Abstract
Pathogenic Yersinia spp (Yersinia pestis, Yersinia pseudotuberculosis, and Yersinia enterocolitica) have evolved an exquisite method for delivering powerful effectors into cells of the host immune system where they inhibit signaling cascades and block the cells' response to infection. Understanding the molecular mechanisms of this system has provided insight into the processes of phagocytosis and inflammation.
Keywords: bacterial pathogenesis; Yersinia; Yops; translocation; type III secretion
Introduction
Yersinia pestis, the infective agent of plague, has caused social devastation on a scale unmatched by any other infectious agent. Although it is not a major public health problem now, Yersinia pestis still infects rodent populations in large endemic zones, and there are cases of human plague reported annually. After inoculation by a flea bite, Y. pestis multiplies in the lymph nodes and finally overwhelms its rodent or human host with massive growth. The closely related food-borne pathogens Yersinia pseudotuberculosis and Yersinia enterocolitica penetrate into the tissues of their host by crossing the intestinal barrier at the lymphoid follicles called Peyer's patches and then multiply in the abdominal lymphoid tissues.
Pathogenic Yersinia species share the Yop virulon, encoded on a 70-kb plasmid, which is the core of the Yersinia pathogenicity machinery designed for close combat with cells of the immune system (Fig. 1). The Yop virulon comprises both the Yop effector proteins and the proteins necessary for injecting them into host cells. The injected Yops perturb the dynamics of the cytoskeleton, disrupting phagocytosis, and block the production of proinflammatory cytokines, thus favoring the survival of the invading Yersinia.
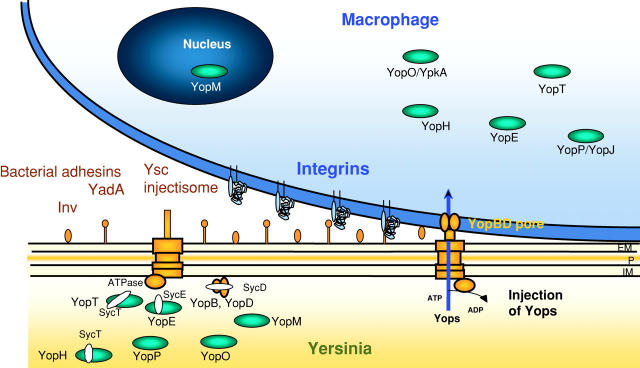
Figure 1.
Secretion of Yops by the Ysc injectisome and translocation across the target cell membrane. When Yersinia are placed at 37°C in a rich environment, the Ysc injectisome is installed and a stock of Yop proteins is synthesized. During their intrabacterial stage, some Yops are capped with their specific Syc chaperone. Upon contact with a eukaryotic target cell, the adhesins YadA or Inv interact with integrins and the bacterium docks at the cell's surface. Then, the secretion channel opens and Yops are exported. YopB and YopD form a pore in the target cell plasma membrane, and the effector Yops are translocated across this membrane into the eukaryotic cell cytosol. YopM migrates to the nucleus. EM, outer membrane; P, peptidoglycen; IM, plasma membrane.
The Yop virulon is an archetype of the type III secretion systems (TTSSs)* now identified in more than a dozen major animal or plant pathogens (Cornelis and Van Gijsegem, 2000; Buttner and Bonas, 2002). Some of these, including Salmonella enterica, have two TTSSs, both of which are essential for pathogenicity and come into play at different stages of infection. One is required for the initial interaction of S. enterica with intestinal epithelial cells, whereas the other is expressed only after S. enterica has gained access to host cells and is required for systemic infection (Galan, 2001). A homologue of the first S. enterica TTSS is found in Shigella flexneri where it also functions to promote invasion of epithelial cells (Sansonetti, 2001). In enteropathogenic Escherichia coli, a different TTSS remodels the cytoskeleton of enterocytes, leading to the formation of a pedestal of unknown function underneath the bacterium (Celli et al., 2000).
In this mini-review, I will consider the Yop virulon as a model of type III secretion but will draw on other examples when their molecular description has gone beyond that of Yersenia. The virulon consists of three basic components: the Ysc injectisome, spanning the bacterial membranes, the Yop effectors, and the Yop translocators, needed to deliver the effectors across the eukaryotic plasma membrane.
The injection of bacterial proteins into a eukaryotic cell
The Ysc injectisome.
Before contact with eukaryotic target cells, Yersinia bacteria incubated at the temperature of their host build several syringe-like organelles at their surface (Fig. 1). These organelles, called the Ysc injectisome, are protein pumps spanning the peptidoglycan layer and the two bacterial membranes topped by a stiff needle-like structure protruding outside the bacterium. The whole organelle comprises 27 proteins. The internal part contains ∼10 proteins, which have counterparts in the basal body of the flagellum, indicating that the two organelles have a common evolutionary origin. An essential part of the pump is an ATPase resembling the α and β subunits of F0F1 proton translocase. The external part of the injectisome, which spans the bacterial outer membrane, is not related to the flagellum and presumably has a different evolutionary origin. It is a homomultimeric ring-shaped structure with a central pore of ∼50 Å (Koster et al., 1997). The monomer is related to the protein that filamentous phage insert into the bacterial outer membrane to allow their extrusion. The Ysc injectisome ends with a 60–80-nm-long and 6–7-nm-wide needle formed by the polymerization of monomers of the 6-kD YscF protein that are secreted by the Ysc apparatus itself (Hoiczyk and Blobel, 2001). Like the length of the hook of the flagellum (Minamino et al., 1999), the length of the needle is genetically controlled (L. Journet, and P. Broz, personal communications; unpublished data).
