Abstract
In yeast, growth and organelle segregation requires formin-dependent assembly of polarized actin cables. These tracks are used by myosin Vs to deliver secretory vesicles for cell growth, organelles for their segregation, and mRNA for fate determination. Several specific receptors have been identified that interact with the cargo-binding tails of the myosin Vs. A recent study implicates specific degradation in the bud of the vacuolar receptor, Vac17, as a mechanism for cell cycle–regulated segregation of this organelle.
Keywords: polarity; transport; actin; myosin; vacuole
The last quarter century saw tremendous strides in our understanding of cell cycle regulation. In addition to being a carefully regulated signal transduction pathway, the cell cycle is a miracle of coordinated duplication of cellular constituents and their spatial segregation to make two identical, or in many important circumstances, two nonidentical cells. After the decision to undertake another cycle, the cell has to select an axis for division along which its duplicated constituents must be segregated. The most visible and intensely studied aspect of organelle segregation is the elaborate process of mitosis, yet all organelles have to be segregated and the mechanism by which this is achieved is only now beginning to emerge.
Much progress has come from studies in the budding yeast. The problem is especially simple in these small cells, since polarized growth and organelle segregation are performed by the actin cytoskeleton, whereas in larger eukaryotic cells it is a cooperation between microtubules and microfilaments. It is now possible to assemble a plausible working model describing how an axis for cell division is selected, how this drives the assembly of a polarized cytoskeleton, and how this guides motors for the delivery of secretory vesicles for polarized growth, for the transport of organelles for their segregation, and for transport of factors that determine distinct fates of mother and daughter cells (Fig. 1). The aim of this mini-review is to highlight some of these advances, especially the recent elegant studies uncovering the molecular basis of yeast vacuole segregation (Ishikawa et al., 2003; Tang et al., 2003). Many of the molecules involved in these processes are conserved from yeast to mammals, suggesting that similar mechanisms are likely to prevail across eukaryotes.
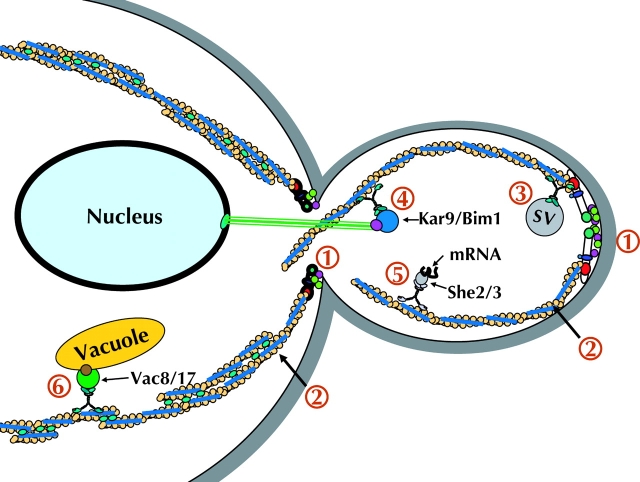
Figure 1.
Diagram summarizing the myosin V–based mechanisms of organelle transport. (1) Formins are localized and activated (Bni1 at the bud tip and Bnr1 at the bud neck), which drive the assembly of polarized actin cables. (2) Actin cables are stabilized by tropomyosin (blue) and cross-linking proteins (e.g., fimbrin, green). (3) Myo2 transports post-Golgi secretory vesicles into the bud. (4) Nuclear orientation involves Myo2-dependent transport of Kar9/Bim1 into the bud. (5) Specific mRNAs are selected by the She2/3 complex and transported into the bud by Myo4. (6) Vacuolar elements are moved by Myo2 into the bud through an interaction with Vac8/17. After transport into the bud, Vac17 is degraded (not depicted).
Polarity determination: selecting an axis for cell division
Rapidly growing unbudded yeast entering the cell cycle appear morphologically unpolarized, yet they are not. An elaborate signaling pathway has already interpreted information from previous budding cycles to select the position of the new bud site (Chant, 1999). Thus in haploids, the bud site is adjacent to the old one, whereas in diploids the cell buds from either end. In the absence of this nonessential machinery, cells still manage to assemble a single bud, albeit at random sites. The bud site selection machinery directs an essential signal transduction cascade necessary for bud emergence. The first known component of this machinery is Cdc24, the exchange factor for the extremely well-conserved Rho family member Cdc42. This system contributes to the generation of a single bud, since mutants defective in GTP hydrolysis can grow in the absence of Cdc24 but simultaneously generate multiple buds (Caviston et al., 2002). Cdc42 activation independently drives the assembly of two distinct cytoskeletal structures at the site of bud emergence: the septin ring necessary for later assembly of the contractile ring and a polarized actin cytoskeleton (Pringle et al., 1995).
