to immunoprecipitation either with the α3 cytoplasmic domain antibody or with the α6 mAb. (C) Coimmunoprecipitation of integrins and CD151. Cell lines were extracted in 1% Triton X-100 buffer and immunoprecipitated with either the α3 or α6 antibody or with the CD151 antibody and immunoblotted with CD151 antibody. The immunoprecipitation and immunoblot antibodies are designated above or below the panels. (D) CD151 shRNA. Wild-type cells were infected with lentivirus containing CD151 shRNA. Cells were extracted in 1% Triton X-100 buffer and tested for CD151 expression and for their association with α3β1 integrin. One of the CD151 shRNAs (CD151 shRNA) blocked the expression and the other one did not (control shRNA) and was used in subsequent experiments as a negative control. (E) Adhesion assay. Plates were coated with laminin-1 or human placental laminin (contains mainly laminin-10 and -11), and adhesion of the cells was analyzed by an MTT assay. Each bar represents the mean of five wells, and SEM bars are shown. 100% adhesion is defined as the adhesion of α6-stalk cells for laminin-1 and wild-type cells for laminin-10 and -11.
To further confirm the specificity of the α3β1–CD151 interaction, it was demonstrated that CD151 could not be coimmunoprecipitated with α6β1 or α6β4 integrins (Fig. 1, B and C). Under other experimental conditions, generally involving the use of mild detergents in the cell extraction, the α6 subunit has been found to interact with CD151 (Zhang et al., 2002). This could also be demonstrated in the cell lines used in this paper (unpublished data), but under the more stringent conditions of Triton X-100 extractions, there was no demonstrable interaction of CD151 with the α6-stalk region.
Multiple tetraspanins interact with α3β1 integrin. Lentiviral-based small hairpin RNA (shRNA; Rubinson et al., 2003) vectors were constructed to allow specific inhibition of CD151 expression (Fig. 1 D). Two of three constructs sufficiently inhibited CD151 expression (Fig. 1 D), no CD151 was found in association with α3β1 integrin. The shRNA vector that did not inhibit CD151 was used in further experiments as a negative control.
α6/α3 chimeric integrins mediate adhesion to laminin-1, -10, and -11
Although α3β1 and α6β1 are both receptors for laminin, α3β1 preferentially binds laminins 5, 10, and 11, whereas α6β1 has been shown to have a less restricted laminin-binding repertoire (Delwel et al., 1994; Kikkawa et al., 1998; Yauch et al., 2000). Cells expressing the α6 extracellular domain (α3-stalk and α6-stalk) showed increased adhesion to laminin-1 in comparison with wild-type and mutant cells (Fig. 1 E). Consistent with the ability of both α3β1 and α6β1 to bind laminin-10 and -11, wild-type and chimeric integrin-expressing cell lines showed increased adhesion to laminin-10 and -11 in comparison with α3β1 integrin–deficient cells. Importantly, the heterodimer of β1 and the α6-stalk–containing subunit mediated adhesion to laminin-10 and -11 despite its inability to interact with CD151. This finding is consistent with observations that integrin–tetraspanin interactions are not involved in modulating the cell–ECM adhesive functions.
α3β1–CD151 complex stimulates E-cadherin–mediated cell–cell adhesion
Cells were trypsinized in the presence of calcium to preserve cadherins (Takeichi, 1977) and kept in suspension, such that the ability of cells to aggregate could be studied independently of cell migration. 50% of the input wild-type cells and α3-stalk–expressing cells became aggregated within 1 h in suspension, and after 3 h, nearly 80% of the cells were found in clusters. In contrast, only 20% of mutant and α6-stalk–expressing cells were found in clusters at 1 h, and only 30% after 3 h in suspension (Fig. 2 A). Similarly, expression of CD151 shRNA decreased the aggregation of wild-type cells down to levels observed for mutant or α6-stalk–expressing cells (Fig. 2 A). Thus, cell aggregation appears to be stimulated by the integrin–tetraspanin interaction.
Figure 2.
Cadherin-mediated cell–cell adhesion. (A) Cell aggregation assay. Cells were kept in suspension for the designated time periods in complete medium, in medium with the addition of the HAV hexapeptide, or in the presence of E-cadherin–blocking antibody. The number of single cells and the number of cells in clusters were counted. The standard curve was used to determine the optimum concentration of HAV hexapeptide. (B) Binding assay. 96-well plates were coated with E-cadherin/Fc recombinant protein and binding of control, E-cadherin–blocking antibody–treated and HAV peptide–treated cells were measured by the MTT assay. Binding of wild-type cells to E-cadherin was considered as 100%, representing binding of over 95% of the input cells. Each bar is the mean of five wells and SEMs are shown.
The hypothesis that integrin–tetraspanin interactions stimulated cadherin-mediated adhesion was examined using an HAV peptide (Makagiansar et al., 2001) or an E-cadherin–blocking antibody (DECMA). Both the HAV peptide and the E-cadherin–blocking antibody were able to eliminate the greater aggregation observed with wild-type or α3-stalk–expressing cells in comparison with mutant and α6-stalk–expressing cells (Fig. 2 A). A control peptide with the HAV sequence reversed to VAH had no effect on cell–cell aggregation. An mAb (Ralph 3.2) known to block binding of α3β1-expressing cells to laminin had no effect on cell aggregation (unpublished data), serving as a negative control for the E-cadherin–blocking antibody. This result also suggested that the laminin binding site of α3β1 integrin was not directly involved in cell aggregation. The role of E-cadherin was confirmed using an adhesion assay in which cells were allowed to adhere to E-cadherin/Fc protein–coated dishes (Fig. 2 B). This assay demonstrated greater binding of wild-type or α3-stalk–expressing cells, and adhesion could be blocked using either the HAV peptide or the E-cadherin–blocking antibody. Cells did not bind significantly to N-cadherin/Fc (Utton et al., 2001)–coated dishes (Fig. 2 B). Thus, the increased aggregation observed in wild-type and α3-stalk–expressing cells appears to be dependent on E-cadherin.
The integrin–tetraspanin interaction is required for the interaction of the cadherin–catenin complex with the subcortical cytoskeleton
Cadherin-mediated cell–cell interactions progress from weak to strong interactions through the assembly of cadherin–catenin complexes and the association of these complexes with the subcortical actin cytoskeleton (Braga, 2000). E-cadherin and β-catenin were present at cell–cell contacts in all cell lines, although cell–cell contacts appeared more diffuse in mutant and α6-stalk cells (Fig. 3 A). α-Actinin is an actin-binding protein shown to be involved in associating the cadherin–catenin complex with the cytoskeleton (Knudsen et al., 1995; Nieset et al., 1997). α-Actinin was present at the cell–cell junction of wild-type and α3-stalk–containing cells but stained diffusely in cells where integrin–tetraspanin interaction was absent, suggesting that the cadherin–catenin complex does not colocalize with α-actinin in these cells. Moreover, a subcortical actin cytoskeleton was present in both wild-type and α3-stalk cells, whereas actin stress fibers were more prominent in mutant and α6-stalk cells (Fig. 3 A) or in cells expressing shRNA for CD151 (Fig. 3 A).
Figure 3.

