Abstract
Membrane growth factors that are processed to produce soluble ligands may function both as soluble factors and as membrane factors. The membrane growth factor Kit-ligand (KL), the ligand of the Kit receptor tyrosine kinase, is encoded at the Sl locus, and mice carrying Sl mutations have defects in hematopoiesis, gametogenesis, and melanogenesis. Two alternatively spliced KL transcripts encode two cell-associated KL protein products, KL-1 and KL-2. The KL-2 protein lacks the major proteolytic cleavage site for the generation of soluble KL, thus representing a more stable cell-associated form of KL. We investigated the consequences of exclusive expression of KL-2 in vivo. The KL gene in embryonic stem cells was modified and KL exon 6 was replaced with a PGKneoNTRtkpA cassette by homologous recombination, and mice carrying the SlKL2 allele were obtained. SlKL2/SlKL2 mice had only slightly reduced levels of soluble KL in their serum, suggesting that in vivo KL-2 may be processed to produce soluble KL-2S. The steady-state characteristics of the hematopoietic system and progenitor numbers were normal, and the mutant animals were not anemic. However, mast cell numbers in the skin and peritoneum were reduced and the mutant animals displayed increased sensitivity to sublethal doses of γ-irradiation. Therefore, KL-2 may substitute for KL-1 in most situations with the exception of the production of mast cells, and induced proteolytic cleavage of KL-1 to produce soluble KL may have a role in the regeneration of hematopoietic tissue after radiation injury.
Ligands of cell surface receptors may be soluble proteins that are produced by secretion, or alternatively they may be produced as membrane molecules, which may or may not be processed to produce soluble proteins. This second class of receptor ligand may function both as soluble and as membrane-bound growth factors. An increasing number of growth factors, which derive from membrane-anchored precursors, and growth factors, which function both as soluble and as membrane growth factors, have been described in the past several years. They include members of the epidermal growth factor family, several hematopoietic growth factors, including the Kit-ligand (KL), colony stimulating factor (CSF)-1, interleukin-1, and tumor necrosis factor (1–3) and the eph ligand family (4). Some of these membrane growth factors release soluble growth factors by proteolytic processing or shedding (2, 5, 6); others are obligate membrane growth factors. The nonprocessed membrane-anchored growth factors themselves possess biological activities and promote cell adhesion and juxtacrine stimulation in adjacent cells, which express cognate receptors on their cell surface (3). The respective roles in vivo of the soluble and membrane forms of membrane growth factors at present is poorly understood. The membrane growth factor KL provides an excellent model to investigate these issues.
Mice carrying mutations in either the Steel (Sl) or dominant White Spotting (W) loci develop similar phenotypic abnormalities, including defects in hematopoiesis, gametogenesis, and melanogenesis (7, 8). The receptor tyrosine kinase c-kit and the ligand of c-kit KL are encoded at the W and Sl loci, respectively (9). KL encodes a membrane protein with an extracellular domain, a transmembrane-spanning segment, and a cytoplasmic domain. Two alternatively spliced KL RNA transcripts encode two cell-associated KL protein products, KL-1 and KL-2, that differ in their sequences N-terminal of the transmembrane segment (2, 12). The KL-2 protein lacks sequences that include the major proteolytic cleavage site in exon 6 for the generation of the soluble KL protein. The KL-1 protein is processed effectively by proteolytic cleavage at a site in exon 6 to produce soluble KL-1 protein (KL-1S); by contrast KL-2 also is processed at a secondary cleavage site, presumably in exon 7, to produce soluble KL-2 protein (KL-2S) but not as effectively. Therefore, KL-2 represents a differentially more stable cell-associated form of KL. Expression of the differentially spliced mRNAs encoding KL-1 and KL-2 occurs in a tissue-specific manner. Whereas, predominant KL-1 expression is associated with fibroblasts, the brain, and thymus, KL-2 expression is prominent in bone marrow, spleen, placenta, cerebellum, and testis (2, 10). Both the soluble and the transmembrane forms of KL have growth factor activities, and consequently, KL may function both as a soluble factor and as membrane growth factor, which delivers its signal upon cell–cell contact (2, 12).
Important insight about the respective roles in vivo of membrane-associated KL and soluble KL have come from the characterization of Sl mutant mice. Two alleles at the Sl locus, Sld and Sl17H, are of particular interest in regards to the function of the transmembrane form of KL (2, 12, 13, 14). Sld encodes a biologically active secreted soluble KL protein and no membrane forms as a result of an intragenic deletion that includes the transmembrane domain and C terminus. Because the phenotypes of Sld/Sld mice are very severe, the membrane form of KL appears to be critical for normal function (7, 8, 15). In the Sl17H allele the extracellular and transmembrane domains of KL are unchanged, but as a result of a frame shift mutation, the cytoplasmic domain is replaced by a nonsense poly-peptide chain of similar length. The phenotype of the Sl17H mutation is noteworthy for a particularly severe effect on the development of male germ cells, although other lineages are affected less severely (14, 16). The nature of the Sl17H mutation, as well as the strong conservation of the cytoplasmic domain sequences of KL in different species, suggests an important functional role for the cytoplasmic domain of KL.
A juxtacrine delivery of the Kit signal is critical in many situations. The in vivo role of soluble KL, although intensively studied in vitro, is not understood very well. The investigation of the functional roles of the soluble and the two cell-associated forms of KL are therefore of great interest. As a first step, we sought to produce a mouse in which the KL-2 protein product would be produced exclusively by modifying the KL gene in embryonic stem (ES) cells and the subsequent production of chimeric mice and germline transmission. We have obtained mice, which produce KL-2 exclusively, by gene targeting and have carried out an extensive analysis of the phenotypes in these mice. Although exclusive KL-2 expression in these mice may substitute for KL-1 in melanogenesis, gametogenesis, and erythropoiesis, SlKL2/SlKL2 mice have diminished numbers of tissue mast cells, and they exhibit an increased sensitivity to sublethal dose of γ-radiation SlKL2.
