Abstract
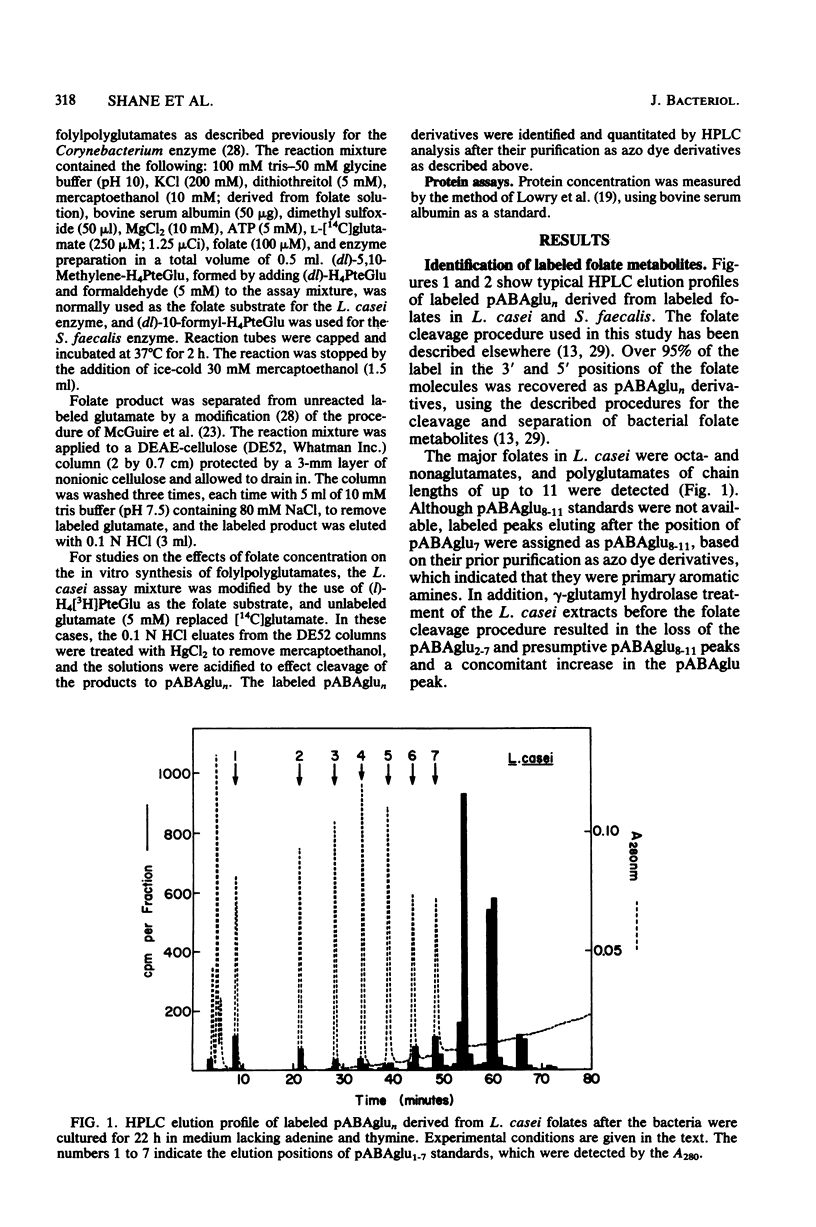
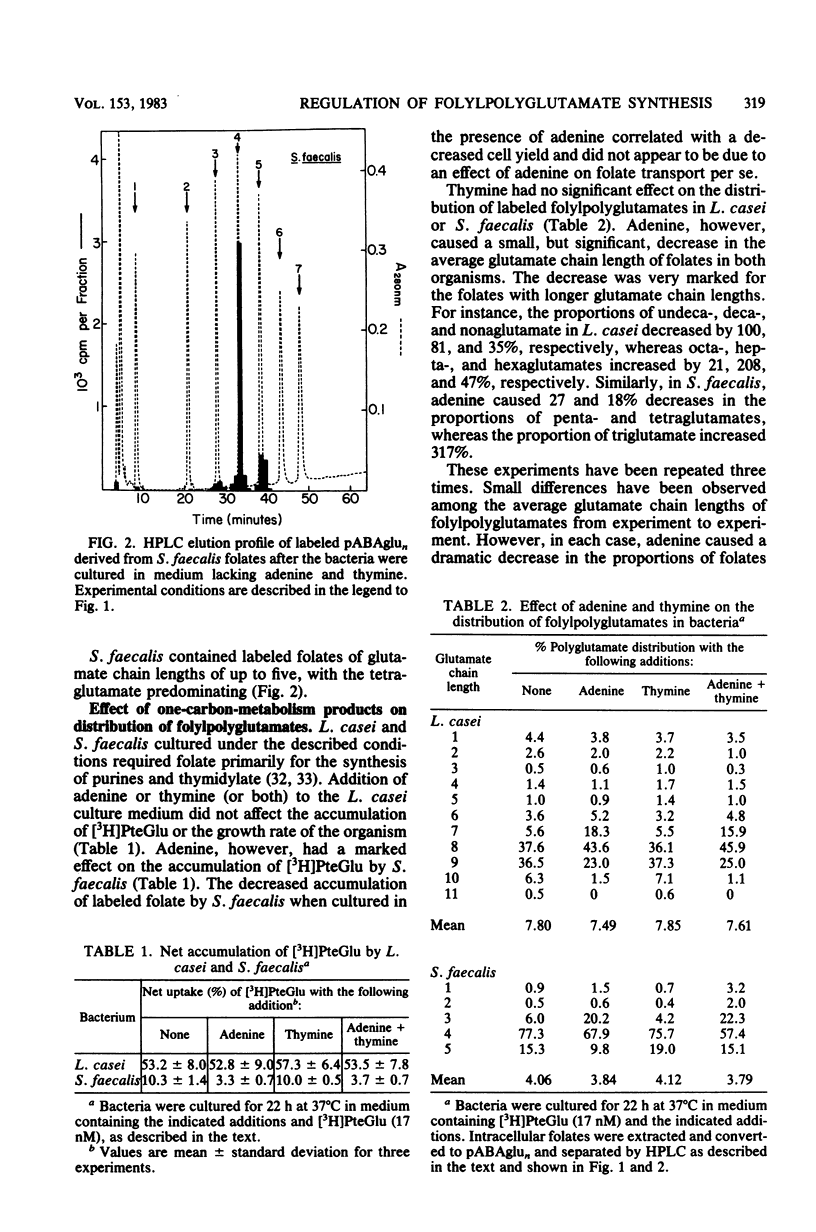
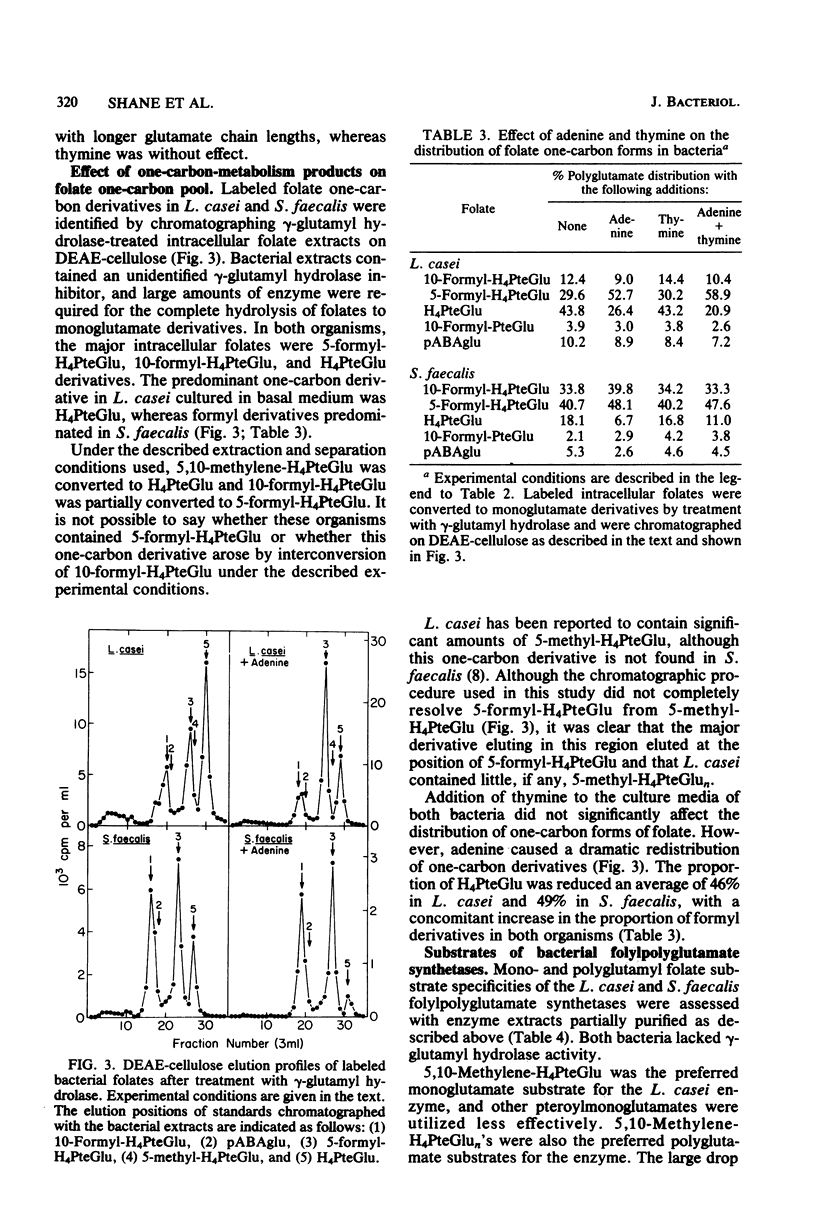
Lactobacillus casei and Streptococcus faecalis accumulated labeled folic acid and metabolized this compound to poly-gamma-glutamates of chain lengths of up to 11 and 5, respectively. Octa- and nonaglutamates predominated in L. casei, and tetraglutamates predominated in S. faecalis. The most effective monoglutamate substrates for the L. casei and S. faecalis folylpoly-gamma-glutamate (folylpolyglutamate) synthetases were methylene- and formyltetrahydrofolate, respectively. Methylenetetrahydropteroylpoly-gamma-glutamates were the preferred poly-gamma-glutamate substrates for both enzymes and, in each case, the highest activity was observed with the diglutamate substrate. The final distribution of folylpolyglutamates in these bacteria appeared to reflect the ability of folates with various glutamate chain lengths to act as substrates for the bacterial folylpolyglutamate synthetases. The proportions of individual folylpolyglutamates were markedly affected by culturing the bacteria in medium containing adenine, whereas thymine was without effect. Adenine did not affect the level of folylpolyglutamate synthetase in either organism but caused a large increase in the proportion of intracellular folates containing one-carbon units at the oxidation level of formate, folates which are substrates for enzymes involved in purine biosynthesis. The folates with shorter glutamate chain lengths in bacteria cultured in the presence of adenine resulted from primary regulation of the de novo purine biosynthetic pathway, regulation which caused an accumulation of formyltetrahydropteroyl-poly-gamma-glutamates (folate derivatives that are ineffective substrates for folylpolyglutamate synthetases), and did not result from regulation of folylpolyglutamate synthetase per se.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baugh C. M., Braverman E., Nair M. G. The identification of poly-gamma-glutamyl chain lengths in bacterial folates. Biochemistry. 1974 Nov 19;13(24):4952–4957. doi: 10.1021/bi00721a012. [DOI] [PubMed] [Google Scholar]