How Cdc42 choreographs these processes is beginning to be understood. Like all GTPases of the Rho family, many potential effectors of Cdc42-GTP are known, including the PAK-like kinases Cla4 and Ste20, Gic1/2, and the formin Bni1p (Johnson, 1999). All of these play a role in establishing or maintaining a polarized actin cytoskeleton, which is the primary cytoskeletal framework of cell polarity in yeast (Pruyne and Bretscher, 2000a). Gladfelter et al. (2002) recently reported genetic evidence that led them to suggest the novel idea that septin ring formation requires rounds of GTP hydrolysis by Cdc42, but the precise mechanism remains to be elucidated.
Two major types of F-actin–based structures are assembled at bud emergence: bundles of actin filaments known as cables that extend from the site of polarization into the cell, and actin patches, which are dense membrane-associated structures that form a necklace-type ring around the site of polarization (Adams and Pringle, 1984; Kilmartin and Adams, 1984; Pruyne and Bretscher, 2000b). Both cables and patches are reorganized during the cell cycle and are focused at or around areas of cell growth. Actin patches are involved in endocytosis and possibly localized cell wall synthesis (for review see Schott et al., 2002) and do not appear to play a direct role in polarized growth or organelle segregation. Therefore, a discussion of them is not included here.
The tracks: actin cables and their regulated assembly
Until recently it was not clear which structural component of the actin cytoskeleton was responsible for directing the delivery of post-Golgi secretory vesicles for polarized growth. This uncertainty was resolved by analysis of cells conditionally defective for tropomyosin function (Pruyne et al., 1998). In budding yeast, tropomyosin is an essential protein that associates with actin cables and not patches (Liu and Bretscher, 1989; Drees et al., 1995). The conditional mutant has normal cables at the permissive temperature, yet selectively looses them within minutes after shifting to the restrictive temperature without apparently affecting the structure, localization, or function of cortical patches. The analysis revealed that it was the cables, not the patches, that are necessary for the polarized delivery of secretory vesicles (Pruyne et al., 1998) and the segregation of organelles (see below). Thus, a key question became, how are polarized actin cables assembled?
Since cables appear to emerge from the site of polarization, it seemed likely that components tightly clustered at that site might be involved. Earlier work had suggested that the formin, Bni1, might play an important role, since it is a target of Cdc42 and other Rho proteins (Kohno et al., 1996; Evangelista et al., 1997) and loss of Bni1p, although not lethal, had some mild morphological defects, including the formation of rounder buds (Ozaki-Kuroda et al., 2001). Subsequently, the related formin Bnr1 was found (Imamura et al., 1997), and loss of both Bni1 and Bnr1 was shown to be lethal (Kamei et al., 1998; Vallen et al., 2000). This allowed for the isolation of conditional mutations in BNI1 in a bnr1Δ background. Remarkably, these mutations confer a phenotype very similar to the tropomyosin conditional mutation: actin cables are lost very rapidly after shifting to the restrictive temperature, again without any immediate effect on cortical patches, and secretion becomes depolarized (Evangelista et al., 2002; Sagot et al., 2002a).
Bni1 and Bnr1 are classified as formins, since they contain conserved FH1 and FH2 domains (Kohno et al., 1996; Imamura et al., 1997). Formins have been recognized as targets of Rho proteins (Watanabe et al., 1997), and recent work on mammalian formins indicates that they are negatively regulated through an intramolecular association involving the NH2- and COOH-terminal regions of the proteins (Alberts, 2001), which is relieved by activated Rho proteins (Watanabe et al., 1999). Studies in both mammalian and yeast cells show that overexpression constructs lacking the NH2-terminal Rho-binding domain but containing the FH1 and FH2 region induce the formation of excessive actin filaments (Evangelista et al., 1997; Watanabe et al., 1999). Yeast cells that overexpress the conserved FH1-FH2 region of Bni1p have excess tropomyosin-containing actin cables, which suggests that formins participate in the assembly of actin cables (Evangelista et al., 2002; Sagot et al., 2002a). This in vivo activity is independent of the Arp2/3 complex (Evangelista et al., 2002), a regulated nucleator of actin filament assembly for cortical patches (Winter et al., 1997, 1999). Thus, yeast has two distinct actin nucleation systems, an Arp2/3-based one for cortical patch assembly and a formin-based one for actin cable assembly.
