Abstract
Although the transport of model proteins across the mammalian ER can be reconstituted with purified Sec61p complex, TRAM, and signal recognition particle receptor, some substrates, such as the prion protein (PrP), are inefficiently or improperly translocated using only these components. Here, we purify a factor needed for proper translocation of PrP and identify it as the translocon-associated protein (TRAP) complex. Surprisingly, TRAP also stimulates vectorial transport of many, but not all, other substrates in a manner influenced by their signal sequences. Comparative analyses of several natural signal sequences suggest that a dependence on TRAP for translocation is not due to any single physical parameter, such as hydrophobicity of the signal sequence. Instead, a functional property of the signal, efficiency of its post-targeting role in initiating substrate translocation, correlates inversely with TRAP dependence. Thus, maximal translocation independent of TRAP can only be achieved with a signal sequence, such as the one from prolactin, whose strong interaction with the translocon mediates translocon gating shortly after targeting. These results identify the TRAP complex as a functional component of the translocon and demonstrate that it acts in a substrate-specific manner to facilitate the initiation of protein translocation.
Keywords: protein translocation; endoplasmic reticulum; secretion; translocon; signal sequence
Introduction
The vast majority of secretory and membrane proteins are transported across or integrated into the membrane of the mammalian ER by a multiprotein assembly termed the translocon (for review see Johnson and van Waes, 1999). An essential function of the translocon is to provide an aqueous protein-conducting channel spanning the membrane bilayer. At the ER, the heterotrimeric Sec61p complex (composed of α, β, and γ subunits) has been shown to be a principal component of this translocation channel (Gorlich et al., 1992b; Musch et al., 1992; Sanders et al., 1992; Mothes et al., 1994). This protein complex can homo-oligomerize into a toroidal channel-like structure (Hanein et al., 1996; Beckmann et al., 1997), bind ribosomes with high affinity (Gorlich et al., 1992b; Kalies et al., 1994), recognize functional signal sequences (Jungnickel and Rapoport, 1995), and allow the lateral partitioning of transmembrane domains into the lipid bilayer (Heinrich et al., 2000).
Notwithstanding this multifunctionality, the Sec61p complex is insufficient for cotranslational translocation. Although the Sec61p complex can recognize signal sequences, efficient nascent chain targeting to the membrane and transfer to the translocation channel additionally requires the coordinated actions of the cytosolic signal recognition particle and its membrane-bound receptor (SR)* (Walter and Johnson, 1994). After targeting to the mammalian ER, another component, TRAM, is necessary for the efficient translocation of most substrates in a manner dependent on structural features of their signal sequences (Gorlich et al., 1992a; Voigt et al., 1996). Thus, reconstitution into lipid vesicles of at least three proteins, the Sec61p complex, SR, and TRAM, is necessary for the translocation of most substrates, defining them as essential translocon components (Gorlich and Rapoport, 1993).
The translocation of several model secretory and membrane proteins has been tested in proteoliposomes containing these three purified components (Gorlich and Rapoport, 1993; Voigt et al., 1996). Although every substrate examined thus far displays at least some level of translocation, the efficiency of transport relative to unfractionated microsomes appears to vary from protein to protein. For instance, the translocation of the secretory hormone prolactin (Prl) into the minimal system approached ∼60–70% of that observed in unfractionated starting microsomes (Gorlich and Rapoport, 1993). By contrast, translocation of the hormone α-factor in the same proteoliposomes was only ∼15–20% of the control, raising the possibility that its transport may require stimulatory factor(s) not needed by Prl. Unfortunately, the difficulties of functionally assaying modestly stimulatory activities using laborious and technically demanding membrane protein reconstitution methods have hindered a biochemical approach to the identification of any such factors.
More recently, a particularly striking example of discrepant translocation in native versus minimal proteoliposomes was described for the prion protein (PrP). In native microsomes, the majority of PrP is ordinarily fully translocated into the lumen in a form termed secPrP, with smaller amounts being made as single spanning membrane proteins in either orientation, termed CtmPrP (a type II or Cout/Nin orientation) and NtmPrP (a type I or Cin/Nout orientation) (Hegde et al., 1998a; Holscher et al., 2001; Stewart and Harris, 2001). However, only the CtmPrP form, whose increased expression is associated with neurodegenerative disease (Hegde et al., 1998a), is made to any appreciable degree in proteoliposomes containing the minimal translocation machinery (Hegde et al., 1998b). Thus, although the currently identified essential translocation machinery is able to mediate a basal level of translocation for many substrates (Gorlich and Rapoport, 1993; Voigt et al., 1996), optimal and proper transport of the entire repertoire of proteins that ordinarily transit the secretory pathway is likely to involve other stimulatory components that act in a substrate-specific manner (Andrews and Johnson, 1996; Hegde and Lingappa, 1999). The identity of such putative factors, the attributes of the substrates for which they are important, or the specific steps in translocation at which they might act all remain elusive.
In this study, we have exploited the inability of PrP to be translocated properly by the minimal translocation machinery to facilitate the purification of a trans-acting stimulatory factor involved in its translocation. We subsequently demonstrate that this factor, identified as the translocon-associated protein (TRAP) complex, is more broadly involved in substrate translocation in a manner dependent on functional features of signal sequences. Our results therefore identify an additional functional component of the translocation machinery, define the characteristics of the substrates that depend on its function, and indicate that it acts at the critical step of initiation of translocation.
Results
A putative translocation accessory factor at the ER
Proteoliposomes reconstituted from an unfractionated detergent extract are able to support the translocation of PrP in the secPrP, CtmPrP, and, to a lesser extent, NtmPrP forms (Hegde et al., 1998b; Fig. 1 A, lane 1). By contrast, proteoliposomes prepared from a glycoprotein-depleted detergent extract show a substantially reduced ability to translocate PrP, particularly in the secPrP form (Fig. 1 A, lane 2). Previous experiments attempting to complement the secPrP translocation defect with purified TRAM, the only glycoprotein with a known functional role in translocation, have been unsuccessful (Hegde et al., 1998b). These observations raised the possibility that nonessential components of the translocon, provisionally termed TrAFs (for translocation accessory factors; Hegde et al., 1998b; Hegde and Lingappa, 1999), may function in a substrate-specific manner to stimulate secPrP translocation.
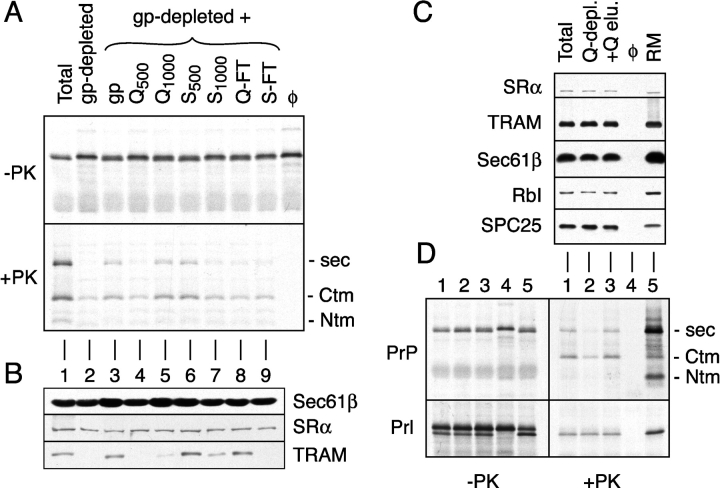
Figure 1.
Detection and fractionation of a translocation accessory factor activity. (A) Analysis of PrP translocation activity in fractionated proteoliposomes. A glycoprotein-depleted detergent extract was mixed with either buffer (lane 2), total glycoproteins (lane 3), or ion exchange fractions of total glycoproteins (lanes 4–9). Proteoliposomes were prepared from each mixture and assayed for their ability to translocate PrP. Shown are translation products before and after digestion with PK. The positions of protease-protected fragments of PrP corresponding to the secPrP, CtmPrP, and NtmPrP forms (Hegde et al., 1998a) are indicated to the right of the autoradiograph. Q-FT and S-FT indicate flowthrough fractions after binding at 200 mM KAc to Q- and S-sepharose, respectively. Fractions resulting from sequential elution of these resins with either 500 mM or 1,000 mM KAc are indicated with a subscript. For comparison, shown are translocation reactions lacking membranes (last lane) and containing proteoliposomes reconstituted from a total unfractionated detergent extract (lane 1). (B) Immunoblots of each of the proteoliposomes from panel A with antibodies against Sec61β, SRα, and TRAM. (C) Proteoliposomes were prepared from a total detergent extract, a detergent extract after depletion of proteins that bind to Q-sepharose (Q-depl.), and a Q-depleted extract replenished with the protein eluted from Q-sepharose (+Q-elu.). Aliquots of each proteoliposome preparation, along with the starting RMs, were immunoblotted with antibodies against the indicated proteins. (D) Translocation of PrP and Prl into the proteoliposomes from panel C. Aliquots of the translation products before and after digestion with PK are shown on the left and right, respectively. Lane 4 is a translocation reaction lacking membranes. The topologic forms of PrP are indicated to the right of the autoradiograph.
