Abstract
Three cell adhesion molecules are present at the axoglial junctions that form between the axon and myelinating glia on either side of nodes of Ranvier. These include an axonal complex of contacin-associated protein (Caspr) and contactin, which was proposed to bind NF155, an isoform of neurofascin located on the glial paranodal loops. Here, we show that NF155 binds directly to contactin and that surprisingly, coexpression of Caspr inhibits this interaction. This inhibition reflects the association of Caspr with contactin during biosynthesis and the resulting expression of a low molecular weight (LMw), endoglycosidase H–sensitive isoform of contactin at the cell membrane, which remains associated with Caspr but is unable to bind NF155. Accordingly, deletion of Caspr in mice by gene targeting results in a shift from the LMw- to a HMw-contactin glycoform. These results demonstrate that Caspr regulates the intracellular processing and transport of contactin to the cell surface, thereby affecting its ability to interact with other cell adhesion molecules.
Keywords: node of Ranvier; transport; cell adhesion; paranodal junction; myelin
Introduction
The myelin sheath produced by Schwann cells and oligodendrocytes closely attaches to the axon at both sides of the nodes of Ranvier, creating a specialized septate-like junction known as the axoglial or the paranodal junction (Rosenbluth, 1995). The axonal membrane at the axoglial junction contains a complex of two cell recognition molecules: contactin (Rios et al., 2000), a GPI-linked protein of the immunoglobulin superfamily; and the neurexin-like protein contacin-associated protein (Caspr; Einheber et al., 1997; Peles et al., 1997), also known as paranodin (Menegoz et al., 1997). The interaction between Caspr and contactin occurs in the ER and is required for the efficient transport of Caspr to the plasma membrane (Faivre-Sarrailh et al., 2000; Boyle et al., 2001). Both Caspr and contactin are essential for the generation of the axoglial junction, and their absence results in the disappearance of septa and widening of the space between the axon and the paranodal loops (Bhat et al., 2001; Boyle et al., 2001).
There is growing evidence that the myelin sheath dictates the localization of the Caspr–contactin complex along the axolemma. During development, these molecules are always associated with the edges of the myelin sheath (Rasband et al., 1999; Rios et al., 2000), accumulating at the paranodes as a spiral that mirrors each turn of the myelin wrap (Pedraza et al., 2001). In addition, in the peripheral nerve system, Caspr and contactin are located along the internodal region in a strand apposing the inner mesaxon of the myelin sheath and in a circumferential ring just below the inner aspect of the Schmidt-Lanterman incisures (Arroyo et al., 1999; Rios et al., 2000). The strict dependence of this localization of Caspr on the glial cell is underscored by its aberrant distribution in several myelin mutants, including the myelin deficient (md) rats (Arroyo et al., 2002), shiverer (Rasband et al., 1999), Jimpy (Jenkins and Bennett, 2002), as well as in mice lacking galactolipids (Dupree et al., 1999; Poliak et al., 2001) and sulfatide (Ishibashi et al., 2002). Finally, the localization of Caspr at the paranodes in myelinating cocultures is perturbed by addition of a soluble RPTPβ protein, which binds contactin, suggesting that the paranodal localization of the Caspr–contactin complex is dictated by its interaction with a glial ligand (Rios et al., 2000). The most likely candidate to serve as a ligand for the Caspr–contactin complex is NF155, a glial isoform of the cell adhesion molecule neurofascin. NF155 is located at the axoglial junction (Tait et al., 2000) and is markedly reduced at this site in contactin (Boyle et al., 2001), galactolipid (Poliak et al., 2001), or Caspr (Bhat et al., 2001)-deficient mice. It was reported recently that a soluble NF155-Fc chimera binds to cells expressing Caspr and contactin and precipitates these proteins from rat brain lysates, suggesting that NF155 serves as a receptor for the Caspr–contactin complex (Charles et al., 2002).
To determine the precise molecular mechanism of the interaction between NF155 and its neuronal partners, we have investigated whether NF155 binds to Caspr, to contactin or to a site formed by the combination of the two proteins. We found that NF155 binds directly to contactin and that, surprisingly, Caspr inhibits this interaction by regulating the intracellular processing, and cell surface expression of contactin.
