Abstract
Listeria monocytogenes has emerged as a remarkably tractable pathogen to dissect basic aspects of cell biology, intracellular pathogenesis, and innate and acquired immunity. In order to maintain its intracellular lifestyle, L. monocytogenes has evolved a number of mechanisms to exploit host processes to grow and spread cell to cell without damaging the host cell. The pore-forming protein listeriolysin O mediates escape from host vacuoles and utilizes multiple fail-safe mechanisms to avoid causing toxicity to infected cells. Once in the cytosol, the L. monocytogenes ActA protein recruits host cell Arp2/3 complexes and enabled/vasodilator-stimulated phosphoprotein family members to mediate efficient actin-based motility, thereby propelling the bacteria into neighboring cells. Alteration in any of these processes dramatically reduces the ability of the bacteria to establish a productive infection in vivo.
Keywords: hemolysins; phagosome; virulence; macrophage; actins
Natural history and murine listeriosis model
Listeria monocytogenes is an ubiquitous, rapidly growing, Gram-positive bacterium with an unusually broad ecological niche and host range. Infection of humans and animals has been traced to contaminated foods and can lead to serious, often fatal disease. In humans, disease is most common among pregnant women, newborns, and immunocompromised individuals (Schlech, 2000).
Murine listeriosis has been studied for the past four decades to examine basic aspects of innate and acquired cellular immunity (North et al., 1997; Unanue, 1997; Finelli and Pamer, 2000; Harty et al., 2000). Critical features of the murine model are that it is relatively rapid and quantitative, either by enumeration of colony forming units in the liver and spleen or by determination of a lethal dose. During experimental listeriosis, laboratory-grown bacteria are commonly injected in the tail vein and internalized by liver and splenic macrophages. In the liver, the majority of bacteria are killed by resident macrophages. Surviving bacteria grow in macrophages and spread into hepatocytes and form foci of infection that attract the influx of neutrophils and activated macrophages and resolve as granulomas. Mice that survive a sublethal dose of live bacteria acquire enhanced resistance to subsequent challenge. Acquired immunity is entirely cell mediated and largely dependent on cytotoxic CD8+ T-cells that recognize and lyse infected cells. Antibodies play little or no role in immunity, and administration of killed bacteria does not induce protective immunity, an observation with obvious implications for the development of vaccines to intracellular pathogens.
In vitro models of listeriosis
L. monocytogenes is particularly amenable to in vitro models of infection, as it enters and grows rapidly in a wide variety of tissue culture cells. However, as a facultative pathogen, it also grows in tissue culture media. To prevent extracellular replication, the aminoglycoside antibiotic gentamicin is added to infected cells subsequent to bacterial internalization. A relatively high concentration of gentamicin is bactericidal for extracellular bacteria, but has no measurable effect on intracellular wild-type L. monocytogenes during the first 8 h of infection (Brundage et al., 1993). Intracellular L. monocytogenes grow with initial doubling times of ∼40 min, which approximates the growth rate in rich media. Importantly, in most cells, intracellular growth continues without causing damage to host cells and, as will be discussed below, mutants that prematurely kill or damage host cells are avirulent. However, whereas cells infected with wild-type bacteria eventually die sometime after 8 h postinfection, bacteria rapidly spread to neighboring cells and thus propagate the infection (Fig. 1). Intracellular growth and cell-to-cell spread can be quantitated by measuring the diameter of macroscopic plaques that form in monolayers of cells. Plaque size has proven to be a good correlate of in vivo virulence (Marquis et al., 1997).
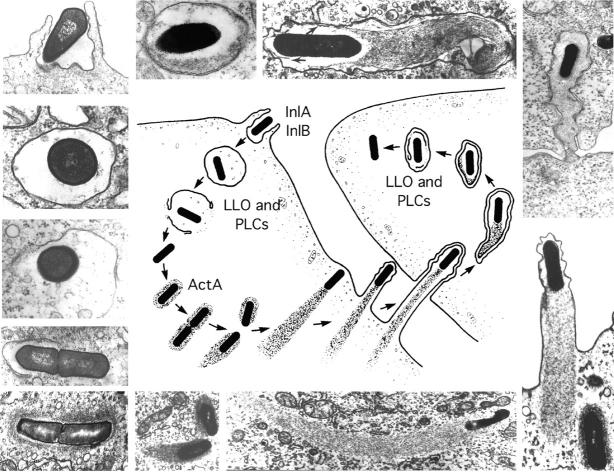
Figure 1.