Cadherin–catenin complexes. (A) Immunocytochemistry. Cells were stained with different antibodies indicated at the top of each panel and cell lines are designated at the left side of each panel. Phalloidin-Texas red was used to stain for actin. (B) E-cadherin immunoprecipitation. Cells were extracted in 1% Triton X-100 buffer and equal amounts of cell extract from each cell lines were immunoprecipitated and blotted. Cell lines and antibodies for immunoprecipitation and immunoblot are shown at the bottom and top of each panel, respectively. (C) Inhibition of CD151 expression with shRNA dissociated α-actinin from the cadherin–catenin complex. (D) Tyrosine phosphorylation of β-catenin. Cells were treated with 1 mM sodium orthovanadate before extraction. All buffers used in this experiment were supplemented with 1 mM sodium orthovanadate. The top panel is the phosphotyrosine blot, and the bottom panel is the reblot with the same antibody used for immunoprecipitation. (E) A model summarizing the results of this figure.
To quantitatively assess the dependency of cadherin–catenin interactions on association of α3β1 with CD151, E-cadherin was immunoprecipitated from the cell lines, and the immunoprecipitate was immunoblotted for α- and β-catenin components of cadherin–catenin complexes (Fig. 3 B). The abundance of E-cadherin and the amount of α- and β-catenin complexed with E-cadherin did not appear to be dependent on the integrin–tetraspanin interaction. In contrast, there was a marked difference between α3- and α6-stalk cells in the amount of actin and α-actinin that could be coimmunoprecipitated with the cadherin–catenin complex (Fig. 3 B). Inhibition of CD151 expression with shRNA also inhibited the association of α-actinin with the cadherin–catenin complex (Fig. 3 C). Thus, in the cell lines under study, the α3β1 integrin–CD151 association appears to regulate the interaction of the assembled cadherin–catenin complex with components of the cytoskeleton.
β-Catenin tyrosine phosphorylation is regulated by the integrin–tetraspanin interaction
The association of E-cadherin with β-catenin can be regulated by the tyrosine phosphorylation of β-catenin (Muller et al., 1999; Roura et al., 1999), and tyrosine phosphorylation of β-catenin generally has been correlated with a loss of epithelial morphology (Ozawa and Kemler, 1998; Provost and Rimm, 1999). Consistent with these observations, loss of the α3β1–CD151 interaction leads to increased tyrosine phosphorylation of β-catenin (Fig. 3 D). It was also observed that the total amount of β-catenin present in wild-type and α3-stalk cells is higher than that of mutant and α6-stalk cells (Fig. 3 D), even though the amount of β-catenin associated with E-cadherin does not differ (Fig. 3 B). However, our work does not support the hypothesis that tyrosine phosphorylation of β-catenin dissociates it from E-cadherin, at least in the cells under study. This is because when cells are pretreated with orthovanadate to preserve phosphorylation (as in Fig. 3 D but not Fig. 3 B), the reblot with an anti–β-catenin antibody shows all the β-catenin in α3β1-deficient and α6-stalk chimera–expressing cells as a higher molecular mass band than that observed in wild-type and α3-stalk chimera–expressing cells (Fig. 3 D), presumably reflecting a hyperphosphorylated state. Combined with our additional observation that the E-cadherin–β-catenin association did not differ depending on the integrin–tetraspanin interaction, our results suggest that tyrosine-phosphorylated β-catenin remains associated with E-cadherin in these cell lines. The results are summarized in a model (Fig. 3 E).
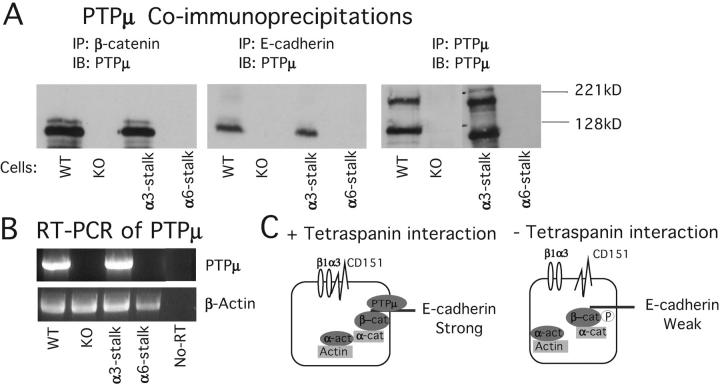
PTPμ expression is regulated by integrin–tetraspanin association
The increased tyrosine phosphorylation observed in mutant cells led us to examine the possibility that a phosphatase activity was decreased in the absence of the integrin–tetraspanin interaction. Several tyrosine phosphatases have been shown to be associated with E-cadherin (Lilien et al., 2002). PTPμ is a transmembrane protein tyrosine phosphatase that can interact with several classical cadherins, including E-cadherin, and can regulate E-cadherin–mediated cell–cell adhesion (Brady-Kalnay et al., 1995, 1998; Hellberg et al., 2002). The proteolytic form of PTPμ (detected at 100 kD) was found to be associated with cadherin–catenin complex only in wild-type and α3-stalk–expressing cells, suggesting a potential role for PTPμ in regulating the tyrosine phosphorylation of the cadherin–catenin complex. Indeed, upon further analysis, it was determined that the full-length (200 kD) and proteolytic (100 kD) forms of PTPμ are expressed only in the cells where the integrin–tetraspanin interaction is present, neither form of PTPμ or PTPμ RNA was found in mutant or α6-stalk–expressing cells (Fig. 4, A and B). This finding indicates a role for the α3β1–CD151 complex in modulating PTPμ gene expression, an area of future studies.
Figure 4.
Association of the cadherin–catenin complex with PTPμ. Antibodies used for immunoprecipitation and immunoblotting are described at the top of each panel and cell lines are designated at the bottom. (A) PTPμ coimmunoprecipitation. PTPμ is expressed only in cells where α3β1–CD151 complex was present and coimmunoprecipitated with E-cadherin and β-catenin. (B) RT-PCR. Total RNA was extracted from the cells and RT-PCR using primers for PTPμ. No-RT, negative control using wild-type RNA but no reverse transcriptase. β-Actin RT-PCR was used as a control. (C) A model summarizing the results of this figure.
The integrin–tetraspanin complex is required for PTPμ regulation of cell–cell adhesion
Because PTPμ is surprisingly absent in mutant and α6-stalk–expressing cells, it became important to evaluate whether PTPμ can regulate cell–cell adhesion in epithelial cells such as those in our work. To first examine whether phosphatases could play a role in integrin–tetraspanin-stimulated cell–cell adhesion, cells were treated with a tyrosine phosphatase inhibitor (bpV/Phen) and cell–cell aggregation, or adhesion of the cells to recombinant E-cadherin protein was studied. Treatment with a tyrosine phosphatase inhibitor blocked the cell aggregation (Fig. 5 A) or E-cadherin/Fc binding of wild-type cells or α3-stalk–expressing cells down to levels observed for mutant or α6-stalk–expressing cells (Fig. 5 B).
Figure 5.