Characterization of recombinant Bni1 FH1-FH2 or FH2 reveals that FH2 can nucleate actin assembly in vitro, albeit rather inefficiently, and the FH1-FH2 is the more efficient nucleator (Pruyne et al., 2002; Sagot et al., 2002b). After nucleation, FH1-FH2 remains bound to the barbed end of the nucleated filament, binding with a high affinity (Kd ∼20 nm) while still allowing growth to continue at that end (Pruyne et al., 2002). Localized and activated formins appear to be perfectly designed to nucleate actin filaments and hold onto the growing barbed end, which means that the filament bundles must grow away from the site of polarization. In remarkable agreement with this model is the recent report that actin cables visualized with GFP-ABP140 can be seen in vivo to constantly move away from their site of assembly in an actin polymerization–dependent manner (Yang and Pon, 2002). Moreover, this implied filament polarity would provide a focal point for transport by myosins, since the vast majority move toward the barbed end of actin filaments, and is consistent with unidirectional myosin-dependent movements along cables (see below). The formin system therefore appears to provide the heart of a regulated machine for the assembly of polarized actin cables. Although Spa2 and other factors are involved in Bni1 localization (Fujiwara et al., 1998; Pruyne and Bretscher, 2000a), the hierarchy of how this localization occurs and the details of Bni1 activation are fascinating topics for future studies.
The myosin V motors, Myo2 and Myo4, and cargo receptors
Secretion is polarized through transport by Myo2.
The myosin V heavy chain encoded by MYO2 was identified by Johnston et al. (1991) based on an analysis of the first conditional allele myo2–66. Cells harboring this mutation undergo depolarized growth at the restrictive temperature, suggesting that the myosin is involved in polarized transport of secretory vesicles (Johnston et al., 1991; Govindan et al., 1995). Myo2 concentrates at sites of cell growth, and this is specifically dependent on the presence of actin cables and on motor activity (Lillie and Brown, 1994; Walch-Solimena et al., 1997; Pruyne et al., 1998; Schott et al., 1999). A demonstration that Myo2p is involved in transport of post-Golgi vesicles came with the isolation and characterization of conditional mutations in the tail of Myo2: these mutations did not affect the ability of the motor to concentrate at the ends of actin cables but resulted in a failure to concentrate post-Golgi secretory vesicles there (Schott et al., 1999). Visualization of Myo2-dependent movement of transport vesicles was provided by imaging GFP-Sec4, a Rab associated with post-Golgi secretory vesicles, where fast directed transport (∼3 μm/s) toward the bud was observed (Schott et al., 2002). In vitro assays show that Myo2 can move actin filaments at about the same rate measured for GFP-Sec4 in vivo (Reck-Peterson et al., 2001). Finally, the maximal rates of secretory vesicle movements in vivo are proportionally increased or reduced by shortening or lengthening the lever arm connecting the Myo2 coiled-coil dimerization domain to the head motor domain (Schott et al., 2002).
Organelle segregation by Myo2.
Although transport of secretory vesicles may be the essential function of Myo2, this motor has also been implicated in the transport of many organelles for their segregation. Orientation of the nucleus in preparation for mitosis is a crucial event to ensure that the mitotic spindle aligns with the cell division axis. Orientation is achieved early in the yeast cell cycle in a process requiring Bni1 for the assembly of actin cables and Myo2 (Lee et al., 1999; Theesfeld et al., 1999; Beach et al., 2000; Yin et al., 2000; Evangelista et al., 2002). This orientation involves an interaction between the Myo2 tail and the nuclear orientation receptor Kar9 (Yin et al., 2000), which binds (at least) Bim1 located at the ends of cytoplasmic microtubules (Korinek et al., 2000; Lee et al., 2000; Miller et al., 2000). A plausible mechanism is that the Myo2–Kar9–Bim1 complex associates with and actively transports the ends of cytoplasmic microtubules to the site of cell polarization (Yin et al., 2000); alternatively, the Myo2–Kar9 complex might act as a capture site for Bim1-coated dynamic microtubules (Beach et al., 2000; Korinek et al., 2000; Lee et al., 2000).
Many other organelles are also transported into the bud by Myo2, including peroxisomes (Hoepfner et al., 2001), late compartments of the Golgi (Rossanese et al., 2001), and a portion of the vacuole (the yeast equivalent of a lysosome). How the vacuole is inherited is especially well understood and provides a fascinating and insightful tale.
The story begins when it was discovered that some actin mutants and myo2–66 are defective in vacuolar inheritance (Hill et al., 1996). A mutation affecting the tail of Myo2 was then characterized that rendered vacuole inheritance defective, although not significantly affecting polarized growth, thus firmly implicating vacuole inheritance as another function of Myo2 (Catlett and Weisman, 1998). Analysis of a panel of mutations in the Myo2 tail identified a region specifically required for vacuolar inheritance, suggesting that this might define a site necessary to bind the vacuole (Catlett et al., 2000). In work reported in this issue (Ishikawa et al., 2003), overexpression and extragenic suppressors of myo2 mutants defective in vacuole inheritance both identified VAC17. Deletion of VAC17 was found to confer a specific defect in vacuole inheritance without affecting any other known function of Myo2. The Myo2 tail interacts with Vac17 as seen by two-hybrid analysis, but no interaction is seen when the constructs are derived from myo2 or vac17 mutants defective in vacuolar inheritance. Moreover, intragenic suppressors of the myo2 tail mutants that restored vacuolar inheritance also restored the two-hybrid interaction between Vac17 and the Myo2 tail. Finally, increasing the level of functional VAC17 enhanced the amount of Myo2 seen on the vacuole. Thus, Vac17 is identified as the receptor linking Myo2 to the vacuole (Ishikawa et al., 2003).