MATERIALS AND METHODS
Targeting Construct to Modify the KL Gene in ES Cells.
A 35-kb fragment of the mouse KL gene containing exons 4–9 was isolated by screening a genomic 129/SvJ mouse library (Stratagene) with a 32P-labeled probe derived from the KL-1 cDNA (exons 5–7) (2, 17, 18). The exon–intron structure of the genomic DNA clones was characterized by restriction mapping and DNA sequencing. The targeting construct was generated by replacing the 6-kb BamHI-PstI fragment containing exon 6 with the pPGK-neoNTRtkpA cassette (obtained from Rudolf Jaenisch, M.I.T., Cambridge) (19). The resulting targeting vector was linearized with ScaI for transfection.
Isolation of Recombinant ES Clones and Generation of SlKL2/SlKL2 Mice.
The 129/Sv W9.5 ES cell line was kindly provided by Colin L. Stewart (National Cancer Institute, Frederick, MD) (20). The ES cells were maintained, by using standard condition, on primary mouse embryonic fibroblasts expressing the neomycin resistance gene (21). Three × 107 ES cells in 0.8 ml of PBS were electroporated with 40 μg of linearized targeting construct in a 0.4-cm Bio-Rad cuvette with a single pulse at 320 V and 250 mF. After 24 hr, the medium was supplemented with 400 mg/ml of G418 (GIBCO), and after 6–7 days, 200–300 ES clones were isolated from each electroporation. Individual clones were expanded, and genomic DNA was prepared and digested with BglII. Recombinant ES cell clones were identified by Southern blot analysis. Heterozygous ES cells were injected into C57BL/6J blastocysts, and chimeric mice were setup for germline transmission by backcrossing with C57BL/6J mice. Resulting F1 mice containing the SlKL2 mutation in the germline were identified by Southern blot analysis and intercrossed to generate honmozygous SlKL2/SlKL2 mice. SlKL2/SlKL2 mice were maintained on an (129/Sv × C57BL/6) F2 background.
RNA Isolation and Analysis.
Total cellular RNA was extracted from mouse tissues by using RNAzol-B (Tel-Test, Friendswood, TX). RNA (10 μg) was fractionated in 1% agarose/formaldehyde gels, transferred to a membrane, hybridized, and bands visualized by phosphoimager. Ribonuclease protection assays were done as described (2).
Determination of KL Serum Levels.
Microtiter plates were coated with 100 μl of 20 μg/ml of goat anti-mouse stem cell factor (KL) polyclonal antibody (R & D Systems) in 0.1 mol/liter borate-buffered saline, pH 8.8, at 4°C for 24 hr. Plates were blocked with 100 μl of 2% BSA in PBS with 0.05% Tween-20 (PBST) for an additional 24 hr at 4°C. Wells were incubated for 24 hr at 4°C with 100 μl of serially diluted mouse serum, washed with PBST, and developed with 100 μl of a 4 μg/ml solution of rabbit anti-mouse stem cell factor (KL) polyclonal antibody (Genzyme) in PBST for 1 hr at 37°C. The wells were washed four times with PBST, and 100 μl of a 1:5,000 dilution of anti-rabbit IgG biotin conjugate antibody in PBST was added. After incubation for 30 min at room temperature, the antibody solution was washed four times with PBST. Subsequently, wells were incubated for 30 min at room temperature with 100 μl of a 1:5,000 dilution of streptavidin-horseradish peroxidase in PBS plus O-phenylenediamine in citrate-phosphate buffer, pH 4.0, containing 0.03% hydrogen peroxide solution for 10 min at room temperature. The reaction was terminated by the addition of 100 μl of 12.5% H2SO4 to each well. Using a microplate reader, the absorbency at 490 nm was determined. Escherichia coli-derived recombinant mouse KL diluted in PBS containing 0.2% BSA was used to generate standard curves. Standard curves were linear from 78 pg/ml to 1,250 pg/ml.
Determination of Peripheral Blood Parameters and Hematopoietic Progenitor Numbers.
Blood samples were drawn from the retroorbital plexus or tail vein with a capillary pipette (Unopette; Becton Dickinson). Platelet and white blood cell numbers were determined by using a hemocytometer under a phase contrast microscope. The hematocrit was measured by using heparinized micro-hematocrit capillary tube (Fisher Scientific). For in vitro progenitor assays, 105 bone marrow cells were resuspended in 1 ml of Iscove’s modified Dulbecco’s medium, 0.8% methylcellulose, 30% fetal bovine serum (HyClone), and 0.2 mM hemin containing 50 ng/ml mouse IL-3 (BioSource International, Camarillo, CA), 3 units/ml human erythropoietin (Amgen Biologicals), and 20 ng/ml recombinant mouse Kit-ligand (KL). Cultures were incubated at 37°C in a 5% CO2. Colonies were scored after 7 days on the basis of gross morphology as erythroid burst (BFU-E), granulocyte/macrophage colony-forming unit (CFU-GM), and mixed colony (CFU-GEMM) (22, 23).