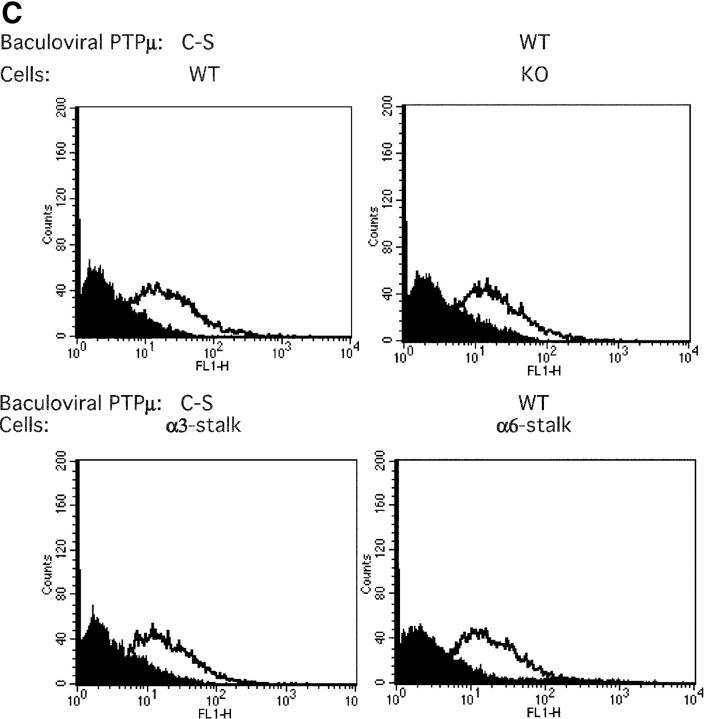
PTPμ baculovirus infection. (A) Cell aggregation assays. Cells were treated with either 1 mM phosphatase inhibitor (bpV [Phen]) or infected with baculovirus expressing either wild type or the C-S mutant of PTPμ, each as a GFP fusion protein. (B) E-cadherin/Fc adhesion assay after baculovirus infection. Each bar is the mean of five wells and SEMs are shown. (C) FACS® analysis after baculovirus expression. The shaded curve shows uninfected cells, and the unshaded curve shows infected cells. (D) Immunoprecipitation. Cells were extracted in 1% Triton X-100 buffer after baculovirus infection. Cadherin–catenin immunoprecipitations and the β-catenin tyrosine phosphorylation assay were performed as described in Materials and methods. (E) Phalloidin staining of baculovirus-infected cells. (F) A model summarizing the results of this figure. When wild-type PTPμ is present in α6-stalk cells, it is not associated with the cadherin–catenin complex (middle model); hence, cadherin-mediated cell–cell adhesion is weak. When the C-S form of PTPμ is overexpressed in wild-type cells (right model), it displaces wild-type PTPμ from its association with the cadherin–catenin complex; β-catenin becomes phosphorylated; and there is weak cadherin-mediated adhesion.
A direct role for PTPμ in α3β1–CD151-stimulated cell–cell adhesion was demonstrated by infecting cells with baculoviruses encoding either wild-type or catalytically inactive PTPμ (C-S mutant) each fused to GFP. Equivalent levels of baculoviral-encoded PTPμ were expressed in each of the cell lines (Fig. 5 C), and direct Western blots after baculoviral infection demonstrated equivalent expression of exogenous PTPμ in all cell lines, that was only in two- to threefold excess of the level of endogenous PTPμ in wild-type cells (Fig. 5 D). Endogenous PTPμ could not be detected in infected cells (Fig. 5 D), suggesting that there is tight regulation of the maximal amount of PTPμ that can be expressed in these cells. Expression of the C-S mutant decreased cell aggregation and adhesion to E-cadherin/Fc of wild-type and α3-stalk cells, dissociated α-actinin from the cadherin–catenin complex, and resulted in disorganization of subcortical actin (Fig. 5, A, B, D, and E). Therefore, these results predict that expression of the wild-type form of PTPμ in mutant or α6-stalk–expressing cells would restore a wild-type phenotype to mutant or α6-stalk–expressing cells. However, expression of wild-type PTPμ did not rescue cell aggregation, adhesion to E-cadherin/Fc, or association of the cadherin–catenin complex with the cytoskeleton in mutant and α6-stalk–expressing cells (Fig. 5, A, B, D, and E), indicating the absolute requirement for the α3β1–CD151 complex to stimulate E-cadherin–mediated cell–cell adhesion. Moreover, expression of the PTPμ C-S mutant in wild-type cells also resulted in tyrosine phosphorylation of β-catenin, but wild-type PTPμ could not decrease tyrosine phosphorylation in α6-stalk or mutant cells. Expression of an enzymatically active baculoviral PTPμ in mutant cells was confirmed by Western blot (Fig. 5 D) and by immunoprecipitation of PTPμ followed by in vitro phosphatase assays (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200306067/DC1). A model summarizing these interactions is provided in Fig. 5 F.
The α3β1–tetraspanin complex stabilizes the interaction of PTPμ with the cadherin–catenin complex
The failure of wild-type PTPμ to rescue mutant or α6-stalk cells suggested that the presence of the integrin–tetraspanin complex on the cell membrane could also affect the association of PTPμ with the cadherin–catenin complex. Previous studies demonstrated an interaction of the α3β1–CD151 complex with PKCβII (Zhang et al., 2001) and an interaction between PTPμ and the adaptor protein RACK1 (Mourton et al., 2001). Because activated PKCβII binds RACK1 (Ron et al., 1994; Mochly-Rosen, 1995; Csukai et al., 1997), these results led us to investigate the possibility that the integrin–tetraspanin complex could stabilize the interaction of PTPμ with the cadherin–catenin complex through the establishment of a complex that contains PKCβII, RACK1, and PTPμ. After cross-linking, PKCβII, RACK1, and PTPμ could be coimmunoprecipitated with α3β1 or the α3-stalk–containing integrin heterodimer, but they were not present in immunoprecipitates from the α6-stalk–expressing cells (Fig. 6 A). Expression of CD151 shRNA also abrogated the association of RACK1 with the integrin–tetraspanin complex (Fig. 6 A). Sequential immunoprecipitations of cross-linked cells were used to demonstrate that the same pool of PTPμ associated with α3β1–CD151 and with the cadherin–catenin complex (Fig. 6 B). As negative controls, none of these components could be coimmunoprecipitated from wild-type cells with α6β1 integrin. As additional negative controls, neither CD44, an abundant membrane protein, nor Ezrin, an abundant peripheral membrane protein associated with the cytoskeleton, could be coimmunoprecipitated with α3β1 integrin, PTPμ, E-cadherin (Fig. 6 A), or RACK1 and PKCβII (not depicted). Additionally, the epidermal growth factor receptor, although present in all cells, was not coimmunoprecipitated with α3β1–CD151 (unpublished data).
Figure 6.
Interactions of the integrin–tetraspanin complex. (A) Coimmunoprecipitations of the integrin–tetraspanin complex. Cell lines and antibodies for immunoprecipitation and immunoblot are shown at the top and bottom of each panel, and cell lines are designated as in Fig. 1. In A–C, cells were cross-linked with 1 mM DSP at 4°C for 1 h before extraction. Cells were extracted in 1% Triton X-100 buffer and subjected to immunoprecipitation either with α3 or α6 antibody. The control α3 reblot of the α3 immunoprecipitation is shown in Fig. 1 C, as this required nonreducing conditions and was run in a separate gel. An α6 immunoprecipitation was used as a negative control. CD151 RNAi experiment. CD151shRNA or control shRNA was expressed in wild-type cells before immunoprecipitation of α3β1 integrin and immunoblotting for RACK1. (B) Sequential immunoprecipitations showing the same pool of PTPμ is associated with α3β1–CD151 complex and the cadherin–catenin complex. Cells were cross-linked before extraction. Proteins were extracted from α3 immunoprecipitates with 1% Triton X-100 buffer containing 0.5% SDS. The second immunoprecipitation was done with PTPμ antibody and the immunoblot was developed either with E-cadherin or β-catenin antibodies. The negative control in the rightmost lane of each panel is a wild-type extract in which the primary antibody is omitted from the second immunoprecipitation. The reblots with PTPμ serve as positive controls for the immunoprecipitations. After obtaining the first blots, the membrane was stripped in buffer containing 62.5 mM Tris-HCl, pH 6.7, 0.7% β-mercaptoethanol, and 2% SDS for 30 min at 50°C and reprobed with a PTPμ antibody. (C and D) Inhibition of PKC–RACK1 interaction. Cells were treated with 1 μM of carrier or PKCβII translocation inhibitor peptide, as designated above the panels, 1 h before beginning the aggregation assay or before extraction. (C) Translocation inhibitor effects on the α3β1 integrin–E-cadherin–PTPμ association. (D) Effect of translocation inhibitor on cell aggregation and cadherin complex association. The inhibitor decreased aggregation of wild-type and α3-stalk cells. Each bar of the aggregation assay histogram is the mean of five wells, and SEMs are shown. Association with α-actinin is lost, but the E-cadherin–β-catenin association is not affected.