Results and discussion
The glial isoform of neurofascin binds directly to contactin
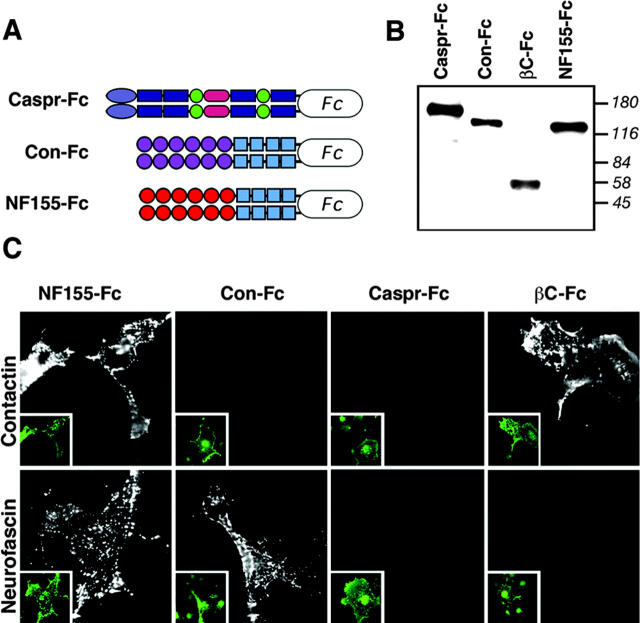
To determine whether NF155 binds to contactin, we used soluble Fc-fusion proteins containing the extracellular domain of these adhesion molecules in binding experiments with cells expressing either contactin or NF155. As depicted in Fig. 1, a soluble contactin-Fc protein bound to cells expressing NF155 but not to contactin, whereas NF155-Fc bound to cells expressing contactin, as well as homophilically to cells expressing NF155. βC-Fc, a fusion protein containing the carbonic anhydrase-like domain of RPTPβ, a known high affinity ligand of contactin (Peles et al., 1995), did not bind to NF155. Nevertheless, βC-Fc pulled down NF155 from brain lysates of both wild-type and Caspr-deficient mice (unpublished data), further confirming that contactin binds NF155 even in the absence of Caspr. Because contactin is required for cell surface expression of Caspr (Faivre-Sarrailh et al., 2000; Boyle et al., 2001), we could not examine the binding of NF155-Fc to cells expressing Caspr alone. Thus, we produced a soluble Caspr-Fc protein to determine whether it could bind to NF155. Although Caspr-Fc was mainly found within the cells, sufficient levels of this fusion protein were present in the medium (Fig. 1 B and not depicted). This soluble Caspr-Fc bound neither NF155, nor contactin expressing cells. The lack of Caspr-Fc binding to the latter was expected because these molecules only interact when both are present on the same membrane (i.e., in cis; Peles et al., 1997). Thus, we concluded that NF155 interacts directly with contactin but not with Caspr. The interaction between contactin and neurofascin is in agreement with previous observations using the chick homologues of these adhesion molecules (Volkmer et al., 1998).
Figure 1.
Binding analysis of the different paranodal junction components. (A) Schematic presentation of the Fc-fusion proteins used. (B) Immunoblot using an anti-Fc antibody showing the expression of the various Fc-fusion proteins in cell supernatants. Molecular mass markers are shown in kilodaltons on the right. βC-Fc contains the carbonic anhydrase domain of RPTPβ fused to human Fc. (C) NF155 directly binds contactin, but not Caspr. Binding was performed between the indicated Fc-fusion proteins (labeled on top of each panel) and COS-7 cells expressing either contactin or NF155, as indicated on the left. Cell surface expression of NF155 or contactin in the transfected cells, as determined by antibody staining to these proteins using nonpermeabilized cells is shown in the insets of each panel. All NF155 expressing cells bound contactin-Fc and NF155-Fc, whereas all contactin expressing cells bound NF155-Fc or βC-Fc.
Caspr inhibits the binding of contactin to neurofascin
Next, we determined whether the presence of Caspr affects the binding of NF155 to contactin. As a control, we used Caspr2, a homologous protein which does not interact with contactin (Poliak et al., 1999 and unpublished data). Surprisingly, binding of NF155 was substantially reduced when contactin was coexpressed with Caspr but not Caspr2 (Fig. 2, A and B). In contrast, βC-Fc bound equally well to cells expressing contactin alone or in combination with Caspr or Caspr2, indicating that contactin was available for binding other ligands at the cell surface. The expression of Caspr together with contactin on the cell surface of these cells resulted in the inhibition of NF155 binding to contactin (Fig. 2 C). It should be realized that because contactin is required for the export of Caspr from the ER (Faivre-Sarrailh et al., 2000), all Caspr-positive cells detected here also express contactin on their surface. The reduction in NF155-Fc binding was proportional to the amount of Caspr used in the transfection (not depicted) and was specific to NF155-Fc, as the presence of Caspr had no affect on βC-Fc binding (Fig. 2 C, bottom). Counting the number of cells expressing Caspr (and therefore also contactin) on their surface that also bound NF155-Fc or βC-Fc (n = 500 and n = 1,000 stained cells in two different experiments), showed that βC-Fc binds to all Caspr-positive cells, whereas NF155-Fc only bound to <40% of these cells. Furthermore, clustering of NF155-Fc using a secondary antibody induced aggregation of contactin, but had no effect on Caspr (Fig. 2 D), suggesting that in Caspr–contactin expressing cells, NF155-Fc binds contactin molecules that are not associated with Caspr. The inability of NF155-Fc to cluster Caspr was in marked contrast to βC-Fc, which coclusters Caspr and contactin in cultured cells (unpublished data) and primary DRG neurons (Rios et al., 2000). Hence, in contrary to a previous report, suggesting that NF155 binds the Caspr–contactin complex (Charles et al., 2002), our findings demonstrate that Caspr negatively regulates the interaction between NF155 and contactin.