To examine this idea, we fractionated total ER glycoproteins by ion exchange chromatography and assayed each of the fractions for an activity capable of stimulating secPrP translocation. Among the ion exchange samples, the highest TrAF activity (comparable to that seen with total glycoproteins) was observed in the Q1000 and S500 fractions (Fig. 1 A, lanes 5 and 6, respectively). By contrast, the other ion exchange fractions were largely inactive in their ability to stimulate secPrP translocation above that seen with the glycoprotein-depleted proteoliposomes. Immunoblotting analyses demonstrated that, as expected, the nonglycoproteins SR and Sec61p complex were present at comparable amounts in each of the proteoliposome preparations. By contrast, the glycoprotein TRAM was efficiently depleted by concanavalin A (ConA), largely replenished by the total glycoprotein fraction, and found predominantly in the S500 and Q-FT fractions after ion exchange chromatography. Thus, the TrAF activity that stimulates secPrP translocation did not appear to cofractionate with any of these components. Of particular note, TrAF activity was observed to remain bound to anion exchange (Q-sepharose) at 500 mM KAc, being eluted only by 1,000 mM KAc.
This property was exploited to prepare a total detergent extract depleted of the very minor population of polypeptides (<5% of total protein; unpublished data) that bind Q-sepharose under these high salt conditions (hereafter termed a Q-depleted extract). Proteoliposomes reconstituted from a Q-depleted extract contained the full complement of SR, Sec61p complex, TRAM, ribophorin I (a component of the oligosaccharyl transferase complex), and signal peptidase complex (Fig. 1 C). Yet, they were essentially devoid of TrAF activity, being largely inactive in generating secPrP (Fig. 1 D, lane 2). This defect was fully replenished upon coreconstitution of the Q-depleted extract with the proteins eluted from Q-sepharose (Fig. 1 D, lane 3). Importantly, proteoliposomes made from the Q-depleted extract showed no discernible defect in the translocation of Prl (Fig. 1 D, bottom), consistent with this substrate needing only SR, Sec61p complex, and TRAM for maximal translocation (Gorlich and Rapoport, 1993). Thus, a TrAF activity that is involved in secPrP translocation, but not required for Prl translocation, could be separated from the currently established essential components of the translocon with Q-sepharose, thereby facilitating its subsequent purification.
Purification and identification of TrAF as the TRAP complex
Elution of the proteins that bound to Q-sepharose with a step gradient of salt, followed by fractionation with ConA, consistently resulted in the substantial enrichment of only five main proteins (Fig. 2 A). These proteins could be identified by immunoblotting, respective sizes, and abundance to be calnexin (CNX) and the α through δ subunits of the TRAP complex (unpublished data; Fig. 2 B). Each fraction was then coreconstituted with a Q-depleted extract into proteoliposomes that were analyzed by immunoblotting (Fig. 2 B) and translocation activity (Fig. 2 C). Whereas Prl translocation was comparable in each of the proteoliposomes, secPrP translocation was diminished by Q-depletion and replenished to varying degrees by the different fractions (Fig. 2 C). We consistently noticed that the highest levels of secPrP translocation corresponded to the fractions containing TRAP, and to a lesser extent CNX (Fig. 2, compare B with C).
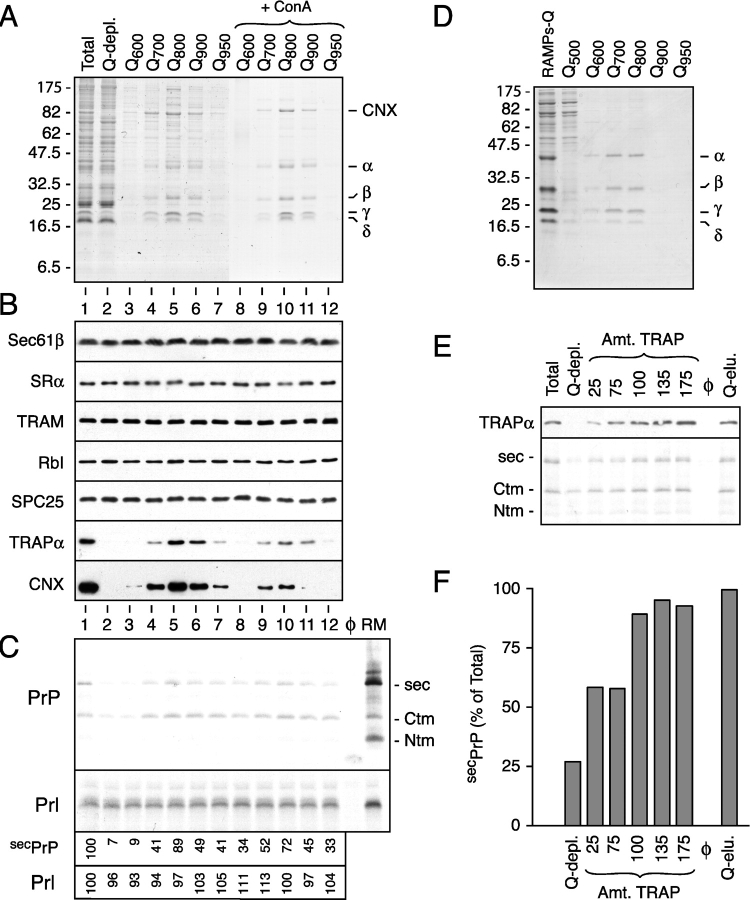
Figure 2.
Purification of TrAF and identification as the TRAP complex. (A) Shown is a Coomassie blue–stained gel of fractions resulting from the separation of membrane proteins by ion exchange and ConA chromatography. The positions of CNX and the α through δ subunits of the TRAP complex are indicated. (B) Immunoblots against various proteins in proteoliposomes prepared using the fractions in panel A. Lanes 1 and 2 contain proteoliposomes reconstituted from the total and Q-depleted detergent extracts, respectively. Lanes 3–12 contain proteoliposomes coreconstituted with the Q-depleted detergent extract plus the respective individual fractions in lanes 3–12 of A. (C) Translocation assays of PrP and Prl using proteoliposomes from panel B. Control reactions lacking membranes or containing RMs are also shown for comparison. Only the translocated material, remaining after digestion of the translation reactions with PK, is shown. The efficiencies of secPrP and Prl translocation, relative to the unfractionated proteoliposomes in lane 1, are shown below the autoradiograph. (D) Purification of the TRAP complex from the RAMP fraction. Shown is the Coomassie blue–stained gel containing the final fractions of the purification. (E) A Q-depleted detergent extract was replenished with varying concentrations of RAMP-purified TRAP or total Q eluate and reconstituted into proteoliposomes. As a control, an unfractionated detergent extract was also reconstituted in parallel. Shown in the top panel is an immunoblot against TRAPα of the different proteoliposomes. The amount of TRAP, as a percent of that found in the unfractionated proteoliposomes, is indicated above the blot. The bottom panel shows the assay for PrP translocation into these proteoliposomes. Only the translocated products remaining after protease digestion are shown. (F) The extent of secPrP translocation in the assay from panel E was quantitated and plotted as a bar graph. The amount of translocation in the Q-depleted proteoliposomes replenished with the total Q-eluate fraction was defined as 100%.
Two observations led us to focus on TRAP, rather than CNX, as the likely active component in these fractions. First, in contrast to TrAF activity and TRAP, CNX is not a glycoprotein and is usually rather poorly depleted by ConA (unpublished data). Thus, its binding to ConA appears to be indirectly mediated by a glycoprotein with whom its association is variable and weak. Consistent with this idea, CNX has been shown in some, but not other, studies to be associated with TRAP (Wada et al., 1991; Hartmann et al., 1993), perhaps explaining its variable degrees of contamination in TRAP-containing fractions. And second, fractions that contained CNX, but not TRAP (e.g., Q-binding nonglycoproteins), were not active in stimulating secPrP translocation (unpublished data). Together, these observations suggested that TrAF activity may be attributable to the TRAP complex.
To test whether the tetrameric TRAP complex alone was the component that stimulates secPrP translocation, we took advantage of its known tight association with the ribosome (Gorlich et al., 1992b; Matlack and Walter, 1995) to separate it from any contaminating CNX. Anion exchange chromatography of a ribosome-associated membrane protein (RAMP) fraction (Gorlich and Rapoport, 1993) resulted in a TRAP preparation (Fig. 2 D) in which CNX was undetectable by immunoblotting (unpublished data). A titration of this RAMP-purified TRAP showed that it could restore secPrP translocation to levels comparable to those seen when a Q-depleted extract was replenished with the total Q eluate (Fig. 2, E and F). These data suggest not only that TRAP is able to stimulate secPrP translocation, but also that it is likely to be the principal, if not only, active component that is depleted by Q-sepharose. Thus, by two independent methods of purification, fractions substantially enriched for TRAP are active in stimulating PrP translocation. We therefore conclude that TrAF, the activity originally defined by its substrate-specific requirement for PrP translocation and topogenesis but not for Prl translocation (Hegde et al., 1998b), is the tetrameric TRAP complex.