The discovery that Vac17 interacts with Vac8 is reported in Tang et al. (2003). Vac8 is a vacuolar membrane protein known to be involved in inheritance (Wang et al., 1998) so it could provide the link from Myo2–Vac17 to the vacuolar membrane. Consistent with this model is the finding that association of Vac17 with vacuolar membranes, as determined both cytologically and biochemically, is lost in cells deleted for Vac8. Moreover, Vac8 coimmunoprecipitates with Myo2 but only in the presence of Vac17. The evidence strongly supports the simple model that the Myo2 tail is linked to the vacuolar membrane through a vacuole-specific Vac17–Vac8 bridge (Tang et al., 2003).
How many cargoes can Myo2 transport?
Myo2 has many cargoes, so how many different binding sites might there be in its tail? Delivery of the vacuole and secretory vesicles can be cleanly separated genetically, implying two distinct receptors (Schott et al., 1999; Catlett et al., 2000). Transport of Kar9 is affected in a subset of Myo2 tail mutants that affect secretion, and kar9Δ cells do not have a defect in secretion, implying that binding the receptor for secretory vesicles and Kar9 are also distinct. Thus, it is likely that Myo2 binds at least three cargo receptors (Vac17, Kar9, and the secretory vesicle receptor). In addition, the tail of Myo2 binds Smy1, a divergent member of the kinesin family whose localization depends on Myo2 and not microtubules (Lillie and Brown, 1992, 1994, 1998) and whose function is yet to be clarified. Finally, the coiled-coil dimerization region of Myo2 also binds Rho3 (Robinson et al., 1999), one of yeast's six Rho proteins, and this interaction might be important for secretory vesicle delivery (Adamo et al., 1999).
Coordination of organelle segregation with the cell cycle.
Given this diversity of ligands, how does the Myo2 tail select between its suitors and in a cell cycle–dependent manner? Again, recent work from the Weisman lab on vacuole inheritance provides key insights into this question. Tang et al. (2003) suggest a model in which cell cycle–regulated expression of Vac17 provides a link between Vac8 and the Myo2 tail for delivery of the vacuole into the bud followed by the subsequent degradation of Vac17. In this way, degradation of Vac17 in the bud ensures the vectorial transport of the vacuole. The key player, Vac17, is a modular protein with a Myo2 tail binding site in the NH2-terminal region, the Vac8-binding domain in the COOH terminus, and a PEST sequence between them. The first clue for a cell cycle–regulated process came with the finding that the expression of Vac17 mRNA (Spellman et al., 1998) and protein (Tang et al., 2003) are cell cycle regulated and peak about the time of vacuolar inheritance. Second, the level of Vac17 protein is elevated when cells are defective in vacuolar targeting, either due to loss of Vac8 or through a Myo2 tail mutation. Third, removal of the Vac17 PEST sequence (a potential signal for protein degradation) stabilizes Vac17 and results in the appearance of Vac17 in the bud, which is not seen in wild-type cells. Moreover, whereas in large budded cells the vacuole is normally found near the bud center, in Vac17-PEST cells it becomes aberrantly dragged to the bud neck where Myo2 normally delivers secretory cargo for septum assembly at this stage of the cell cycle. This suggests that the vacuole is deliberately deposited at a specific destination. Although the machinery that might degrade Vac17 in the bud is not yet known, the results strongly support a model of vectorial transport of Vac17 followed by site-specific degradation (Tang et al., 2003).
mRNA transport by Myo4.
Budding yeast has a second myosin V encoded by Myo4 (Haarer et al., 1994). The first insight into its function emerged from a genetic screen to identify mutations affecting mating-type switching.
