Abstract
Membranous and nonmembranous cargoes are transported along axons in the fast and slow components of axonal transport, respectively. Recent observations on the movement of cytoskeletal polymers in axons suggest that slow axonal transport is generated by fast motors and that the slow rate is due to rapid movements interrupted by prolonged pauses. This supports a unified perspective for fast and slow axonal transport based on rapid movements of diverse cargo structures that differ in the proportion of the time that they spend moving. A Flash feature (http://www.jcb.org/cgi/content/full/jcb.200212017/DC1) accompanies this Mini-Review.
Introduction
Intracellular movement is the life blood of the eukaryotic cell. In the bustling macromolecular environment of cytoplasm, nothing can function without the constant shuttling of intracellular components from place to place. In recent years we have witnessed a revolution in our understanding of the extent of these movements inside cells and their underlying molecular mechanisms. We now know that intracellular transport is orchestrated by a diverse constellation of molecular motor proteins that bind specific cargoes and convey them in a particular direction along cytoskeletal polymer tracks (Vale and Milligan, 2000). Moreover, the cargoes themselves are far more varied than previously imagined, including every type of membranous organelle and species of transport vesicle, as well as nonmembranous cargoes such as cytoskeletal polymers, cytosolic protein complexes, ribosomes, and messenger RNAs.
Fast and slow axonal transport
In the world of intracellular transport, the journey along the axons of nerve cells is truly an epic one. These slender cylindrical processes can extend for distances in excess of one meter in large animals, yet they are dependent on the cell body for the synthesis of many of their components. Materials destined for the axon are transported anterogradely, toward the axon tip, and materials destined to return are transported retrogradely, toward the cell body. This bidirectional transport process, known as axonal transport, is not fundamentally different from the pathways of macromolecular and membrane traffic that occur in all eukaryotic cells, but it is remarkable for its scale: essentially all of the macromolecules and membranous compartments that comprise axonal cytoplasm are transported along the entire length of the axon throughout the life of the neuron.
Most of what we know about the composition and kinetics of axonal transport comes from studies on laboratory animals using radioisotopic pulse labeling (Grafstein and Forman, 1980). Several decades of work using this experimental paradigm has demonstrated that proteins and other molecules are transported along axons in association with distinct membranous and nonmembranous cargo structures that move at different rates (Tytell et al., 1981). Membranous organelles move most rapidly, in the fast components of axonal transport, whereas cytoskeletal polymers and cytosolic protein complexes move more slowly, in the slow components. The difference in the rate of fast and slow axonal transport has long been assumed to indicate that membranous and nonmembranous cargoes move by fundamentally distinct mechanisms, but direct observations on the movement of these cargoes in living cells now indicate that they are all transported by fast motors and that the principle difference between fast and slow transport is not the rate of movement per se, but rather the manner in which the movement is regulated.
Movement of membranous organelles
Membranous organelles are the principal cargoes of fast axonal transport. The many proteins, lipids, and polysaccharides that move along the axon at fast rates do so by virtue of their association with one or more subclasses of organelle or vesicle, either because they are sequestered within its lumen, embedded in its membrane, or bound to its surface. Golgi-derived transport vesicles move anterogradely at maximal rates of ∼200–400 mm/d (or ∼2–5 μm/s), whereas endocytic vesicles, lysosomes, and autophagosomes move retrogradely at maximal rates of ∼100–250 mm/d (or ∼1–3 μm/s) (Table I). Each of these components of fast axonal transport represents the movement of a diverse fleet of organelles, each type of organelle conveying a unique molecular consignment, and each the product of a distinct packaging and sorting pathway (Almenar-Queralt and Goldstein, 2001).
