Abstract
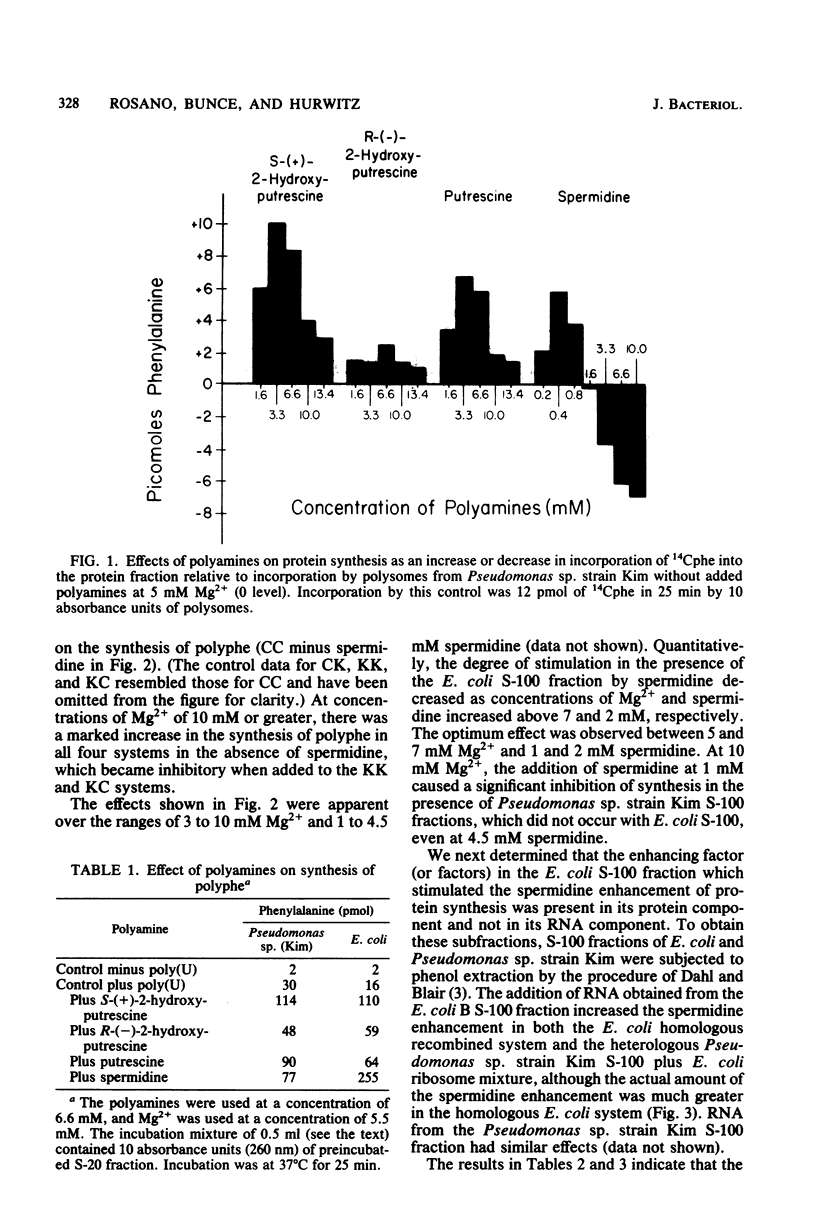
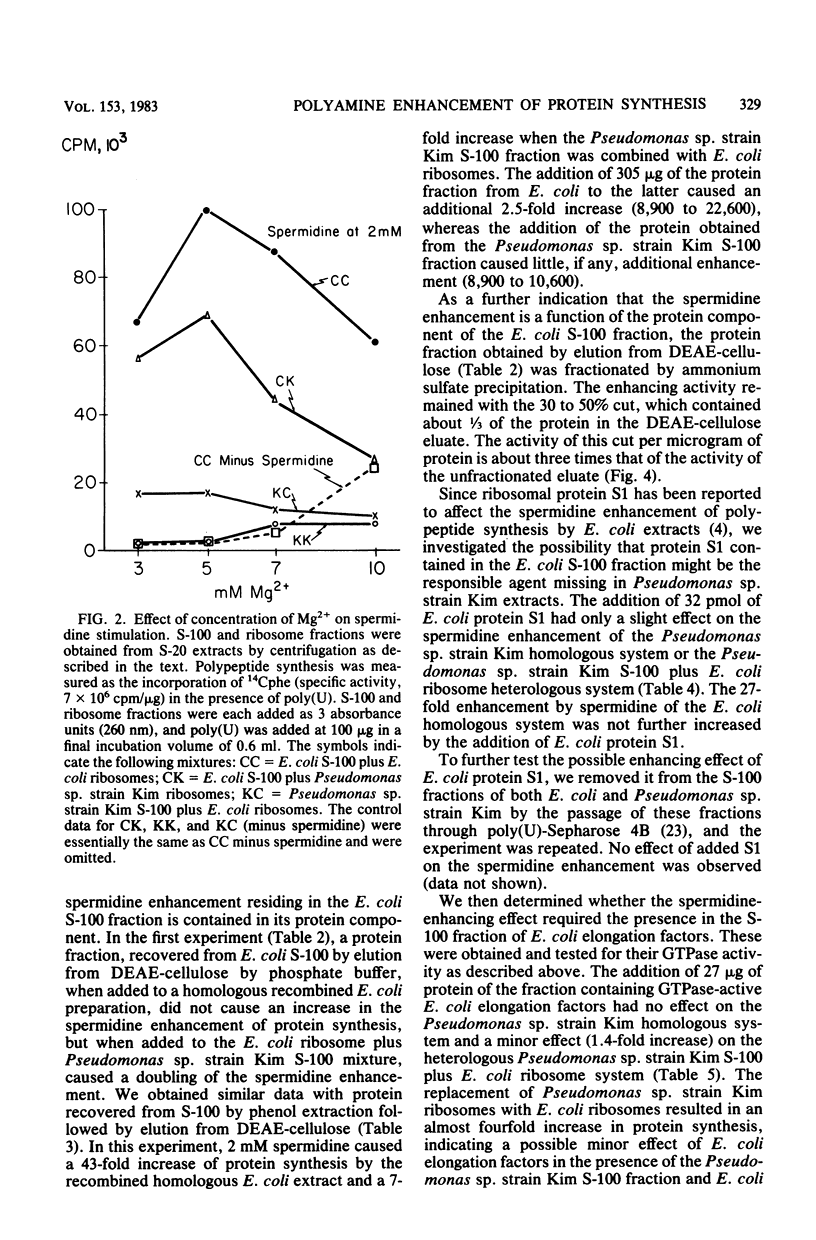
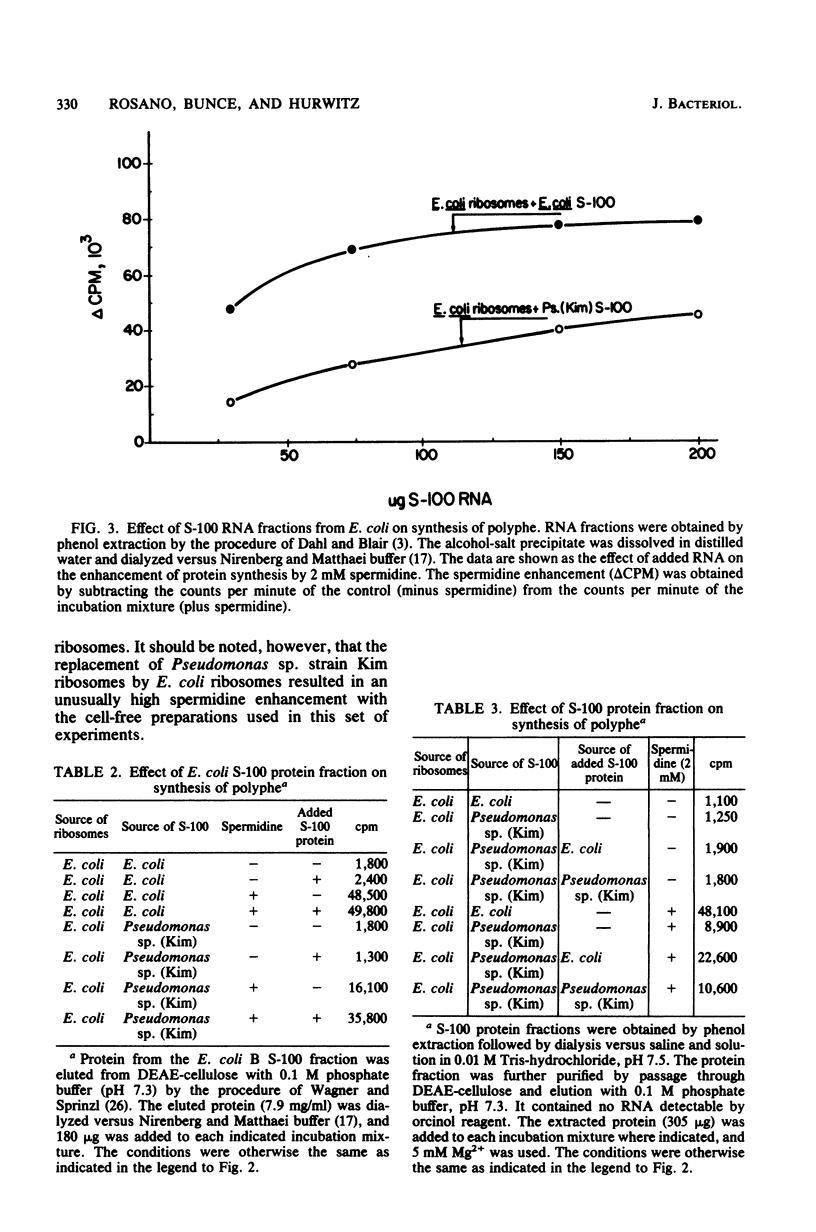
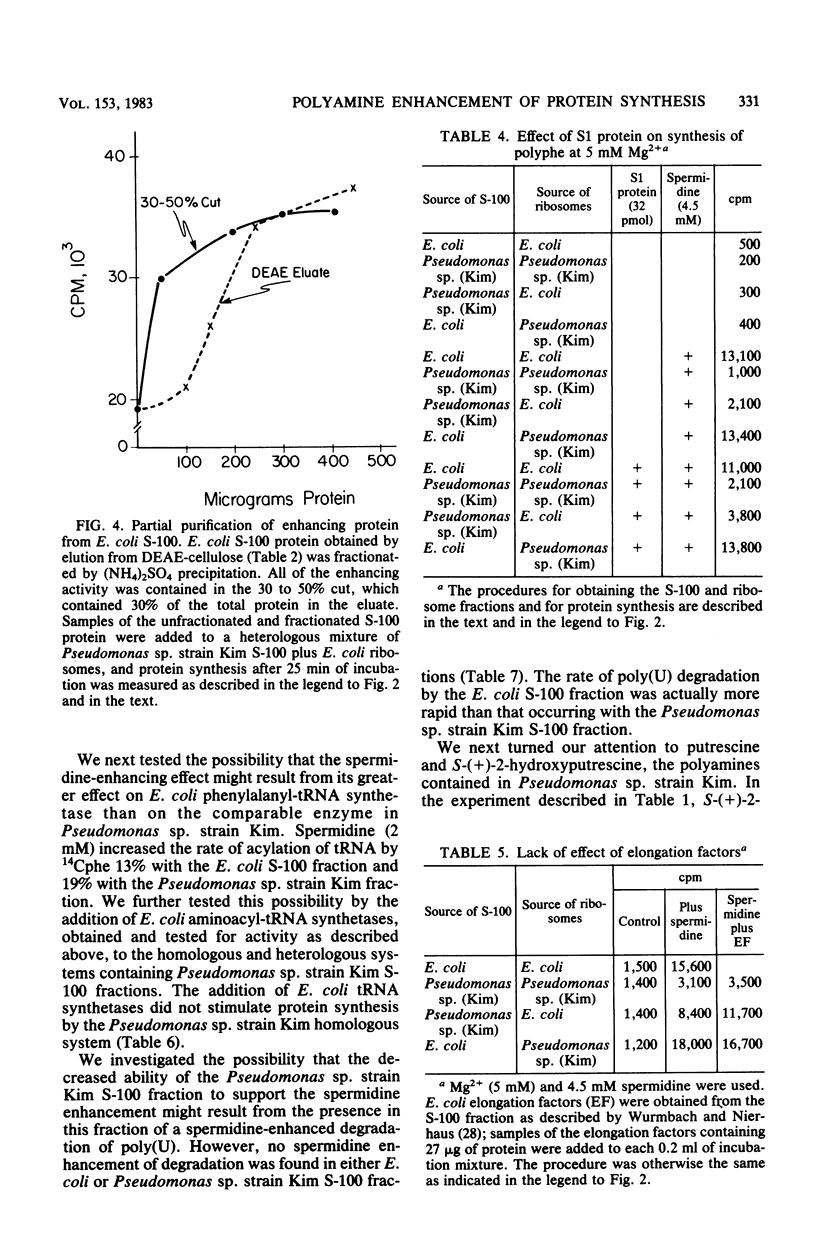
At 5 mM Mg2+, spermidine stimulation of polyphenylalanine synthesis by cell-free extracts of Escherichia coli was found to be about 30 times greater than that by extracts of Pseudomonas sp. strain Kim, a unique organism which lacks detectable levels of spermidine. By means of reconstitution experiments, the target of spermidine stimulation was localized to the protein fraction of the highspeed supernatant component (S-100) of E. coli and was absent from, or deficient in, the S-100 fraction of Pseudomonas sp. strain Kim. The spermidine stimulation did not appear to be due to the presence in the E. coli S-100 fraction of ribosomal protein S1, elongation factors, or E. coli aminoacyl-tRNA synthetases. The failure to observe spermidine stimulation by the Pseudomonas sp. strain Kim S-100 fraction was also not due to a spermidine-enhanced polyuridylic acid degradation. The synthesis of polyphenylalanine by Pseudomonas sp. strain Kim extracts was stimulated by putrescine and by S-(+)-2-hydroxyputrescine to a greater degree than was synthesis by E. coli extracts. The enhancement by putrescine and by S-(+)-2-hydroxyputrescine with Pseudomonas sp. strain Kim extracts was found to be due to effects on its ribosomes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Algranati I. D., Goldemberg S. H. Initiation, elongation and termination of polypeptide synthesis in cell-free systems from polyamine-deficient bacteria. Biochem Biophys Res Commun. 