In the wild, yeast is naturally a diploid. Under conditions of stress it undergoes meiosis to generate four haploid spores, two of each mating type, a and α. Upon return to favorable conditions, haploid a and α spores can germinate and mate to restore the diploid state. However, even a single germinating spore can restore the diploid state through mating-type switching. When a single haploid spore germinates, it will grow a bud that retains the mother's mating type, whereas the mother will switch mating type because it selectively expresses a nuclease called HO. A genetic screen to identify genes that are required for HO expression in the mother netted mutations in five genes designated SHE1–5. Remarkably, SHE1 turned out to be MYO4, and SHE5 turned out to be BNI1, thereby implicating the actin cytoskeleton (Jansen et al., 1996). With our current understanding of the role of Bni1, this implies transport of something by Myo4 down actin cables assembled by the formin Bni1. Myo4 in fact transports the mRNA for a repressor of HO expression called Ash1 (Bobola et al., 1996; Sil and Herskowitz, 1996; Takizawa et al., 1997). Thus, mating-type switching is suppressed in the daughter by transport of the ASH1 mRNA into the bud where Ash1 is synthesized and shuts off HO expression in the daughter nucleus. In the absence of this system, i.e., in she mutants, Ash1 mRNA is not transported into the bud, and HO expression is switched off in both the mother and daughter. Subsequent work has shown that the receptor protein She3 binds to the tail of Myo4p and to She2, which binds ASH1 mRNA (Bohl et al., 2000; Long et al., 2000). This polarized transport system also delivers other mRNAs, including one for a daughter-specific plasma membrane protein (Ist2) (Takizawa et al., 2000). Interestingly, Myo4 polarization is defective in cells lacking either mRNA cargo or the She2 or She3 proteins. In addition, the polarized distribution of Myo4 appears to involve a ribonucleoprotein-dependent retention mechanism in the bud (Kruse et al., 2002).
Thus, the myosin V encoded by Myo4 seems to use the same tracks and polarity system used by Myo2 but transports mRNAs, whereas Myo2 transports protein complexes and membrane-bound compartments.
Organelle segregation without myosin Vs.
Like other organelles in yeast, mitochondria use actin cables for segregation (Simon et al., 1995, 1997; Hermann et al., 1997; Singer et al., 2000). This segregation does not show a dependence on myosin motors but appears to be driven by Arp2/3-dependent actin polymerization along actin cables (Simon et al., 1997; Boldogh et al., 2001). However, a recent report concluded that Ypt11, another yeast Rab protein, binds to the tail of Myo2 and through this interaction can influence the accumulation of mitochondria in the bud (Itoh et al., 2002). How direct a role Ypt11–Myo2 plays in mitochondrial motility or retention in the bud remains to be determined.
Are the processes uncovered in yeast conserved in large eukaryotes?
Many of the proteins discussed in this review, actin, Rho proteins, myosin Vs, formins, Bim1p, Rabs, etc., are conserved from yeast to mammals. Interestingly, this conservation does not so far extend to the receptor proteins that bind to the tails of the myosin Vs, Kar9, Vac17, and She3. An example of a reciprocal result has been found in the well-studied mammalian melanocyte system. Here, melanosomes are transported along microtubules to the cell periphery where they are then captured by a myosin V (myosin Va) through an interaction with the nonconserved receptor protein melanophilin (Hume et al., 2001; Nagashima et al., 2002; Wu et al., 2002). Thus, core principles for the spatial assembly of microfilament arrangements and the involvement of myosin V motors seem to be conserved, yet the adapters for cell-specific processes appear to be more variable.
Although the formin system directs the assembly of actin cables, a large fraction of the F-actin in yeast is localized in cortical patches that are assembled by the Arp2/3-dependent pathway. In animal cells, the Arp2/3 system is involved in the assembly of branched and dynamic actin filaments in structures such as lamellipodia and endosome-associated clouds, whereas it remains to be determined what structures the formin family contribute to the assembly of, with stress fibers so far being the strongest candidate. An important and interesting question is how a cell partitions actin between these different systems.
Perspectives
Although these major advances have provided the framework for a simple model explaining how secretion is polarized and organelles are segregated, it also raises a new round of fascinating questions. We now need to know how signaling pathways can coordinate the location of actin cables during the cell cycle and respond to cell stresses and changing environmental cues. Once the appropriate tracks have been assembled, the motor proteins Myo2 and Myo4 have to select the appropriate cargoes for transport at appropriate times, and as described above, the first insights into how this might be done for vacuole segregation are emerging. Since the tail of Myo2 seems to carry multiple cargoes, it will be important to determine how a single type of motor protein can distribute its duties between multiple functions and whether the precedent of localized degradation of a cargo receptor is a general or specific mechanism for cargo release.
Acknowledgments
I am grateful to the wonderful colleagues in my lab for comments on the manuscript, and in addition to David Pruyne for help with the figure.