Table I. Motile behavior of axonally transported cargoes.
| Cargo structures | Overall rate (pulse labeling) |
Instantaneous rate (light microscopy) | Directionality | Duty ratio |
|---|---|---|---|---|
| Golgi-derived vesicles (fast anterograde) |
200–400 mm/da (2–5 µm/s) | 1–5 µm/sb | Anterograde | High |
| Endocytic vesicles, lysosomes, autophagosomes (fast retrograde) | 100–250 mm/da (1–3 µm/s) | 1–3 µm/sb | Retrograde | High |
| Mitochondria | <70 mm/dc (<0.8 µm/s) | 0.3–0.7 µm/sd | Bidirectional | Intermediate |
| Microfilaments, cytosolic protein complexes (slow component b) | 2–8 mm/de (0.02–0.09 µm/s) | Unknown | Unknown | Unknown |
| Microtubules, neurofilaments (slow component a) |
0.2–1 mm/de (0.002–0.01 µm/s) | 0.3–1 µm/sf | Bidirectional | Low |
Each rate component of axonal transport corresponds to a distinct group of cargo structures. The overall rate is the average or maximum rate (including movements and pauses) determined by radioisotopic pulse labeling (for technical reasons, the rates of fast axonal transport are generally quoted as maximal rates, whereas the rates of slow axonal transport are generally quoted as average rates). Note that these rates are approximate and that there is considerable variation between different cell types and different stages of development and maturation. The instantaneous rate is the actual rate of movement of the cargo structures (between pauses) determined by light microscopy. The duty ratio is the proportion of the time that the structures spend moving, inferred by comparison of the overall and instantaneous rates. Other axonally transported cargos, such as endoplasmic reticulum, mRNAs, and ribonucleoprotein particles are not included in this table because there is insufficient information about their overall rate of movement at this time. Note that actin may also move in slow component a in some neurons, and tubulin may also move in slow component b (Oblinger et al., 1987), but the significance of this is not clear.
Though much remains to be learned about the molecular mechanisms of organelle movement in axons, the general principles underlying this transport process are now clearly established. Membranous organelles move along both microtubule and microfilament tracks powered by molecular motor proteins (kinesins, dyneins, and myosins). Microtubules appear to be the principal tracks for long-range movements along the axis of the axon. Plus end–directed kinesin motors propel organelles along microtubules anterogradely, whereas dynein (and possibly also minus end–directed kinesin motors) propel organelles retrogradely (Goldstein and Yang, 2000). Each motor binds its cargo by direct interaction with transmembrane proteins on the organelle surface, or by indirect interaction with the organelle surface via scaffolds of cytosolic linker proteins (Karcher et al., 2002).
Direct observations on the movement of membranous organelles in living axons indicate that many organelles move in a continuous and unidirectional manner at instantaneous rates that are comparable to the maximal rate of fast axonal transport determined by radioisotopic pulse labeling (Table I) and also to the maximal rates reported for microtubule motors in vitro (e.g., Woehlke and Schliwa, 2000). These observations indicate that many membranous organelles move along axons in a highly efficient manner, pausing only infrequently during their journey. One way to express this efficiency is in terms of the duty ratio. In the context of intracellular transport, the duty ratio is the proportion of time that a cargo structure spends actually moving. Thus, we can say that the axonal transport of many membranous organelles is characterized by a high duty ratio.
One exception to the high duty ratio of membranous organelles are mitochondria (Hollenbeck, 1996, Fig. 1). These organelles move less rapidly than most Golgi-derived and endocytic vesicles, forming a kinetically distinct component of axonal transport (Table I). The maximal rate of mitochondrial movement is generally quoted to be ∼20–70 mm/d, which is comparable to the instantaneous rates observed in living cells, but the average rate appears to be considerably slower (Lorenz and Willard, 1978; Grafstein and Forman, 1980). The explanation for this transport behavior appears to be that mitochondria move in an intermittent and bidirectional manner, with differences in the balance of anterograde and retrograde movements and pauses giving rise to a broad range of overall rates (Morris and Hollenbeck, 1993; Ligon and Steward, 2000). Although some axonal mitochondria may move rapidly and continuously for long distances, resulting in the maximal rate of movement, most exhibit frequent pauses and reversals, resulting in much slower rates of movement (Blaker et al., 1981). Though not extensively studied, it appears that endoplasmic reticulum may also be transported along axons in this manner (Ellisman and Lindsey, 1983).