More is known about the needle complex of the TTSS that is common to S. enterica and S. flexneri (Kimbrough and Miller, 2000; Kubori et al., 2000; Blocker et al., 2001). The base appears as two pairs of rings that are anchored to the inner and outer membranes of the bacterial envelope and are joined by a central rod. Not surprisingly, this architecture resembles that of the flagellar hook–basal body complex. Protruding outward from the base is the needle itself with a length of ∼8 nm and a central diameter of 2–3 nm. Enteropathogenic Escherichia coli possess a similar complex, but in this case the needle extends as a larger filament of 10–12 nm diameter and a variable length of 75–260 nm (Daniell et al., 2001).
It is generally assumed that the injectisome serves as a hollow conduit, allowing the exported proteins to travel across the two membranes and the peptidoglycan barrier in one step. If so, they have to travel at least partially unfolded because the internal diameter of the needle would not allow the passage of fully folded globular proteins. The injectisome, as described here, is sufficient for bacteria to secrete Yops into their environment but it is not sufficient for the injection of Yops into target animal cells (see below).
Regulation of Yop secretion.
Effector Yops destined for secretion through the injectisome have no classical signal sequence (Michiels et al., 1990). Nevertheless, a minimum of 15 residues at the NH2 terminus are necessary for Yop secretion (Sory et al., 1995; Anderson and Schneewind, 1997). No sequence similarity with other known proteins could be detected in this region, and systematic mutagenesis of the secretion signal (Anderson and Schneewind, 1997, 1999) led to doubts about the proteinaceous nature of this signal. Indeed, no point mutation was identified that specifically abolished secretion of YopE, YopN, or YopQ, and moreover, some frameshift mutations that completely altered the peptide sequences of the signals also failed to prevent secretion. Anderson and Schneewind (1997)(1999) concluded from these observations that the signal for secretion of these Yops could be in the 5′ end of the messenger RNA and that Yop secretion may be regulated at the level of translation. Translation of yop mRNA might be inhibited either by its own structure or as a result of its binding to other regulatory elements (Anderson and Schneewind, 1997, 1999). If this is correct, one would not expect to detect Yop proteins inside bacteria in conditions that are not permissive for secretion. However, although this may be true for some Yops (Anderson and Schneewind, 1999), it is not true for others, such as YopE (Bliska and Black, 1995), implying that regulation of secretion is at least partially posttranslational.
Kinetic studies also raise questions about the mRNA signal hypothesis, since the bacterial antiphagocytic response needs to be extremely fast. Recently, Lloyd et al. (2001) showed that secretion of YopE was not impaired after the first 11 codons of YopE were mutated to modify the mRNA but not the amino acid sequence. This suggested that it is indeed the NH2-terminus of YopE protein and not the 5′ end of yopE mRNA that serves as a targeting signal. They proposed that an amphipathic peptide helix is recognized by the injectisome. Thus, although the nature of the signal remains controversial (Ramamurthi and Schneewind, 2002), it is currently thought that the signal for initial secretion is likely to be in the protein rather than in mRNA. However, this does not preclude the possibility that secretion may be cotranslational at a later stage of infection.
Keeping the Yops fit for secretion.
Secretion of some Yops requires the presence of small cytosolic chaperones of a family called the Syc proteins (for specific Yop chaperone) (Wattiau and Cornelis, 1993; Wattiau et al., 1994). These are small acidic proteins with little or no sequence similarity between them but with a common, putative COOH-terminal amphiphilic α-helix. They bear no sequence or functional resemblance to ATP-dependent chaperones, such as heat shock protein 70 (Hsp70). They only bind only to a specific partner Yop, and in their absence Yop secretion is severely reduced, if not abolished. In general, Syc proteins are encoded by a gene located beside the gene encoding the protein they serve.
The exact function of the Syc proteins remains mysterious. Hans Wolf-Watz and colleagues (Lloyd et al., 2001) showed recently that the immediate injection of stored YopE requires the SycE chaperone, whereas long term cotranslational delivery of YopE does not. This observation suggests that SycE maintains preformed, stored YopE in a “secretion-competent” (presumably unfolded or partially folded) state. Crystal structure analyses of the chaperone-binding domain of YopE (Birtalan et al., 2002) and SptP from S. enterica (Stebbins and Galan, 2001) bound to their respective chaperones revealed striking stereochemical conservation, despite the absence of sequence similarity, indicative of a universal mode of interaction between TTSS chaperones and effectors. However, there is no agreement on the interpretation of these findings: although it is proposed that the chaperone of SptP maintains the whole protein in a partially unfolded, secretion-competent state, it appears that the SycE–YopE complex is catalytically active and thus folded. At this stage, we are therefore left with at least two hypotheses regarding the role of TTSS chaperones: either they prevent folding (Stebbins and Galan, 2001) or they provide a three-dimensional “helper” secretion signal (Birtalan et al., 2002), perhaps orchestrating secrection of effectors in a defined order as suggested by other experiments (Boyd et al., 2000; Wulff-Strobel et al., 2002). An additional intriguing question regarding these chaperones is which protein strips the chaperone off of its partner as it enters the injectisome channel?
Preparing to fire.