- Baugh C. M., Stevens J. C., Krumdieck C. L. Studies on gamma-glutamyl carboxypeptidase. I. The solid phase synthesis of analogs of polyglutamates of folic acid and their effects on human liver gamma-glutamyl carboxypeptidase. Biochim Biophys Acta. 1970 Jul 15;212(1):116–125. doi: 10.1016/0005-2744(70)90184-1. [DOI] [PubMed] [Google Scholar]
- Brody T., Shane B., Stokstad E. L. Separation and identification of pteroylpolyglutamates by polyacrylamide gel chromatography. Anal Biochem. 1979 Jan 15;92(2):501–509. doi: 10.1016/0003-2697(79)90691-2. [DOI] [PubMed] [Google Scholar]
- Buehring K. U., Tamura T., Stokstad E. L. Folate coenzymes of Lactobacillus casei and Streptococcus faecalis. J Biol Chem. 1974 Feb 25;249(4):1081–1089. [PubMed] [Google Scholar]
- Cichowicz D. J., Foo S. K., Shane B. Folylpoly-gamma-glutamate synthesis by bacteria and mammalian cells. Mol Cell Biochem. 1981 Sep 25;39:209–228. doi: 10.1007/BF00232575. [DOI] [PubMed] [Google Scholar]
- Covey J. M. Polyglutamate derivatives of folic acid coenzymes and methotrexate. Life Sci. 1980 Mar 3;26(9):665–678. doi: 10.1016/0024-3205(80)90256-8. [DOI] [PubMed] [Google Scholar]
- Dev I. K., Harvey R. J. Sources of one-carbon units in the folate pathway of Escherichia coli. J Biol Chem. 1982 Feb 25;257(4):1980–1986. [PubMed] [Google Scholar]
- Foo S. K., Cichowicz D. J., Shane B. Cleavage of naturally occurring folates to unsubstituted p-aminobenzoylpoly-gamma-glutamates. Anal Biochem. 1980 Sep 1;107(1):109–115. doi: 10.1016/0003-2697(80)90499-6. [DOI] [PubMed] [Google Scholar]