The PKCβII–RACK1 interaction, known to be dependent on activation of PKCβII (Ron et al., 1994), is only observed in wild-type or α3-stalk cells, indicating that association of PKCβII with α3β1 integrin–CD151 may activate PKCβII. To further establish the importance of the PKCβII–RACK1 interaction in cell aggregation, cells were treated with a PKCβII translocation inhibitor peptide that blocks the association of PKCβII with RACK1 (Stebbins and Mochly-Rosen, 2001; Fig. 6 C). This showed that the association of PTPμ with E-cadherin or α3β1 integrin was dependent on the association of PKCβII with RACK1 (Fig. 6 C). Moreover, blocking the interaction of PKCβII and RACK1 decreased cell aggregation of integrin–tetraspanin-expressing cells and dissociated α-actinin from the cadherin–catenin complex (Fig. 6 D). In contrast, association of PTPμ with RACK1 was constitutive and not affected by the PKCβII translocation inhibitor peptide (unpublished data). The translocation inhibitor peptide also did not affect the interaction of E-cadherin and β-catenin (Fig. 6 D). Together, these results indicate that the association of α3β1 with CD151 is required to stabilize an interaction between PKCβII and RACK1–PTPμ that regulates the interaction of the cadherin–catenin complex with the cytoskeleton.
α3β1 integrins are expressed on the lateral surface of the epithelial cells and can act as members of the cell–cell adhesion complex
Some integrins, including α3β1 integrin, are often found in a basolateral distribution, particularly in developing epithelia. Confocal microscopy demonstrated the lateral localization of α3β1 in α3-stalk and α6-stalk–expressing cells (Fig. 7 A). Together with the preceding data, this suggests a model in which α3β1 in basal membranes mediates cell–matrix adhesion, whereas α3β1–CD151 in lateral membranes modulates cell–cell adhesion. To further examine the hypothesis that α3β1 molecules associated with cadherin–catenin complexes were separate from those binding laminin, and the converse situation, sequential immunoprecipitations were performed. DiPersio et al. (1995) had shown that it is possible to cross-link α3β1 integrin to the underlying ECM before immunofluorescent staining. Using a similar approach, cells plated on a laminin-5–rich matrix were cross-linked and extracted, and the immunoprecipitate obtained with an anti–α3 integrin antibody was reimmunoprecipitated with anti–laminin-5, and then blotted for E-cadherin, which was not detected (Fig. 7 B). Laminin-5 could be detected after reblot, serving as a positive control for the immunoprecipitations. In the converse experiment, cells were sequentially immunoprecipitated with anti–α3 integrin and anti–E-cadherin, and then blotted for laminin-5, which was not detected, with the reblotting for E-cadherin serving as a positive control (Fig. 7 B). Both experiments showed that distinct pools of α3β1 integrin receptors were either binding laminin-5 or associated with E-cadherin, but not both. To further determine whether the ECM ligand-binding properties of α3β1 integrin affected its association with E-cadherin and PTPμ, cells were plated on fibronectin instead of laminin-5 or -10 and -11. Plating on fibronectin had no effect on the association of α3β1 with CD151, PKCβII, RACK1, or PTPμ, nor did it affect the expression or activity of total PTPμ or that associated with α3β1 integrin (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200306067/DC1), supporting the conclusion that cell–cell interaction is regulated by α3β1 situated at the cell–cell junction and not dependent on interaction of α3β1 with components of the ECM.
Figure 7.
Expression of α3β1 integrin on the lateral surface of epithelial cells. (A) Immunofluorescence staining of α3β1. The xz axis is shown. Staining for α3β1 integrin showed lateral expression along the entire z-axis. Cell lines are shown above each panel. (B) Sequential immunoprecipitations. Cells were treated with 1 mM DSP before extraction. Cell lines and antibodies for immunoprecipitations and immunoblots are shown above each panel. Lane C is the negative control in which the primary antibody is omitted from the second immunoprecipitation.
Discussion
This paper elucidates the mechanism through which the α3β1 integrin–CD151 complex stimulates cadherin-mediated cell–cell adhesion. In contrast to previously identified roles for α3β1 integrin as a receptor for the ECM, we demonstrate that a distinct pool of α3β1 is located along lateral membranes, and is associated with the cadherin–catenin complex. Previous studies have not identified the mechanism whereby cell–cell adhesion is affected by an interaction of integrin and tetraspanin proteins. In this work, we provide direct evidence that cell–cell adhesion regulated by the α3β1–CD151 complex is mediated by the cadherin family of cell adhesion molecules. One major pathway through which the integrin–tetraspanin complex affects cadherin-mediated adhesion is the regulation of expression of PTPμ. PTPμ expression and activity is crucial for stable interaction of the cadherin–catenin complex with the cytoskeleton and for maintaining β-catenin in a hypophosphorylated state. It is not yet proven whether phosphorylation of β-catenin is directly responsible for regulating the interaction of the cadherin–catenin complex with α-actinin or other components of the cytoskeleton. The α3β1–CD151 complex also organizes the multimolecular association of PKCβII, RACK1, PTPμ, E-cadherin, and β-catenin. Because it has previously been demonstrated that purified PTPμ binds E-cadherin in vitro in the absence of integrin–tetraspanin complexes (Brady-Kalnay et al., 1995, 1998), it is likely that this multimolecular association involving integrin–tetraspanin complexes PKCβII and RACK1 stabilizes the interaction of PTPμ with the cadherin–catenin complex. A model that summarizes these results is shown in Fig. 8.
Figure 8.
A model for α3β1 as a component of the cell–cell adhesion complex. The integrin–tetraspanin complex present on the lateral surface of the cells induced expression of PTPμ and can organize a multimolecular complex containing α3β1–CD151–PKCβII–RACK1–PTPμ–β-catenin–E-cadherin.
Integrin function is becoming increasingly complex, a traditional view of integrins as receptors for the ECM represents only a subset of integrin function. Recent studies on α3β1 integrin in keratinocytes suggested a role as a transdominant inhibitor of other integrins (Hodivala-Dilke et al., 1998). In this case, increased adhesion to fibronectin and type IV collagen, which is assumed to be mediated by other integrins, was observed in cells deficient for α3β1. Might the decreased cell–cell adhesion observed in the absence of the integrin–tetraspanin interaction be due to similar loss of inhibitory influences on other integrins? Certainly, promigratory signals from integrins may have the consequence of increasing tyrosine kinase activity and inhibiting cadherin-mediated adhesion. Regulatory cross-talk between these pathways and the regulation of cadherin-mediated adhesion by the integrin–tetraspanin complex is a fertile ground for future investigations.
There has been relatively little study of how expression of specific integrin repertoires may generate specific patterns of gene expression. Previously, the expression of MMP9 was shown to be activated in immortalized keratinocytes in the absence of α3β1 integrin (DiPersio et al., 2000), providing at least one example of a gene expression difference related to α3β1 expression. The dependence of PTPμ expression on the α3β1–tetraspanin interaction demonstrates how epithelial morphology and adhesive behavior can be dramatically affected by differences in gene expression. Activation of phosphatase and kinase expression based on interactions of integrins with ECM ligands or other cell surface molecules, as shown here, provides an indication of how the integrin repertoire may affect cell migration or cell–cell interaction during development or tumorigenesis.