Dependence on TRAP for translocation is influenced by the signal sequence
We next sought to determine the basis for the substrate-specificity of TRAP function that allows Prl, but not PrP, to be translocated completely independently of TRAP. Because the most apparent difference between Prl and PrP is that the latter, but not the former, is synthesized in multiple topologic forms, we analyzed the behavior of a PrP mutant that eliminates this property. PrP(G123P), which disrupts the potential membrane-spanning domain in PrP, is made exclusively in the secPrP form (Hegde et al., 1998a) and is therefore topologically equivalent to Prl. When analyzed in proteoliposomes lacking and containing TRAP, we found that the translocation of PrP(G123P) was influenced by the presence of TRAP in a manner similar to the secPrP form of wild-type PrP (Fig. 3 B). Similar results were observed with other mutants of PrP that abolished or diminished its topologic heterogeneity (unpublished data), arguing that the topologic heterogeneity of PrP is unlikely to be the basis of its requirement for TRAP.
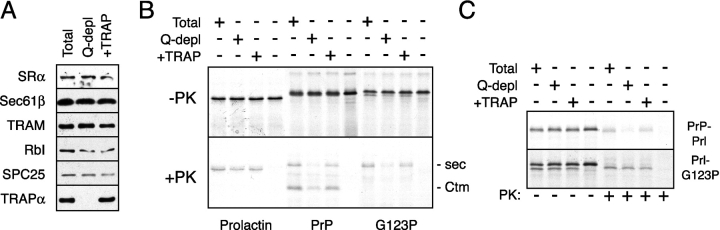
Figure 3.
Dependence on TRAP for translocation is influenced by the signal sequence. (A) Immunoblots against various proteins of proteoliposomes prepared from an unfractionated detergent extract (Total), a Q-depleted detergent extract, and a Q-depleted extract replenished with RAMP-purified TRAP at a level comparable to that in the unfractionated extract. (B) Translocation of Prl, PrP, and PrP(G123P) into the proteoliposomes from panel A, or a control reaction lacking proteoliposomes. Aliquots of the translation reaction before (top) and after (bottom) digestion with PK are shown. (C) Prl–G123P (which contains the signal sequence of Prl fused to the mature domain of PrP[G123P]) and PrP–Prl (containing the signal of PrP fused to the mature domain of Prl) were assayed for their TRAP dependence, as in B.
To investigate whether the different translocation behavior of these two substrates could be attributed to differences in their signal sequences, we prepared chimeric molecules in which the signal of Prl was fused to the mature domain of PrP(G123P) (termed Prl–G123P) and the signal of PrP was fused to the mature domain of Prl (termed PrP–Prl). Remarkably, simply swapping the signal sequences of these two substrates was sufficient to confer TRAP-independent translocation to PrP(G123P) and TRAP-dependent translocation to Prl (Fig. 3 C). Thus, the requirement for TRAP during substrate transport is influenced by the signal sequence used to direct its translocation.
Correlation of TRAP dependence with post-targeting signal sequence function
The PrP and Prl signal sequences differ in numerous physical and functional characteristics, including length, hydrophobicity, charge, amino acid composition, and effect on substrate translocation (Rutkowski et al., 2001; Kim et al., 2002). To gain insight into whether any of these features influence dependence on TRAP for translocation, we took advantage of a series of constructs encoding each of eight mammalian signal sequences fused to the PrP mature domain (Kim et al., 2002). A comparative analysis of these natural signal sequences revealed that the translocation of constructs containing the signals from Prl, growth hormone (GH), and osteopontin (Ost) was influenced to a lesser degree than PrP by the presence or absence of TRAP (Fig. 4 A). By contrast, the other four signals analyzed displayed more dependence on TRAP for directing translocation of the PrP mature domain.
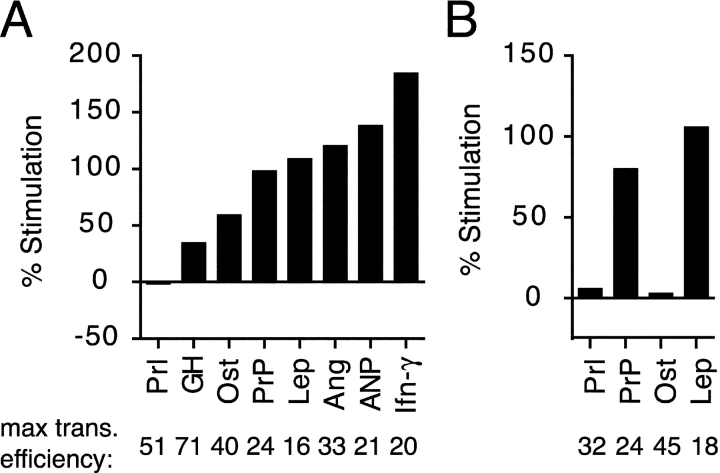
Figure 4.
Signal sequences from different substrates vary in their TRAP dependence. (A) Constructs containing the signal sequences of various mammalian proteins fused to PrP (Kim et al., 2002) were tested for their translocation into Q-depleted proteoliposomes lacking or containing RAMP-purified TRAP (as in Fig. 3 A). To facilitate direct comparisons, all constructs were analyzed in parallel using the same batch of proteoliposomes.The percent increase in overall translocation into the TRAP-containing proteoliposomes, relative to the membranes lacking TRAP, is plotted. The maximal overall translocation efficiency for each substrate (as a percent of total synthesized translation product) into proteoliposomes containing TRAP is indicated below the graph. The sequences and properties of the signals used in this experiment are depicted in Fig. 5. (B) The indicated signal sequences fused to the mature domain of bovine Prl were analyzed for their degree of dependence on TRAP for translocation, as in A.
The Ost and leptin signals displayed qualitatively similar properties when fused to the Prl mature domain: the leptin, but not Ost, signal sequence required TRAP to direct maximal translocation of the substrate (Fig. 4 B). In addition, it should be noted that some differences are observed in TRAP dependence for the same signals fused to PrP versus Prl (Fig. 4; unpublished data), perhaps indicating that features of the mature domain may also influence TRAP dependence to some degree. Although this remains to be investigated, the results in Fig. 4 demonstrate that an important determinant of TRAP dependence is the signal sequence, which ranges widely in this feature from substrate to substrate.
The sequences of the eight signals analyzed for TRAP dependence were compared by several criteria to elucidate which, if any, physical property may be an important determinant (Fig. 5). However, no single feature of the signal sequence correlated with their respective functional dependence on TRAP. This included overall length, maximal hydrophobicity, length of the hydrophobic domain, charge of the n-domain (the nonhydrophobic domain preceding the hydrophobic core of the signal; von Heijne, 1985), or charge differential across the hydrophobic domain. A qualitative assessment of the hydropathy profiles of the signals also did not reveal a simple correlation. And finally, a systematic difference was not observed in the amino acid composition of the hydrophobic core of the signal (distinguishing between leucine/isoleucine/valine versus alanine/phenylalanine/tryptophan). Together, these observations suggest that TRAP dependence is determined by either a combination of multiple physical features of the signal sequence or by a feature that was not analyzed here.
Figure 5.