Figure 2.
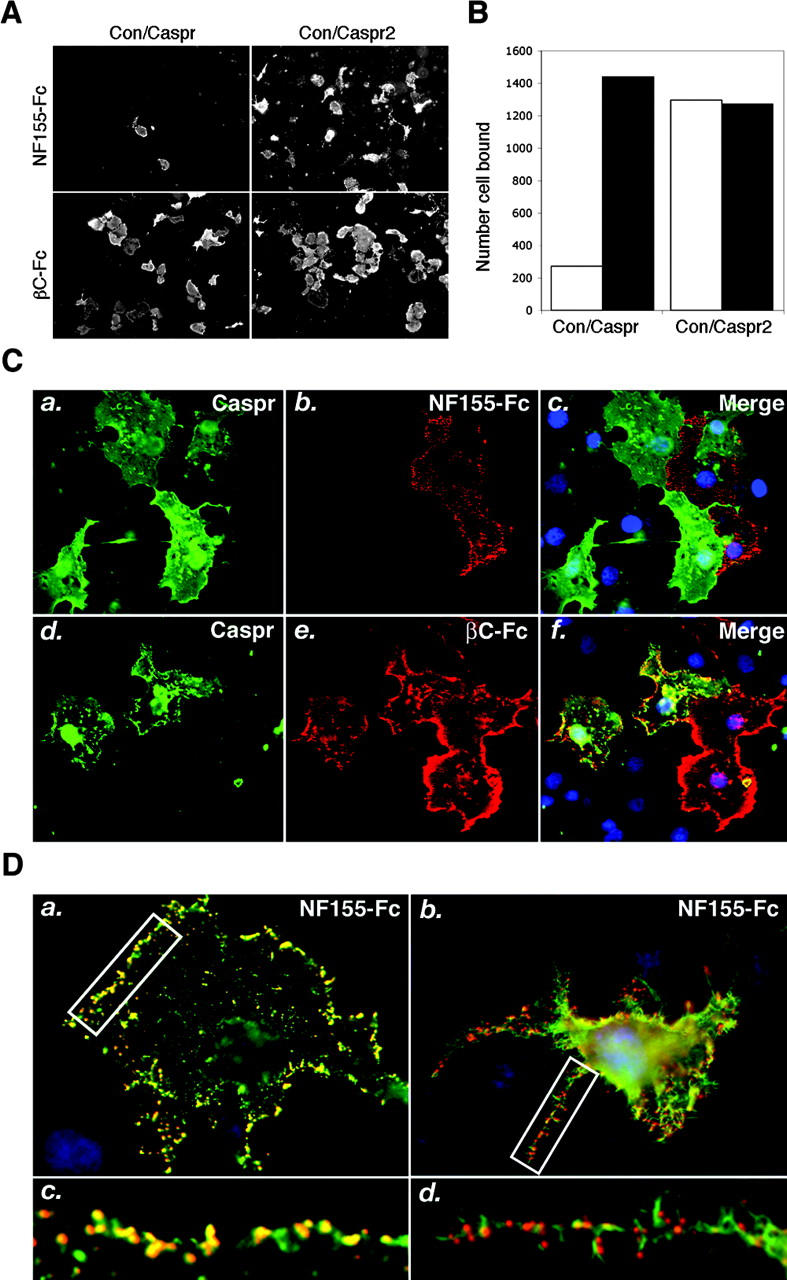
Caspr reduces binding of NF155-Fc to contactin. (A) Binding of NF155-Fc or βC-Fc to COS-7 cells transfected with equal amounts (2 μg) of contactin and Caspr cDNA, or contactin and Caspr2 as indicated on top. Note that the presence of Caspr reduces the binding of NF155-Fc, but not of βC-Fc to contactin. (B) Quantitation of the binding results shown in A; white bar, NF155-Fc; black bar, βC-Fc. (C) Double staining of cells coexpressing Caspr and contactin using an antibody to the extracellular domain of Caspr (green) and NF155-Fc (red) or βC-Fc (red). The merge images are shown on the right. Note that all Caspr-positive cells express contactin because it is required for its cell surface expression. Although βC-Fc binding overlapped Caspr staining, NF55-Fc binding was only detected on cells that did not express Caspr or only expressed low levels of this protein. (D) Clustering of NF155-Fc after binding to cells expressing contactin (a), or Caspr and contactin (b). NF155-Fc binding is shown in red, whereas staining with antibodies to contactin (a and c), or Caspr (b and d) are shown in green. Higher magnification of the labeled areas are shown below each panel (c and d). Note that there is hardly any overlap between Caspr and NF155 clusters, which is best detected in the cell processes; some overlap of nonclustered molecules is seen in the cell bodies.