Regulation of cadherin–catenin association
Cadherin-mediated adhesion is regulated through the assembly of cadherin–catenin complexes at the cadherin cytoplasmic domain. The assembly of these complexes is essential for the transition from weak to strong cell–cell contacts. There are different observations with regard to the specific molecular interactions that are affected by signaling events regulating cell–cell interaction and cell morphology. For example, several studies that either increased kinase activity through stimulation with EGF or decreased phosphatase activity using pervanadate or phosphatase mutants demonstrated decreased interaction between a cadherin–catenin complex and the cytoskeleton (Balsamo et al., 1998; Hazan and Norton, 1998; Ozawa and Kemler, 1998). This decreased interaction was correlated with increased tyrosine phosphorylation of β-catenin. Other studies have shown that tyrosine phosphorylation of β-catenin results in decreased interaction of β-catenin with E-cadherin (Muller et al., 1999; Roura et al., 1999). In Wang et al. (1999), we observed yet another level of regulation between the cadherin–catenin complex and α-actinin shown here to be dependent on the integrin–tetraspanin interaction. Wang et al. also detected no integrin-dependent difference in the α-catenin–β-catenin association, confirmed in this work. It is possible that these different observations reflect the distinct cell types used in the respective studies and the different kinase and phosphatase activities present therein. As discussed previously, our paper does not support the hypothesis that tyrosine phosphorylation of β-catenin dissociates it from E-cadherin. However, it is important to note that there are several tyrosine residues in β-catenin, and it is not known if the tyrosine residues phosphorylated in our cell lines are the same identified in a previous paper (Roura et al., 1999).
The HAV sequence is conserved among several members of the cadherin family, and HAV-containing peptides have historically been used to block homophilic interaction between cadherin molecules. Renaud-Young and Gallin recently published a paper in which mutation of the HAV sequence did not affect homophilic adhesion, leading them to suggest that the HAV sequence may not be involved in the initial cadherin homophilic interaction (Renaud-Young and Gallin, 2002). This possibility is consistent with our results because the HAV peptide and the phosphatase inhibitor blocked adhesion of wild-type cells to E-cadherin/Fc down to levels observed for knockout cells, whereas the E-cadherin–blocking antibody entirely blocked adhesion to E-cadherin/Fc.
Integrins, tetraspanins, and cell transformation
Our results lead us to hypothesize that neoplastic transformation of a cell is due to both the activation of specific oncogenes and the loss of signaling molecules from integrin–tetraspanin complexes. In a normal epithelial cell, integrin–tetraspanin complexes direct expression of PTPμ, which binds RACK1 and establishes an integrin–tetraspanin-dependent link to the cadherin–catenin complex, thereby stimulating cadherin-mediated cell–cell adhesion. The RACK1 scaffolding protein binds to the Src tyrosine kinase, and binding of RACK1 to PTPμ or Src is mutually exclusive (Mourton et al., 2001). In a neoplastic cell, increased levels of activated Src may displace PTPμ from RACK1 and suppress cadherin-mediate adhesion, or loss of α3β1 integrin expression may result in a complete loss of PTPμ expression, exacerbating the affect of activated oncogenes.
Hellberg et al. (2002) recently studied the role of PTPμ in conferring cadherin-dependent cell–cell adhesion in prostate carcinoma cells. Both Hellberg et al. (2002) and the present work demonstrate the importance of PTPμ in regulating E-cadherin–mediated adhesion. In contrast to the observations of Hellberg et al., we demonstrated that the phosphatase activity of PTPμ is required to increase E-cadherin–dependent adhesion. Because that work used prostate carcinoma cells, this difference may reflect different levels of activated tyrosine kinases or different integrin–tetraspanin complexes within the respective cell types that rendered the cells more or less sensitive to the phosphatase activity of PTPμ. In support of this hypothesis, N-cadherin–dependent neurite outgrowth does require PTPμ tyrosine phosphatase activity (Burden-Gulley and Brady-Kalnay, 1999). As demonstrated in Hellberg et al., PTPμ expression is variable among carcinoma cells lines. It will be of interest to examine various transformed cell lines and determine if expression of PTPμ correlates with the presence of integrin–tetraspanin complexes. Not all epithelial cells in mammals express α3β1 integrin, and it is likely that other closely related integrins that are also known to associate with tetraspanins may function similarly in other cell types.
In summary, this paper identifies a new role for α3β1 and perhaps other integrins as components of cell–cell adhesion complexes. Association with tetraspanins appears essential for this function, and integrin–tetraspanin complexes may direct specific patterns of gene expression in addition to directing protein–protein interactions at the membrane. In the future, consideration of the role of integrins in disease processes that involved changes in cell morphology, such as epithelial to mesenchymal transitions in fibrosis, or tumor progression, will need to consider this new role for integrins.
Materials and methods
Antibodies, peptides, and other materials
Rabbit polyclonal anti-α3β1 antibody was obtained from R. Hynes (Massachusetts Institute of Technology, Cambridge, MA; DiPersio et al., 1995); rabbit polyclonal anti-GFP was obtained from P. Silver (Dana-Farber Cancer Center, Boston, MA); monoclonal anti-CD151 11b1-G4 (Sincock et al., 1997). mAbs against intracellular domain of PTPμ (SK15 and SK18; Brady-Kalnay et al., 1993). Anti–integrin α6 A6ELE was obtained from M. Hemler (Dana-Farber Cancer Center; Lee et al., 1995). E-cadherin antibody ECCD-2 for immunofluorescence was purchased from Zymed Laboratories; E-cadherin–blocking antibody (monoclonal antiuvomorulin, clone DECMA-1) and anti–α-actinin clone BM-75.2 antibody was purchased from Sigma-Aldrich; E-cadherin (clone 36), β-catenin (clone 14), α-catenin (clone 5), and RACK1 (clone 20) antibodies were purchased from BD Biosciences; antiphosphotyrosine antibody 4G10 was purchased from Upstate Biotechnology; and anti-PTPμ (C-20), anti-α6 (N-19), anti-PKCβII (C-18), and anti-α3β1 (Ralph 3.2) were purchased from Santa Cruz Biotechnology, Inc. Anti-α6 (MA6) for immunoprecipitation and monoclonal anti–laminin-5 (epiligrin, clone P3H9–2) were obtained from Chemicon International. All secondary antibodies were obtained from Jackson ImmunoResearch Laboratories.
E-cadherin–blocking peptide Ac-SHAVAS-NH2 and control peptide (Ac-SVAHAS-NH2) were obtained from New England Peptide, Inc. PKC regulator peptides pp94 and pp96 were obtained from the D. Mochly-Rosen (Stanford University, Stanford, CA).
NHS biotinylation reagent and DSP were obtained from Pierce Chemical Co.; recombinant mouse E-cadherin/Fc chimeric protein, Trichostatin A, and MTT were obtained from Sigma-Aldrich; matrigel was obtained from BD Biosciences; tyrosine phosphatase inhibitor bpV(phen) was obtained from Calbiochem; human placental laminin (contains mainly laminin-10 and -11) was obtained from Chemicon International; and laminin-1 was obtained from BD Biosciences. N-Cadherin/Fc recombinant protein plasmid was obtained from P. Doherty (Kings College, London, UK; Utton et al., 2001).