Once bacteria reach a temperature of 37°C, the injectisomes are assembled and a stock of intracellular Yops is synthesized. However, the secretion channel remains closed, and feedback inhibition prevents a deleterious accumulation of Yops (Cornelis et al., 1987). This retrocontrol, which involves several Yops and chaperones, is very complex and still not fully understood (Francis et al., 2002). The idea that some intrabacterial chaperones participate in the feedback regulatory mechanism is particularly appealing. Indeed, the concentration of a specific chaperone is directly linked to the rate of secretion of its partner Yop. This concept has been nicely documented in TTSS from S. enterica (Darwin and Miller, 2001) and Shigella flexneri (Mavris et al., 2002).
Upon close contact with a target cell, some injectisome channels become unplugged and secretion starts. The required contact is not achieved by the needle itself but by the interaction between bacterial outer membrane proteins called adhesins and integrins at the surface of a target cell. In the case of phagocytes, the adhesins are dispensable because phagocytic receptors provide the necessary intimate contact. Pettersson et al. (1996) provided a nice visual demonstration of the contact dependency phenomenon. By expressing luciferase under control of a yop promoter, these authors showed that active transcription of yop genes is indeed limited to bacteria that are in close contact with eukaryotic cells.
Whether lipids or specific host protein receptors are involved in regulating secretion is not known, at least in the case of Yersinia. It seems unlikely that protein receptors are involved, since Yops are injected into every cell type to which Yersinia can dock. A few mutants can secrete Yops in a contact-independent manner, indicating that some proteins constitute one or more plugs along the channel. One of these proteins is YopN, which is secreted but is not a translocator or effector protein (Forsberg et al., 1991; Boland et al., 1996; Cheng and Schneewind, 2000). So far, no interaction between YopN and any cellular surface component has been reported.
Translocation of effectors across animal cell membranes.
Purified secreted effector Yops have no cytotoxic effect on cultured cells unless they are microinjected into them (Rosqvist et al., 1991). This suggested that Yop effectors are injected into the eukaryotic cell cytosol by extracellular adhering bacteria. This hypothesis was demonstrated by confocal laser scanning microscopy (Rosqvist et al., 1994) and by following the accumulation of cAMP in eukaryotic cells infected with bacteria expressing a Yop–calmodulin-dependent adenylate cyclase hybrid (Sory and Cornelis, 1994). Translocation of the Yop effectors into the host cell requires the secretion of the translocator Yops–YopB, YopD, and LcrV (Rosqvist et al., 1994; Sory and Cornelis, 1994; Persson et al., 1995; Boland et al., 1996; Pettersson et al., 1999). YopB and YopD have hydrophobic domains, suggesting that they could act as transmembrane proteins (Rosqvist et al., 1994; Sory and Cornelis, 1994; Boland et al., 1996). In agreement with this, the membrane of cells infected with a mutant Y. enterocolitica making translocators but not effectors becomes permeable to small dyes (Neyt and Cornelis, 1999). If the cells are preloaded with a low molecular weight fluorescent marker before the infection, they release the fluorescent marker but not cytosolic proteins, indicating that there is no membrane lysis but rather insertion of a small pore (diameter 16–23 Å) into the cell plasma membrane (Neyt and Cornelis, 1999). In addition, artificial liposomes that have been incubated with Y. enterocolitica producing YopB, YopD, and LcrV contain channels detectable by electrophysiology (Tardy et al., 1999). Since these pores are only detectable in the absence of secreted Yop effectors, it is generally believed that trafficking Yop effectors obstruct the pore. Clearly, if the pore formers are not present at the tip of the needle before contact, they need to be the first Yop proteins to be secreted immediately after contact is established with a target cell. However, the hierarchy of secretion remains an open question.
The fact that the Ysc secretion apparatus ends up with a long needle protruding from the bacterium raises the question of the relationship between the needle and the pore formers. Because the injection process requires the very tight adhesin-mediated contact, the needle must either retract, break down, or pierce the target cell membrane as suggested by some EM observations (Hoiczyk and Blobel, 2001). It is possible that YopB, YopD, and LcrV destabilize the host cell membrane, enabling the needle to pierce it, driven by the pressure that gradually builds up as a result of the increased docking of the bacterium at the cell surface. Whether this happens or not, there are good arguments to believe that the Yop effectors are guided in a continuous channel from the bacterial cytosol to the target cell cytosol. Indeed, the minimal signal required for their recognition by the Ysc secretion apparatus is sufficient to ensure translocation across the eukaryotic cell plasma membrane.
Intracellular action of the Yop effectors
Inhibition of phagocytosis by YopH, YopE, YopT, and YopO/YpkA.
Once inside the eukaryotic cell, the Yop effectors inhibit signaling cascades and block the ability of the cell to respond to infection. Out of six effectors identified so far, four inhibit cytoskeleton dynamics (YopH, YopE, YopT, and YopO/YpkA). By doing so, they contribute to the strong resistance of pathogenic Yersinia to phagocytosis by macrophages (Fig. 2) (Rosqvist et al., 1990; Bliska and Black, 1995; Fallman et al., 1995; Grosdent et al., 2002) and polymorphonuclear leukocytes (Visser et al., 1995; Andersson et al., 1999; Grosdent et al., 2002). Y. enterocolitica lacking only one of these four Yops are more efficiently phagocytosed by polymorphonuclear leukocytes and by J774 cultured macrophages, indicating that there is no redundancy between the Yops but rather synergy (Grosdent et al., 2002).