Caspr regulates the glycosylation and cell surface expression of contactin
The inhibitory effect of Caspr on the binding of contactin to neurofascin may result from direct competition for the same binding site and/or a modification of contactin by Caspr within the cells. We have shown previously that two contactin isoforms, differing in their extent of glycosylation, are found in neurons and that only the low molecular weight (LMw) form specifically associates with Caspr in the paranodes (Rios et al., 2000). Immunoprecipitation using a contactin antibody revealed that both isoforms are found in contactin-transfected HEK-293 cells (Fig. 3 A), but only the high molecular weight (HMw) isoform is expressed at the cell surface (Fig. 3 A, middle). In contrast, in the presence of Caspr, both the LMw- and HMw-contactin isoforms are detected at the cell surface. The amount of the LMw contactin present on the cell surface was directly proportional to the level of Caspr expressed in the cells (Fig. 3 B). Increasing the levels of Caspr resulted in a gradual increase of the LMw contactin and a gradual decrease in the amount of the HMw-contactin isoform present on the cell surface. The effect of Caspr on the cell surface expression of contactin was specific and was not detected when the latter was coexpressed with the closely related Caspr2 (Fig. 3 B). These results demonstrate that Caspr allows the transport of LMw contactin to the plasma membrane and reduces the amount of the HMw isoform present on the cell surface. Consistently, an increase in the expression of Caspr during the development of sciatic nerve correlates well with a detected shift between the HMw to the LMw-contactin isoform (Einheber et al., 1997).
Figure 3.
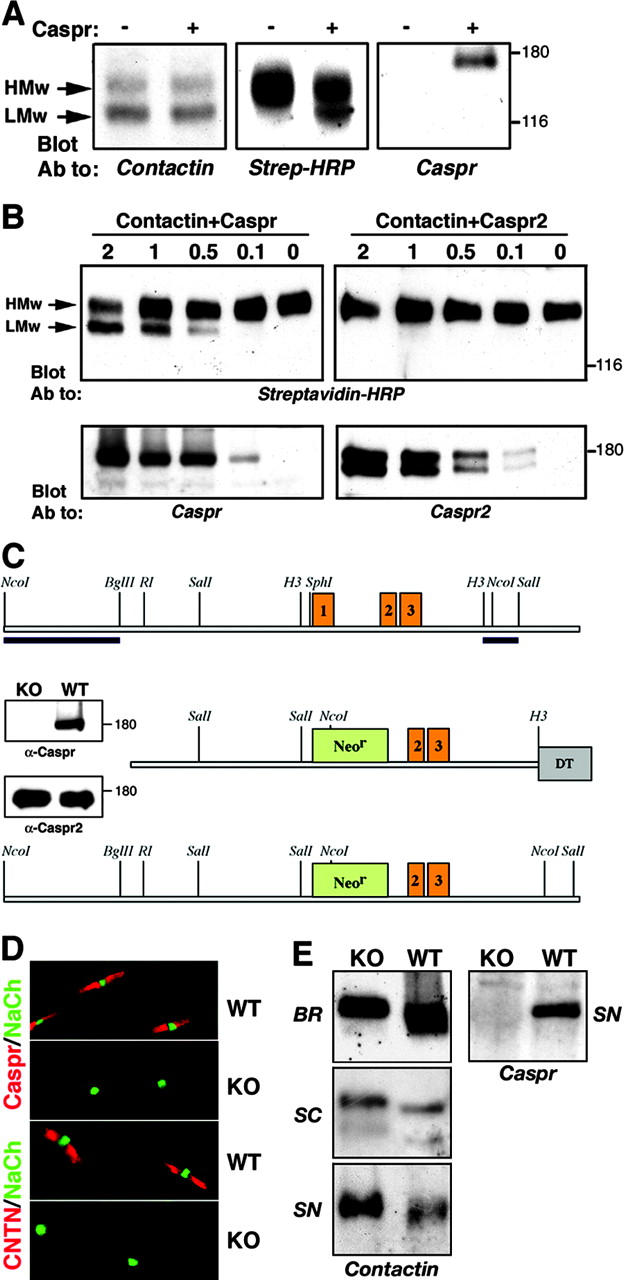
Caspr induces the expression of an LMw isoform of contactin on the cell surface. (A) Contactin was expressed in HEK-293 cells either alone (−) or together with Caspr (+). Cell surface proteins were biotinylated and cell lysates were subjected to immunoprecipitation with an antibody to contactin. Western blot analysis was performed using streptavidin-HRP to detect cell surface proteins or with antibodies to Caspr or contactin as indicated. Note that the LMw isoform of contactin is found on the cell surface only in the presence of Caspr. (B) The amount of LMw contactin present on the cell surface depends on the expression level of Caspr and not of Caspr2. HEK-293 cells were transfected with contactin and increasing amounts (micrograms of plasmids DNA) of Caspr or Caspr2, followed by cell surface biotinylation. Cell lysates were immunoprecipitated using an antibody to contactin and immunoblotted with streptavidin-HRP (top) to detect proteins that were expressed on the plasma membrane. The expression of Caspr and Caspr2 in total cell lysates is shown in the bottom. Note that increasing levels of Caspr, but not of Caspr2, reduces the HMw and concomitantly increases the LMw isoform on the cell surface. (C) Generation of Caspr null mice. Schematic map of a genomic DNA fragment containing exons 1–3, the targeting construct, and the resulted allele in which exon 1 was replaced by a Neo gene are presented. Western blot analysis of brain protein extracts from wild-type (WT) or knockout (KO) mice using antibodies to Caspr or Caspr2 is presented as an inset on the left. (D) Adult-teased sciatic nerves isolated from wild-type (WT) or Caspr-deficient (KO) mice were labeled with antibodies to Na+ channels (NaCh; green) and Caspr (Caspr; red), or contactin (CNTN; red) as indicated. Note the absence of contactin from the paranodal junction in Caspr null mice. (E) Deletion of Caspr results in a shift in the molecular weight of contactin. Proteins extracts from brain (BR), spinal cord (SC), and sciatic nerve (SN) of wild-type (WT), or Caspr null mice (KO) were immunoblotted using antibodies to contactin or Caspr, as indicated at the bottom.