Stages in the intracellular life-cycle of Listeria monocytogenes. (Center) Cartoon depicting entry, escape from a vacuole, actin nucleation, actin-based motility, and cell-to-cell spread. (Outside) Representative electron micrographs from which the cartoon was derived. LLO, PLCs, and ActA are all described in the text. The cartoon and micrographs were adapted from Tilney and Portnoy (1989).
L. monocytogenes enters almost all adherent cells, but the efficiency of uptake can vary over 10,000-fold. Macrophages and macrophage-like cell lines can internalize up to 20 bacteria per cell, whereas fibroblast cell lines internalize less than one bacterium per cell. However, significant levels of internalization by nonprofessional phagocytic cells is often mediated by one or more bacterial surface proteins, collectively named internalins, of which Internalin A and B are the best characterized (Braun and Cossart, 2000). Internalin A promotes binding and internalization by E-cadherin, whereas Internalin B binds to the Met receptor tyrosine kinase and mediates internalization via PI3-kinase activation (Cossart, 2001). The remainder of this review will focus on events that occur subsequent to internalization.
Escape from a vacuole
Upon phagocytosis by macrophages, there are a number of possible fates awaiting a bacterium (Duclos and Desjardins, 2000). In the case of L. monocytogenes, internalized bacteria are either killed or escape into the cytosol. Mutants that fail to escape from a vacuole may survive in tissue culture cell lines, but do not grow. The pore-forming protein listeriolysin O (LLO)* is largely responsible for mediating escape from the vacuole, and is consequently an essential determinant of pathogenicity (Vazquez-Boland et al., 2001). Mutants lacking LLO fail to escape from a vacuole in most cells, and synthesis of LLO by other organisms such as Bacillus subtilis is sufficient to mediate escape from a vacuole (Bielecki et al., 1990). Thus, it is clear that the role of LLO is to mediate vacuolar escape from a phagosome and from a secondary vacuole formed upon cell-to-cell spread (Gedde et al., 2000).
In addition to LLO, L. monocytogenes secretes two phospholipases C (PLCs) that contribute to escape: a phosphatidylinositol-specific PLC (PI-PLC) and a broad-spectrum PLC (PC-PLC) that is synthesized as a proenzyme activated by a secreted L. monocytogenes metalloprotease (Vazquez-Boland et al., 2001). Mutants lacking both PLCs show a marked defect in vacuolar escape, and in human epithelial cells such as HeLa cells, PC-PLC and metalloprotease mediate escape from a vacuole in the absence of LLO (Marquis et al., 1995).
The precise mechanism by which L. monocytogenes escapes from a vacuole is not clear but is consistent with the following model: upon phagocytosis, the L. monocytogenes–containing vacuole acquires markers of a maturing endosome/phagosome and acidifies to an average pH of 5.9 (Alvarez-Dominguez et al., 1997; Beauregard et al., 1997). Agents, such as bafilomycin that block acidification, inhibit vacuolar perforation and bacterial escape (Conte et al., 1996; Beauregard et al., 1997; Glomski et al., 2002). We propose that LLO insertion into the phagosomal membrane has two functions: one is to dissipate the pH gradient and thereby halt the maturation of the phagosome, and the other is act as a channel for the passage of proteins from the vacuole. The bacterial phospholipases and/or host vacuolar constituents then pass through the channel and act on the vacuole, leading to its dissolution. A direct role played by the host cell, if any, in mediating vacuolar dissolution is yet to be appreciated, although a host protease can activate PC-PLC during cell-to-cell spread (Marquis et al., 1997).