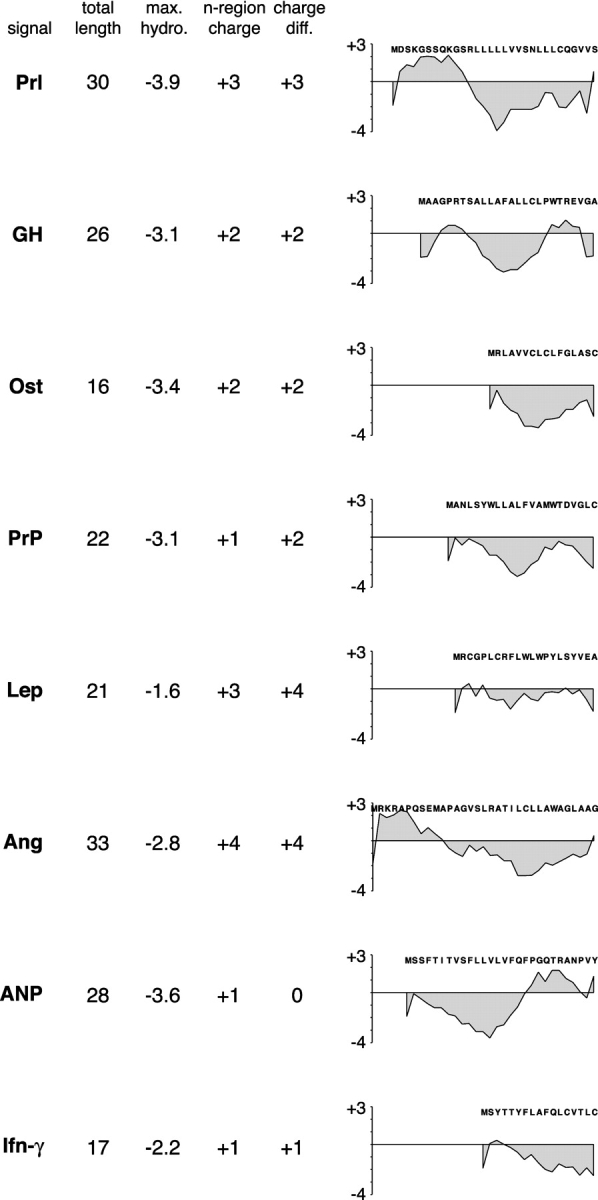
Physical properties of signal sequences that differ in their TRAP dependence. Shown are several parameters for eight signal sequences (human Prl, pig GH, rat Ost, hamster PrP, pig leptin [Lep], human angiotensinogen [Ang], pig atrial naturetic peptide [ANP], and pig interferon-γ [Ifn-γ]). Hydropathy was determined by the method of Kyte and Doolittle (1982) using a window of seven residues. In calculating the net charge of the n-region (the domain preceding the hydrophobic core) or the charge difference flanking the hydrophobic domain, the amino terminus, lysine, and arginine were each taken to contribute a net +1 charge, whereas aspartate and glutamate were taken to contribute a net −1 charge. All of the hydropathy plots are shown on the same scale to allow direct comparisons to be made.
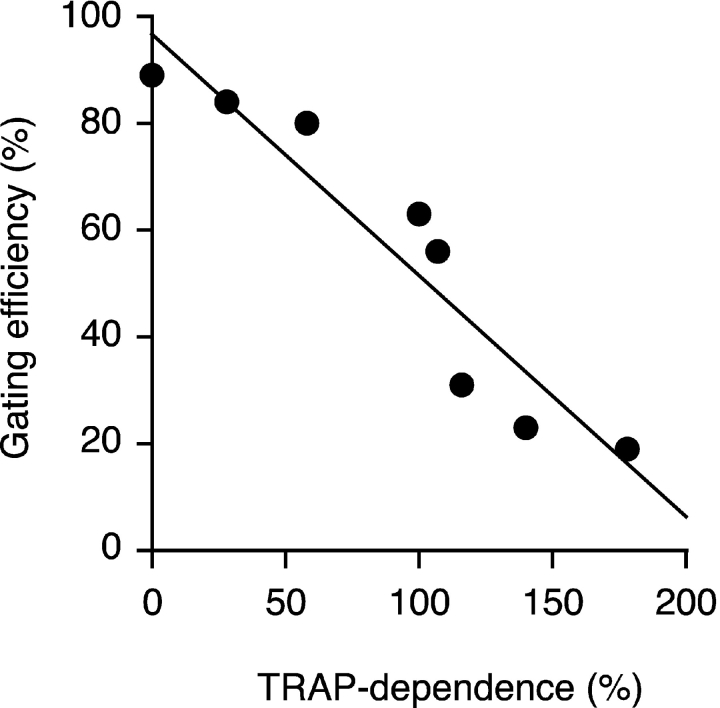
We therefore asked whether a functional feature of signal sequences that differs among substrates could be correlated to its dependence on TRAP for translocation. Signal sequences have at least two principal functions: signal recognition particle–mediated targeting of nascent chains to the translocon (Walter and Johnson, 1994) and a decisive post-targeting translocon “gating” step that commits the substrate to initiate translocation of its NH2 terminus (Jungnickel and Rapoport, 1995). In a recent study by Kim et al. (2002), the ability of a downstream transmembrane domain to compete with signal-mediated initiation of NH2 terminus translocation was exploited to demonstrate that the relative gating efficiencies of different mammalian signal sequences vary substantially. When we compared the relative gating efficiencies of eight signal sequences with their TRAP dependences, we noticed a remarkable inverse correlation (Fig. 6). Thus, substrates containing signal sequences with weaker gating activity (as measured by Kim et al., 2002) have a greater requirement for TRAP during their translocation than those with stronger gating activity.
Figure 6.
Correlation of a signal's TRAP dependence and post-targeting gating function. The eight signal sequences from Fig. 5 are plotted on a graph that shows their relative dependence on TRAP for translocation on the x axis and their post-targeting gating activity on the y axis. Each signal sequence's gating activity was taken from data reported in previously published work (Kim et al., 2002). In this study, gating was defined as a signal's ability to initiate substrate translocation at the translocon and was measured based on an assay using the topology of PrP as a reporter of the efficiency of NH2-terminal translocation (Kim et al., 2002).
TRAP-dependent signals interact weakly with the translocon
Translocon gating by a signal sequence appears to involve its recognition by Sec61α and TRAM (Jungnickel and Rapoport, 1995; Voigt et al., 1996; Mothes et al., 1998), and results in several measurable changes to the ribosome–nascent chain–translocon complex: the ribosome–translocon interaction becomes resistant to high salt (Jungnickel and Rapoport, 1995), the channel is opened toward the ER lumen (Crowley et al., 1994), the nascent chain becomes shielded from the cytosol (Crowley et al., 1994; Jungnickel and Rapoport, 1995), and the nascent chain is committed to forward transport into the ER lumen. We reasoned that the strength or manner of a signal sequence's interaction with the translocon can influence its efficiency in directing some or all of these key steps during the initiation of translocation. Based on the correlation in Fig. 6, putative differences among substrates in one or more of these events may be a key determinant of whether TRAP is subsequently needed during translocation. To examine this idea, translocation intermediates of matched substrates, differing only in the TRAP dependence of their signal sequences, were analyzed in native ER microsomes for salt-resistant binding, protection from cytosolically added protease, and commitment to forward translocation into the lumen.
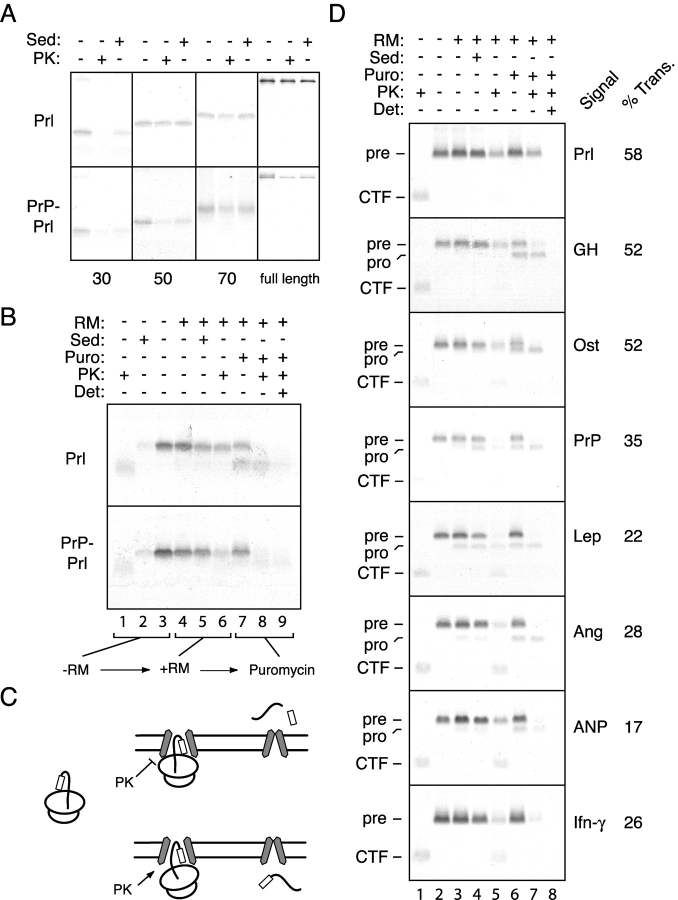
We first analyzed progressively longer translocation intermediates of Prl containing either its own or the PrP signal sequence. At translocation intermediates of only 30 residues beyond the signal sequence, both Prl and PrP–Prl displayed incomplete salt-resistant binding and complete exposure to cytosolic protease (Fig. 7 A). At an intermediate that was 20 residues longer, Prl displayed quantitative salt-resistant binding and was well shielded from cytosolic protease. By contrast, PrP–Prl, while bound to the ER in a salt-resistant manner, was observed to largely be accessible to cytosolic protease. Only after the synthesis of an additional 20 residues did the PrP–Prl intermediate display both salt- and protease-resistant binding comparable to that seen for Prl (Fig. 7 A). At subsequent lengths, Prl and PrP–Prl appeared to be similar in their salt- and protease-resistant binding characteristics (unpublished data), and synthesis of the full-length products resulted in the translocation of both substrates (Fig. 7 A).
Figure 7.