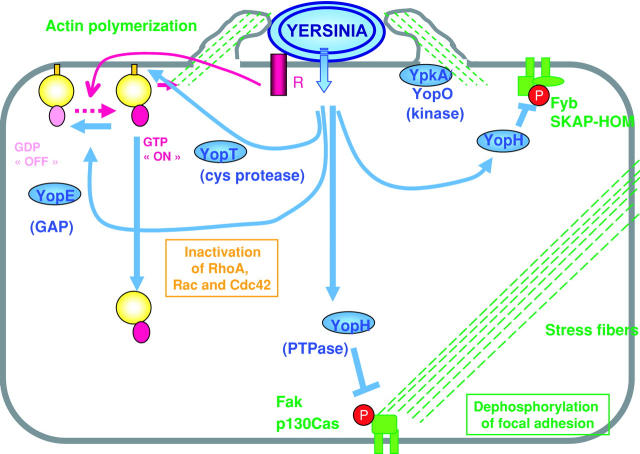
Figure 2.
Antiphagocytic action of the Yops. Upon contact with a phagocyte receptor (R), a signaling cascade is triggered and GTP-bound Rho family members (RhoA, Rac-1, Cdc42) promote actin polymerization. YopE, acting as a GAP, down-regulates Rac-1, Cdc42, and RhoA. The YopT protease cleaves the COOH terminus of RhoA, Rac, and Cdc42, liberating them from the plasma membrane. The YpkA/YopO kinase becomes autophosphorylated upon contact with actin and interacts with RhoA and Rac-1. The PTPase YopH is targeted to focal adhesions and to other protein complexes where it dephosphorylates proteins such as the focal adhesion kinase (Fak), p130Cas, and SKAP-HOM.
The effector YopH is among the most powerful phosphotyrosine phosphatases (PTPase) known (Zhang et al., 1992) and also acts to inhibit phagocytosis. When injected into J774 macrophages, YopH dephosphorylates p130Cas and disrupts focal adhesions (Hamid et al., 1999). It also dephosphorylates the Fyn-binding protein Fyb (Hamid et al., 1999) and the scaffolding protein SKAP-HOM. These proteins interact with each other and become tyrosine phosphorylated in response to macrophage adhesion (Black et al., 2000).
The action of YopH against macrophages is extremely fast. When a macrophage contacts Y. pseudotuberculosis, Fyb and SKAP-HOM become phosphorylated within 30 s. Within 2 min, YopH-mediated dephosphorylation is visible already (Andersson et al., 1996). YopH also protects Yersinia against phagocytosis by polymorphonuclear leukocytes (Visser et al., 1995; Grosdent et al., 2002). In this case, the mechanism of antiphagocytosis has not been investigated as well as in macrophages, but YopH has been shown to abrogate calcium signaling in these cells within seconds (Andersson et al., 1999).
The three other Yop effectors counteracting phagocytosis, YopE, YopT, and YpkA/YopO, act on monomeric GTPases of the Rho family, which are known to control the dynamics of the cytoskeleton. YopE (Rosqvist et al., 1990) acts as a GTPase-activating protein (GAP), switching RhoA, Rac1, and Cdc42 to the inactive state by accelerating GTP hydrolysis (Black and Bliska, 2000; Von Pawel-Rammingen et al., 2000). Rho family members are anchored to the inner side of the cell's plasma membrane by a prenyl group attached at their COOH terminus, and attachment is essential for their function (for a review see Ridley, 2001). YopT (Iriarte and Cornelis, 1998) has been shown recently to be a cysteine protease that cleaves Rho, Rac, and Cdc42 near their COOH terminus, releasing them from the membrane (Shao et al., 2002). YpkA (for Yersinia protein kinase A) is an 80-kD autophosphorylating serine-threonine kinase (Galyov et al., 1993) that shows some sequence and structural similarity to RhoA-binding kinases (Dukuzumuremyi et al., 2000). YpkA is activated by actin binding (Juris et al., 2000), and actin can also function as an in vitro substrate of the kinase. Although binding of YpkA/YopO to actin and to RhoA and Rac-1 is clearly relevant in the context of phagocytosis inhibition, the kinase target and the exact mode of action of YpkA/YopO remain unknown.
Blockade of the immune system by YopP/J and by YopH.
Yop effectors also promote the intracellular survival of Yersinia by counteracting the normal proinflammatory response of cells to infection (Fig. 3). YopJ (YopP in Y. enterocolitica) reduces the release of TNF-α (Boland and Cornelis, 1998) by macrophages and of IL-8 by epithelial (Schesser et al., 1998) and endothelial cells (Denecker et al., 2002). It also reduces the presentation of adhesion molecules ICAM-1 and E-selectin at the surface of endothelial cells (Denecker et al., 2002) and presumably reduces neutrophil recruitment to the site of infection. All of these events result from the inhibition of activation of NF-κB, a transcription factor known to be of central importance in the onset of inflammation (Boland and Cornelis, 1998; Schesser et al., 1998). YopP/YopJ inhibits IKKβ, a kinase that phosphorylates IkB, the inhibitor of NF-κB (Orth et al., 1999). By preventing phosphorylation of IκB, YopP/YopJ prevents its degradation and thus prevents the translocation of NF-κB to the nucleus.