Compartmentalization of LLO activity
LLO is one of 22 members of the cholesterol-dependent family of cytolysins (CDCs) secreted by Gram-positive bacteria (Tweten et al., 2001). The best characterized of the CDCs are perfringolysin O (PFO) and streptolysin O, which are normally secreted by extracellular pathogens and presumably act on cells from outside. Replacement of LLO with PFO in L. monocytogenes results in a strain that is able to escape from a vacuole, albeit at a reduced efficiency, but that kills the infected cells from within (Jones and Portnoy, 1994). Thus, LLO is apparently unique in that it acts in a vacuole, but does not kill the host cell upon growth in the cytosol. The properties of LLO as a vacuole-specific lysin can be exploited to deliver macromolecules to the cytosol of macrophages either by incorporation of LLO into acid sensitive liposomes or by expression of recombinant proteins in Escherichia coli expressing LLO (Lee et al., 1996; Higgins et al., 1999).
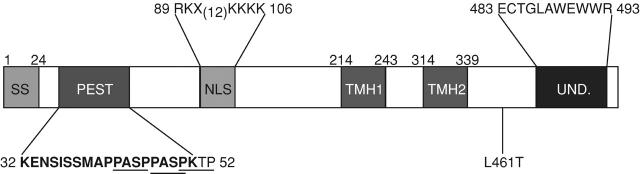
One unique feature of LLO that contributes to its lack of toxicity is a PEST-like sequence near its NH2 terminus, rich in proline, glutamate, serine, and threonine residues (Fig. 2) (Decatur and Portnoy, 2000; Lety et al., 2001). Removal of the PEST-like sequence does not affect LLO activity or vacuolar escape, but results in a strain that is extremely toxic to infected host cells and is 10,000-fold less virulent in mice. During in vitro infection, bacteria expressing the PEST-minus LLO permeabilize the host cell membrane after several bacterial generations, resulting in the influx of gentamicin and killing of the intracellular bacteria. Interestingly, most of the infected host cells apparently repair the LLO-mediated lesion and survive. In contrast, in the absence of gentamicin, intracellular PEST-minus bacteria continue to grow intracellularly and cause the rapid necrotic death of the infected host. Although it is tempting to assume that the PEST-like sequence is acting by targeting LLO for proteolytic degradation, the actual mechanism by which the PEST-like sequence acts to negate LLO toxicity is still not clear. However, the presence of three consensus mitogen-activated protein kinase sites (Fig. 2) suggests that it may be acting by interaction with host kinases. Mutation of the predicted phosphate acceptor residues results in a strain that is toxic to host cells and 100-fold less virulent (Decatur and Portnoy, 2000).
Figure 2.
Schematic representation of sequences of interest within LLO. The full length of the box represents the entire translated polypeptide of LLO expressed from the hly gene of Listeria monocytogenes. Shaded regions within the box represent stretches of amino acids of particular interest, but are not depicted to scale. The boundaries of the shaded regions are either indicated by the amino acid number directly above their depicted edge, or on either end of the detailed amino acid sequences contained within the region. MAPK consensus sites within and adjacent to the PEST-like sequence are underlined; NLS, a putative nuclear localization sequence, as predicted by the PSORTII program (http://psort.nibb.ac.jp/); PEST, the PEST-like region defined by PESTFind (http://at.embnet.org/embnet/tools/bio/PESTfind/), is represented as bold characters; SS, the signal sequence cleaved from the propeptide upon secretion; TMH1 and TMH2, transmembrane helixes discovered in PFO that pass through the target membrane upon pore formation (Tweten et al., 2001); UND, the conserved undecapeptide found in all cholesterol-dependent cytolysins. The mutation L461T increases hemolytic activity 10-fold at neutral pH (Glomski et al., 2002).
LLO is unique among the CDCs in that it has a pronounced acidic pH optimum. Mutation of a single LLO residue (L461T) results in an increase in LLO activity at neutral pH and leads to a 100-fold loss in virulence (Glomski et al., 2002). This mutation does not affect escape of L. monocytogenes from a vacuole, but causes premature permeabilization of infected host cells after about five bacterial generations. Thus, the acidic pH optimum of LLO, similar to the PEST-like sequence, restricts LLO activity to a vacuolar compartment.
Surprisingly, transcription and synthesis of LLO continues in the host cytosol (Bubert et al., 1999; Moors et al., 1999). However, a number of other potential mechanisms may be in place to prevent toxicity to the infected host. For example, PC-PLC secretion is acid dependent and occurs preferentially in host vacuoles (Marquis and Hager, 2000), although this is yet to be documented for LLO. Another intriguing observation is that LLO contains a consensus dipartite nuclear localization signal (Fig. 2). Although it has not been examined experimentally, shuttling of LLO to the nucleus, which has very little membrane cholesterol, would be yet another mechanism to prevent LLO from breaching the cytoplasmic membrane. Clearly, a molecule as potentially toxic as LLO has multiple regulatory mechanisms to prevent damage to host cells.