Movement of cytoskeletal polymers
In radioisotopic pulse labeling studies, cytoskeletal and cytosolic proteins can be resolved into two kinetically distinct groups (Lasek et al., 1984). Neuronal intermediate filament proteins, tubulin, and a number of other cytosolic proteins generally move at rates of ∼0.2–1 mm/d in slow component a, whereas actin and several hundred other cytosolic proteins generally move at slightly faster rates of ∼2-8 mm/d in slow component b (Table I). The mechanism by which these proteins move has been the subject of intense interest and controversy for more than two decades, due largely to the failure of numerous attempts to observe slow axonal transport directly in living cells (Brown, 2000). Most attention has been focused on the cytoskeletal subunit proteins, and most of the controversy has centered on the form in which these proteins move. Some have argued that cytoskeletal proteins move in the form of assembled polymers (Baas and Brown, 1997), whereas others have argued that cytoskeletal polymers are stationary and that cytoskeletal proteins move in the form of free subunits or small oligomers (Hirokawa et al., 1997).
Recently the movement of neurofilaments and microtubules has been observed in axons, and these observations indicate that the solution to the slow axonal transport controversy is disarmingly simple. Cytoskeletal polymers do move in axons, but their movements are not slow after all (Fig. 1). Both neurofilaments and microtubules move at fast rates, approaching the rate of movement of membranous organelles, but the average rate of movement is slow because the movements are both infrequent and bidirectional (Roy et al., 2000; Wang et al., 2000; Wang and Brown, 2001). Remarkably, the key to observing this movement was simply a matter of experimental design; previous studies that did not detect movement were designed with the explicit expectation of a slow and synchronous movement, and it now appears that they were probably not capable of detecting the rapid and asynchronous movement (Wang and Brown, 2001). Thus, the overall speed and direction of neurofilament and microtubule movement is a temporal summation of anterograde and retrograde movements and pauses, perhaps not fundamentally dissimilar from the behavior of mitochondria in axons described above. As is the case for mitochondria, the slow overall rate of movement of neurofilaments and microtubules suggests that these structures move with a low duty ratio, spending most of their time not moving. For example, it has been estimated that neurofilaments in mature axons (such as those used for radioisotopic pulse labeling studies) may spend as much as 99% of their time pausing during their journey along the axon (Brown, 2000). It seems likely that microfilaments may exhibit a similar behavior, but the movement of these cytoskeletal polymers has not yet been observed.
Figure 1.
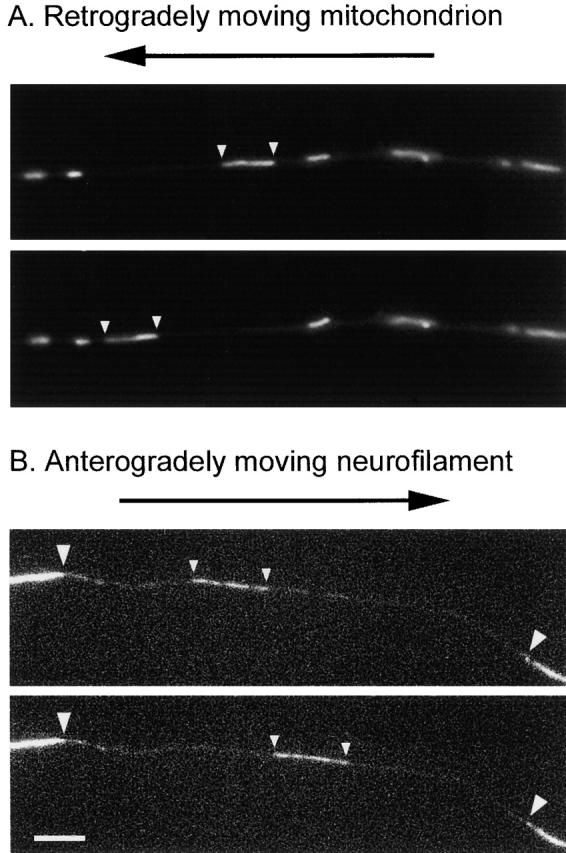