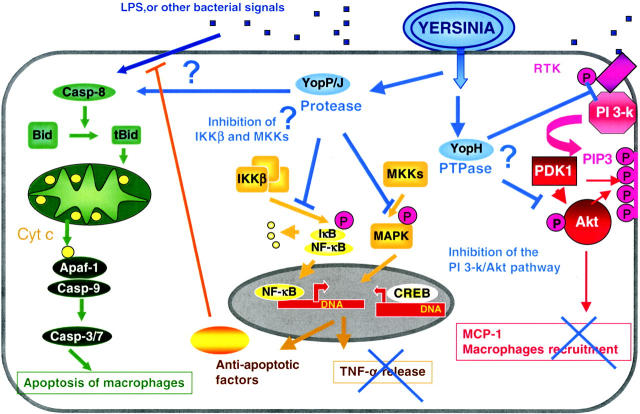
Figure 3.
Down-regulation of the inflammatory response. YopP binds and blocks the IkB kinase β (IKKβ), which inhibits the phosphorylation and degradation of IkB, the inhibitor of NF-kB. This in turn prevents the migration of the transcription factor NF-κB to the nucleus. The absence of NF-κB in the nucleus prevents transcription of antiapoptotic genes and several proinflammatory genes including the TNF-α gene. YopP also blocks the MKKs, inhibiting the activation of MAPK, which abrogates activation of CREB, another transcription factor involved in the immune response. YopP induces apoptosis in macrophages, either directly by acting upstream of Bid or indirectly by blocking the synthesis of NF-κB–dependent antiapoptotic factors. Yop H inhibits the production of monocyte chemoattractant protein 1 (MCP-1) by dephosphorylating key players in the phosphatidylinositol 3-kinase/Akt pathway.
Macrophages infected with Yersinia secreting YopP/YopJ also lack the usual activation of mitogen-activated protein kinases (MAPKs), c-jun–N-terminal kinase, p38, and extracellular signal-regulated kinase 1 and 2 (Ruckdeschel et al., 1997; Boland and Cornelis, 1998) due to the inhibition of upstream MAPK kinases (MKKs) (Orth et al., 1999). Inhibition of the MAPK pathways abrogates phosphorylation of CREB, another transcription factor involved in the immune response (Meijer et al., 2000). Last but not least, YopP/YopJ induces apoptosis of macrophages but not of other cell types (Denecker et al., 2001). It is not clear yet whether apoptosis results from a YopP-induced early cell death signal or from the YopP-induced loss of NF-κB activity known to protect cells from apoptosis (Ruckdeschel et al., 2001). Recently, it was suggested that YopJ/P is a cysteine protease, possibly a SUMO protease (SUMO are ubiquitin-like proteins that are involved in posttranslational modification) (Orth et al., 2000). However, this result is not easy to link to the previous findings that YopJ/P interacts with the MKKs and IKKβ pathways, preventing their phosphorylation.
Recent observations have shown that YopH also contributes to the down-regulation of the inflammatory response. Indeed, YopH exerts an inhibitory effect on the synthesis of the monocyte chemoattractant protein 1, a chemokine involved in the recruitment of other macrophages to the sites of infection (Sauvonnet et al., 2002a). In the same vein, Yao et al. (1999) observed that T and B cells transiently exposed to Y. pseudotuberculosis are impaired in their ability to be activated through their antigen receptors. These events result from the inhibition by YopH of early phosphorylation events (Yao et al., 1999) and of the subsequent activation of the phosphatidylinositol 3-kinase pathway (Sauvonnet et al., 2002a). Thus, YopH not only contributes to Yersinia's evasion of the innate immune response by inhibiting phagocytosis, but it also incapacitates the host adaptive immune response.
The YopM enigma.
YopM is a strongly acidic protein containing 13–20 repeats of a 19-residue leucine-rich repeated motive (Leung and Straley, 1989) folded in a crescent shape (Evdokimov et al., 2001). Although YopM is an important Yop effector in mouse infection (Leung et al., 1990; Boland et al., 1996), its function has not yet been defined yet. It has been shown to migrate to the nucleus of target cells by means of a vesicle-associated pathway (Skrzypek et al., 1998), and recent microarray experiments (Sauvonnet et al., 2002b) indicate that it influences gene transcription, leading to new working hypotheses now being tested.
Conclusion
The Ysc–Yop system is a powerful integrated weapon that combines an organelle-like secretion apparatus and a set of protein effectors. Although the former is typically prokaryotic and related to the bacterial flagellum, the latter has probably been taken up from eukaryotic cells. Indeed, Yop effectors have more sequence similarity to eukaryotic proteins than to bacterial house-keeping proteins, and they act in a physiological way on targets that do not exist in bacteria. The most remarkable element may not be how the complex nanosyringe of the injectisome has evolved but rather how the effectors have been recruited. If it is easily conceivable that eukaryotic DNA was taken up by bacteria during evolution, it is more difficult to understand how these genes have been selected for their role in TTS. Indeed, to function they needed to integrate in the bacterial genome in such a way that they received a type III secretion signal and became expressed at the right time. This may have occurred in different steps and involved different bacterial hosts.
The Ysc–Yop system makes use of half a dozen effectors and at least five of them work in a cooperative way to incapacitate phagocytosis and inflammation. Moreover, some of them, like YopH, even play a role in both events. This concentration of effort on two key events is a sign of extreme sophistication. The recent elucidation of the targets of the Yops not only leads to a better understanding of the mechanism of Yersinia infection but also provides valuable insights into the intracellular signaling response to infection.