To further examine whether this shift is directly dependent on Caspr, we have used mice lacking Caspr that were generated by a gene targeting approach (Fig. 3, C and D). As reported previously for another Caspr mutant mouse line (Bhat et al., 2001), these mice showed paranodal abnormalities (not depicted), including the absence of both Caspr and contactin from this site (Fig. 3 D). Immunoblot analysis of brain, spinal cord, and sciatic nerves from wild-type and Caspr null mice revealed that in the absence of Caspr, similar to results in transfected cells (Fig. 3, A and B), there was a clear shift to the HMw isoform (Fig. 3 E). In accordance with previous observations that noted the presence of HMw contactin in Caspr-deficient sciatic nerve (Bhat et al., 2001), these results demonstrate that Caspr is required for the generation of the LMw-contactin isoform normally present in the wild-type axolemma.
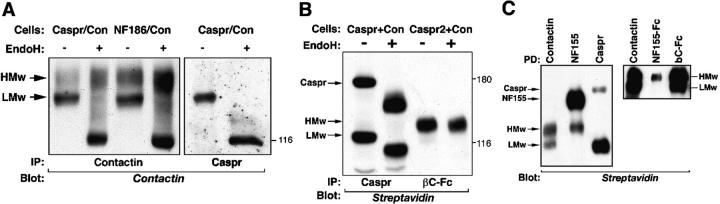
We have suggested previously the HMw- and LMw-contactin isoforms differed in their extent of glycosylation (Rios et al., 2000). To further characterize this difference, we treated contactin immunocomplexes with endoglycosidase H (EndoH), an enzyme that cleaves high mannose structures usually found in ER-resident proteins, but has no effect on complex oligosaccharides present on mature glycosylated proteins. As shown in Fig. 4, EndoH treatment decreases the apparent molecular weight of the LMw, but not the HMw-contactin isoform. The LMw-EndoH sensitive form was also precipitated using an antibody to Caspr, confirming that Caspr interacts with this contactin glycoform. Surface biotinylation of cells expressing Caspr and contactin showed that a complex of Caspr and the LMw contactin was present on the cell surface and that both molecules were EndoH sensitive (Fig. 4 B). In contrast, the HMw-contactin glycoform found on the plasma membrane of cells expressing contactin and Caspr2 was EndoH resistant (Fig. 4 B), indicating that this contactin glycoform was further processed and carried complex oligosaccharides. These results demonstrate that contactin exists as two glycoforms, out of which only the LMw binds Caspr. Furthermore, although the HMw-contactin glycoform can reach the plasma membrane in the absence of Caspr, the latter is required for the transport of the LMw contactin to the cell surface.
Figure 4.
Biochemical analysis of contactin isoforms. (A) Differential sensitivity of HMw and LMw contactin to endoglycosidase H (EndoH). HEK-293 cells expressing contactin with Caspr (Caspr/Con) or with neurofascin 186 as a control (NF186/Con) were subjected to immunoprecipitation with an antibody to contactin or Caspr. Immunocomplexes were incubated in the absence (−) or in the presence (+) of EndoH, following by Western blot analysis using anticontactin antibody. The Caspr-associated LMw but not the HMw-contactin isoform is EndoH sensitive. (B) Both LMw contactin and Caspr expressed on the cell surface contain high mannose structures, which are cleaved by EndoH. Intact HEK-293 cells expressing contactin with Caspr (Caspr + Con) or with Caspr2 (Caspr2 + Con) were biotinylated and subjected to immunoprecipitation with an antibody to Caspr or βC-Fc. The washed immunocomplexes were treated with EndoH as indicated (−/+), and blotted using streptavidin-HRP. (C) Preferential binding of Caspr and NF155 to the two contactin isoforms. Cells expressing Caspr, contactin and NF155 (left) or cells expressing Caspr and contactin (right) were biotinylated and subjected to immunoprecipitation (left; right, first lane), or Fc-pulldown (right) using the indicated antibodies and Fc proteins (PD). Precipitated material was blotted using streptavidin-HRP to detect cell surface proteins. In both panels, the location of the two forms of contactin, as well as Caspr and NF155, is indicated. In the left panel, only 20% of the lysates was used to precipitate contactin.