Growth in the cytosol
Intracellular pathogens can be broadly divided into those that grow within a modified vacuole of the host cell (Duclos and Desjardins, 2000) and those like L. monocytogenes that grow in the host cytosol. There is compelling evidence to suggest that the cytosol is a favorable environment for bacterial growth: Bacillus subtilis expressing LLO or E. coli precoated with LLO can escape from a vacuole and grow in the cytosol of tissue culture cell lines (Bielecki et al., 1990; Monack and Theriot, 2001). However, it was recently shown that an L. monocytogenes hexose phosphate transporter was virulence-regulated and necessary for growth on glucose-6-phosphate and optimal cytosolic growth (Chico-Calero et al., 2002). Thus, although nonpathogens can grow in the cytosol under some circumstances, intracytosolic bacteria have clearly evolved specific mechanisms to enhance intracellular growth. In addition, as discussed below for L. monocytogenes, most intracytosolic bacterial pathogens have evolved mechanisms of actin-based motility to spread from cell to cell (Goldberg, 2001). For a more detailed discussion of factors affecting cytosolic growth, see O'Riordan and Portnoy (2002).
Actin-based motility
Shortly after entry into the mammalian cytosol, L. monocytogenes induces the polymerization of host actin filaments and uses the force generated by actin polymerization to move, first intracellularly and then from cell to cell (Fig. 1; for video images, see: http://cmgm.stanford.edu/theriot/movies.htm). Remarkably, a single bacterial protein, ActA, is responsible for mediating actin nucleation, actin-based motility, and is necessary for pathogenicity. ActA-minus mutants escape normally from vacuoles, but grow in the host cytosol as microcolonies and do not spread cell to cell or form plaques in tissue culture cell monolayers. The ActA protein provides multiple binding sites for host cytoskeletal components, thereby acting as a scaffold to assemble the cell's actin polymerization machinery (Cameron et al., 2000). The NH2 terminus of ActA binds to monomeric actin and acts as a constitutively active nucleation promoting factor by stimulating the intrinsic actin nucleation activity of the Arp2/3 complex by a mechanism similar to that proposed for host WASP family proteins (Welch et al., 1998; Pistor et al., 2000; Skoble et al., 2000; Boujemaa-Paterski et al., 2001; Zalevsky et al., 2001). Although ActA and WASP family proteins share little overall amino acid similarity, they do share a region that binds the Arp2/3 complex, referred to as the connector sequence that spans 15 residues containing the core sequence KKRRK. Mutations in this site confer an ActA-null phenotype in cells (Pistor et al., 2000; Skoble et al., 2000; Boujemaa-Paterski et al., 2001; Lauer et al., 2001). ActA and WASP family proteins also share similarity in a short acidic stretch and both contain an actin binding region (WH2 region in WASP family proteins) that is unrelated.