Functional analysis of signal sequence–translocon interactions. (A) Translocation intermediates of 30, 50, or 70 residues beyond the signal sequence of either Prl or PrP–Prl were prepared and examined by protease protection and salt-resistant binding assays. Equal aliquots of untreated, PK-digested, and salt-resistant samples are shown for each intermediate. In addition, the full-length proteins were analyzed in parallel. (B) Translocation intermediates at 56 residues beyond the signal of either Prl or PrP–Prl were examined for protease protection, salt-resistant binding, and ability to translocate upon release from the ribosome with puromycin. (C) Shown is a schematic diagram of the experimental protocol and interpretation of the experiment in B. Nascent chains that are protected from protease digestion appear to translocate into the lumen (with concomitant signal sequence cleavage) upon release with puromycin, whereas protease-accessible nascent chains slip into the cytosol upon puromycin release. (D) Translocation intermediates of 101 residues beyond the signal sequence were examined for cytosolic accessibility, salt-resistant binding, and translocation, as in B. Each substrate is the mature region of PrP containing the indicated signal sequences. The positions of the precursor and processed (i.e., signal cleaved) forms for each substrate are indicated to the left. Also indicated is the position of the COOH-terminal fragment (CTF) that represents the segment of the nascent chain within the ribosome and, hence, is protected from protease digestion. The percent of translocon-bound chains that are translocated (and, hence, protease protected) upon puromycin release was calculated by dividing the amount of substrate in lane 7 by that in lane 4, and is indicated to the right of each autoradiograph.
We next asked if, at a point where Prl and PrP–Prl can be distinguished by their cytosolic accessibility, their respective translocation properties also differ. We therefore examined translocation intermediates of 56 residues beyond the signal sequence to determine whether release of the nascent chain with puromycin and dissociation of the ribosome with high salt would result in the completion of substrate translocation into the ER lumen (Fig. 7 B). We found that mimicking the termination of translation at this critical stage of translocation resulted in Prl, but not PrP–Prl, being translocated into the lumen (Fig. 7 B). This was evidenced by signal sequence cleavage of Prl, but not PrP–Prl, and subsequent protection of the processed species from protease in the absence, but not presence, of detergent (Fig. 7 B, lanes 7–9). Thus, the TRAP-independent Prl signal can be distinguished from the TRAP-dependent PrP signal on the basis of its increased efficiency in directing nascent chain shielding from the cytosol and commitment to forward translocation at an early stage of biogenesis.
We also performed a similar analysis of the various signal sequence constructs from Fig. 4 A. Translocation intermediates of each construct containing 101 amino acids beyond the signal sequence were generated and assayed for salt-resistant binding, cytosolic accessibility to protease, and ability to translocate into the lumen upon release from the ribosome (e.g., as diagrammed in Fig. 7 C). Although each of the nascent chain intermediates was comparably membrane bound in a salt-resistant manner (Fig. 7 D, lanes 3 and 4), substantial differences were seen in their protease accessibility (lane 5) and translocation competence (lanes 6–8). The translocation intermediates containing the Prl, GH, or Ost signal sequences were protected from protease digestion and translocated into the lumen upon puromycin release to a greater degree than the other translocation intermediates.
Because shielding of the nascent chain from the cytosol and commitment to translocation into the lumen are both measures of a properly gated translocon, these data are consistent with and corroborate the previous analysis of signal gating activity based on topogenic sequence competition (Kim et al., 2002). More importantly, these observations provide specific insight into the characteristics of TRAP-dependent and -independent signals. Signals that interact strongly with the translocon to mediate efficient formation of a protease-protected ribosome–membrane junction and achieve a translocation-committed state early in synthesis tend to be TRAP independent. By contrast, TRAP-dependent signals are characterized by their weaker interactions with the translocon, as evidenced by their relatively poor ability to commit the nascent chain to forward translocation at an early stage in synthesis.
It is additionally worth noting that although the signal is a principal determinant of a substrate's efficiency in initiating translocation, a role for the mature domain should not be entirely excluded. Indeed, in comparing translocation intermediates of the same signals fused to different mature domains, some differences in the timing of the achievement of protease resistance and commitment to translocation have been observed (e.g., Fig. 7, A versus D; unpublished data). It may be that sequences in the initial portion of the mature domain also influence signal sequence gating and translocation activities (Kim et al., 2002), although this remains to be investigated in a systematic manner. Regardless of this possibility, when the mature domain is held constant (as in Fig. 7 D), differences can readily be demonstrated between signal sequences in their post-targeting gating function, and this activity is inversely correlated with its dependence on TRAP for translocation.
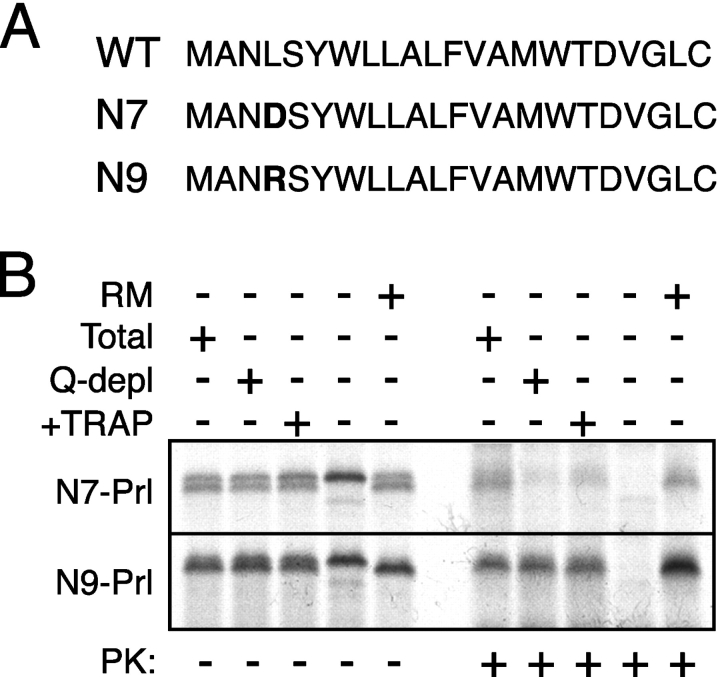
If this functional feature of the signal sequence is the basis of its TRAP dependence, we reasoned that it should be manipulable in predictable ways. To test this, we analyzed the TRAP dependence of two point mutations in the PrP signal sequence (termed N7 and N9 [Fig. 8 A], which replace a leucine at position 4 with either an aspartate or arginine, respectively). The N7 and N9 mutations were shown previously to decrease and increase, respectively, the post-targeting gating function of the PrP signal (Kim et al., 2002). In addition, a recent analysis of serial translocation intermediates revealed that the N9 signal mediates nascent chain protection from the cytosol and access to the ER lumen at an earlier stage in translocation than the N7 signal (Kim and Hegde, 2002). As would be predicted from our working hypothesis above, we found that when fused to Prl, the N9, but not N7, signal substantially reduced TRAP dependence (Fig. 8 B). Thus, a single amino acid mutation that increases the strength of a signal sequence's post-targeting interaction with the translocon is sufficient to decrease TRAP dependence. Taken together with the analysis of natural signal sequences, these data argue that TRAP is particularly important for the translocation of substrates whose signal sequences interact relatively weakly with the native translocon and are thus less efficient at directing closure of the ribosome–translocon junction and committing the nascent chain to forward translocation early in biogenesis.
Figure 8.
Modulation of TRAP dependence by changing signal–translocon interactions. (A) Sequences of the wild-type, N7 mutant, and N9 mutant signal sequences of PrP. The mutated residue is indicated in bold. (B) The N7 and N9 signal sequences fused to Prl were assayed for their dependence on TRAP for translocation, as in Fig. 3 B. Control reactions containing RMs or lacking membranes are also shown for comparison.
Discussion
In this study, we have combined membrane protein fractionation and reconstitution methods with functional complementation of transport activity to purify and identify an additional component of the translocation machinery that is needed in a substrate-specific manner. Several principal conclusions can be drawn from the experiments presented here. First, the data demonstrate that TRAP, an abundant protein complex that has long been suggested to be at or near the site of translocation, is functionally involved in protein transport. Second, the requirement for TRAP in achieving efficient translocation is substrate specific and ranges from completely dispensable to modestly stimulatory to all but essential. Third, the TRAP dependence of a substrate is substantially influenced by the choice of signal sequence. And fourth, the extent to which TRAP is needed for translocation is inversely related to a signal sequence's efficiency at its post-targeting function in initiating substrate translocation early in biogenesis. Taken together, our findings indicate that TRAP is an integral component of the mammalian translocon that acts at a post-targeting stage of protein translocation to facilitate the initiation of substrate transport.