In contrast to Caspr, which exclusively interacts with the LMw-contactin glycoform, NF155-Fc binds to the HMw form of contactin at the cell surface. Antibodies to NF155 or Caspr selectively immunoprecipitated the HMw- and LMw-contactin isoforms, respectively, from the cell surface of HEK-293 cells expressing all three proteins, (Fig. 4 C, left). The aim of this triple transfection was to examine the interaction of NF155 with contactin under conditions where both forms of contactin are present in the cell and that allow NF155 to interact with LMw contactin before it assembles with Caspr. Although the interaction between HMw contactin and NF155 under these conditions could thus occur in trans or cis, they clearly show that NF155 bind the HMw-contactin isoform. Similarly, NF155-Fc pulled down the HMw but not the LMw-contactin isoform from cells transfected with contactin and a moderate amount of Caspr, which allows the expression of both contactin forms on the cell surface (Fig. 4 C, right). In contrast, βC-FC interacted with both HMw and LMw. Hence, we conclude that NF155 preferentially interacts with the higher molecular weight form of contactin, which is not associated with Caspr. Whether the inability of NF155 to bind the LMw-contactin glycoform results from the immature glycosylation of the latter or steric inhibition secondary to its existence in a protein complex with Caspr on the plasma membrane will require further study.
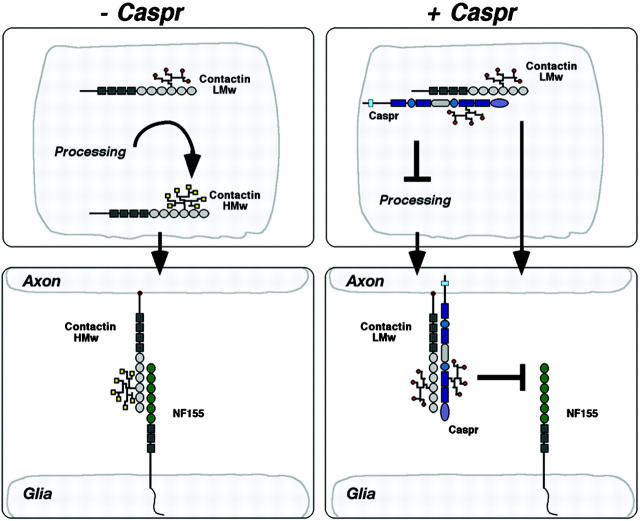
During the biosynthesis of membrane glycoproteins, N-linked oligosaccharides are added cotranslationally in the ER and then terminal glucose and mannose residues are removed to generate a simple core-glycans (Helenius and Aebi, 2001). Properly folded proteins carrying the core-glycans then undergo further trimming and terminal glycosylation in the Golgi complex before they reach their final destination at the cell surface. The interaction between Caspr and contactin regulate each other's transport to the cell surface. Contactin is required for the exit of Caspr from the ER (Faivre-Sarrailh et al., 2000), whereas Caspr regulates the intracellular processing and cell surface transport of contactin glycoforms (Fig. 5). Contactin is found as two different glycoforms, of which only the mature, EndoH-resistance HMw reaches the cell surface. The expression of Caspr in the cell chaperones the EndoH-sensitive contactin glycoform to the cell surface, where it is found in a complex with Caspr.
Figure 5.
A schematic model describing the role of Caspr in the processing and transport of contactin. In the absence of Caspr, the high mannose residues (red dots) attached to contactin in the ER are being trimmed and replaced by complex oligosaccharide side chains (yellow dots) in the Golgi complex, resulting in the formation of an EndoH-resistant high molecular weight isoform (HMw) of contactin (only a single prototypic glycosylation site is illustrated). HMw contactin is being transported to the cell surface where it binds neurofascin and other ligands such as RPTPβ. The presence of Caspr in the cell allows the transport of an EndoH-sensitive LMw-contactin isoform to the plasma membrane. This may result from the inhibition of further processing of contactin in the Golgi complex or the transport of the Caspr–LMw-contactin complex to the cell membrane through an alternative pathway. The Caspr–LMw-contactin complex found at the cell surface binds RPTPβ but not neurofascin. The levels of Caspr determine the ratio between the HMw- and LMw-contactin isoforms present on the cell surface.