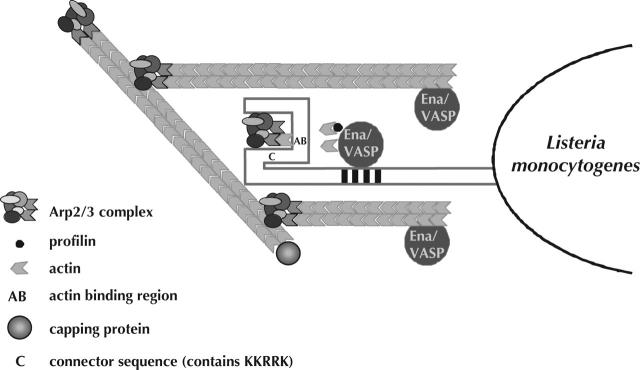
The central region of ActA consists of three or four proline-rich repeats (consensus DFPPPPTDEEL) that bind the enabled/vasodilator-stimulated phosphoprotein (Ena/VASP) homology (EVH)1 domain of Ena/VASP family members with an affinity higher than that of all known EVH1 ligands such as vinculin and zyxin (Prehoda et al., 1999). Ena/VASP family members are important for many actin-based processes including cell motility, platelet shape change, axon guidance, and T-cell activation (Renfranz and Beckerle, 2002); however, a clear picture of their mechanistic role has yet to emerge. L. monocytogenes expressing ActA lacking Ena/VASP binding sites move more slowly in cells (Lasa et al., 1995; Smith et al., 1996), and wild-type L. monocytogenes fail to move at all in cells microinjected with an excess of EVH1-binding peptides (Southwick and Purich, 1994). Paradoxically, fibroblasts lacking Ena/VASP proteins crawl faster than cells expressing Mena (mouse Ena) (Bear et al., 2000). Ena/VASP proteins contain a proline-rich domain that binds profilin and an EVH2 domain that interacts with F-actin. Profilin specifically binds ATP monomeric actin and promotes actin polymerization from the barbed end of actin filaments (Pantaloni et al., 2001). Cells expressing Mena/VASP derivatives lacking profilin binding exhibit a decreased rate of L. monocytogenes motility but exhibit normal rates of cellular motility. In contrast, cells expressing Mena/VASP derivatives lacking the F-actin binding site move more rapidly than cells expressing wild-type Mena and support faster than wild-type rates of L. monocytogenes movement (Geese et al., 2002; Loureiro et al., 2002). However, in these cells, bacterial movement shows a significant difference in the directional persistence of actin-based motility resulting in less successful interaction with the cellular membrane and a corresponding decrease in plaque size (unpublished data). An explanation for these observations may be that Ena/VASP proteins, via their F-actin binding site, interact with the barbed ends of actin filaments and delay F-actin capping, resulting in longer, less branched actin filaments in vivo (Bear et al., 2002) and in vitro (Skoble et al., 2001). Thus, the role of Ena/VASP proteins is to augment actin polymerization and to regulate the architecture of the actin filament network (Fig. 3). A possible explanation for Ena/VASP's effect on branching may relate to a recent paper showing that the Arp2/3 complex displays a branching preference at or near the barbed end (Ichetovkin et al., 2002). Perhaps Ena/VASP's interaction with F-actin decreases the likelihood of Arp2/3-mediated branching.
Figure 3.
Model of L. monocytogenes actin-based motility. The eukaryotic Arp2/3 complex is activated by the NH2 terminus of the L. monocytogenes surface protein ActA (Welch et al., 1998). ActA, Mena/VASP proteins, and profilin may all contribute to Arp2/3-mediated nucleation by delivering monomer to the complex. At this point, actin clouds can be observed around the bacterium. Once an actin filament is nucleated, profilin may increase elongation rates. The Arp2/3 complex caps the pointed ends of filaments and eventually dissociates from ActA. Mena/VASP proteins interact with the barbed ends of growing filaments and compete with capping protein (Bear et al., 2002). Mena/VASP proteins (which bind ActA and barbed ends) remain concentrated at the bacterial-tail interface, whereas capping protein can be found throughout the tail. Rapidly growing barbed ends (protected from capping protein by Mena/VASP) are concentrated at the site necessary for force generation. At this point, the bacterium can be observed moving throughout the cytosol.
Immunological perspectives
One of the most striking results from the studies described above is that mutations in LLO that render strains prematurely cytotoxic are avirulent. Thus, just as it is commonly stated that successful pathogens have evolved to avoid killing their host, it is not beneficial for intracellular pathogens to kill their host cell. Indeed, the host has evolved innate and acquired mechanisms, including induction of apoptosis and the generation of antigen-specific cytotoxic T-cells, that result in killing of infected cells (Harty et al., 2000). Lysis by cytotoxic T-cells is an important acquired immunological effector mechanism to eliminate L. monocytogenes (Finelli and Pamer, 2000). This may provide an explanation for the observations that L. monocytogenes cytotoxic mutants are avirulent: premature killing of infected host cells may lead to extracellular bacteria that are readily killed by infiltrating phagocytes. This also provides a framework with which to understand why L. monocytogenes spreads from cell to cell; i.e., to avoid cytotoxic T-cells and phagocytes. Consistent with this notion, L. monocytogenes mutants that cannot recruit Ena/VASP peoteins show a small virulence defect in naïve mice, but show a 400-fold virulence defect in the liver of immune mice (Auerbuch et al., 2001) and (unpublished data). Presumably, efficient cell-to-cell spread is necessary during a cellular immune response. Lastly, it should be noted that an immunodominant epitope recognized by Listeria-immune cytotoxic T-cells is derived from LLO (Vijh and Pamer, 1997). Perhaps the fail-safe properties of LLO that are necessary for pathogenesis, such as rapid degradation in the cytosol, also lead to entry of LLO into the host's cytosolic pathway of antigen processing and presentation. Thus, LLO lies at the interface of bacterial pathogenesis and cell-mediated immunity.