Although our understanding of the TTS system is becoming more coherent, many intriguing questions remain. In particular, by what mechanism does contact trigger secretion? Is there a hierarchy of secretion, and how is it orchestrated? How is the pore assembled, and what is its role in relation to the needle? Among the effectors, what is the function of YopM? A complete understanding of the complex intrabacterial regulatory network that orchestrates the attack will still require much concerted effort.
Acknowledgments
I thank my postdoctoral fellows Geertrui Denecker, Mario Feldman, Laure Journet, and Jaime Mota for a constant flow of information and stimulating discussions. Due to the restricted number of references allowed, I could not cite all of the excellent work that has been done in this field. I apologize to my colleagues who may feel that their work is underevaluated.
My laboratory in Brussel was supported by the Belgian Fonds National de la Recherche Scientifique Médicale (Convention 3.4595.97) and the Direction générale de la Recherche Scientifique-Communauté Française de Belgique (Action de Recherche Concertée 94/99-172). In Switzerland, my laboratory is supported by the Swiss National Science Foundation (contract Nr 32-65393.01). The laboratory is also member of a European Union network (HPRN-CT-2000-00075).
Footnotes
Abbreviations used in this paper: GAP, GTPase-activating protein; MKK, MAPK kinase; TTSS, type III secretion system.
References
- Anderson, D.M., and O. Schneewind. 1997. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 278:1140–1143. [DOI] [PubMed] [Google Scholar]
- Anderson, D.M., and O. Schneewind. 1999. Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol. Microbiol. 31:1139–1148. [DOI] [PubMed] [Google Scholar]
- Andersson, K., N. Carballeira, K.E. Magnusson, C. Persson, O. Stendahl, H. Wolf-Watz, and M. Fallman. 1996. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol. Microbiol. 20:1057–1069. [DOI] [PubMed] [Google Scholar]
- Andersson, K., K.E. Magnusson, M. Majeed, O. Stendahl, and M. Fallman. 1999. Yersinia pseudotuberculosis-induced calcium signaling in neutrophils is blocked by the virulence effector YopH. Infect. Immun. 67:2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtalan, S.C., R.M. Phillips, and P. Ghosh. 2002. Three-dimensional secretion signals in chaperone-effector complexes of bacterial pathogens. Mol. Cell. 9:971–980. [DOI] [PubMed] [Google Scholar]
- Black, D.S., and J.B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515–527. [DOI] [PubMed] [Google Scholar]
- Black, D.S., A. Marie-Cardine, B. Schraven, and J.B. Bliska. 2000. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell Microbiol. 2:401–414. [DOI] [PubMed] [Google Scholar]
- Bliska, J.B., and D.S. Black. 1995. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun. 63:681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri “needle complex”, a part of its type III secreton. Mol. Microbiol. 39:652–663. [DOI] [PubMed] [Google Scholar]
- Boland, A., and G.R. Cornelis. 1998. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect. Immun. 66:1878–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland, A., M.P. Sory, M. Iriarte, C. Kerbourch, P. Wattiau, and G.R. Cornelis. 1996. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- Boyd, A.P., I. Lambermont, and G.R. Cornelis. 2000. Competition between the Yops of Yersinia enterocolitica for delivery into eukaryotic cells: role of the SycE chaperone binding domain of YopE. J. Bacteriol. 182:4811–4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner, D., and U. Bonas. 2002. Port of entry—the type III secretion translocon. Trends Microbiol. 10:186–192. [DOI] [PubMed] [Google Scholar]
- Celli, J., W. Deng, and B.B. Finlay. 2000. Enteropathogenic Escherichia coli (EPEC) attachment to epithelial cells: exploiting the host cell cytoskeleton from the outside. Cell Microbiol. 2:1–9. [DOI] [PubMed] [Google Scholar]
- Cheng, L.W., and O. Schneewind. 2000. Yersinia enterocolitica TyeA, an intracellular regulator of the type III machinery, is required for specific targeting of YopE, YopH, YopM, and YopN into the cytosol of eukaryotic cells. J. Bacteriol. 182:3183–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis, G.R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735–774. [DOI] [PubMed] [Google Scholar]
- Cornelis, G., J.C. Vanootegem, and C. Sluiters. 1987. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb. Pathog. 2:367–379. [DOI] [PubMed] [Google Scholar]
- Daniell, S.J., N. Takahashi, R. Wilson, D. Friedberg, I. Rosenshine, F.P. Booy, R.K. Shaw, S. Knutton, G. Frankel, and S. Aizawa. 2001. The filamentous type III secretion translocon of enteropathogenic Escherichia coli. Cell Microbiol. 3:865–871. [DOI] [PubMed] [Google Scholar]
- Darwin, K.H., and V.L. Miller. 2001. Type III secretion chaperone-dependent regulation: activation of virulence genes by SicA and InvF in Salmonella typhimurium. EMBO J. 20:1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecker, G., W. Declercq, C.A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M.P. Sory, P. Vandenabeele, and G.R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276:19706–19714. [DOI] [PubMed] [Google Scholar]