1981 Nov 16;103(1):8–15. doi: 10.1016/0006-291x(81)91653-3. [DOI] [PubMed] [Google Scholar]
- COHEN S. S., LICHTENSTEIN J. Polyamines and ribosome structure. J Biol Chem. 1960 Jul;235:2112–2116. [PubMed] [Google Scholar]
- DUBIN D. T., ROSENTHAL S. M. The acetylation of polyamines in Escherichia coli. J Biol Chem. 1960 Mar;235:776–782. [PubMed] [Google Scholar]
- Dahl H. H., Blair G. E. Purification of four eukaryotic initiation factors required for natural mRNA translation. Methods Enzymol. 1979;60:87–101. doi: 10.1016/s0076-6879(79)60009-5. [DOI] [PubMed] [Google Scholar]
- Dijk J., Littlechild J. Purification of ribosomal proteins from Escherichia coli under nondenaturing conditions. Methods Enzymol. 1979;59:481–502. doi: 10.1016/0076-6879(79)59109-5. [DOI] [PubMed] [Google Scholar]
- Dionne P., Rosano C. L., Hurwitz C. Effect of tetracycline on puromycin-induced polysome degradation: influence of magnesium and polyamines. Antimicrob Agents Chemother. 1975 May;7(5):571–577. doi: 10.1128/aac.7.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J Biol Chem. 1979 Dec 25;254(24):12419–12426. [PubMed] [Google Scholar]
- Hurwitz C., Rosano C. L. The intracellular concentration of bound and unbound magnesium ions in Escherichia coli. J Biol Chem. 1967 Aug 25;242(16):3719–3722. [PubMed] [Google Scholar]