Acknowledgments
We thank Laurel Lenz, Mary O'Riordan, and Matt Welch for critical reading of the review, and Darren Higgins for Fig. 1.
This work was supported by National Institutes of Health grants AI27655 and AI29619 (D.A. Portnoy).
Footnotes
Abbreviations used in this paper: CDC, cholesterol-dependent cytolysin; EVH, Ena/VASP homology; LLO, listeriolysin O; PFO, perfringolysin O; PLC, phospholipase C; Mena, mouse Ena; Ena/VASP, enabled/vasodilator-stimulated phosphoprotein.
References
- Alvarez-Dominguez, C., R. Roberts, and P.D. Stahl. 1997. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J. Cell Sci. 110:731–743. [DOI] [PubMed] [Google Scholar]
- Auerbuch, V., L. Lenz, and D. Portnoy. 2001. Developmment of a competitive index assay to evaluate the virulence of Listeria monocytogenes actA mutants during primary and secondary infection of mice. Infect. Immun. 69:5953–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear, J.E., J.J. Loureiro, I. Libova, R. Fassler, J. Wehland, and F.B. Gertler. 2000. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 101:717–728. [DOI] [PubMed] [Google Scholar]
- Bear, J.E., T.M. Svitkina, M. Krause, D.A. Schafer, J.J. Loureiro, G.A. Strasser, I.V. Maly, O.Y. Chaga, J.A. Cooper, G.G. Borisy, and F.B. Gertler. 2002. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 109:509–521. [DOI] [PubMed] [Google Scholar]
- Beauregard, K.E., K. Lee, R.J. Collier, and J.A. Swanson. 1997. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J. Exp. Med. 186:1159–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki, J., P. Youngman, P. Connelly, and D.A. Portnoy. 1990. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 345:175–176. [DOI] [PubMed] [Google Scholar]
- Boujemaa-Paterski, R., E. Gouin, G. Hansen, S. Samarin, C. Le Clainche, D. Didry, P. Dehoux, P. Cossart, C. Kocks, M.F. Carlier, and D. Pantaloni. 2001. Listeria protein ActA mimics WASp family proteins: it activates filament barbed end branching by Arp2/3 complex. Biochemistry. 40:11390–11404. [DOI] [PubMed] [Google Scholar]
- Braun, L., and P. Cossart. 2000. Interactions between Listeria monocytogenes and host mammalian cells. Microbes Infect. 2:803–811. [DOI] [PubMed] [Google Scholar]
- Brundage, R.A., G.A. Smith, A. Camilli, J.A. Theriot, and D.A. Portnoy. 1993. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc. Natl. Acad. Sci. USA. 90:11890–11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubert, A., Z. Sokolovic, S.K. Chun, L. Papatheodorou, A. Simm, and W. Goebel. 1999. Differential expression of Listeria monocytogenes virulence genes in mammalian host cells. Mol. Gen. Genet. 261:323–336. [DOI] [PubMed] [Google Scholar]
- Cameron, L.A., P.A. Giardini, F.S. Soo, and J.A. Theriot. 2000. Secrets of actin-based motility revealed by a bacterial pathogen. Nat. Rev. Mol. Cell Biol. 1:110–119. [DOI] [PubMed] [Google Scholar]
- Chico-Calero, I., M. Suarez, B. Gonzalez-Zorn, M. Scortti, J. Slaghuis, W. Goebel, and J.A. Vazquez-Boland. 2002. Hpt, a bacterial homolog of the microsomal glucose-6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proc. Natl. Acad. Sci. USA. 99:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte, M.P., G. Petrone, C. Longhi, P. Valenti, R. Morelli, F. Superti, and L. Seganti. 1996. The effects of inhibitors of vacuolar acidification on the release of Listeria monocytogenes from phagosomes of Caco-2 cells. J. Med. Microbiol. 44:418–424. [DOI] [PubMed] [Google Scholar]
- Cossart, P. 2001. Met, the HGF-SF receptor: another receptor for Listeria monocytogenes. Trends Microbiol. 9:105–107. [DOI] [PubMed] [Google Scholar]
- Decatur, A.L., and D.A. Portnoy. 2000. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science. 290:992–995. [DOI] [PubMed] [Google Scholar]
- Duclos, S., and M. Desjardins. 2000. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell Microbiol. 2:365–377. [DOI] [PubMed] [Google Scholar]
- Finelli, A. and E.G. Pamer. 2000. Immune and inflammatory responses to Listeria monocytogenes infection. In Gram-Positive Pathogens. V.A. Fischetti, editor. American Society for Microbiology Press, Washington, DC. 480–487.