- Denecker, G., S. Tötemeyer, L.J. Mota, P. Troisfontaines, I. Lambermont, C. Youta, I. Stainier, and G.R. Cornelis. 2002. Effect of low- and high-virulence Yersinia enterocolitica strains on the inflammatory response of human umbilical vein endothelial cells. Infect Immun. 70. [DOI] [PMC free article] [PubMed]
- Dukuzumuremyi, J.M., R. Rosqvist, B. Hallberg, B. Akerstrom, H. Wolf-Watz, and K. Schesser. 2000. The Yersinia protein kinase A is a host factor inducible RhoA/Rac-binding virulence factor. J. Biol. Chem. 275:35281–35290. [DOI] [PubMed] [Google Scholar]
- Evdokimov, A.G., D.E. Anderson, K.M. Routzahn, and D.S. Waugh. 2001. Unusual molecular architecture of the Yersinia pestis cytotoxin YopM: a leucine-rich repeat protein with the shortest repeating unit. J. Mol. Biol. 312:807–821. [DOI] [PubMed] [Google Scholar]
- Fallman, M., K. Andersson, S. Hakansson, K.E. Magnusson, O. Stendahl, and H. Wolf-Watz. 1995. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect. Immun. 63:3117–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg, A., A.M. Viitanen, M. Skurnik, and H. Wolf-Watz. 1991. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol. Microbiol. 5:977–986. [DOI] [PubMed] [Google Scholar]
- Francis, M.S., H. Wolf-Watz, and A. Forsberg. 2002. Regulation of type III secretion systems. Curr. Opin. Microbiol. 5:166–172. [DOI] [PubMed] [Google Scholar]
- Galan, J.E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53–86. [DOI] [PubMed] [Google Scholar]
- Galyov, E.E., S. Hakansson, A. Forsberg, and H. Wolf-Watz. 1993. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 361:730–732. [DOI] [PubMed] [Google Scholar]
- Grosdent, N., I. Maridonneau-Parini, M.P. Sory, and G.R. Cornelis. 2002. Role of the Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70:4165–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid, N., A. Gustavsson, K. Andersson, K. McGee, C. Persson, C.E. Rudd, and M. Fallman. 1999. YopH dephosphorylates Cas and Fyn-binding protein in macrophages. Microb. Pathog. 27:231–242. [DOI] [PubMed] [Google Scholar]
- Hoiczyk, E., and G. Blobel. 2001. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl. Acad. Sci. USA. 98:4669–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriarte, M., and G.R. Cornelis. 1998. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29:915–929. [DOI] [PubMed] [Google Scholar]
- Juris, S.J., A.E. Rudolph, D. Huddler, K. Orth, and J.E. Dixon. 2000. A distinctive role for the Yersinia protein kinase: actin binding, kinase activation, and cytoskeleton disruption. Proc. Natl. Acad. Sci. USA. 97:9431–9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough, T.G., and S.I. Miller. 2000. Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc. Natl. Acad. Sci. USA. 97:11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster, M., W. Bitter, H. de Cock, A. Allaoui, G.R. Cornelis, and J. Tommassen. 1997. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol. Microbiol. 26:789–797. [DOI] [PubMed] [Google Scholar]
- Kubori, T., A. Sukhan, S.I. Aizawa, and J.E. Galan. 2000. Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc. Natl. Acad. Sci. USA. 97:10225–10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, K.Y., and S.C. Straley. 1989. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPIb alpha. J. Bacteriol. 171:4623–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, K.Y., B.S. Reisner, and S.C. Straley. 1990. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect. Immun. 58:3262–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, S.A., M. Norman, R. Rosqvist, and H. Wolf-Watz. 2001. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol. Microbiol. 39:520–531. [DOI] [PubMed] [Google Scholar]
- Mavris, M., A.L. Page, R. Tournebize, B. Demers, P. Sansonetti, and C. Parsot. 2002. Regulation of transcription by the activity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 43:1543–1553. [DOI] [PubMed] [Google Scholar]
- Meijer, L.K., K. Schesser, H. Wolf-Watz, P. Sassone-Corsi, and S. Pettersson. 2000. The bacterial protein YopJ abrogates multiple signal transduction pathways that converge on the transcription factor CREB. Cell Microbiol. 2:231–238. [DOI] [PubMed] [Google Scholar]
- Michiels, T., P. Wattiau, R. Brasseur, J.M. Ruysschaert, and G. Cornelis. 1990. Secretion of Yop proteins by Yersiniae. Infect. Immun. 58:2840–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino, T., B. Gonzalez-Pedrajo, K. Yamaguchi, S.I. Aizawa, and R.M. Macnab. 1999. FliK, the protein responsible for flagellar hook length control in Salmonella, is exported during hook assembly. Mol. Microbiol. 34:295–304. [DOI] [PubMed] [Google Scholar]
- Neyt, C., and G.R. Cornelis. 1999. Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol. 33:971–981. [DOI] [PubMed] [Google Scholar]
- Orth, K., L.E. Palmer, Z.Q. Bao, S. Stewart, A.E. Rudolph, J.B. Bliska, and J.E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science. 285:1920–1923. [DOI] [PubMed] [Google Scholar]
- Orth, K., Z. Xu, M.B. Mudgett, Z.Q. Bao, L.E. Palmer, J.B. Bliska, W.F. Mangel, B. Staskawicz, and J.E. Dixon. 2000. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science. 290:1594–1597. [DOI] [PubMed] [Google Scholar]
- Persson, C., R. Nordfelth, A. Holmstrom, S. Hakansson, R. Rosqvist, and H. Wolf-Watz. 1995. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol. Microbiol. 18:135–150. [DOI] [PubMed] [Google Scholar]