- Igarashi K., Kishida K., Kashiwagi K., Tatokoro I., Kakegawa T., Hirose S. Relationship between methylation of adenine near the 3' end of 16-S ribosomal RNA and the activity of 30-S ribosomal subunits. Eur J Biochem. 1981 Jan;113(3):587–593. doi: 10.1111/j.1432-1033.1981.tb05103.x. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Yabuki M., Yoshioka Y., Eguchi K., Hirose S. Mechanism of stimulation of polyphenylalanine synthesis by spermidine. Biochem Biophys Res Commun. 1977 Mar 7;75(1):163–171. doi: 10.1016/0006-291x(77)91304-3. [DOI] [PubMed] [Google Scholar]
- Kim K. H. Properties and distribution of intracellular putrescine in a pseudomonas. J Bacteriol. 1966 Jan;91(1):193–197. doi: 10.1128/jb.91.1.193-197.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G., Odom O. W., Hardesty B. Polyamines in eukaryotic peptide initiation. Methods Enzymol. 1979;60:555–566. doi: 10.1016/s0076-6879(79)60053-8. [DOI] [PubMed] [Google Scholar]
- Kullnig R. K., Rosano C. L., Coulter M. E., Hurwitz C. Configuration of 2-hydroxyputrescine. J Biol Chem. 1973 Apr 10;248(7):2487–2488. [PubMed] [Google Scholar]
- Kullnig R., Rosano C. L., Hurwitz C. Identification of 2-hydroxyputrescine in a pseudomonad lacking spermidine. Biochem Biophys Res Commun. 1970;39(6):1145–1148. doi: 10.1016/0006-291x(70)90679-0. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Niveleau A., Wahba A. J. Inhibition of synthetic and natural messenger translation. I. Purification and properties of a protein isolated from Escherichia coli MRE 600 ribosomes. J Biol Chem. 1974 Jun 25;249(12):3803–3807. [PubMed] [Google Scholar]