The conclusions of our study can be readily reconciled with earlier work suggesting that TRAP may not be functionally essential for protein translocation. In one study, proteoliposomes reconstituted from a detergent extract immunodepleted of TRAP had failed to reveal a translocation defect (Migliaccio et al., 1992). Because Prl was the principal substrate analyzed by Migliaccio et al. (1992), the inability to detect a consequence of TRAP depletion is consistent with our finding that it contains a TRAP-independent signal sequence. In other studies, reconstitution with purified components that did not include TRAP still supported translocation of numerous model proteins, albeit to varying degrees of efficiency (Gorlich and Rapoport, 1993; Voigt et al., 1996). This can be explained by our observation that for most substrates, the requirement for TRAP is not absolute and varies in a signal sequence–specific manner. Indeed, we have recently confirmed that α-factor is at least partially TRAP dependent (unpublished data), providing an explanation for its relatively poor translocation (in comparison to Prl) into proteoliposomes containing only the Sec61p complex, SR, and TRAM (Gorlich and Rapoport, 1993). Thus, while our study clearly demonstrates a role for TRAP in translocation, its modest stimulatory effect on the vast majority of substrates may partly explain why its function had previously remained enigmatic and difficult to demonstrate.
Although the molecular mechanism of TRAP function is not clear at present, the role of the signal sequence in determining TRAP dependence suggests that TRAP is likely to improve transport by acting at a signal-mediated step in translocation. The demonstration that proteoliposomes lacking TRAP are able to mediate targeting and salt-resistant binding of both TRAP-dependent (PrP) and -independent (PrP) substrates with equal efficiency (Hegde et al., 1998b) argues against a role for TRAP in modulating targeting. Instead, TRAP is likely to influence the signal sequence's post-targeting function of gating the translocation channel to initiate substrate translocation into the ER. However, TRAP does not appear to be directly involved in signal sequence recognition. Extensive site-specific cross-linking studies using probes in the signal sequence have demonstrated that it interacts with the translocon at a specific binding site at the membrane formed by a combination of the Sec61p complex, TRAM, and membrane lipids (High et al., 1993; Jungnickel and Rapoport, 1995; Martoglio et al., 1995; Voigt et al., 1996; Mothes et al., 1998). Only at substantially later steps in translocation are cross-links seen between TRAP and the nascent chain, probably to regions of the mature domain (Gorlich et al., 1992a; Mothes et al., 1994).
If TRAP does not interact with the signal directly, how then might it influence translocation in a signal-dependent manner? In one model, TRAP could interact with the mature domain of the nascent chain on the lumenal side of the translocon to help stabilize the newly inserted nascent chain in the proper orientation at the translocation channel. Thus, signals whose strong interaction with Sec61α and/or TRAM allow the nascent chain to be firmly held in the proper “loop” orientation would not additionally need TRAP to stabilize the mature domain. This may explain why the strength of signal–translocon interactions early in translocation is a key determinant of TRAP dependence. Consistent with such a model, the α and β subunits of TRAP have large, conserved lumenal domains (Hartmann et al., 1993), cross-links to TRAPα are not seen until nascent chain lengths are relatively long (Gorlich et al., 1992a; Mothes et al., 1994), and mutations in a signal's n-domain, which can modulate interactions with TRAM, can influence TRAP dependence (Fig. 8).
An alternative model is one in which TRAP stabilizes the nascent chain indirectly by an effect on Sec61p structure, function, or oligomerization. Given that TRAP appears to be as abundant as Sec61p and is found tightly associated with membrane-bound ribosomes (Gorlich et al., 1992b; Matlack and Walter, 1995), it would seem reasonable to conclude that at least one copy of TRAP is present at every translocon. These observations, coupled with the fact that the “native” translocon appears to be larger and structurally different than a translocon composed only of the Sec61p complex (Hanein et al., 1996; Menetret et al., 2000), raises the possibility that incorporation of TRAP into a Sec61p translocon changes its overall dimensions. Perhaps the slightly larger translocon, with a bigger and differently shaped pore, is more easily able to accommodate certain nascent chains than the more restrictive translocon lacking TRAP. This could have the consequence of allowing nascent chains easier access to the ER lumen, where they can either be partially folded or perhaps interact with lumenal chaperones, thereby biasing their forward transport. Substrates that are tightly inserted into the translocation site and do not have access to the cytosol (due to a closed ribosome–translocon junction) would not need lumenal chaperones or protein folding to bias translocation, perhaps explaining why they do not require TRAP for efficient transport. Future studies examining the consequences of TRAP on the structure and/or function of the Sec61p channel will be required to address these issues.
From a physiological standpoint, it is particularly noteworthy that dependence on the function of accessory factors (such as TRAP and TRAM) is influenced to varying degrees by topogenic elements, such as the signal sequence, that are highly divergent from one substrate to the next. This raises the intriguing possibility that modulation of the machinery that is involved in signal recognition or function at the ER could be exploited by the cell to regulate translocation (and hence secretion) in a substrate-specific manner. It is tempting to speculate that the observed phosphorylation of TRAP (Prehn et al., 1990; Ou et al., 1992) and other translocon components (Gruss et al., 1999) may play yet unappreciated regulatory roles in translocation. Similarly, our discovery of a key role for TRAP during PrP translocation could have potential implications for disease pathogenesis, given that defects in the topogenesis of PrP lead to the development of neurodegeneration (Hegde et al., 1998a, 1999). Thus, substrate-specific translocation factors, in contrast to the core translocation machinery, are more likely to be involved in either physiologic regulation and/or disease pathogenesis because modulation of their function may influence the biogenesis of a relative minority of secretory and membrane proteins.
Materials and methods
Plasmids, antibodies, and other materials
Construction schemes for plasmids that encode the following chimeric or mutant translation products have been described previously: PrP(G123P) (Hegde et al., 1998b); various signal sequences fused to the mature domain of PrP (Rutkowski et al., 2001; Kim et al., 2002); and Ost, leptin, and PrP signals fused to the mature domain of Prl (Kim et al., 2002). The N7 and N9 mutant signals of PrP (Kim et al., 2001) were fused to the mature domain of Prl using the scheme described by Kim et al. (2002). Prl–G123P was generated from Prl–PrP by replacing the segment between the Bsu361 and EcoR1 sites of PrP with the corresponding region from PrP(G123P). The Prl and PrP coding regions were bovine and hamster in origin. All constructs contained either an SP6 or T7 promoter for in vitro transcription and were in vectors of either pSP64 (Promega) or pCDNA3.1 (Invitrogen) origin. The following rabbit antisera were prepared (Lampire Biological Laboratories) against synthetic peptides conjugated to keyhole limpet hemocyanin using standard protocols: Sec61β (residues 2–10 plus a cysteine); TRAM (residues 2–18 plus a cysteine); SRα (residues 137–150 plus a cysteine); and TRAPα (cysteine plus residues 272–286). Antibodies against the 25-kD subunit of the signal peptidase complex, ribophorin I, and PrP (3F4 mouse monoclonal antibody) were the generous gifts of Tom Rapoport (Harvard Medical School, Boston, MA), Kennan Kellaris and Reid Gilmore (University of Massachusetts Medical School, Worcester, MA), and Stanley Prusiner (University of California San Francisco, San Francisco, CA). Anti-CNX (against the COOH terminus) was from StressGen Biotechnologies, and anti-Prl was from ICN Biomedicals. DeoxyBigCHAP (DBC) and digitonin were from Calbiochem. Saponin was from Sigma-Aldrich. Digitonin and saponin were dissolved and further purified as described previously (Gorlich and Rapoport, 1993; Panzner et al., 1995). Rabbit reticulocyte lysate and rough microsomal membranes (RMs) from either dog or pig pancreas were prepared as described previously (Jackson and Hunt 1983; Walter and Blobel, 1983). Lipids were from Avanti Polar Lipids, Inc. In some experiments, a 20 mg/ml mixture of phosphatidyl choline, phosphatidyl ethanolamine, phosphatidyl serine, and phosphatidyl inositol was prepared in a detergent-containing buffer (50 mM Hepes, pH 7.4, 15% glycerol, 2% DBC) as described previously (Gorlich and Rapoport, 1993). In more recent experiments, a 20 mg/ml preparation containing a 4:1 mixture of phosphatidyl choline and phosphatidyl ethanolamine has been used without any noticeable differences in reconstitution efficiencies or translocation activities. Biobeads SM2 were from Bio-Rad Laboratories. Prior to use, they were washed in methanol, rinsed extensively in distilled water, and equilibrated in extraction buffer (EB, see below). Chromatography resins (ConA sepharose, S- and Q-sepharose Fast Flow) and HRP-conjugated secondary antibodies for immunoblots were from Amersham Biosciences.