Oligonucleotides for PTPμ RT-PCR were obtained from Invitrogen. CD151 RNAi oligonucleotides were obtained from IDT. All other common chemicals were obtained from Sigma-Aldrich and Bio-Rad Laboratories.
cDNA constructs
Construction of α6/α3 chimeric integrins was performed as described in Yauch et al. (2000). α6/α3 chimeric integrins were subcloned in pcDNA3.1 hygro (−) vector.
Cell lines
Generation of wild-type and knockout immortalized cell lines from wild-type and α3 mutant mouse kidney collecting ducts was performed as described previously (B7 and B12 cells in Wang et al., 1999). To obtain the α3- and α6-stalk cells, knockout cells were transfected with α6/α3 chimeric integrins in pCDNA3.1 hygro using calcium phosphate transfections and selected for hygromycin resistance. Pools of transfected cells were FACS® sorted using anti–human α6 (A6-ELE) antibody. To culture cells on laminin-5, SCC25 cells (which produce a laminin-5–rich matrix) were grown to confluence and removed (Xia et al., 1996) before plating cells under study.
Cell lysis and immunoprecipitation
Cells were grown in 10-cm dishes precoated with a laminin-5–rich matrix. For immunoprecipitation and blotting of cadherin–catenin complexes, cells were washed with PBS and lysed in lysis buffer (20 mM Tris, pH 7.6, 1% Triton X-100, 2 mM CaCl2, 1 mM benzamidine, 0.1 mM ammonium molybdate, 1 mM PMSF, 20 μg/ml aprotinin, and 10 μg/ml leupeptin). For β-catenin tyrosine phosphorylation assay, cells were pretreated with 1 mM sodium orthovanadate before lysis, and all the buffers for immunoprecipitation and immunoblot were supplemented with 2 mM sodium orthovanadate.
In experiments where the interaction of α3β1–CD151 complex with PKCβII, RACK1, and PTPμ was studied (Fig. 6) and in sequential immunoprecipitations (Figs. 6 and 7), cells were incubated in 1 mM DSP for 1 h at 4°C (to cross-link the proteins) and treated as described by Berditchevski et al. (1995). Integrin surface labeling and immunoprecipitations were conducted as described previously (Wang et al., 1999).
Laminin adhesion assay
96-well plates were coated with human placental laminin (predominantly laminin-10 and -11) or laminin-1 for 2 h at RT, and blocked with 1% BSA in PBS containing 100 mM Ca2+ and 100 mM Mg2+ for 1 h. 2.5 × 104 cells prepared by trypsinization in 200 μl of medium containing 1% FCS were added in each well for 1 h at RT, this being the maximal number of cells that can adhere to a coated well. After washing out nonadherent cells, adherent cells were incubated 3 h in medium containing 800 μg/ml MTT solution. The reaction product was measured at 595 nm. Each data point is the mean of five wells, and SEMs are shown at the top of each bar in the figures. The background level of binding, defined as the number of wild-type cells adhering to a BSA-only well, usually 1–2% of the level of wild-type cells binding to placental laminin, was subtracted from all results. In pilot experiments, absorbance at 595 nm was directly proportional to the number of adherent cells.
E-cadherin/Fc adhesion assay
Cells were treated as previously described (Higgins et al., 1998), 96-well plates were coated with 1.5 μg/ml recombinant mouse E-cadherin/Fc chimeric or with N-cadherin/Fc chimeric proteins and blocked with 1% BSA. To test the effect of E-cadherin–blocking antibody or HAV peptide on cell binding, cells were incubated in 5 μg/ml antibody or 400 μM HAV peptide before adding to the wells. Adhesion was measured as described above for the laminin binding assay.
Cell aggregation assay
Cells were trypsinized in the presence of calcium as described in the preceding section. A single cell suspension was obtained and 2.5 × 104 cells were placed in a 0.2-ml tube and incubated on a rotation apparatus for 0, 1, or 3 h at RT. At the end of the incubation, cells were diluted into single wells of a 6-well plate to prevent further aggregation. After allowing cells to settle for 10 min at 33°C, the number of single cells and cells in clusters were manually counted, counting 10 low-power fields using an inverted tissue culture microscope. The percentage of cells in clusters was calculated as the number in clusters of five or more cells, divided by the total number of single cells and cells in clusters. To study the effect of phosphatase inhibitor or PKC inhibitor peptides on cell–cell aggregation, cells were treated with 1 mM bpV (phen) or 1 μM PKC-regulating or control peptide before trypsinization. In the case of HAV peptide (or control peptide) or antibody treatment, cells were kept in suspension in the presence of 5 μg/ml anti–E-cadherin–blocking antibody or 400 μM HAV peptide (or control peptide).
Immunofluorescent staining
For immunofluorescent staining, the cells were grown overnight in 8-well glass chamber slides coated with human placental laminin (source of laminin-10 and -11). Cells were washed, fixed in 3% PFA, permeabilized with 5% NP-40, and blocked with 10% sheep serum. After blocking, the cells were incubated with E-cadherin, β-catenin, or α-actinin antibodies, followed by FITC-coupled IgG. For actin staining, cells were reacted with Texas red–coupled phalloidin.
RT-PCR
Total RNA was isolated from cells as described previously (Chomczynski and Sacchi, 1987). 7 μg of total RNA was used for reverse transcription reaction using Prostar first stand RT-PCR kit. First strand cDNA was synthesized as described by the manufacturer (Stratagene). The resulting cDNA was subjected to PCR amplification reaction using primers 5′-ACCTCCTCCAACACATCAC-3′ and 5′-TCACGGACACTGTAGAACTC-3′ and following protocol supplied by QIAGEN. The PCR product was visualized using ethidium bromide in 1% agarose gel.
Baculovirus production
Using the pBacMam-2 vector obtained from Novagen, the following constructs were generated: wild-type PTPμ and a catalytically inactive (C-S) mutant form of PTPμ. This vector system allows expression of exogenous genes in mammalian cells using recombinant baculoviruses. The wild-type PTPμ tagged with the GFP at the COOH terminus and the catalytically inactive (C-S) mutant PTPμ-GFP have been described previously (Burden-Gulley and Brady-Kalnay, 1999). The pBPSTR1 plasmids were digested with NotI and the PTPμ-GFP–encoding DNA was ligated into the pBacMAM-2 vector (Novagen) that had been digested with NotI. The recombinant baculoviruses were made using the BaculoGold Transfection System (Invitrogen). In brief, recombinant baculoviruses were generated by calcium phosphate-mediated cotransfection of Sf9 cells with plasmid and viral DNA. Four rounds of virus amplification were performed. The virus was harvested from the Sf9 cells 4 d after infection. To infect mammalian cells, 500 μl of viral supernatant was added to a 10-cm dish of cells containing 4 ml of media and incubated at 37°C for 2 h. After incubation all the culture media was removed and fresh media containing a final concentration of 150 nM Trichostatin A (Sigma-Aldrich) was added to the cultures. 16 to 24 h after virus addition, PTPμ expression was analyzed by FACS®. Exogenous PTPμ expression was also verified by immunoblotting lysates from infected cells with antibodies to PTPμ.
CD151 RNAi
Three sequences were selected from mouse CD151 gene, which were predicted to form CD151 shRNA. The oligos were inserted into pLentilo×3.7 vector obtained from L. van Parijs (Massachusetts Institute of Technology; Rubinson et al., 2003) to generate pLL CD151–1, pLL-CD151–2, and pLL-CD151–3, and cotransfected with packaging vectors into 293T cells. This vector expresses shRNA and GFP, each from distinct promoters. The lentivirus in the supernatant was collected after 24 h and used directly to infect the wild-type cell line; GFP expression was maximum after 48 h of infection. Immunoprecipitation, immunofluorescence, and cell–cell aggregation assay were done after 48 h of infection.