- Furness R. A., Loewen P. C. Detection of p-aminobenzoylpoly(gamma-glutamates) using fluorescamine. Anal Biochem. 1981 Oct;117(1):126–135. doi: 10.1016/0003-2697(81)90702-8. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Dev I. K. Regulation in the folate pathway of Escherichia coli. Adv Enzyme Regul. 1975;13:99–124. doi: 10.1016/0065-2571(75)90010-2. [DOI] [PubMed] [Google Scholar]
- Kisliuk R. L., Gaumont Y., Lafer E., Baugh C. M., Montgomery J. A. Polyglutamyl derivatives of tetrahydrofolate as substrates for Lactobacillus casei thymidylate synthase. Biochemistry. 1981 Feb 17;20(4):929–934. doi: 10.1021/bi00507a044. [DOI] [PubMed] [Google Scholar]
- Kisliuk R. L. Pteroylpolyglutamates. Mol Cell Biochem. 1981 Sep 25;39:331–345. doi: 10.1007/BF00232583. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mansouri A., Decter J. B., Silber R. Studies on the regulation of one-carbon metabolism. II. Repression-derepression of serine hydroxymethyltransferase by methionine in Escherichia coli 113-3. J Biol Chem. 1972 Jan 25;247(2):348–352. [PubMed] [Google Scholar]
- McBurney M. W., Whitmore G. F. Isolation and biochemical characterization of folate deficient mutants of Chinese hamster cells. Cell. 1974 Jul;2(3):173–182. doi: 10.1016/0092-8674(74)90091-9. [DOI] [PubMed] [Google Scholar]
- McGuire J. J., Bertino J. R. Enzymatic synthesis and function of folylpolyglutamates. Mol Cell Biochem. 1981 Aug 11;38(Spec No)(Pt 1):19–48. doi: 10.1007/BF00235686. [DOI] [PubMed] [Google Scholar]
- McGuire J. J., Hsieh P., Coward J. K., Bertino J. R. Enzymatic synthesis of folylpolyglutamates. Characterization of the reaction and its products. J Biol Chem. 1980 Jun 25;255(12):5776–5788. [PubMed] [Google Scholar]
- Miller B. A., Newman E. B. Control of serine transhydroxymethylase synthesis in Escherichia coli K12. Can J Microbiol. 1974 Jan;20(1):41–47. doi: 10.1139/m74-007. [DOI] [PubMed] [Google Scholar]