Movement of mitochondria and neurofilaments in axons. (A) Retrograde movement of a mitochondrion stained with Rhodamine-123 in the axon of a cultured neuron. The small arrowheads mark the position of the moving organelle. The other mitochondria in the field of view remain paused. The time interval between these two images is 3 s. (B) Anterograde movement of a GFP-tagged neurofilament in a photobleached axon of a cultured neuron. The large arrowheads mark the edges of the photobleached region, and the small arrowheads mark the leading and trailing ends of the filament. The time interval between these two images is 8 s. See Wang and Brown (2001) for details. Both neurofilaments and microtubules move in a rapid, intermittent, and bidirectional manner, which underscores the similarity in the underlying mechanism of movement of membranous and nonmembranous cargoes. Bar, 5 μm. Images in A were provided by Sunita R. Chada and Peter J. Hollenbeck. Images in B are reprinted from Molecular Biology of the Cell, 12:3257–3267, Wang, L., and A. Brown, Copyright 2001, with permission from American Society for Cell Biology.
The rapid rate of movement of neurofilaments and microtubules in axons indicates that they are transported by fast motors, perhaps similar or identical to motors that move membranous organelles, but the identity of these motors and the tracks along which they move are not known. Several lines of evidence suggest that dynein may transport axonal microtubules anterogradely, perhaps relative to the microfilament matrix (Susalka and Pfister, 2000; Baas, 2002), and that dynein and kinesin may transport axonal neurofilaments bidirectionally along microtubules by the same mechanism that is thought to move vimentin along microtubules in nonneuronal cells (Prahlad et al., 1998; Shea and Flanagan, 2001; Helfand et al., 2002). Interestingly, axonal neurofilaments also interact with myosin Va, but the role of this interaction in neurofilament movement is presently unclear (Rao et al., 2002). Although much remains to be learned about the movement of cytoskeletal polymers in axons, it seems likely that microtubules, microfilaments, and neurofilaments each interact with a number of different motor proteins and that these motors act cooperatively to translocate and organize these polymers in both the longitudinal and radial dimensions of the axon.
Cytoskeletal polymers as carrier structures
Cytoskeletal proteins have been the exclusive focus of studies on slow axonal transport in recent years, but it is important to remember that several hundred other proteins also move in this rate group, representing the entire spectrum of cytosolic proteins that comprise axoplasm. Some examples include proteins involved in vesicle dynamics such as clathrin and synapsin; regulatory proteins such as calmodulin; metabolic enzymes such as creatine kinase, aldolase, and enolase; cytoskeletal proteins such as spectrin, tau, and dynactin; and motor proteins such as dynein and myosin (Lasek et al., 1984; Paggi and Petrucci, 1992; Dillman et al., 1996; Rao et al., 2002). The sheer number and diversity of these proteins and the fact that they move together for days, weeks, or months as they travel down the axon, suggest that they associate with a common class of nonmembranous carrier structures analogous to the membranous organelles of fast axonal transport, but the identity of these carrier structures is not known. One hypothesis, first proposed more than 15 years ago, is that cytoskeletal polymers fulfill this role and that other cytosolic proteins are transported by riding piggyback on the moving polymers (Lasek et al., 1984, Lasek, 1986). According to this hypothesis, the transport rate of cytosolic proteins is determined by the proportion of the time that they spend in association with the cytoskeletal polymers, as well as by the rate and frequency of movement of the polymers themselves.