These results have important implications on the molecular mechanisms involved in the generation of distinct domains at and around the nodes of Ranvier. First, by regulating which glycoform of contactin is present on the cell surface, Caspr controls the ability of this cell adhesion molecule to interact with additional ligands. Contactin can bind several nodal proteins, such as the β1-subunit of Na+ channels, NrCAM and neurofascin-186 (for review see Falk et al., 2002). The increase in Caspr expression and subsequently, the transition between HMw to LMw contactin observed during development (Einheber et al., 1997), may also regulate the ability of contactin to bind these nodal components. Second, by controlling the relative expression of contactin glycoforms at the cell surface, Caspr determines the localization of contactin in myelinated axons. Thus, in Caspr-deficient neurons, the HMw predominates (Fig. 3) and is preferentially expressed at nodes of central nervous system neurons rather than the paranodes (Bhat et al., 2001). Our results further suggest that Caspr may regulate this differential localization by directing contactin through distinct biosynthetic pathways. Finally, a major finding of this paper is the demonstration that NF155 does not bind directly to the Caspr–contactin complex. Because Caspr and contactin can be pulled down by NF155-Fc from rat brain (Charles et al., 2002), our results indicate that most likely other components are required to bridge between these proteins at the axoglial junction. Future experiments focused on identification of additional junctional components that may stabilize this tripartite complex should provide important new insights into the mechanism of paranodal junction formation.
Materials and methods
Generation of Caspr −/− mice
A mouse genomic fragment, corresponding to the first three exons of caspr gene was isolated and used to generate a replacement-type vector, in which a neomycin resistant gene replaced an SphI-BssHII fragment containing the first exon of Caspr including the initiator methionine and the signal sequence. ES clones were screened by Southern blot using a 2.4-kb NcoI–BglII and a 0.8-kb SpeI–SalI fragment as probes. Mice were generated as described previously for Caspr2 gene (Poliak et al., 2003). All experiments were performed in compliance with the relevant laws and institutional guidelines and were approved by the Weizmann's Institutional Animal Care and Use Committee.
Binding and immunofluorescence
βC-Fc and Hcon-Fc were described previously (Peles et al., 1995). For Caspr-Fc and NF155-Fc, the extracellular domain of rat Caspr (aa 1–1278) or rat NF155 (aa 1–1024) were fused to the hinge region of human IgG1-Fc. Similar results were obtained with a NF155-Fc construct containing an HA-tag after the signal sequence (provided by S. Lambert, University of Edinburgh, Edinburgh, UK), or with the NF155-Fc protein described previously (Charles et al., 2002; aa 1–1040, provided by P. Brophy, University of Massachusetts Medical Center, Worcester, MA). For binding experiments, conditioned media containing 0.5–1 μg/ml of the various Fc-fusion proteins were mixed with a Cy3-conjugated anti–human-Fc antibody for 30 min and incubated with the transfected cells indicated in each figure for 20 min at RT. Unbound proteins were removed by three washes with PBS and the cells were fixed with 4% PFA. Cell transfection, Fc-fusion clustering, and antibody labeling were described previously (Gollan et al., 2002). Fluorescence images were acquired on a microscope (20× 0.5NA and 60× 1.4NA; model Eclipse E600; Nikon), using a Spot-II camera and further processed using Photoshop software.
Immunoprecipitation and immunoblot analysis
Preparation of tissue lysates, cell surface biotinylation immunoprecipitation, and Western blot analysis was performed as described previously (Poliak et al., 2001, 2003; Gollan et al., 2002). When indicated, immunocomplexes were incubated with 8 mU of EndoH (NEB) for 2 h at 37°C in 50 mM Na-citrate buffer, pH 5.7. Control samples were incubated in the same buffer without the enzyme.
Acknowledgments
We thank Steve Lambert and Peter Brophy for cDNA constructs and Steve Scherer for ideas and discussions.
This work was supported by the National Multiple Sclerosis Society (grants RG-3102 and RG3439-A-2), the National Institutes of Health (NS43474), the United States-Israel Science Foundation (BSF), Jerusalem, Israel, and the Minerva Foundation. E. Peles is an Incumbent of the Madeleine Haas Russell Career Development Chair.
Abbreviations used in this paper: Caspr, contacin-associated protein; EndoH, endoglycosidase H; HMw, high molecular weight; LMw, low molecular weight.