- Gedde, M.M., D.E. Higgins, L.G. Tilney, and D.A. Portnoy. 2000. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect. Immun. 68:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geese, M., J.J. Loureiro, J.E. Bear, J. Wehland, G. F.B., and A.S. Sechi. 2002. The contribution of Ena/VASP proteins to the intracellular motility of Listeria requires phosphorylation and the proline-rich core but not F-actin binding or multimerisation. Mol. Biol. Cell. 13:2383–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomski, I.J., M.M. Gedde, A.W. Tsang, J.A. Swanson, and D.A. Portnoy. 2002. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 156:1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, M.B. 2001. Actin-based motility of intracellular microbial pathogens. Microbiol. Mol. Biol. Rev. 65:595–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty, J.T., A.R. Tvinnereim, and D.W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275–308. [DOI] [PubMed] [Google Scholar]
- Higgins, D.E., N. Shastri, and D.A. Portnoy. 1999. Delivery of protein to the cytosol of macrophages using Escherichia coli K-12. Mol. Microbiol. 31:1631–1641. [DOI] [PubMed] [Google Scholar]
- Ichetovkin, I., W. Grant, and J. Condeelis. 2002. Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp2/3 complex. Curr. Biol. 12:79–84. [DOI] [PubMed] [Google Scholar]
- Jones, S., and D.A. Portnoy. 1994. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect. Immun. 62:5608–5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa, I., V. David, E. Gouin, J.B. Marchand, and P. Cossart. 1995. The amino-terminal part of ActA is critical for the actin-based motility of Listeria monocytogenes; the central proline-rich region acts as a stimulator. Mol. Microbiol. 18:425–436. [DOI] [PubMed] [Google Scholar]
- Lauer, P., J.A. Theriot, J. Skoble, M.D. Welch, and D.A. Portnoy. 2001. Systematic mutational analysis of the amino-terminal domain of the Listeria monocytogenes ActA protein reveals novel functions in actin-based motility. Mol. Microbiol. 42:1163–1177. [DOI] [PubMed] [Google Scholar]
- Lee, K.D., O.Y.K. Oh, D.A. Portnoy, and J.A. Swanson. 1996. Delivery of macromolecules into cytosol using liposomes containing hemolysin from Listeria monocytogenes. J. Biol. Chem. 271:7249–7252. [PubMed] [Google Scholar]
- Lety, M.A., C. Frehel, I. Dubail, J.L. Beretti, S. Kayal, P. Berche, and A. Charbit. 2001. Identification of a PEST-like motif in listeriolysin O required for phagosomal escape and for virulence in Listeria monocytogenes. Mol. Microbiol. 39:1124–1139. [DOI] [PubMed] [Google Scholar]
- Loureiro, J.J., D.A. Rubinson, J.E. Bear, G.A. Baltus, A.V. Kwiatkowski, and F.B. Gertler. 2002. Critical roles of phosphorylation and actin binding motifs, but not the central proline-rich region, for Ena/VASP function during cell migration. Mol. Biol. Cell. 13:2533–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis, H., and E.J. Hager. 2000. pH-regulated activation and release of a bacteria-associated phospholipase C during intracellular infection by Listeria monocytogenes. Mol. Microbiol. 35:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis, H., V. Doshi, and D.A. Portnoy. 1995. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect. Immun. 63:4531–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis, H., H. Goldfine, and D.A. Portnoy. 1997. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J. Cell Biol. 137:1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack, D.M., and J.A. Theriot. 2001. Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell Microbiol. 3:633–647. [DOI] [PubMed] [Google Scholar]