The idea that cytoskeletal polymers are carrier structures for slow axonal transport was initially met with skepticism, but it is now clear that cytoskeletal polymers do move in axons and that many of the cytosolic proteins that are conveyed by slow axonal transport can bind, directly or indirectly, to cytoskeletal polymers. For example, tau protein and spectrin both move in slow component a along with neurofilament proteins and tubulin. Spectrin is known to interact with neurofilaments (Macioce et al., 1999), and tau is a well characterized microtubule-associated protein. Thus, it is possible that neurofilaments and microtubules could be carrier structures for slow component a of axonal transport. A prediction of this hypothesis is that other proteins that move in this rate component, which have yet to be identified, will also be found to bind to these cytoskeletal polymers. By the same logic, it is also possible that microfilaments could be carrier structures for slow component b. In support of this hypothesis, microfilaments are known to interact with a wide range of different cytosolic proteins, including many that are not traditionally thought of as cytoskeleton-associated proteins (e.g., Knull and Walsh, 1992). However, it is unlikely that the several hundred different proteins that move in slow component b all bind directly to microfilaments. More probably, many of these proteins form functional complexes that in turn associate with the moving filaments. It is also possible that some cytosolic protein complexes may move by binding directly to motor proteins. The identification and characterization of these various protein complexes is likely to provide fundamental insights into the supramolecular interactions that organize the cytosolic compartment of cytoplasm, not just in axons, but in all eukaryotic cells.
Beyond fast and slow: a unified perspective
The existence of distinct fast and slow components of axonal transport has been known for more than 25 years, but the mechanistic significance underlying these different rates of movement has been obscure for most of that time. The principal reason for this protracted period of uncertainty has been our inability to observe slow axonal transport directly in living cells. The recent discovery that cytoskeletal polymers conveyed by slow axonal transport actually move as fast as membranous organelles indicates that both fast and slow axonal transport may be generated by fast motors; cargoes as diverse as vesicles, mitochondria, and neurofilaments all move at comparable rates but they differ in the proportion of the time that they spend moving.
According to this unified perspective, membranous and nonmembranous cargoes are all transported along axons by the same underlying mechanism but they move at different rates due to differences in their duty ratio. Membranous organelles on the secretory and endocytic pathways, which function primarily to deliver membrane and protein components to sites along the axon and at the axon tip, move rapidly in a unidirectional manner, pausing for only brief periods of time. The high duty ratio of these organelles ensures that they are delivered rapidly to their destination. In contrast, cytoskeletal polymers, mitochondria, and possibly also endoplasmic reticulum, move in an intermittent and bidirectional manner, pausing more often and for longer periods of time, and sometimes reversing during their journey along the axon. Although we refer to these structures as cargoes, they are not simply the luggage of intracellular transport; these organelles and macromolecular assemblies are preassembled functional units that fulfill their architectural, physiological, and metabolic roles in the axon during their transit. For these cargoes, the journey is perhaps more important than the ultimate destination, and this may explain their unique motile behavior.
Based on these considerations, a central question underlying the difference between fast and slow axonal transport is the mechanism by which the movement of membranous and nonmembranous cargoes is regulated. For example, what determines whether a particular cytoskeletal polymer or membranous organelle moves or pauses, or how frequently it does so? And when movement does occur, what determines its direction and duration? Since the motile behavior of axonally transported cargoes determines the efficiency with which they are transported and the manner in which they are distributed along the axon, the regulation of this behavior is likely to be critical for many aspects of axonal structure and function. For example, in the case of mitochondria, the balance of anterograde and retrograde movements and pauses is regulated during axon growth in order to recruit these organelles to sites of metabolic demand (Morris and Hollenbeck, 1993). Likewise, in the case of neurofilaments and microtubules, the balance of anterograde and retrograde movements and pauses is likely to be the principal determinant of their steady-state distribution along the axon, and thus the regulation of the axonal transport of these structures is probably essential for local and long-range remodeling of the neuronal cytoskeleton during axon growth and maturation. Since axonal transport continues throughout the life of the neuron, it is likely that active regulation of the movement of its membranous and nonmembranous components is an ongoing process as fundamental to the biology of axons as metabolism itself.
Acknowledgments
Thanks to Sunita R. Chada and Peter J. Hollenbeck for the images of mitochondrial movement used in Fig. 1 A, and to Kitty Jensen for helpful comments on this manuscript.