- Ohara O., Silber R. Studies on the regulation of one-carbon metabolism. The effects of folate concentration in the growth medium on the activity of three folate-dependent enzymes in Lactobacillus casei. J Biol Chem. 1969 Apr 25;244(8):1988–1993. [PubMed] [Google Scholar]
- Scott J. M., Weir D. G. Folate composition, synthesis and function in natural materials. Clin Haematol. 1976 Oct;5(3):547–568. [PubMed] [Google Scholar]
- Shane B. High performance liquid chromatography of folates: identification of poly-gamma-glutamate chain lengths of labeled and unlabeled folates. Am J Clin Nutr. 1982 Mar;35(3):599–608. doi: 10.1093/ajcn/35.3.599. [DOI] [PubMed] [Google Scholar]
- Shane B. Pteroylpoly(gamma-glutamate) synthesis by Corynebacterium species. In vivo synthesis of folates. J Biol Chem. 1980 Jun 25;255(12):5649–5654. [PubMed] [Google Scholar]
- Shane B. Pteroylpoly(gamma-glutamate) synthesis by Corynebacterium species. Purification and properties of folypoly(gamma-glutamate) synthetase. J Biol Chem. 1980 Jun 25;255(12):5655–5662. [PubMed] [Google Scholar]
- Shane B., Stokstad E. L. Transport and metabolism of folates by bacteria. J Biol Chem. 1975 Mar 25;250(6):2243–2253. [PubMed] [Google Scholar]
- Shane B., Stokstad E. L. Transport and utilization of methyltetrahydrofolates by Lactobacillus casei. J Biol Chem. 1976 Jun 10;251(11):3405–3410. [PubMed] [Google Scholar]
- Taylor R. T., Hanna M. L. Folate-dependent enzymes in cultured Chinese hamster cells: folypolyglutamate synthetase and its absence in mutants auxotrophic for glycine + adenosine + thymidine. Arch Biochem Biophys. 1977 May;181(1):331–334. doi: 10.1016/0003-9861(77)90512-4. [DOI] [PubMed] [Google Scholar]
- Whiteley J. M., Henderson G. B., Russell A., Singh P., Zevely E. M. The isolation of dihydrofolate reductases by affinity chromatography on folate-sepharose. Anal Biochem. 1977 May 1;79(1-2):42–51. doi: 10.1016/0003-2697(77)90376-1. [DOI] [PubMed] [Google Scholar]