- Moors, M.A., B. Levitt, P. Youngman, and D.A. Portnoy. 1999. Expression of listeriolysin O and ActA by intracellular and extracellular Listeria monocytogenes Infect. Immun. 45:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North, R.J., P.L. Dunn, and J.W. Conlan. 1997. Murine listeriosis as a model of antimicrobial defense. Immunol. Rev. 158:27–36. [DOI] [PubMed] [Google Scholar]
- O'Riordan, M., and D.A. Portnoy. 2002. The host cytosol: Front-line or home front. Trends Microbiol. In press. [DOI] [PubMed] [Google Scholar]
- Pantaloni, D., C. Le Clainche, and M.F. Carlier. 2001. Mechanism of actin-based motility. Science. 292:1502–1506. [DOI] [PubMed] [Google Scholar]
- Pistor, S., L. Grobe, A.S. Sechi, E. Domann, B. Gerstel, L.M. Machesky, T. Chakraborty, and J. Wehland. 2000. Mutations of arginine residues within the 146-KKRRK-150 motif of the ActA protein of Listeria monocytogenes abolish intracellular motility by interfering with the recruitment of the Arp2/3 complex. J. Cell Sci. 113:3277–3287. [DOI] [PubMed] [Google Scholar]
- Prehoda, K.E., D.J. Lee, and W.A. Lim. 1999. Structure of the enabled VASP homology 1 domain-peptide complex: a key component in the spatial control of actin assembly. Cell. 97:471–480. [DOI] [PubMed] [Google Scholar]
- Renfranz, P.J., and M.C. Beckerle. 2002. Doing (F/L)PPPPs: EVH1 domains and their proline-rich partners in cell polarity and migration. Curr. Opin. Cell Biol. 14:88–103. [DOI] [PubMed] [Google Scholar]
- Schlech, W.F., III. 2000. Epidemiology and clinical manifestations of Listeria monocytogenes infection. In Gram-Positive Pathogens. V.A. Fischetti, editor. American Society for Microbiology Press, Washington, D.C. 473–479.
- Skoble, J., D.A. Portnoy, and M.D. Welch. 2000. Three regions within ActA promote Arp2/3 complex-mediated actin nucleation and Listeria monocytogenes motility. J. Cell Biol. 150:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoble, J., V. Auerbuch, E.D. Goley, M.D. Welch, and D.A. Portnoy. 2001. Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility. J. Cell Biol. 155:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G.A., J.A. Theriot, and D.A. Portnoy. 1996. The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin-based motility, the percentage of moving bacteria, and the localization of vasodilator-stimulated phosphoprotein and profilin. J. Cell Biol. 135:647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick, F.S., and D.L. Purich. 1994. Arrest of Listeria movement in host cells by a bacterial ActA analogue: implications for actin-based motility. Proc. Natl. Acad. Sci. USA. 91:5168–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L.G., and D.A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten, R.K., M.W. Parker, and A.E. Johnson. 2001. The cholesterol-dependent cytolysins. Curr. Top. Microbiol. Immunol. 257:15–33. [DOI] [PubMed] [Google Scholar]
- Unanue, E.R. 1997. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 158:11–25. [DOI] [PubMed] [Google Scholar]
- Vazquez-Boland, J.A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijh, S., and E.G. Pamer. 1997. Immunodominant and subdominant CTL responses to Listeria monocytogenes infection. J. Immunol. 158:3366–3371. [PubMed] [Google Scholar]
- Welch, M.D., J. Rosenblatt, J. Skoble, D.A. Portnoy, and T.J. Mitchison. 1998. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science. 281:105–108. [DOI] [PubMed] [Google Scholar]
- Zalevsky, J., I. Grigorova, and R.D. Mullins. 2001. Activation of the Arp2/3 complex by the Listeria ActA protein. ActA binds two actin monomers and three subunits of the Arp2/3 complex. J. Biol. Chem. 276:3468–3475. [DOI] [PubMed] [Google Scholar]