References
- Adamo, J.E., G. Rossi, and P. Brennwald. 1999. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell. 10:4121–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, A.E., and J.R. Pringle. 1984. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 98:934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts, A.S. 2001. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J. Biol. Chem. 276:2824–2830. [DOI] [PubMed] [Google Scholar]
- Beach, D.L., J. Thibodeaux, P. Maddox, E. Yeh, and K. Bloom. 2000. The role of the proteins Kar9 and Myo2 in orienting the mitotic spindle of budding yeast. Curr. Biol. 10:1497–1506. [DOI] [PubMed] [Google Scholar]
- Bobola, N., R.P. Jansen, T.H. Shin, and K. Nasmyth. 1996. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell. 84:699–709. [DOI] [PubMed] [Google Scholar]
- Bohl, F., C. Kruse, A. Frank, D. Ferring, and R.P. Jansen. 2000. She2p, a novel RNA-binding protein tethers ASH1 mRNA to the Myo4p myosin motor via She3p. EMBO J. 19:5514–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh, I.R., H.C. Yang, W.D. Nowakowski, S.L. Karmon, L.G. Hays, J.R. Yates, III, and L.A. Pon. 2001. Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proc. Natl. Acad. Sci. USA. 98:3162–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett, N.L., and L.S. Weisman. 1998. The terminal tail region of a yeast myosin-V mediates its attachment to vacuole membranes and sites of polarized growth. Proc. Natl. Acad. Sci. USA. 95:14799–14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett, N.L., J.E. Duex, F. Tang, and L.S. Weisman. 2000. Two distinct regions in a yeast myosin-V tail domain are required for the movement of different cargoes. J. Cell Biol. 150:513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston, J.P., S.E. Tcheperegine, and E. Bi. 2002. Singularity in budding: a role for the evolutionarily conserved small GTPase Cdc42p. Proc. Natl. Acad. Sci. USA. 99:12185–12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant, J. 1999. Cell polarity in yeast. Annu. Rev. Cell Dev. Biol. 15:365–391. [DOI] [PubMed] [Google Scholar]
- Drees, B., C. Brown, B.G. Barrell, and A. Bretscher. 1995. Tropomyosin is essential in yeast, yet the TPM1 and TPM2 products perform distinct functions. J. Cell Biol. 128:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista, M., K. Blundell, M.S. Longtine, C.J. Chow, N. Adames, J.R. Pringle, M. Peter, and C. Boone. 1997. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 276:118–122. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., D. Pruyne, D.C. Amberg, C. Boone, and A. Bretscher. 2002. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4:32–41. [DOI] [PubMed] [Google Scholar]
- Fujiwara, T., K. Tanaka, A. Mino, M. Kikyo, K. Takahashi, K. Shimizu, and Y. Takai. 1998. Rho1p-Bni1p-Spa2p interactions: implication in localization of Bni1p at the bud site and regulation of the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell. 9:1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter, A.S., I. Bose, T.R. Zyla, E.S. Bardes, and D.J. Lew. 2002. Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J. Cell Biol. 156:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan, B., R. Bowser, and P. Novick. 1995. The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol. 128:1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer, B.K., A. Petzold, S.H. Lillie, and S.S. Brown. 1994. Identification of MYO4, a second class V myosin gene in yeast. J. Cell Sci. 107:1055–1064. [DOI] [PubMed] [Google Scholar]
- Hermann, G.J., E.J. King, and J.M. Shaw. 1997. The yeast gene, MDM20, is necessary for mitochondrial inheritance and organization of the actin cytoskeleton. J. Cell Biol. 137:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, K.L., N.L. Catlett, and L.S. Weisman. 1996. Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J. Cell Biol. 135:1535–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner, D., M. van den Berg, P. Philippsen, H.F. Tabak, and E.H. Hettema. 2001. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J. Cell Biol. 155:979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume, A.N., L.M. Collinson, A. Rapak, A.Q. Gomes, C.R. Hopkins, and M.C. Seabra. 2001. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 152:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura, H., K. Tanaka, T. Hihara, M. Umikawa, T. Kamei, K. Takahashi, T. Sasaki, and Y. Takai. 1997. Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16:2745–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, K., N.L. Catlett, J.L. Novak, F. Tang, J.J. Nau, and L.S. Weisman. 2003. Identification of an organelle-specific myosin V receptor. J. Cell Biol. 160:887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, T., A. Watabe, E.A. Toh, and Y. Matsui. 2002. Complex formation with Ypt11p, a rab-type small GTPase, is essential to facilitate the function of Myo2p, a class V myosin, in mitochondrial distribution in Saccharomyces cerevisiae. Mol. Cell. Biol. 22:7744–7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, R.P., C. Dowzer, C. Michaelis, M. Galova, and K. Nasmyth. 1996. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin myo4p and other cytoplasmic proteins. Cell. 84:687–697. [DOI] [PubMed] [Google Scholar]