CFU-S (spleen) day 12 were assessed by injection of 105 donor bone marrow cells suspended in MEM supplemented with 3-(N-morpholino)propanesulfonic acid and 10% fetal bovine serum into the lateral tail vein of lethally irradiated (9.5 Gy) recipient mice. Mice were killed 12 days later, and their spleens fixed in Bouin’s solution and macroscopically visible colonies on the surface of the spleens were counted (22, 24). Determination of mast cell numbers in the skin and peritoneum of control and SlKL2/SlKL2 mice was as described (22, 25).
For long-term bone marrow cultures (LTBMC) BM cells from one femur were flushed into a T-25 flask in Fisher’s medium containing 20% horse serum and 1 mM hydrocortisone (Sigma) (22, 26). Cultures were incubated at 33°C and fed weekly by removal of 50% of the medium and cells with addition of fresh medium. Nonadherent cells collected at each weekly feeding were used for cytospin preparations and for plating in progenitor cultures.
Irradiation of Mice.
Wild-type mice and SlKL2/SlKL2 mice were subjected to γ-irradiation from a cesium source (6.5 Gy/mouse). In control mice, irradiation with 6.5 Gy caused negligible lethality, transient anemia, thrombocytopenia, granulocytopenia, and minimal weight loss or other symptoms in control mice. Hematocrit, white blood cell, granulocyte, and platelet numbers were measured at weekly intervals.
RESULTS
Deletion of KL Exon 6 in the KL Gene of ES Cells (SlKL2) and Production of Mice Containing this Mutation in the Germline.
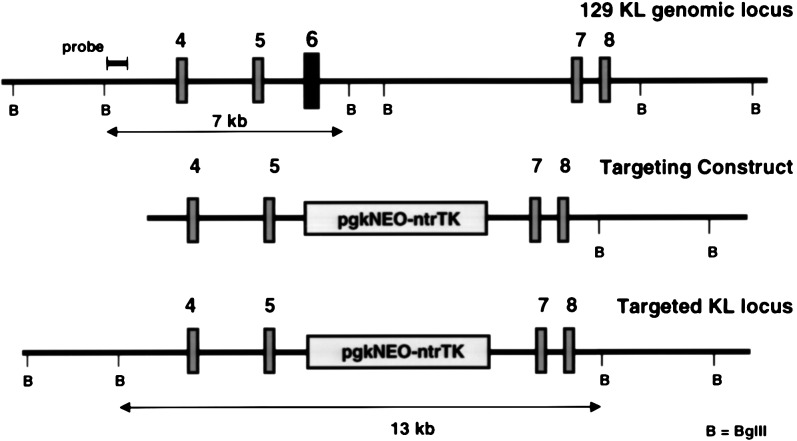
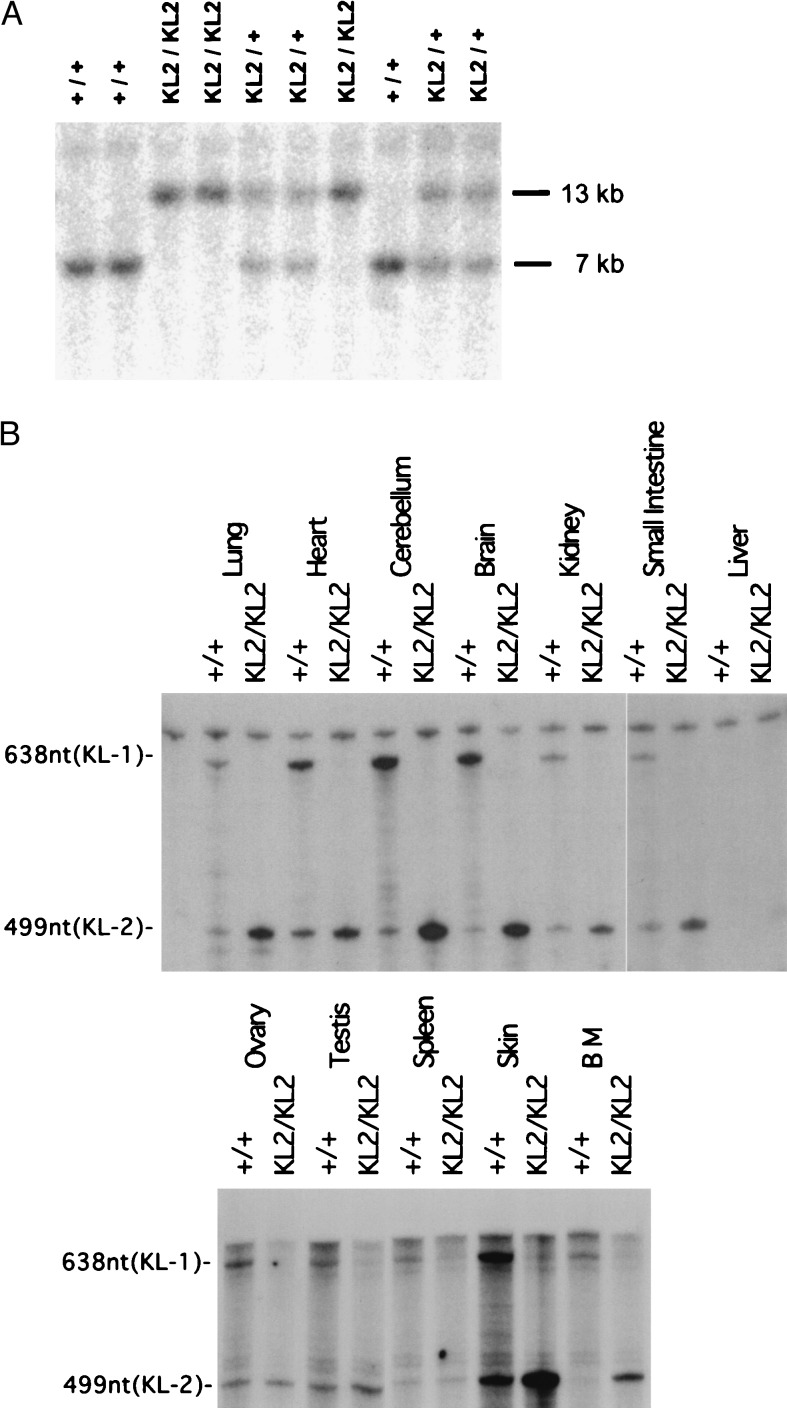
To evaluate the role of KL2 in vivo, we set out to derive a mouse expressing KL-2 only, using homologous recombination technology in ES cells. To modify the germ line KL gene in the ES cell genome, the relevant region of the KL gene from a 129/Sv mouse genomic library (obtained from Stratagene) was isolated and characterized, and a targeting construct in which exon 6 was replaced with a pgkNEOntrTKpA double cassette was derived (Fig. 1). W9.5 ES cells were transfected with the linearized targeting construct, and selection with G418 (400 mg/ml) produced 562 G418-resistant ES cell clones, of which six were correctly targeted. Injection of one of these clones into C57BL/6J blastocysts produced chimeric mice and mating with C57BL/6J mice gave rise to offspring heterozygous for the targeted KL gene. Heterozygous SlKL2/+ mice were then intercrossed to generate homozygous SlKL2/SlKL2 mice in the mixed 129/Sv–C57BL/6J background. The genotypes of wild-type, heterozygous mutant SlKL2/+, and homozygous mutant SlKL2/SlKL2 mice were identified by Southern blotting (Fig. 2A). Sequencing of amplified reverse transcription–PCR products obtained from mRNA confirmed correct splicing from exon 5 to exon 7 in the mutant KL transcripts (data not shown). Furthermore, RNase protection assays were used to monitor expression of KL-1 (638 nt) and KL-2 (449 nt) transcripts. As anticipated, SlKL2/SlKL2 mice expressed only KL-2 (449 nt) transcripts as determined by RNase protection assay (Fig. 2B), and analysis of the distribution of KL transcripts in various tissues in homozygous mutant and wild-type mice by RNase protection assay and Northern blotting (not shown) suggested that the level of KL transcripts (KL1 + KL2) in normal and SlKL2/SlKL2 mice were quite similar and that there were no major differences (Fig. 2B). Therefore, the SlKL2 mutation did not affect the KL expression pattern significantly in various tissues analyzed.