- Morris D. R., Jorstad C. M. Growth and macromolecular composition of a mutant of Escherichia coli during polyamine limitation. J Bacteriol. 1973 Jan;113(1):271–277. doi: 10.1128/jb.113.1.271-277.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M., Hapke B., Schreier M. H., Noll H. Structural dynamics of bacterial ribosomes. I. Characterization of vacant couples and their relation to complexed ribosomes. J Mol Biol. 1973 Apr 5;75(2):281–294. doi: 10.1016/0022-2836(73)90021-1. [DOI] [PubMed] [Google Scholar]
- Pestka S. Peptidyl-puromycin synthesis on polyribosomes from Escherichia coli. Proc Natl Acad Sci U S A. 1972 Mar;69(3):624–628. doi: 10.1073/pnas.69.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Teeter M. M., Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C. L., Hurwitz C. Antagonistic action between spermidine and putrescine on association and dissociation of purified, run-off ribosomes from Escherichia coli. J Biol Chem. 1977 Jan 25;252(2):652–654. [PubMed] [Google Scholar]
- Rosano C. L., Hurwitz C. Interrelationship between magnesium and polyamines in a pseudomonad lacking spermidine. Biochem Biophys Res Commun. 1969 Nov 6;37(4):677–683. doi: 10.1016/0006-291x(69)90864-x. [DOI] [PubMed] [Google Scholar]