References
- Arroyo, E.J., Y.T. Xu, L. Zhou, A. Messing, E. Peles, S.Y. Chiu, and S.S. Scherer. 1999. Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvbeta2, and Caspr. J. Neurocytol. 28:333–347. [DOI] [PubMed] [Google Scholar]
- Arroyo, E.J., T. Xu, J. Grinspan, S. Lambert, S.R. Levinson, P.J. Brophy, E. Peles, and S.S. Scherer. 2002. Genetic dysmyelination alters the molecular architecture of the nodal region. J. Neurosci. 22:1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, M.A., J.C. Rios, Y. Lu, G.P. Garcia-Fresco, W. Ching, M. St Martin, J. Li, S. Einheber, M. Chesler, J. Rosenbluth, et al. 2001. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 30:369–383. [DOI] [PubMed] [Google Scholar]
- Boyle, M.E., E.O. Berglund, K.K. Murai, L. Weber, E. Peles, and B. Ranscht. 2001. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 30:385–397. [DOI] [PubMed] [Google Scholar]
- Charles, P., S. Tait, C. Faivre-Sarrailh, G. Barbin, F. Gunn-Moore, N. Denisenko-Nehrbass, A.M. Guennoc, J.A. Girault, P.J. Brophy, and C. Lubetzki. 2002. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr. Biol. 12:217–220. [DOI] [PubMed] [Google Scholar]
- Dupree, J.L., J.A. Girault, and B. Popko. 1999. Axo–glial interactions regulate the localization of axonal paranodal proteins. J. Cell Biol. 147:1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber, S., G. Zanazzi, W. Ching, S. Scherer, T.A. Milner, E. Peles, and J.L. Salzer. 1997. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J. Cell Biol. 139:1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Sarrailh, C., F. Gauthier, N. Denisenko-Nehrbass, A. Le Bivic, G. Rougon, and J.A. Girault. 2000. The glycosylphosphatidyl inositol-anchored adhesion molecule F3/contactin is required for surface transport of paranodin/contactin-associated protein (caspr). J. Cell Biol. 149:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk, J., C. Bonnon, J.A. Girault, and C. Faivre-Sarrailh. 2002. F3/contactin, a neuronal cell adhesion molecule implicated in axogenesis and myelination. Biol. Cell. 94:327–334. [DOI] [PubMed] [Google Scholar]
- Gollan, L., H. Sabanay, S. Poliak, E.O. Berglund, B. Ranscht, and E. Peles. 2002. Retention of a cell adhesion complex at the paranodal junction requires the cytoplasmic region of Caspr. J. Cell Biol. 157:1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius, A., and M. Aebi. 2001. Intracellular functions of N-linked glycans. Science. 291:2364–2369. [DOI] [PubMed] [Google Scholar]
- Ishibashi, T., J.L. Dupree, K. Ikenaka, Y. Hirahara, K. Honke, E. Peles, B. Popko, K. Suzuki, H. Nishino, and H. Baba. 2002. A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J. Neurosci. 22:6507–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins, S.M., and V. Bennett. 2002. Developing nodes of Ranvier are defined by ankyrin-G clustering and are independent of paranodal axoglial adhesion. Proc. Natl. Acad. Sci. USA. 99:2303–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegoz, M., P. Gaspar, M. Le Bert, T. Galvez, F. Burgaya, C. Palfrey, P. Ezan, F. Arnos, and J.A. Girault. 1997. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 19:319–331. [DOI] [PubMed] [Google Scholar]
- Pedraza, L., J.K. Huang, and D.R. Colman. 2001. Organizing principles of the axoglial apparatus. Neuron. 30:335–344. [DOI] [PubMed] [Google Scholar]
- Peles, E., M. Nativ, P.L. Campbell, T. Sakurai, R. Martinez, S. Lev, D.O. Clary, J. Schilling, G. Barnea, G.D. Plowman, et al. 1995. The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell. 82:251–260. [DOI] [PubMed] [Google Scholar]
- Peles, E., M. Nativ, M. Lustig, M. Grumet, J. Schilling, R. Martinez, G.D. Plowman, and J. Schlessinger. 1997. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 16:978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak, S., L. Gollan, R. Martinez, A. Custer, S. Einheber, J.L. Salzer, J.S. Trimmer, P. Shrager, and E. Peles. 1999. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 24:1037–1047. [DOI] [PubMed] [Google Scholar]
- Poliak, S., L. Gollan, D. Salomon, E.O. Berglund, R. Ohara, B. Ranscht, and E. Peles. 2001. Localization of Caspr2 in myelinated nerves depends on axon-glia interactions and the generation of barriers along the axon. J. Neurosci. 21:7568–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak, S., D. Salomon, H. Elhanany, H. Sabanay, B. Kiernan, L. Pevny, C.L. Stewart, X. Xu, S.Y. Chiu, P. Shrager, et al. 2003. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J. Cell Biol. 162:1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband, M.N., E. Peles, J.S. Trimmer, S.R. Levinson, S.E. Lux, and P. Shrager. 1999. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J. Neurosci. 19:7516–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios, J.C., C.V. Melendez-Vasquez, S. Einheber, M. Lustig, M. Grumet, J. Hemperly, E. Peles, and J.L. Salzer. 2000. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J. Neurosci. 20:8354–8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth, J. 1995. Glial membranes and axoglial junctions. Neuroglia. H. Kettenmann and B.R. Ransom, editors. Oxford University Press, New York. 613–633.
- Tait, S., F. Gunn-Moore, J.M. Collinson, J. Huang, C. Lubetzki, L. Pedraza, D.L. Sherman, D.R. Colman, and P.J. Brophy. 2000. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo–glial junction. J. Cell Biol. 150:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmer, H., U. Zacharias, U. Norenberg, and F.G. Rathjen. 1998. Dissection of complex molecular interactions of neurofascin with axonin-1, F11, and tenascin-R, which promote attachment and neurite formation of tectal cells. J. Cell Biol. 142:1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]