Figure 1.
Targeted modification of the KL gene by homologous recombination. (A) Segment of the 129 mouse KL gene including exons 4–8. (B) Targeting construct in which exon 6 is replaced by a pgkNEOntrTK expression cassette. (C) Structure of targeted KL-[Δexon6] locus.
Figure 2.
(A) Southern blot analysis of DNA from mice obtained by intercross breeding of heterozygous SlKL2/+ mice. Blots containing DNA digested with BglII were hybridized with the probe indicated in Fig. 1. The approximate size of normal and mutant fragments is 7 and 13 kb, respectively. (B) Expression of KL-1 and KL-2 transcripts in tissues of +/+ and SlKL2/SlKL2 mice determined by RNase protection assay. Fifty micrograms of total RNA isolated from various tissues was hybridized with a 700-nt 32-P-labeled antisense probe. Protected fragments for KL-1 and KL-2 are 638 and 449 nt, respectively.
Much of what is known about the phenotypes of Sl and W mutant mice derives from the analysis of mice from a few distinct genetic backgrounds: WCB6 F1 mice (Sl/Sld), C57BL6/J mice (Sld/Sld and many of the W alleles including Wv isolated at the Jackson laboratories), and WBB6 F1 mice (W/Wv). For analysis of the SlKL2 mutation on a homogeneous genetic background, the 129/Sv/C57BL/6J F1 SlKL2/+ mice have been backcrossed with C57BL/6J mice.
SlKL2/SlKL2 Mice Have No Pigmentary and Germ Cell Deficits.
The homozygous mutant SlKL2/SlKL2 mice displayed no pigmentation phenotype and were healthy and fertile. The distribution of offspring of 234 mice born form matings of SlKL2/+ mice, 74 (31.6%) were +/+, 105 (44.9%) were SlKL2/+, and 55 (23.5%) were SlKL2/SlKL2, implying that +/+, SlKL2/+, and SlKL2/SlKL2 mice were equally viable. Moreover, matings between male and female SlKL2/ SlKL2 mice produced normal offspring. Therefore, the pigmentary system and gametogenesis do not appear to be affected in these mice (not shown).
Reduced Levels of Soluble KL in Serum of SlKL2 Mice.
In vitro analysis had shown that the KL-2 membrane protein is more resistant to proteolytic processing than the KL-1 protein (2). It therefore seemed important to determine whether proteolytic processing of KL-2 occurs in vivo. To assess proteolytic processing of the KL-2 protein in vivo, we determined the level of soluble KL-2 protein in the serum of mutant mice. An ELISA assay sensitive to 0.1 ng/ml was used to quantitate soluble KL in the serum of normal and mutant mice. Both normal and SlKL2/SlKL2 mice contained soluble KL in their serum, but in the SlKL2/SlKL2 mice, the level of soluble KL was reduced (0.45 ± 0.13 vs. 0.31 ± 0.09; P = 0.027; n = 8). These results suggest that in vivo KL-2 may be processed to produce soluble KL-2S. It is probable that soluble KL-2S is produced via a secondary proteolytic cleavage site, presumably located in exon 7 near the exon boundary (2, 27).