- Pettersson, J., R. Nordfelth, E. Dubinina, T. Bergman, M. Gustafsson, K.E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science. 273:1231–1233. [DOI] [PubMed] [Google Scholar]
- Pettersson, J., A. Holmstrom, J. Hill, S. Leary, E. Frithz-Lindsten, A. von Euler-Matell, E. Carlsson, R. Titball, A. Forsberg, and H. Wolf-Watz. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961–976. [DOI] [PubMed] [Google Scholar]
- Ramamurthi, K.S., and O. Schneewind. 2002. Yersinia enterocolitica type iii secretion: mutational analysis of the yopQ secretion signal. J. Bacteriol. 184:3321–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J. 2001. Rho family proteins: coordinating cell responses. Trends Cell Biol. 11:471–477. [DOI] [PubMed] [Google Scholar]
- Rosqvist, R., A. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol. Microbiol. 4:657–667. [DOI] [PubMed] [Google Scholar]
- Rosqvist, R., A. Forsberg, and H. Wolf-Watz. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59:4562–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist, R., K.E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel, K., J. Machold, A. Roggenkamp, S. Schubert, J. Pierre, R. Zumbihl, J.P. Liautard, J. Heesemann, and B. Rouot. 1997. Yersinia enterocolitica promotes deactivation of macrophage mitogen- activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. Correlation with its inhibitory effect on tumor necrosis factor-alpha production. J. Biol. Chem. 272:15920–15927. [DOI] [PubMed] [Google Scholar]
- Ruckdeschel, K., O. Mannel, K. Richter, C.A. Jacobi, K. Trulzsch, B. Rouot, and J. Heesemann. 2001. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-kappa B pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J. Immunol. 166:1823–1831. [DOI] [PubMed] [Google Scholar]
- Sansonetti, P.J. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions III. Shigellosis: from symptoms to molecular pathogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G319–G323. [DOI] [PubMed] [Google Scholar]
- Sauvonnet, N., I. Lambermont, P. Van der Bruggen, and G.R. Cornelis. 2002. a. YopH prevents monocyte chemoattractant protein 1 expression in macrophages and T-cell proliferation trough inactivation of the phosphatidylinositol 3-kinase pathway. Mol Microbiol. In press. [DOI] [PubMed] [Google Scholar]
- Sauvonnet, N., B. Pradet-Balade, J.A. Garcia-Sanz, and G.R. Cornelis. 2002. b. Regulation of mRNA expression in macrophages following Yersinia enterocolitica infection: role of different Yop effectors. J. Biol. Chem. 227:25133–25142. [DOI] [PubMed] [Google Scholar]
- Schesser, K., A.K. Spiik, J.M. Dukuzumuremyi, M.F. Neurath, S. Pettersson, and H. Wolf-Watz. 1998. The yopJ locus is required for Yersinia-mediated inhibition of NF- kappaB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 28:1067–1079. [DOI] [PubMed] [Google Scholar]
- Shao, F., P.M. Merritt, Z. Bao, R.W. Innes, and J.E. Dixon. 2002. A yersinia effector and a pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell. 109:575–588. [DOI] [PubMed] [Google Scholar]
- Skrzypek, E., C. Cowan, and S.C. Straley. 1998. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol. Microbiol. 30:1051–1065. [DOI] [PubMed] [Google Scholar]
- Sory, M.P., and G.R. Cornelis. 1994. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14:583–594. [DOI] [PubMed] [Google Scholar]
- Sory, M.P., A. Boland, I. Lambermont, and G.R. Cornelis. 1995. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. USA. 92:11998–12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins, C.E., and J.E. Galan. 2001. Maintenance of an unfolded polypeptide by a cognate chaperone in bacterial type III secretion. Nature. 414:77–81. [DOI] [PubMed] [Google Scholar]
- Tardy, F., F. Homble, C. Neyt, R. Wattiez, G.R. Cornelis, J.M. Ruysschaert, and V. Cabiaux. 1999. Yersinia enterocolitica type III secretion-translocation system: channel formation by secreted Yops. EMBO J. 18:6793–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser, L.G., A. Annema, and R. van Furth. 1995. Role of Yops in inhibition of phagocytosis and killing of opsonized Yersinia enterocolitica by human granulocytes. Infect. Immun. 63:2570–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Pawel-Rammingen, U., M.V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737–748. [DOI] [PubMed] [Google Scholar]
- Wattiau, P., and G.R. Cornelis. 1993. SycE, a chaperone-like protein of Yersinia enterocolitica involved in Ohe secretion of YopE. Mol. Microbiol. 8:123–131. [DOI] [PubMed] [Google Scholar]
- Wattiau, P., B. Bernier, P. Deslee, T. Michiels, and G.R. Cornelis. 1994. Individual chaperones required for Yop secretion by Yersinia. Proc. Natl. Acad. Sci. USA. 91:10493–10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff-Strobel, C.R., A.W. Williams, and S.C. Straley. 2002. LcrQ and SycH function together at the Ysc type III secretion system in Yersinia pestis to impose a hierarchy of secretion. Mol. Microbiol. 43:411–423. [DOI] [PubMed] [Google Scholar]
- Yao, T., J. Mecsas, J.I. Healy, S. Falkow, and Y. Chien. 1999. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J. Exp. Med. 190:1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z.Y., J.C. Clemens, H.L. Schubert, J.A. Stuckey, M.W. Fischer, D.M. Hume, M.A. Saper, and J.E. Dixon. 1992. Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J. Biol. Chem. 267:23759–23766. [PubMed] [Google Scholar]