Fractionation and reconstitutions
Saponin-extracted, EDTA- and high salt–washed RMs were prepared as previously described (Hegde et al., 1998b) and resuspended at 1 eq/μl (see Walter and Blobel, 1983, for definition) in EB (350 mM KAc, 50 mM Hepes, pH 7.4, 5 mM MgCl2, 15% glycerol, 5 mM 2-mercaptoethanol, and a protease inhibitor cocktail [EDTA-free complete inhibitor; Roche Applied Sci.]). DBC was added to 0.8% from a 10% wt/vol stock solution and particles larger than ∼30s were removed by centrifugation in a TL100 ultracentrifuge (Beckman Instruments) to yield a DBC extract of RMs. Glycoprotein-depleted extract was prepared by incubation, with gentle mixing, in batch at 4°C for 8–12 h of the DBC extract with ConA sepharose (equilibrated in EB) at a ratio of 150 μl packed ConA beads per 1,000 eq DBC extract. Q-depleted extract was prepared using 150 μl Q-sepharose Fast Flow (equilibrated in EB) per 1,000 eq DBC extract, incubated in batch for 4–12 h at 4°C. Prior to elution, resins were washed four times with 6–10 volumes each with EB containing 0.5% DBC. Elution of glycoproteins from ConA was performed by incubation at 25°C for 90 min in 500 mM KAc, 50 mM Hepes, pH 7.4, 10 mM EDTA, 0.25 M α-methyl-mannopyrannoside, 15% glycerol, 5 mM 2-mercaptoethanol, 0.5% DBC. Elution of proteins from Q-sepharose was performed in EB containing 0.5% DBC and the appropriate KAc concentration, as indicated in the figure legends. Reconstitutions of extracts into proteoliposomes was typically performed in a 200-μl volume containing 100 μl at 1 eq/μl of either a DBC extract, glycoprotein-depleted extract, or Q-depleted extract. The remainder of the volume was used to add crude protein fractions and/or purified proteins and contained between 300 and 1,000 mM KAc, 50 mM Hepes, pH 7.4, 15% glycerol, and 0.5% DBC. Eluates from ConA also contained up to 10 mM EDTA and 0.25 M α-methyl-mannopyrannoside. EB containing 0.5% DBC was used to bring the final volume to 200 μl as needed, after which 100 μg of lipids (from a 20 mg/ml stock solution) was added, and the entire mixture was added to 150 mg Biobeads SM2 prepared as described above. After incubation at 4°C with mixing for 12–18 h, the fluid was separated from the beads and diluted to 1 ml with ice cold water, and the proteoliposomes were sedimented at 70,000 rpm for 15 min in a TL100.3 rotor with microcentrifuge tube adaptors. The pellets were resuspended in a final volume of between 20 and 40 μl, depending on the experiment, in 50 mM Hepes, pH 7.4, 100 mM KAc, 2 mM MgCl2, 250 mM sucrose, 1 mM DTT, frozen in liquid nitrogen, and stored at −80°C until their use in translocation reactions. Typically, 0.5–1 μl of these proteoliposomes was used per 10-μl translation reaction.
Purification of TRAP
A dialyzed RAMP fraction was prepared from ∼30,000 equivalents of pig pancreas microsomes exactly as described previously (Gorlich and Rapoport, 1993). This was bound to a 6-ml column of Q-sepharose Fast Flow equilibrated in 20 mM Hepes, pH 7.6, 5 mM MgAc2, 1% digitonin, 5 mM 2-mercaptoethanol, washed with 20 ml of equilibration buffer, and step eluted with 10 ml of 1,000 mM KAc, 50 mM Hepes, pH 7.6, 2.5% digitonin, 10% glycerol, 5 mM 2-mercaptoethanol. The peak fractions of this crude TRAP fraction (∼3.5 ml) were pooled and frozen in aliquots in liquid nitrogen and stored at −80°C until needed for reconstitutions. Prior to its use in reconstitutions, 500 μl of the crude TRAP was diluted with an equal volume of 50 mM Hepes, pH 7.4, 5 mM MgCl2, 15% glycerol, bound in batch (60 min at 4°C with mixing) to 50–70 μl Q-sepharose Fast Flow, and washed twice with 1 ml each of EB containing 0.5 M KAc and 0.5% DBC to exchange the detergent and remove contaminants. Pure TRAP was subsequently eluted in sequential 250-μl steps of EB containing 600, 700, 800, 900, and 950 mM KAc and 0.5% DBC. Peak fractions were pooled and used in reconstitutions as described above. The protein profile of these final purification–detergent exchange steps is shown in Fig. 2 D. This RAMP-purified TRAP was used for the replenishment of Q-depleted proteoliposomes analyzed in Figs. 3, 4, 6, and 8.
Cell-free translocation assays
In vitro transcription with SP6 or T7 RNA polymerase, translation in rabbit reticulocyte lysate, and translocation using dog pancreas RMs have been described previously (Hegde et al., 1998b and references therein). The analysis of PrP and Prl translocation and topology in RMs and reconstituted proteoliposomes by protease protection was as previously described (Hegde et al., 1998b). Truncated transcripts lacking a stop codon were prepared in one of two ways. For the serial truncations in Fig. 7 A, 5′ and 3′ oligos annealing to the SP6 promoter and the site of truncation, respectively, were used to PCR amplify the appropriate segment of the plasmids (using Pfu polymerase; Stratagene) before transcription. Alternatively, truncations in Fig. 7 (B and D) used Pvu2-digested plasmids (which cleaves at codon 56 of mature Prl) and EcoO109-digested plasmids (which cuts at codon 101 of mature PrP), respectively. Translation reactions encoding truncated products were synchronized by the addition of aurin tricarboxylic acid after 5 min at 26°C, after which translation was allowed to proceed for an additional 20 min at 26°C. Translocation was performed by either including RMs (at 0.1 eq/μl) in the translation reaction (Fig. 7 A) or adding them (at 0.3 eq/μl) after synthesis of the ribosome–nascent chains and incubating an additional 15 min at 26°C. Sedimentation analysis for salt-resistant binding was performed at 4°C by diluting translation reactions (usually 5 μl) with 20 volumes of 50 mM Hepes, pH 7.4, 500 mM KAc, 5 mM MgCl2, layering onto a 100-μl cushion of the same buffer containing 0.5 M sucrose, and centrifugation at 70,000 rpm for 5 min at 4°C in a Beckman TL100.3 rotor with microcentrifuge tube adapters. The supernatants were removed by aspiration before subsequent analysis of the pellets. Protease protection of truncated nascent chains was with 0.5 mg/ml proteinase K (PK) on ice for 60 min as described previously (Hegde et al., 1998b). Release of nascent chains with puromycin and high salt was performed by adjusting the translation reaction to 0.5 M KAc and 1 mM puromycin before incubation at 26°C for 15 min. In many of the experiments shown, translocated products were immunoprecipitated with the appropriate antibodies against the translation products because many of the truncated products and the NtmPrP and CtmPrP fragments migrate close to the highly abundant endogenous globin in reticulocyte lysate, which often distorts that region of the gel.
Miscellaneous
Separation of proteins by SDS-PAGE was on 12% Tris-tricine gels. Immunoblotting was performed after transfer to nitrocellulose. Primary antibodies were diluted between 1:2,000 and 1:10,000. HRP-conjugated secondary antibodies were used at 1:5,000, and the blot was developed with SuperSignal chemiluminescence reagents from Pierce Chemical Co. Quantitative analysis of translocation experiments used a Molecular Dynamics phosphorimager with accompanying software, whereas autoradiographs made on Kodak BioMax film were digitized for display in the figures (prepared using Adobe Photoshop® and Adobe Illustrator®).
Acknowledgments
We thank Soo Jung Kim, Erik Snapp, Tom Rapoport, Sandy Simon and members of his laboratory, Harris Bernstein, and Doug Lowy for valuable discussions; Soo Jung Kim, Jeff Salerno, and Devarati Mitra for help preparing some of the constructs used in this study; and T. Rapoport, K. Kellaris, R. Gilmore, and S. Prusiner for antibody reagents. In addition, we thank the Laboratory of Cellular Oncology of the National Cancer Institute, where much of this work was performed.
This work was supported by the Intramural Research Program of the National Institutes of Health.
Footnotes
Abbreviations used in this paper: ConA, concanavalin A; CNX, calnexin; DBC, DeoxyBigCHAP; EB, extraction buffer; GH, growth hormone; Ost, osteopontin; PK, proteinase K; Prl, prolactin; PrP, prion protein; RAMP, ribosome-associated membrane protein; RM, rough microsomal membrane; SR, signal recognition particle receptor; TrAF, translocation accessory factors; TRAP, translocon-associated protein.