Online supplemental material
Fig. S1 shows in vitro phosphatase assays demonstrating the activity of endogenous and baculoviral PTPμ. Fig. S2 demonstrates similar behavior of cells plated on fibronectin or laminin. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200306067/DC1.
Supplemental Material
Acknowledgments
S.M. Brady-Kalnay acknowledges the technical assistance of Tracy Mourton and Carol Luckey. The authors thank Douglas Cotanche for assistance with confocal microscopy and Mary Taglienti for technical assistance. The confocal microscope facility is supported by the Sarah Fuller Fund. Daria Mochly-Rosen is acknowledged for the PKC inhibitor peptides, Martin Hemler for chimeric integrin constructs, and Luk van Parijs for the Lentiviral shRNA vectors. The authors have no commercial interest in this work.
This work was supported by a National Institute of Diabetes and Digestive and Kidney Diseases grant DK57604 to J.A. Kreidberg and National Institutes of Health grant EY12251 to S.M. Brady-Kalnay. S.M. Brady-Kalnay acknowledges additional support from a Visual Sciences Research Center Core grant from the National Eye Institute (grant P0-EY11373).
The online version of this article contains supplemental material.
Abbreviation used in this paper: shRNA, small hairpin RNA.
References
- Balsamo, J., C. Arregui, T. Leung, and J. Lilien. 1998. The nonreceptor protein tyrosine phosphatase PTP1B binds to the cytoplasmic domain of N-cadherin and regulates the cadherin–actin linkage. J. Cell Biol. 143:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski, F. 2001. Complexes of tetraspanins with integrins: more than meets the eye. J. Cell Sci. 114:4143–4151. [DOI] [PubMed] [Google Scholar]
- Berditchevski, F., and E. Odintsova. 1999. Characterization of integrin–tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J. Cell Biol. 146:477–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski, F., G. Bazzoni, and M.E. Hemler. 1995. Specific association of CD63 with the VLA-3 and VLA-6 integrins. J. Biol. Chem. 270:17784–17790. [DOI] [PubMed] [Google Scholar]
- Berditchevski, F., M.M. Zutter, and M.E. Hemler. 1996. Characterization of novel complexes on the cell surface between integrins and proteins with 4 transmembrane domains (TM4 proteins). Mol. Biol. Cell. 7:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski, F., E. Gilbert, M.R. Griffiths, S. Fitter, L. Ashman, and S.J. Jenner. 2001. Analysis of the CD151-alpha3beta1 integrin and CD151-tetraspanin interactions by mutagenesis. J. Biol. Chem. 276:41165–41174. [DOI] [PubMed] [Google Scholar]
- Boucheix, C., and E. Rubinstein. 2001. Tetraspanins. Cell. Mol. Life Sci. 58:1189–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay, S.M., A.J. Flint, and N.K. Tonks. 1993. Homophilic binding of PTPμ, a receptor-type protein tyrosine phosphatase, can mediate cell–cell aggregation. J. Cell Biol. 122:961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay, S.M., D.L. Rimm, and N.K. Tonks. 1995. Receptor protein tyrosine phosphatase PTPμ associates with cadherins and catenins in vivo. J. Cell Biol. 130:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay, S.M., T. Mourton, J.P. Nixon, G.E. Pietz, M. Kinch, H. Chen, R. Brackenbury, D.L. Rimm, R.L. Del Vecchio, and N.K. Tonks. 1998. Dynamic interaction of PTPμ with multiple cadherins in vivo. J. Cell Biol. 141:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga, V. 2000. Epithelial cell shape: cadherins and small GTPases. Exp. Cell Res. 261:83–90. [DOI] [PubMed] [Google Scholar]
- Burden-Gulley, S.M., and S.M. Brady-Kalnay. 1999. PTPμ regulates N-cadherin–dependent neurite outgrowth. J. Cell Biol. 144:1323–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, W.G., E.A. Wayner, T.S. Bouchard, and P. Kaur. 1990. The role of integrins α2β1 and α3β1 in cell–cell and cell–substrate adhesion of human epidermal cells. J. Cell Biol. 110:1387–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156–159. [DOI] [PubMed] [Google Scholar]
- Csukai, M., C.H. Chen, M.A. De Matteis, and D. Mochly-Rosen. 1997. The coatomer protein beta'-COP, a selective binding protein (RACK) for protein kinase Cepsilon. J. Biol. Chem. 272:29200–29206. [DOI] [PubMed] [Google Scholar]
- Delwel, G.O., A.A. de Melker, F. Hogervorst, L.H. Jaspars, D.L. Fles, I. Kuikman, A. Lindblom, M. Paulsson, R. Timpl, and A. Sonnenberg. 1994. Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol. Biol. Cell. 5:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio, C.M., S. Shah, and R.O. Hynes. 1995. alpha 3A beta 1 integrin localizes to focal contacts in response to diverse extracellular matrix proteins. J. Cell Sci. 108:2321–2336. [DOI] [PubMed] [Google Scholar]
- DiPersio, C.M., M. Shao, L. Di Costanzo, J.A. Kreidberg, and R.O. Hynes. 2000. Mouse keratinocytes immortalized with large T antigen acquire alpha3beta1 integrin-dependent secretion of MMP-9/gelatinase B. J. Cell Sci. 113:2909–2921. [DOI] [PubMed] [Google Scholar]
- Fitter, S., P.M. Sincock, C.N. Jolliffe, and L.K. Ashman. 1999. Transmembrane 4 superfamily protein CD151 (PETA-3) associates with beta 1 and alpha IIb beta 3 integrins in haemopoietic cell lines and modulates cell-cell adhesion. Biochem. J. 338:61–70. [PMC free article] [PubMed] [Google Scholar]
- Hazan, R.B., and L. Norton. 1998. The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. J. Biol. Chem. 273:9078–9084. [DOI] [PubMed] [Google Scholar]
- Hellberg, C.B., S.M. Burden-Gulley, G.E. Pietz, and S.M. Brady-Kalnay. 2002. Expression of the receptor protein-tyrosine phosphatase, PTPmu, restores E-cadherin-dependent adhesion in human prostate carcinoma cells. J. Biol. Chem. 277:11165–11173. [DOI] [PubMed] [Google Scholar]
- Higgins, J.M., D.A. Mandlebrot, S.K. Shaw, G.J. Russell, E.A. Murphy, Y.T. Chen, W.J. Nelson, C.M. Parker, and M.B. Brenner. 1998. Direct and regulated interaction of integrin αEβ7 with E-cadherin. J. Cell Biol. 140:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodivala-Dilke, K.M., C.M. DiPersio, J.A. Kreidberg, and R.O. Hynes. 1998. Novel roles for α3β1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J. Cell Biol. 142:1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa, Y., N. Sanzen, and K. Sekiguchi. 1998. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J. Biol. Chem. 273:15854–15859. [DOI] [PubMed] [Google Scholar]
- Knudsen, K.A., A.P. Soler, K.R. Johnson, and M.J. Wheelock. 1995. Interaction of α-actinin with the cadherin/catenin cell–cell adhesion complex via α-catenin. J. Cell Biol. 130:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava, H., J. Peltonen, S.K. Akiyama, S.S. Yamada, H.R. Gralnick, J. Uitto, and K.M. Yamada. 1990. Novel function for β1 integrins in keratinocyte cell–cell interactions. J. Cell Biol. 110:803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, R.T., F. Berditchevski, G.C. Cheng, and M.E. Hemler. 1995. Integrin-mediated collagen matrix reorganization by cultured human vascular smooth muscle cells. Circ. Res. 76:209–214. [DOI] [PubMed] [Google Scholar]