Hematopoietic Characteristics of SlKL2 Mice.
The hematopoietic system in mice with mutations at the Sl locus is affected in several ways. Effects are seen in the erythroid cell lineage, in the stem cell compartment, and in mast cells (7, 15). To evaluate whether the hematopoietic system was affected in SlKL2/SlKL2 mice, hematopoietic parameters were determined. There was no significant difference between SlKL2/SlKL2 mice and wild-type mice in regards to bone marrow cellularity, mean red blood cell volume and red blood cell, white blood cell, neutrophil, and platelet numbers (Table 1). Also, lymphocyte subsets in the thymus and peripheral lymphoid organs in SlKL2/SlKL2 mice appear to be represented normally when analyzed by flow cytometry for CD3, CD4, CD8, and B220 (data not shown). Because the microenvironment of early hematopoietic progenitors is impaired in mice with mutations at the Sl locus, we analyzed the stem cell populations in the bone marrow and thymus by flow cytometry (28). The Lin-,c-kit+,ScaI+ cells in the bone marrow (presumed hematopoietic stem cells) did not differ significantly between SlKL2/SlKL2 and wild-type mice, and the numbers of CD25, c-kit+, CD4/CD8 double negative cells in the thymus (precursors of thymocytes) were comparable in SlKL2/SlKL2 and wild-type mice.
Table 1.
Peripheral blood cell counts in SlKL2/SlKL2, SlKL2/+, and +/+ mice
| Mice | Hematocrit, % | Red blood cell × 106/mm3 | White blood cell × 103/mm3 | ANC × 103/mm3 | Platelets × 103/mm3 |
|---|---|---|---|---|---|
| B6/129 F2 | 56.0 ± 0.47 | 6.92 ± 0.890 | 11.43 ± 0.87 | 2.02 ± 0.26 | 1.86 ± 0.13 |
| 129Sv | 54.0 ± 1.35 | 8.68 ± 0.66 | 1.36 ± 0.16 | ||
| C57Bl/6 | 52.8 ± 1.34 | 10.48 ± 0.90 | 1.56 ± 0.30 | ||
| SlKL2/+ | 6.04 ± 0.975 | 11.60 ± 1.29 | 1.90 ± 0.25 | 1.60 ± 0.12 | |
| SlKL2/SlKL2 F2 | 54.1 ± 0.92 | 7.81 ± 0.846 | 10.90 ± 0.93 | 2.40 ± 0.30 | 1.45 ± 0.84 |
| Sl/Sld (WCB6 F1) | 29.75 ± 4.6 | 2.30 ± 1.08 |
In addition, hematopoietic progenitors in the bone marrow of SlKL2/SlKL2 and control mice were examined by using biological assays. In agreement with the observed normal hematopoietic parameters, in vitro analysis of hematopoietic progenitors from the bone marrow of SlKL2/SlKL2 C57BL/6 (fifth backcross generation) mice revealed normal numbers of BFU-E, mixed colonies (GEMM) and CFU-GM (Table 2). In contrast, in Sl/Sld mice, bone marrow cellularity was approximately 30% of normal and progenitor numbers were reduced accordingly. Furthermore, the CFU-Sd12 (early myeloid erythroid progenitors) in SlKL2/SlKL2 were also normal, 19.4 ± 2.2 and 19.6 ± 1.6 colonies per 105 cells in C57BL6 and SlKL2/SlKL2 (N5 C57BL6) mice respectively. Thus, the parameters of steady-state hematopoiesis in SlKL2/SlKL2 mice did not deviate from normal.
Table 2.
Myeloid and erythroid progenitors in bone marrow of SlKL2/SlKL2 and +/+ mice
| Genotype | Mouse strain | CFU/femur (KL + IL3 + Epo)
(methylcellulose)
|
CFU/femur, agarose
|
|||
|---|---|---|---|---|---|---|
| GEMM | BFU-E | GM | GM-CSF | GM-KL | ||
| +/+ | C57Bl/6 | 1796 ± 341 | 5198 ± 774 | 19278 ± 3269 | 33264 ± 189 | 39312 ± 1323 |
| SlKL2/SlKL2 | N5 (C57Bl/6) | 1635 ± 102 | 5824 ± 468 | 27994 ± 715 | 35145 ± 737 | 34737 ± 737 |
| Sl/Sld | WCB6 | 601 ± 122 | 1573 ± 202 | 9577 ± 848 | 11197 ± 370 | 11197 ± 1363 |
A role for KL/c-kit in mediating lodging and or homing of hematopoieitc progenitors to sites of hematopoiesis has been implied (22). To investigate whether lodging and/or homing of hematopoietic progenitors is affected in SlKL2/SlKL2 mice, we performed spleen colony assays by using irradiated SlKL2/SlKL2 and normal mice as recipients and donor bone marrow cells from normal mice. In this experiment CFU-Sd12 numbers in SlKL2/SlKL2 and normal recipients were indistinguishable (data not shown), implying that lodging and or homing of hematopoietic progenitors to the spleen of irradiated mutant mice was not affected.