- Sobura J. E., Chowdhury M. R., Hawley D. A., Wahba A. J. Requirement of chain initiation factor 3 and ribosomal protein S1 in translation of synthetic and natural messenger RNA. Nucleic Acids Res. 1977 Jan;4(1):17–29. doi: 10.1093/nar/4.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. W. Escherichia coli mutants completely deficient in adenosylmethionine decarboxylase and in spermidine biosynthesis. J Biol Chem. 1978 May 25;253(10):3671–3676. [PubMed] [Google Scholar]
- Tobari J., Tchen T. T. Identification of (+)-hydroxyputrescine (1,4-diaminobutan-2-ol) from a Pseudomonas species. J Biol Chem. 1971 Mar 10;246(5):1262–1265. [PubMed] [Google Scholar]
- Wagner T., Sprinzl M. Enzymic binding of aminoacyl-tRNA to Escherichia coli ribosomes using modified tRNA species and tRNA fragments. Methods Enzymol. 1979;60:615–628. doi: 10.1016/s0076-6879(79)60058-7. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Morris D. R. Cations and ribosome structure. I. Effects on the 30S subunit of substituting polyamines for magnesium ion. Biochemistry. 1973 Jan 30;12(3):435–441. doi: 10.1021/bi00727a012. [DOI] [PubMed] [Google Scholar]
- Wurmbach P., Nierhaus K. H. Isolation of the protein synthesis elongation factors EF-Tu, EF-Ts, and EF-G from Escherichia coli. Methods Enzymol. 1979;60:593–606. doi: 10.1016/s0076-6879(79)60056-3. [DOI] [PubMed] [Google Scholar]
- Zak R., Prior G., Rabinowitz M. Assessment of protein synthesis by the use of aminoacyl-tRNA as precursor. Methods Enzymol. 1979;59:310–321. doi: 10.1016/0076-6879(79)59093-4. [DOI] [PubMed] [Google Scholar]