The work in the author's laboratory is supported by the National Institute of Neurological Disorders and Stroke.
References
- Almenar-Queralt, A., and L.S. Goldstein. 2001. Linkers, packages and pathways: new concepts in axonal transport. Curr. Opin. Neurobiol. 11:550–557. [DOI] [PubMed] [Google Scholar]
- Baas, P.W. 2002. Microtubule transport in the axon. Int. Rev. Cytol. 212:41–62. [DOI] [PubMed] [Google Scholar]
- Baas, P.W., and A. Brown. 1997. Slow axonal transport: the polymer transport model. Trends Cell Biol. 7:380–384. [DOI] [PubMed] [Google Scholar]
- Blaker, W.D., J.F. Goodrum, and P. Morell. 1981. Axonal transport of the mitochondria-specific lipid, diphosphatidylglycerol, in the rat visual system. J. Cell Biol. 89:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer, A.C., M.P. Lynn, M.B. Atkinson, S.M. Chou, A.J. Wilbourn, K.E. Marks, J.E. Culver, and E.J. Fleegler. 1987. Fast axonal transport in amyotrophic lateral sclerosis: an intra-axonal organelle traffic analysis. Neurology. 37:738–748. [DOI] [PubMed] [Google Scholar]
- Brown, A. 2000. Slow axonal transport: stop and go traffic in the axon. Nat. Rev. Mol. Cell Biol. 1:153–156. [DOI] [PubMed] [Google Scholar]
- Dillman, J.F., L.P. Dabney, S. Karki, B.M. Paschal, E.L. Holzbaur, and K.K. Pfister. 1996. Functional analysis of dynactin and cytoplasmic dynein in slow axonal transport. J. Neurosci. 16:6742–6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman, M.H., and J.D. Lindsey. 1983. The axoplasmic reticulum within myelinated axons is not transported rapidly. J. Neurocytol. 12:393–411. [DOI] [PubMed] [Google Scholar]
- Goldstein, L.S.B., and Z.H. Yang. 2000. Microtubule-based transport systems in neurons: The roles of kinesins and dyneins. Annu. Rev. Neurosci. 23:39–71. [DOI] [PubMed] [Google Scholar]
- Grafstein, B., and D.S. Forman. 1980. Intracellular transport in neurons. Physiol. Rev. 60:1167–1283. [DOI] [PubMed] [Google Scholar]
- Helfand, B.T., A. Mikami, R.B. Vallee, and R.D. Goldman. 2002. A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J. Cell Biol. 157:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa, N., S. Terada, T. Funakoshi, and S. Takeda. 1997. Slow axonal transport: the subunit transport model. Trends Cell Biol. 7:384–388. [DOI] [PubMed] [Google Scholar]
- Hollenbeck, P.J. 1996. The pattern and mechanism of mitochondrial transport in axons. Front. Biosci. 1:d91–d102. [DOI] [PubMed] [Google Scholar]
- Kaether, C., P. Skehel, and C.G. Dotti. 2000. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol. Biol. Cell. 11:1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher, R.L., S.W. Deacon, and V.I. Gelfand. 2002. Motor-cargo interactions: the key to transport specificity. Trends Cell Biol. 12:21–27. [DOI] [PubMed] [Google Scholar]
- Knull, H.R., and J.L. Walsh. 1992. Association of glycolytic enzymes with the cytoskeleton. Curr. Top. Cell. Regul. 33:15–30. [DOI] [PubMed] [Google Scholar]
- Lasek, R.J. 1986. Polymer sliding in axons. J. Cell Science. 5(Suppl.):161–179. [DOI] [PubMed] [Google Scholar]
- Lasek, R.J., J.A. Garner, and S.T. Brady. 1984. Axonal transport of the cytoplasmic matrix. J. Cell Biol. 99:212s–221s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon, L.A., and O. Steward. 2000. Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J. Comp. Neurol. 427:340–350. [DOI] [PubMed] [Google Scholar]