LTBMC are a useful tool for the study of hematopoiesis in vitro. To investigate the effect of the SlKL2 mutation on hematopoiesis in vitro, LTBMCs were established and output of hematopoietic cells was monitored at weekly intervals. These experiments showed that cultures from SlKL2/SlKL2 bone marrow were able to sustain long-term production of hematopoietic cells (Fig. 3). In contrast LTBMCs from Sl/Sld and Sl17H/Sl17H mice failed to sustain the long-term production of hematopoietic cells (22, 35). The output of hematopoietic proigenitors by cultures from SlKL2/SlKL2 BM differed from that of the control cultures in several ways. First, while the output of cells in control cultures peaks at 6 weeks, cultures derived from mutant BM display a more gradual increase in cell output reaching a maximum at 8 weeks and declining thereafter. Importantly, the cultures from SlKL2/SlKL2 BM exhibited a prolonged and increased production of CFU-GEMM, BFU-E and CFU-GM. Furthermore, we determined the production of soluble KL by these LTBMCs during the course of the experiments. Whereas, the control cultures produced between 0.3 and 0.2 ng/ml of soluble KL as determined by ELISA assay, the cultures from SlKL2/SlKL2 BM produced 0.1 or less ng/ml. These results may suggest that sustained expression of KL2 in LTBMCs facilitates the prolonged production of hematopoietic progenitors.
Figure 3.
Long-term culture of bone marrow cells from SlKL2/SlKL2 mice. Hematopoietic output of LTBMC, including the number of nonadherent cells, CFU-GEMM, BFU-E, and CFU-GM, was determined at weekly intervals. Colony assays were carried out in triplicate; mean and standard deviation are shown. The production of soluble KL in culture supernatant was monitored as indicated.
Mast Cell Deficit in SlKL2/SlKL2 Mice.
Mice with Sl mutations typically lack tissue and mucosal mast cells (29). Therefore, the effect of the SlKL2 mutation on mast cells was determined. First, mast cell progenitors in BM of SlKL2/SlKL2 mice were analyzed by flow-cytometry. Slightly increased numbers of Lin−,c-kit+,FcγRII/III+ cells (mast cell progenitors) were reproducibly observed in BM of mutant mice (1.23% vs. 0.87%; data not shown) (30). In contrast, the number of tissue mast cells in skin sections and peritoneal mast cells were reduced significantly in SlKL2/SlKL2 mice (Table 3). The skin of 15-week-old SlKL2/SlKL2 mice contained only 8% of the number of tissue mast cells compared with that in 16-week-old control mice, and the number of peritoneal mast cells was <2% of the number of mast cells in age-matched controls (Table 3; Fig. 4). A similar reduction in numbers of tissue mast cells was observed in SlKL2/SlKL2 (N5 C57BL6) mice (data not shown).
Table 3.
Mast cell numbers in the skin and peritoneum of SlKL2/SlKL2 and +/+ mice
| Age | Genotype | Skin mast cells, CTMC per cm | Percentage of +/+ skin mast cells | Peritoneal mast cells, CTMC per 103 peritoneal cells | Percentage of +/+ peritoneal mast cells |
|---|---|---|---|---|---|
| 4–5 weeks | +/+ | 162.5 ± 2.6 | 49.5 ± 7.0 | ||
| SlKL2/SlKL2 | 48.0 ± 9.2 | 29.6 | 1.9 ± 0.9 | 3.8 | |
| 9–16 weeks | +/+ | 282.2 ± 38.6 | 76.7 ± 3.4 | ||
| SlKL2/SlKL2 | 20.3 ± 1.8 | 7.1 | 1.7 ± 1.0 | 2.2 |
Figure 4.
Histological analysis of mast cells in normal and SlKL2/SlKL2 10-week-old mice. (A) Peritoneal cells stained with toluidine blue. (B) Histological sections of skin stained with toluidine blue.
Sensitivity to Sublethal Doses of γ-Irradiation of SlKL2/SlKL2 Mice.
Both W and Sl mutant mice display an increased sensitivity to the lethal effects of irradiation (7, 31, 32). We, therefore, sought to investigate whether the SlKL2 mutation increases the sensitivity of mice to irradiation. SlKL2/SlKL2 mice, and controls were subjected to a sublethal dose of 6.5 Gy, animals were then observed for up to 4 weeks, and hematopoietic parameters of irradiated animals were monitored (Fig. 5). SlKL2/SlKL2 mice had a survival rate of only 50–60% and onset of mortality invariably was between 12 and 20 days. In contrast, control animals had a survival rate of 95 to 100%. Hematocrit values in mutant animals may suggest delayed onset of hematopoietic regeneration, although white blood cell and neutrophil counts in mutant and control mice were indistinguishable up to 3 weeks after irradiation. Determination of serum levels of soluble KL after irradiation revealed that, in normal mice after irradiation, serum levels of soluble KL increased from 0.4 ng/ml before irradiation to 2.2 ng/ml at 7 days after irradiation followed by a decline to normal levels by 3 to 4 weeks. In contrast, in SlKL2/SlKL2 mice the increase of KL serum levels upon irradiation is quite modest, from 0.3 ng/ml to 0.7 ng/ml (Fig. 5). This observation may suggest that the production of soluble serum KL may be a contributory factor for successful hematopoietic recovery after irradiation.
Figure 5.
Sensitivity to sublethal doses of γ-irradiation of SlKL2/SlKL2 mice. Nine SlKL2/SlKL2, +/+, and 129Sv mice each were subjected to a sublethal dose of 6.5 Gy and survival, hematopoietic parameters and KL serum levels were monitored as indicated. Measurements included five animals for each group; mean and standard deviation are shown.