References
- Andrews, D.W., and A.E. Johnson. 1996. The translocon: more than a hole in the ER membrane? Trends Biochem. Sci. 21:365–369. [PubMed] [Google Scholar]
- Beckmann, R., D. Bubeck, R. Grassucci, P. Penczek, A. Verschoor, G. Blobel, and J. Frank. 1997. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science. 278:2123–2126. [DOI] [PubMed] [Google Scholar]
- Crowley, K.S., S. Liao, V.E. Worrell, G.D. Reinhart, and A.E. Johnson. 1994. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell. 78:461–471. [DOI] [PubMed] [Google Scholar]
- Gorlich, D., and T.A. Rapoport. 1993. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 75:615–630. [DOI] [PubMed] [Google Scholar]
- Gorlich, D., E. Hartmann, S. Prehn, and T.A. Rapoport. 1992. a. A protein of the endoplasmic reticulum involved early in polypeptide translocation. Nature. 357:47–52. [DOI] [PubMed] [Google Scholar]
- Gorlich, D., S. Prehn, E. Hartmann, K.U. Kalies, and T.A. Rapoport. 1992. b. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 71:489–503. [DOI] [PubMed] [Google Scholar]
- Gruss, O.J., P. Feick, R. Frank, and B. Dobberstein. 1999. Phosphorylation of components of the ER translocation site. Eur. J. Biochem. 260:785–793. [DOI] [PubMed] [Google Scholar]
- Hanein, D., K.E. Matlack, B. Jungnickel, K. Plath, K.U. Kalies, K.R. Miller, T.A. Rapoport, and C.W. Akey. 1996. Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell. 87:721–732. [DOI] [PubMed] [Google Scholar]
- Hartmann, E., D. Gorlich, S. Kostka, A. Otto, R. Kraft, S. Knespel, E. Burger, T.A. Rapoport, and S. Prehn. 1993. A tetrameric complex of membrane proteins in the endoplasmic reticulum. Eur. J. Biochem. 214:375–381. [DOI] [PubMed] [Google Scholar]
- Hegde, R.S., and V.R. Lingappa. 1999. Regulation of protein biogenesis at the endoplasmic reticulum membrane. Trends Cell Biol. 9:132–137. [DOI] [PubMed] [Google Scholar]
- Hegde, R.S., J.A. Mastrianni, M.R. Scott, K.A. DeFea, P. Tremblay, M. Torchia, S.J. DeArmond, S.B. Prusiner, and V.R. Lingappa. 1998. a. A transmembrane form of the prion protein in neurodegenerative disease. Science. 279:827–834. [DOI] [PubMed] [Google Scholar]
- Hegde, R.S., S. Voigt, and V.R. Lingappa. 1998. b. Regulation of protein topology by trans-acting factors at the endoplasmic reticulum. Mol. Cell. 2:85–91. [DOI] [PubMed] [Google Scholar]
- Hegde, R.S., P. Tremblay, D. Groth, S.J. DeArmond, S.B. Prusiner, and V.R. Lingappa. 1999. Transmembrane and genetic prion diseases share a common pathway of neurodegeneration involving transmembrane prion protein. Nature. 402:822–826. [DOI] [PubMed] [Google Scholar]
- Heinrich, S.U., W. Mothes, J. Brunner, and T.A. Rapoport. 2000. The Sec61p complex mediates the integration of a membrane protein by allowing lipid partitioning of the transmembrane domain. Cell. 102:233–244. [DOI] [PubMed] [Google Scholar]
- High, S., B. Martoglio, D. Görlich, S.S. Andersen, A.J. Ashford, A. Giner, E. Hartmann, S. Prehn, T.A. Rapoport, B. Dobberstein, and J. Brunner. 1993. Site-specific photocross-linking reveals that Sec61p and TRAM contact different regions of a membrane-inserted signal sequence. J. Biol. Chem. 268:26745–26751. [PubMed] [Google Scholar]
- Holscher, C., U.C. Bach, and B. Dobberstein. 2001. Most pathogenic mutations do not alter the membrane topology of the prion protein. J. Biol. Chem. 276:13388–13394. [DOI] [PubMed] [Google Scholar]
- Jackson, R.J., and T. Hunt. 1983. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 96:50–74. [DOI] [PubMed] [Google Scholar]
- Johnson, A.E., and M.A. van Waes. 1999. The translocon: a dynamic gateway at the ER membrane. Annu. Rev. Cell Dev. Biol. 15:799–842. [DOI] [PubMed] [Google Scholar]
- Jungnickel, B., and T.A. Rapoport. 1995. A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell. 82:261–270. [DOI] [PubMed] [Google Scholar]
- Kalies, K.U., D. Gorlich, and T.A. Rapoport. 1994. Binding of ribosomes to the rough endoplasmic reticulum mediated by the Sec61p-complex. J. Cell Biol. 126:925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.J., and R.S. Hegde. 2002. Cotranslational partitioning of nascent prion protein into multiple populations at the translocation channel. Mol. Biol. Cell. 13:3775–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.J., R. Rahbar, and R.S. Hegde. 2001. Combinatorial control of prion protein biogenesis by the signal sequence and transmembrane domain. J. Biol. Chem. 276:26132–26140. [DOI] [PubMed] [Google Scholar]
- Kim, S.J., D. Mitra, J.R. Salerno, and R.S. Hegde. 2002. Signal sequences control gating of the protein translocation channel in a substrate-specific manner. Dev. Cell. 2:207–217. [DOI] [PubMed] [Google Scholar]
- Kyte, J., and R.F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105–132. [DOI] [PubMed] [Google Scholar]
- Martoglio, B., M.W. Hofmann, J. Brunner, and B. Dobberstein. 1995. The protein-conducting channel in the membrane of the endoplasmic reticulum is open laterally toward the lipid bilayer. Cell. 81:207–214. [DOI] [PubMed] [Google Scholar]
- Matlack, K., and P. Walter. 1995. The 70 carboxyl-terminal amino acids of nascent secretory proteins are protected from proteolysis by the ribosome and the protein translocation apparatus of the endoplasmic reticulum membrane. J. Biol Chem. 270:6170–6180. [DOI] [PubMed] [Google Scholar]
- Menetret, J.F., A. Neuhof, D.G. Morgan, K. Plath, M. Rademacher, T.A. Rapoport, and C.W. Akey. 2000. The structure of ribosome-channel complexes engaged in protein translocation. Mol. Cell. 6:1219–1232. [DOI] [PubMed] [Google Scholar]
- Migliaccio, G., C.V. Nicchitta, and G. Blobel. 1992. The signal sequence receptor, unlike the signal recognition particle receptor, is not essential for protein translocation. J. Cell Biol. 117:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes, W., S. Prehn, and T.A. Rapoport. 1994. Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J. 13:3973–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes, W., B. Jungnickel, J. Brunner, and T.A. Rapoport. 1998. Signal sequence recognition in cotranslational translocation by protein components of the endoplasmic reticulum membrane. J. Cell Biol. 142:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch, A., M. Wiedmann, and T.A. Rapoport. 1992. Yeast Sec proteins interact with polypeptides traversing the endoplasmic reticulum membrane. Cell. 69:343–352. [DOI] [PubMed] [Google Scholar]
- Ou, W.J., D.Y. Thomas, A.W. Bell, and J.J. Bergeron. 1992. Casein kinase II phosphorylation of signal sequence receptor alpha and the associated membrane chaperone calnexin. J. Biol. Chem. 267:23789–23796. [PubMed] [Google Scholar]
- Panzner, S., L. Dreier, E. Hartmann, S. Kostka, and T.A. Rapoport. 1995. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 81:561–570. [DOI] [PubMed] [Google Scholar]
- Prehn, S., J. Herz, E. Hartmann, T.V. Kurzchalia, R. Frank, K. Roemisch, B. Dobberstein, and T.A. Rapoport. 1990. Structure and biosynthesis of the signal sequence receptor. Eur. J. Biochem. 188:439–445. [DOI] [PubMed] [Google Scholar]
- Rutkowski, D.T., V.R. Lingappa, and R.S. Hegde. 2001. Substrate-specific regulation of the ribosome-translocon junction by N-terminal signal sequences. Proc. Natl. Acad. Sci. USA. 98:7823–7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, S.L., K.M. Whitfield, J.P. Vogel, M.D. Rose, and R.W. Schekman. 1992. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 69:353–365. [DOI] [PubMed] [Google Scholar]
- Stewart, R.S., and D.A. Harris. 2001. Most pathogenic mutations do not alter the membrane topology of the prion protein. J. Biol. Chem. 276:2212–2220. [DOI] [PubMed] [Google Scholar]
- Voigt, S., B. Jungnickel, E. Hartmann, and T.A. Rapoport. 1996. Signal sequence–dependent function of the TRAM protein during early phases of protein transport across the endoplasmic reticulum membrane. J. Cell Biol. 134:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne, G. 1985. Signal sequences. The limits of variation. J. Mol. Biol. 184:99–105. [DOI] [PubMed] [Google Scholar]
- Wada, I., D. Rindress, P.H. Cameron, W.J. Ou, J.J. Doherty, D. Louvard, A.W. Bell, D. Dignard, D.Y. Thomas, and J.J. Bergeron. 1991. SSRα and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 266:19599–19610. [PubMed] [Google Scholar]
- Walter, P., and G. Blobel. 1983. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 96:84–93. [DOI] [PubMed] [Google Scholar]
- Walter, P., and A.E. Johnson. 1994. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu. Rev. Cell Biol. 10:87–119. [DOI] [PubMed] [Google Scholar]