- Lilien, J., J. Balsamo, C. Arregui, and G. Xu. 2002. Turn-off, drop-out: functional state switching of cadherins. Dev. Dyn. 224:18–29. [DOI] [PubMed] [Google Scholar]
- Makagiansar, I.T., M. Avery, Y. Hu, K.L. Audus, and T.J. Siahaan. 2001. Improving the selectivity of HAV-peptides in modulating E-cadherin-E-cadherin interactions in the intercellular junction of MDCK cell monolayers. Pharm. Res. 18:446–453. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen, D. 1995. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 268:247–251. [DOI] [PubMed] [Google Scholar]
- Mourton, T., C.B. Hellberg, S.M. Burden-Gulley, J. Hinman, A. Rhee, and S.M. Brady-Kalnay. 2001. The PTPmu protein-tyrosine phosphatase binds and recruits the scaffolding protein RACK1 to cell-cell contacts. J. Biol. Chem. 276:14896–14901. [DOI] [PubMed] [Google Scholar]
- Muller, T., A. Choidas, E. Reichmann, and A. Ullrich. 1999. Phosphorylation and free pool of beta-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J. Biol. Chem. 274:10173–10183. [DOI] [PubMed] [Google Scholar]
- Nieset, J.E., A.R. Redfield, F. Jin, K.A. Knudsen, K.R. Johnson, and M.J. Wheelock. 1997. Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J. Cell Sci. 110:1013–1022. [DOI] [PubMed] [Google Scholar]
- Ozawa, M., and R. Kemler. 1998. Altered cell adhesion activity by pervanadate due to the dissociation of alpha-catenin from the E-cadherin.catenin complex. J. Biol. Chem. 273:6166–6170. [DOI] [PubMed] [Google Scholar]
- Provost, E., and D.L. Rimm. 1999. Controversies at the cytoplasmic face of the cadherin-based adhesion complex. Curr. Opin. Cell Biol. 11:567–572. [DOI] [PubMed] [Google Scholar]
- Renaud-Young, M., and W.J. Gallin. 2002. In the first extracellular domain of E-cadherin, heterophilic interactions, but not the conserved His-Ala-Val motif, are required for adhesion. J. Biol. Chem. 277:39609–39616. [DOI] [PubMed] [Google Scholar]
- Ron, D., C.H. Chen, J. Caldwell, L. Jamieson, E. Orr, and D. Mochly-Rosen. 1994. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc. Natl. Acad. Sci. USA. 91:839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura, S., S. Miravet, J. Piedra, A. Garcia de Herreros, and M. Dunach. 1999. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J. Biol. Chem. 274:36734–36740. [DOI] [PubMed] [Google Scholar]
- Rubinson, D.A., C.P. Dillon, A.V. Kwiatkowski, C. Sievers, L. Yang, J. Kopinja, M. Zhang, M.T. McManus, F.B. Gertler, M.L. Scott, and L. Van Parijs. 2003. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 33:401–406. [DOI] [PubMed] [Google Scholar]
- Serru, V., F. Le Naour, M. Billard, D.O. Azorsa, F. Lanza, C. Boucheix, and E. Rubinstein. 1999. Selective tetraspan-integrin complexes (CD81/alpha4beta1, CD151/alpha3beta1, CD151/alpha6beta1) under conditions disrupting tetraspan interactions. Biochem. J. 340:103–111. [PMC free article] [PubMed] [Google Scholar]
- Sincock, P.M., G. Mayrhofer, and L.K. Ashman. 1997. Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissues: comparison with CD9, CD63, and alpha5beta1 integrin. J. Histochem. Cytochem. 45:515–525. [DOI] [PubMed] [Google Scholar]
- Stebbins, E.G., and D. Mochly-Rosen. 2001. Binding specificity for RACK1 resides in the V5 region of beta II protein kinase C. J. Biol. Chem. 276:29644–29650. [DOI] [PubMed] [Google Scholar]
- Sterk, L.M., C.A. Geuijen, L.C. Oomen, J. Calafat, H. Janssen, and A. Sonnenberg. 2000. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin α6β4 and may regulate the spatial organization of hemidesmosomes. J. Cell Biol. 149:969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterk, L.M., C.A. Geuijen, J.G. van den Berg, N. Claessen, J.J. Weening, and A. Sonnenberg. 2002. Association of the tetraspanin CD151 with the laminin-binding integrins alpha3beta1, alpha6beta1, alpha6beta4 and alpha7beta1 in cells in culture and in vivo. J. Cell Sci. 115:1161–1173. [DOI] [PubMed] [Google Scholar]
- Takeichi, M. 1977. Functional correlation between cell adhesive properties and some cell surface proteins. J. Cell Biol. 75:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utton, M.A., B. Eickholt, F.V. Howell, J. Wallis, and P. Doherty. 2001. Soluble N-cadherin stimulates fibroblast growth factor receptor dependent neurite outgrowth and N-cadherin and the fibroblast growth factor receptor co-cluster in cells. J. Neurochem. 76:1421–1430. [DOI] [PubMed] [Google Scholar]
- Wang, Z., J.M. Symons, S.L. Goldstein, A. McDonald, J.H. Miner, and J.A. Kreidberg. 1999. (Alpha)3(beta)1 integrin regulates epithelial cytoskeletal organization. J. Cell Sci. 112:2925–2935. [DOI] [PubMed] [Google Scholar]
- Xia, Y., S.G. Gil, and W.G. Carter. 1996. Anchorage mediated by integrin α6β4 to laminin 5 (epiligrin) regulates tyrosine phosphorylation of a membrane-associated 80-kD protein. J. Cell Biol. 132:727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Mo, M., R. Tejedor, P. Rousselle, and F. Sanchez-Madrid. 2001. Tetraspanins in intercellular adhesion of polarized epithelial cells: spatial and functional relationship to integrins and cadherins. J. Cell Sci. 114:577–587. [DOI] [PubMed] [Google Scholar]
- Yang, X., C. Claas, S.K. Kraeft, L.B. Chen, Z. Wang, J.A. Kreidberg, and M.E. Hemler. 2002. Palmitoylation of tetraspanin proteins: modulation of CD151 lateral interactions, subcellular distribution, and integrin-dependent cell morphology. Mol. Biol. Cell. 13:767–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch, R.L., F. Berditchevski, M.B. Harler, J. Reichner, and M.E. Hemler. 1998. Highly stoichiometric, stable, and specific association of integrin alpha3beta1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol. Biol. Cell. 9:2751–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch, R.L., A.R. Kazarov, B. Desai, R.T. Lee, and M.E. Hemler. 2000. Direct extracellular contact between integrin alpha(3)beta(1) and TM4SF protein CD151. J. Biol. Chem. 275:9230–9238. [DOI] [PubMed] [Google Scholar]
- Zhang, X.A., A.L. Bontrager, and M.E. Hemler. 2001. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J. Biol. Chem. 276:25005–25013. [DOI] [PubMed] [Google Scholar]
- Zhang, X.A., A.R. Kazarov, X. Yang, A.L. Bontrager, C.S. Stipp, and M.E. Hemler. 2002. Function of the tetraspanin CD151-alpha6beta1 integrin complex during cellular morphogenesis. Mol. Biol. Cell. 13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.