DISCUSSION
We have modified the KL gene in the mouse to prevent the production of the KL-1 protein product instead of exclusive expression of the KL-2 protein products. The SlKL2 mutation produced several notable in vivo phenotypes. First, homozygous SlKL2/SlKL2 mice are viable, and the pigmentary system and gametogenesis do not appear to be affected by the mutation. Second, in steady-state hematopoiesis, the composition of the stem cell compartment (hematopoietic stem cells) and the erythroid compartment are normal and SlKL2/SlKL2 mice are not anemic. Third, whereas committed mast cell progenitors were slightly increased in number, connective tissue mast cell and peritoneal mast cell numbers were reduced by >90%. Fourth, in vivo SlKL2/SlKL2 mice produce soluble KL-2S, but the serum level is reduced compared with wild-type mice. Therefore, KL-2 may substitute for KL-1 in most situations with the exception of the mast cell compartment. A recent study using transgenic mice expressing a membrane obligate form of KL and KL-1 under the control of a ubiquitous promoter suggested that the membrane obligate KL transgene may ameliorate the anemia and runting in Sl/Sld mice but not the pigmentation deficiency (37). It is difficult to compare these results with the study reported here because the expression pattern of the transgenes differs from that of the endogenous KL gene and because potential KL cleavage sites have been altered in the membrane obligate transgene.
Death and septicemia caused by total body irradiation result primarily from damage to the hematopoietic and lymphoid systems, although radiation induced damage of the epithelium lining of the intestine is thought to be a contributory factor as well. Sl and W mutant mice exhibit an increased sensitivity to radiation (7, 31, 32). Whereas, the LD50/30 of W/Wv animals is 365 rad, the LD50/30 of Sl/Sld animals is 150 rad. The increased radiation sensitivity in mutant mice may stem either from increased sensitivity of progenitors and/or from an effect on the regeneration of the hematopoietic tissue. Therefore, the slightly increased radiation sensitivity of the SlKL2/SlKL2 mice may suggest a role for KL-1 and/or soluble KL in the recovery of the hematopoietic system from radiation damage. In agreement with a role for soluble KL in the recovery of the hematopoietic system from radiation damage, administration of soluble KL has been shown to have radioprotective properties (33). Also, KL has been shown to suppress apoptosis induced by both factor deprivation and irradiation (34). Our observation that, upon irradiation, SlKL2/SlKL2 mice fail to produce elevated levels of serum KL implies that induced proteolytic cleavage of KL-1 to produce soluble KL may be a mechanism to produce an important survival factor for hematopoietic progenitors that were released into the periphery from the bone marrow. Therefore, elevated levels of soluble KL in the serum in response to irradiation may promote mobilization of stem cells and progenitors in combination with other cytokines, e.g., granulocyte (G)-CSF, and thus facilitate their homing to the spleen, the main site of hematopoietic regeneration in rodents after radiation injury, and soluble KL may preserve the viability of mobilized stem cells and progenitors in the periphery.
LTBMC provide a unique means to investigate hematopoiesis in vitro. LTBMC are a complex and dynamic culture system consisting of stromal cells, adipocytes, hematopoietic cell types, and extracellular matrix. Cell–cell interactions in LTBMC have been recognized to play a critical role for the establishment of this culture system as well as for its hematopoietic output. Particularly, membrane-associated KL appears to play a critical role for the establishment of LTBMC (22, 35). Our observation of altered/prolonged hematopoietic output of LTBMCs derived from the bone marrow of SlKL2/SlKL2 mice illustrates the important role of cell–cell interactions and signaling in the bone marrow. Different composition/architecture of stromal elements in the bone marrow compared with LTBMC could be responsible for the altered hematopoietic output characteristics of LTBMCs derived from SlKL2/SlKL2 mice. Alternatively, slower processing characteristics of KL-2 in LTBMC stromal elements reflected by the low levels of soluble KL in culture supernatant, may result in higher membrane KL levels critical for the production of early progenitors.
Both the KL-1 and KL-2 proteins are produced as membrane proteins, which may be processed to produce soluble KL protein products. Tissue-specific splicing to produce the respective mRNAs encoding KL-1 and KL-2, as well as KL-1- and KL-2-specific proteolytic processing mechanisms determine the production of soluble KL in various cell types. In SlKL2/SlKL2 mice, KL-2 protein products are expressed exclusively; hence the KL-2 protein processing machinery determines the production of soluble KL in these mice. Previous investigations concerning the mechanism of cleavage of the KL-1 and KL-2 protein products to produce the respective soluble forms by using various protease inhibitors have suggested that KL-1 and KL-2 are processed by different proteases (36). More recent studies indicate that KL-1 and KL-2 are processed by distinct proteases of the metalloprotease family (Y.T. and P.B., unpublished observation). It is not known whether these proteases are expressed in specific cell types to facilitate tissue specific proteolytic processing. However, the subtle and/or restricted phenotypes observed in SlKL2/SlKL2 mice might suggest that the KL-2 protease is expressed ubiquitously and may compensate for lack of processing of KL-1 in the mutant mice. Except that because of the lower cleavage rate of the KL-2 protease radiation induced release of soluble KL into serum is minimal in mutant animals thus affecting recovery from radiation injury.
Acknowledgments
We would like to thank Harry Satterwhite and Maureen Sullivan for expert assistance with hematological assays and Dr. Katia Manova for help with histology and numerous discussions, Dr. Jeffrey Ravetch for letting us use his cell sorter and discussions, and Dr. Shin-ichi Nishikawa for generously providing ACK2 antibody. Support by grants from the American Cancer Society and the National Institutes of Health is acknowledged.