- Johnson, D.I. 1999. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63:54–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, G.C., J.A. Prendergast, and R.A. Singer. 1991. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J. Cell Biol. 113:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei, T., K. Tanaka, T. Hihara, M. Umikawa, H. Imamura, M. Kikyo, K. Ozaki, and Y. Takai. 1998. Interaction of Bnr1p with a novel Src homology 3 domain-containing Hof1p. Implication in cytokinesis in Saccharomyces cerevisiae. J. Biol. Chem. 273:28341–28345. [DOI] [PubMed] [Google Scholar]
- Kilmartin, J.V., and A.E. Adams. 1984. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J. Cell Biol. 98:922–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno, H., K. Tanaka, A. Mino, M. Umikawa, H. Imamura, T. Fujiwara, Y. Fujita, K. Hotta, H. Qadota, T. Watanabe, et al. 1996. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:6060–6068. [PMC free article] [PubMed] [Google Scholar]
- Korinek, W.S., M.J. Copeland, A. Chaudhuri, and J. Chant. 2000. Molecular linkage underlying microtubule orientation toward cortical sites in yeast. Science. 287:2257–2259. [DOI] [PubMed] [Google Scholar]
- Kruse, C., A. Jaedicke, J. Beaudouin, F. Bohl, D. Ferring, T. Guttler, J. Ellenberg, and R.P. Jansen. 2002. Ribonucleoprotein-dependent localization of the yeast class V myosin Myo4p. J. Cell Biol. 159:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, L., S.K. Klee, M. Evangelista, C. Boone, and D. Pellman. 1999. Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J. Cell Biol. 144:947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, L., J.S. Tirnauer, J. Li, S.C. Schuyler, J.Y. Liu, and D. Pellman. 2000. Positioning of the mitotic spindle by a cortical-microtubule capture mechanism. Science. 287:2260–2262. [DOI] [PubMed] [Google Scholar]
- Lillie, S.H., and S.S. Brown. 1992. Suppression of a myosin defect by a kinesin-related gene. Nature. 356:358–361. [DOI] [PubMed] [Google Scholar]
- Lillie, S.H., and S.S. Brown. 1994. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J. Cell Biol. 125:825–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie, S.H., and S.S. Brown. 1998. Smy1p, a kinesin-related protein that does not require microtubules. J. Cell Biol. 140:873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H.P., and A. Bretscher. 1989. Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell. 57:233–242. [DOI] [PubMed] [Google Scholar]
- Long, R.M., W. Gu, E. Lorimer, R.H. Singer, and P. Chartrand. 2000. She2p is a novel RNA-binding protein that recruits the Myo4p-She3p complex to ASH1 mRNA. EMBO J. 19:6592–6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, R.K., S.C. Cheng, and M.D. Rose. 2000. Bim1p/Yeb1p mediates the Kar9p-dependent cortical attachment of cytoplasmic microtubules. Mol. Biol. Cell. 11:2949–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima, K., S. Torii, Z. Yi, M. Igarashi, K. Okamoto, T. Takeuchi, and T. Izumi. 2002. Melanophilin directly links Rab27a and myosin Va through its distinct coiled-coil regions. FEBS Lett. 517:233–238. [DOI] [PubMed] [Google Scholar]
- Ozaki-Kuroda, K., Y. Yamamoto, H. Nohara, M. Kinoshita, T. Fujiwara, K. Irie, and Y. Takai. 2001. Dynamic localization and function of Bni1p at the sites of directed growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle, J.R., E. Bi, H.A. Harkins, J.E. Zahner, C. De Virgilio, J. Chant, K. Corrado, and H. Fares. 1995. Establishment of cell polarity in yeast. Cold Spring Harb. Symp. Quant. Biol. 60:729–744. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., and A. Bretscher. 2000. a. Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113:571–585. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., and A. Bretscher. 2000. b. Polarization of cell growth in yeast. II. The role of the cortical actin cytoskeleton. J. Cell Sci. 113:365–375. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., M. Evangelista, C. Yang, E. Bi, S. Zigmond, A. Bretscher, and C. Boone. 2002. Role of formins in actin assembly: nucleation and barbed end association. Science. 297:612–615. [DOI] [PubMed] [Google Scholar]
- Pruyne, D.W., D.H. Schott, and A. Bretscher. 1998. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 143:1931–1945. [DOI] [PubMed] [Google Scholar]