- Lorenz, T., and M. Willard. 1978. Subcellular fractionation of intra-axonally transported polypeptides in the rabbit visual system. Proc. Natl. Acad. Sci. USA. 75:505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macioce, P., N. Gandolfi, C.L. Leung, S.S. Chin, F. Malchiodi-Albedi, M. Ceccarini, T.C. Petrucci, and R.K. Liem. 1999. Characterization of NF-L and betaIISigma1-spectrin interaction in live cells. Exp. Cell Res. 250:142–154. [DOI] [PubMed] [Google Scholar]
- Morris, R.L., and P.J. Hollenbeck. 1993. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J. Cell Sci. 104:917–927. [DOI] [PubMed] [Google Scholar]
- Nakata, T., S. Terada, and N. Hirokawa. 1998. Visualization of the dynamics of synaptic vesicle and plasma membrane proteins in living axons. J. Cell Biol. 140:659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblinger, M.M., S.T. Brady, I.G. McQuarrie, and R.J. Lasek. 1987. Cytotypic differences in the protein composition of the axonally transported cytoskeleton in mammalian neurons. J. Neurosci. 7:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paggi, P., and T.C. Petrucci. 1992. Neuronal compartments and axonal transport of synapsin I. Mol. Neurobiol. 6:239–251. [DOI] [PubMed] [Google Scholar]
- Prahlad, V., M. Yoon, R.D. Moir, R.D. Vale, and R.D. Goldman. 1998. Rapid movements of vimentin on microtubule tracks: Kinesin-dependent assembly of intermediate filament networks. J. Cell Biol. 143:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, M.V., L.J. Engle, P.S. Mohan, A. Yuan, D. Qiu, A. Cataldo, L. Hassinger, S. Jacobsen, V.M. Lee, A. Andreadis, et al. 2002. Myosin Va binding to neurofilaments is essential for correct myosin Va distribution and transport and neurofilament density. J. Cell Biol. 159:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, S., P. Coffee, G. Smith, R.K.H. Liem, S.T. Brady, and M.M. Black. 2000. Neurofilaments are transported rapidly but intermittently in axons: implications for slow axonal transport. J. Neurosci. 20:6849–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea, T.B., and L.A. Flanagan. 2001. Kinesin, dynein and neurofilament transport. Trends Neurosci. 24:644–648. [DOI] [PubMed] [Google Scholar]
- Susalka, S.J., and K.K. Pfister. 2000. Cytoplasmic dynein subunit heterogeneity: implications for axonal transport. J. Neurocytol. 29:819–829. [DOI] [PubMed] [Google Scholar]
- Tytell, M., M.M. Black, J.A. Garner, and R.J. Lasek. 1981. Axonal transport: each major component reflects the movement of distinct macromolecular complexes. Science. 214:179–181. [DOI] [PubMed] [Google Scholar]
- Vale, R.D., and R.A. Milligan. 2000. The way things move: looking under the hood of molecular motor proteins. Science. 288:88–95. [DOI] [PubMed] [Google Scholar]
- Viancour, T.A., and N.A. Kreiter. 1993. Vesicular fast axonal transport rates in young and old rat axons. Brain Res. 628:209–217. [DOI] [PubMed] [Google Scholar]
- Wang, L., and A. Brown. 2001. Rapid intermittent movement of axonal neurofilaments observed by fluorescence photobleaching. Mol. Biol. Cell. 12:3257–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., and A. Brown. 2002. Rapid movement of microtubules in axons. Curr. Biol. 12:1496–1501. [DOI] [PubMed] [Google Scholar]
- Wang, L., C.-L. Ho, D. Sun, R.K.H. Liem, and A. Brown. 2000. Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat. Cell Biol. 2:137–141. [DOI] [PubMed] [Google Scholar]
- Woehlke, G., and M. Schliwa. 2000. Walking on two heads: the many talents of kinesin. Nat. Rev. Mol. Cell Biol. 1:50–58. [DOI] [PubMed] [Google Scholar]