ABBREVIATIONS
- KL
Kit-ligand
- ES
embryonic stem
- BM
bone marrow
- LTBMC long-term BM cultures
CFU
- colony-forming unit
BFU-E, burst forming unit-erythroid
- GM
granulocyte/macrophage
- GEMM
granulocyte/erythroid/macrophage/megakaryocyte
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Lee D C, Fenton S E, Berkowitz E A, Hissong M A. Pharmacol Rev. 1995;47:51–85. [PubMed] [Google Scholar]
- 2.Huang E J, Nocka K H, Buck J, Besmer P. Mol Biol Cell. 1992;3:349–362. doi: 10.1091/mbc.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosenberg M W, Massague J. Curr Opin Cell Biol. 1993;5:823–838. doi: 10.1016/0955-0674(93)90032-l. [DOI] [PubMed] [Google Scholar]
- 4.Pandey A, Lindberg R A, Dixit V M. Curr Biol. 1995;5:986–989. doi: 10.1016/s0960-9822(95)00195-3. [DOI] [PubMed] [Google Scholar]
- 5.Rettenmier C W. Curr Top Microbiol Immunol. 1989;149:129–141. doi: 10.1007/978-3-642-74623-9_12. [DOI] [PubMed] [Google Scholar]
- 6.Arribas J, Lopez-Cassilas F, Massague J. J Biol Chem. 1997;272:17160–17165. doi: 10.1074/jbc.272.27.17160. [DOI] [PubMed] [Google Scholar]
- 7.Russel E S. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- 8.Silvers W K. The Coat Colors of Mice. New York: Springer; 1979. [Google Scholar]
- 9.Besmer P. Curr Opin Cell Biol. 1991;3:939–946. doi: 10.1016/0955-0674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 10.Manova K, Huang E J, Angeles M, Deleon V, Sanchez S, Pronovost S M, Besmer P, Bachvarova R F. Dev Biol. 1993;157:85–99. doi: 10.1006/dbio.1993.1114. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan J G, Chan D C, Leder P. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 12.Brannan C I, Lyman S D, Williams D E, Eisenman J, Anderson D M, Cosman D, Bedell M A, Jenkins N A, Copeland N G. Proc Natl Acad Sci USA. 1991;88:4671–4674. doi: 10.1073/pnas.88.11.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brannan C I, Bedell M A, Resnick J L, Eppig J J, Handel M A, Williams D E, Lyman S D, Donovan P J, Jenkins N A, Copeland N G. Genes Dev. 1992;6:1832–1842. doi: 10.1101/gad.6.10.1832. [DOI] [PubMed] [Google Scholar]
- 14.Besmer P. Colony Stimulating Factors. New York: Dekker; 1997. pp. 369–404. [Google Scholar]
- 15.Peters J, Ball S T, Loutit J F. Mouse News Lett. 1987;77:125–126. [Google Scholar]
- 16.Huang E, Nocka K, Beier D R, Chu T Y, Buck J, Lahm H W, Wellner D, Leder P, Besmer P. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 17.Martin F H, Suggs S V, Langley K E, Lu H S, Ting J, Okino K H, Morris F H, McNiece I K, Jacobsen F W, Mendiaz E A, et al. Cell. 1990;63:203–211. doi: 10.1016/0092-8674(90)90301-t. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Liu X, Jaenisch R. Proc Natl Acad Sci USA. 1994;91:2819–2823. doi: 10.1073/pnas.91.7.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau M M, Stewart C E, Liu Z, Rotwein P, Stewart C L. Genes Dev. 1994;8:2953–2963. doi: 10.1101/gad.8.24.2953. [DOI] [PubMed] [Google Scholar]
- 20.Stewart C L, Schuetze S, Vanek M, Wagner E F. EMBO J. 1987;6:383–388. doi: 10.1002/j.1460-2075.1987.tb04766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tajima Y, Huang E J, Vosseller K, Ono M, Moore M A S, Besmer P. J Exp Med. 1998;187:1451–1461. doi: 10.1084/jem.187.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muench M O, Firpo M Y, Moore M A S. Blood. 1993;81:3463–3473. [PubMed] [Google Scholar]
- 23.Till J E, McCulloch E A. Radiat Res. 1961;14:216–222. [PubMed] [Google Scholar]
- 24.Kitamura Y, Go S, Hatanaka K. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 25.Dexter T M, Allen T D, Lajtha L G. J Cell Physiol. 1977;91:335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 26.Majumdar M K, Feng L, Medlock E, Tokosz D, Williams D A. J Biol Chem. 1994;269:1237–1242. [PubMed] [Google Scholar]
- 27.Ikuta K, Weissman I L. Proc Natl Acad Sci USA. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitamura Y. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- 29.Rodewald H R, Dessing M, Dvorak A M, Galli S J. Science. 1996;271:818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein S E. Science. 1962;127:428–429. doi: 10.1126/science.137.3528.428. [DOI] [PubMed] [Google Scholar]
- 31.Harrison D E, Russel E S. Br J Haematol. 1972;22:155–168. doi: 10.1111/j.1365-2141.1972.tb08797.x. [DOI] [PubMed] [Google Scholar]
- 32.Zsebo K M, Smith K A, Hartley C A, Greenblatt M, Cooke K, Rich W, McNiece I K. Proc Natl Acad Sci USA. 1992;89:9464–9468. doi: 10.1073/pnas.89.20.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee N S, Paek I, Besmer P. J Exp Med. 1994;179:1777–1787. doi: 10.1084/jem.179.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dexter T M, Moore M A S. Nature (London) 1977;269:412–414. doi: 10.1038/269412a0. [DOI] [PubMed] [Google Scholar]
- 35.Pandiella A, Bosenberg M W, Huang E J, Besmer P, Massague J. J Biol Chem. 1992;267:24028–24033. [PubMed] [Google Scholar]
- 36.Kapur R, Majumder M, Xiao X, McAndrews-Hill M, Schindler K, Williams D A. Blood. 1998;91:879–889. [PubMed] [Google Scholar]