- Reck-Peterson, S.L., M.J. Tyska, P.J. Novick, and M.S. Mooseker. 2001. The yeast class V myosins, Myo2p and Myo4p, are nonprocessive actin-based motors. J. Cell Biol. 153:1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, N.G., L. Guo, J. Imai, E.A. Toh, Y. Matsui, and F. Tamanoi. 1999. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol. Cell. Biol. 19:3580–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossanese, O.W., C.A. Reinke, B.J. Bevis, A.T. Hammond, I.B. Sears, J. O'Connor, and B.S. Glick. 2001. A role for actin, Cdc1p, and Myo2p in the inheritance of late Golgi elements in Saccharomyces cerevisiae. J. Cell Biol. 153:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot, I., S.K. Klee, and D. Pellman. 2002. a. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4:42–50. [DOI] [PubMed] [Google Scholar]
- Sagot, I., A.A. Rodal, J. Moseley, B.L. Goode, and D. Pellman. 2002. b. An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 4:626–631. [DOI] [PubMed] [Google Scholar]
- Schott, D., J. Ho, D. Pruyne, and A. Bretscher. 1999. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 147:791–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott, D., T. Huffaker, and A. Bretscher. 2002. Microfilaments and microtubules: the news from yeast. Curr. Opin. Microbiol. 5:564–574. [DOI] [PubMed] [Google Scholar]
- Sil, A., and I. Herskowitz. 1996. Identification of asymmetrically localized determinant, Ash1p, required for lineage-specific transcription of the yeast HO gene. Cell. 84:711–722. [DOI] [PubMed] [Google Scholar]
- Simon, V.R., S.L. Karmon, and L.A. Pon. 1997. Mitochondrial inheritance: cell cycle and actin cable dependence of polarized mitochondrial movements in Saccharomyces cerevisiae. Cell Motil. Cytoskeleton. 37:199–210. [DOI] [PubMed] [Google Scholar]
- Simon, V.R., T.C. Swayne, and L.A. Pon. 1995. Actin-dependent mitochondrial motility in mitotic yeast and cell-free systems: identification of a motor activity on the mitochondrial surface. J. Cell Biol. 130:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, J.M., G.J. Hermann, and J.M. Shaw. 2000. Suppressors of mdm20 in yeast identify new alleles of ACT1 and TPM1 predicted to enhance actin-tropomyosin interactions. Genetics. 156:523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman, P.T., G. Sherlock, M.Q. Zhang, V.R. Iyer, K. Anders, M.B. Eisen, P.O. Brown, D. Botstein, and B. Futcher. 1998. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell. 9:3273–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa, P.A., A. Sil, J.R. Swedlow, I. Herskowitz, and R.D. Vale. 1997. Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature. 389:90–93. [DOI] [PubMed] [Google Scholar]
- Takizawa, P.A., J.L. DeRisi, J.E. Wilhelm, and R.D. Vale. 2000. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 290:341–344. [DOI] [PubMed] [Google Scholar]
- Tang, F., E. Kauffman, J.L. Novak, J.N. Nau, N.L. Catlett, and L.S. Weisman. 2003. Regulated degradation of a class V myosin receptor determines the direction of movement of the yeast vacuole. Nature. In press. [DOI] [PubMed] [Google Scholar]
- Theesfeld, C.L., J.E. Irazoqui, K. Bloom, and D.J. Lew. 1999. The role of actin in spindle orientation changes during the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 146:1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallen, E.A., J. Caviston, and E. Bi. 2000. Roles of Hof1p, Bni1p, Bnr1p, and Myo1p in cytokinesis in Saccharomyces cerevisiae. Mol. Biol. Cell. 11:593–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena, C., R.N. Collins, and P.J. Novick. 1997. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 137:1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.X., N.L. Catlett, and L.S. Weisman. 1998. Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasm to vacuole. J. Cell Biol. 140:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N., P. Madaule, T. Reid, T. Ishizaki, G. Watanabe, A. Kakizuka, Y. Saito, K. Nakao, B.M. Jockusch, and S. Narumiya. 1997. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 16:3044–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N., T. Kato, A. Fujita, T. Ishizaki, and S. Narumiya. 1999. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1:136–143. [DOI] [PubMed] [Google Scholar]
- Winter, D., A.V. Podtelejnikov, M. Mann, and R. Li. 1997. The complex containing actin-related proteins Arp2 and Arp3 is required for the motility and integrity of yeast actin patches. Curr. Biol. 7:519–529 (published erratum appears in Curr. Biol. 1997. 79:R593). [DOI] [PubMed] [Google Scholar]
- Winter, D.C., E.Y. Choe, and R. Li. 1999. Genetic dissection of the budding yeast Arp2/3 complex: a comparison of the in vivo and structural roles of individual subunits. Proc. Natl. Acad. Sci. USA. 96:7288–7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X.S., K. Rao, H. Zhang, F. Wang, J.R. Sellers, L.E. Matesic, N.G. Copeland, N.A. Jenkins, and J.A. Hammer, III. 2002. Identification of an organelle receptor for myosin-Va. Nat. Cell Biol. 4:271–278. [DOI] [PubMed] [Google Scholar]
- Yang, H.C., and L.A. Pon. 2002. Actin cable dynamics in budding yeast. Proc. Natl. Acad. Sci. USA. 99:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H., D. Pruyne, T.C. Huffaker, and A. Bretscher. 2000. Myosin V orientates the mitotic spindle in yeast. Nature. 406:1013–1015. [DOI] [PubMed] [Google Scholar]