Abstract
Phagosomes containing the bacterial pathogen Legionella pneumophila are transported to the ER after macrophage internalization. To modulate phagosome transport, Legionella use a specialized secretion system that injects bacterial proteins into eukaryotic cells. This review will focus on recent studies that have identified bacterial proteins and host processes that play a concerted role in transporting Legionella to the ER.
Keywords: phagosome lysosome fusion; ADP ribosylation factor; type IV secretion; immune evasion; intracellular replication
Legionnaires' disease
In the summer of 1976, a story topping news headlines across America was on an outbreak of pneumonia claiming the lives of many attendees of a Legionnaires' convention in Philadelphia (Fraser et al., 1977). What made this outbreak so terrifying was that scientists assigned to the case could not determine the cause of these deaths. It wasn't until 1977 that investigators from the Center for Disease Control in Atlanta announced that a new a gram-negative bacterium had been isolated from both infected patients and the air conditioning system of the hotel at which many of these individuals were staying (McDade et al., 1977). Their conclusion was that this newly isolated organism was responsible for the deadly outbreak of pneumonia that occurred at the Legionnaires' convention in Philadelphia. This new bacterium was appropriately named Legionella pneumophila. Human infections most often occur when aerosols containing Legionella are inhaled. Legionella that gain access to the lung are internalized by alveolar macrophages where they multiply intracellularly (Horwitz and Silverstein, 1980). If infected macrophages are not cleared quickly from the lung, a serious infection can result that leads to pneumonia and in severe instances death.
Legionella alter phagosome transport
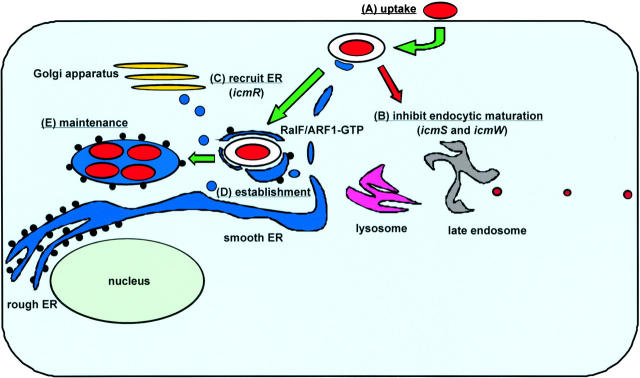
It is after Legionella are internalized by a phagocytic host that their pathogenic behavior is revealed (Fig. 1, step A). Phagosomes containing Legionella evade endocytic maturation (Horwitz, 1983b). Proteins residing in late endosomes or lysosomes are not acquired by phagosomes harboring virulent strains of Legionella (Clemens and Horwitz, 1995; Roy et al., 1998). The lumenal pH of vacuoles in which Legionella reside remains neutral (Horwitz and Maxfield, 1984; Sturgill-Koszycki and Swanson, 2000). It is not known how Legionella prevent vacuole acidification. These bacteria may block host vacuolar ATPase function either by excluding the complex or inhibiting its activity. Alternatively, Legionella may take steps to neutralize their compartments as the vacuolar ATPase pumps protons into the lumen.
Figure 1.
Transport of a phagosome-containing Legionella to the ER. (A) Legionella resides in a plasma membrane-derived phagosome after macrophage internalization. (B) The Dot/Icm secretion system sends a signal that inhibits the fusion of endocytic organelles with the phagosome-containing Legionella. The icmS and icmW products play specific roles in this event. (C) The phagosome-containing Legionella recruits ER vesicles by a process that requires the icmR product. ER vesicles are seen attached to the phagosome-containing Legionella shortly after uptake. At this time, the RalF protein is being injected into host cells by the Dot/Icm secretion system. RalF activates the host protein ARF1, and ARF1–GTP begins to accumulate on this phagosome. (D) Legionella has established a vacuole with ER vesicles covering its surface. (E) An ER-like vacuole studded with ribosomes is created by Legionella, and it is within this organelle that bacterial replication is observed. Maintenance of this vacuole does not require continuous signaling by the Dot/Icm secretion system (Coers et al., 1999), suggesting that host factors involved in ER biogenesis are providing these functions.
One of the most remarkable features observed for phagosomes containing Legionella is that 2–4 h after uptake the vacuoles in which Legionella reside look nothing like the young phagosomes containing these bacteria initially. These older compartments have ribosomes on their surface and stain positive for resident proteins of the ER (Horwitz, 1983a; Swanson and Isberg, 1995). It is within these ribosome-lined vacuoles that Legionella replicate (Katz and Hashemi, 1982).
The morphological similarities between replicative vacuoles harboring Legionella and the host ER indicate that these bacteria have devised a way to enter the ER lumen by altering phagosome transport. Once in the ER, they reside in an organelle rich in peptides, the primary carbon source for Legionella, and are safe from future lysosome degradation. Other bacterial pathogens, such as Brucella, have adopted a similar strategy and replicate within the ER, indicating that the ER is a desirable organelle for bacterial proliferation (Anderson and Cheville, 1986; Pizarro-Cerda et al., 1998). But how are these bacteria able to trick eukaryotic cells into transporting them to the ER? Recent studies have revealed bacterial proteins and host processes that are essential for transport of Legionella to the ER.
A bacterial microinjection device controls Legionella phagosome transport
Screens for intracellular growth mutants of Legionella have identified genes that play an essential role in phagosome transport. These genes are called dot (defective organelle trafficking) or icm (intracellular multiplication) (Segal et al., 1998; Vogel et al., 1998). The dot/icm products comprise a specialized secretion apparatus that can transfer proteins from the cytosol of Legionella into the cytosol of their eukaryotic hosts (Nagai et al., 2002).
To inject proteins into eukaryotic hosts, the Dot/Icm secretion system must translocate substrates across the inner and outer bacterial membranes and through a host cellular membrane. It is thought that the Dot/Icm secretion system must first assemble a pore in the host cellular membrane to serve as a conduit for protein transfer. A dot/icm-dependent activity that forms pores in host membranes has been detected during Legionella infection (Kirby et al., 1998). This pore-forming activity allows membrane-impermeable probes added extracellularly to diffuse into eukaryotic host cells during Legionella infection. Because the Dot/Icm secretion system is ancestrally related to type IV secretion systems that mediate conjugal DNA transfer between bacteria (Christie, 2001), the pore that Legionella form in the eukaryotic cell membrane is probably functioning similar to a mating channel that allows the transfer of genetic material between two cells during conjugation.
It is not currently known which Legionella proteins form the pores in host cellular membranes. One potential candidate is the DotA protein (Berger et al., 1994). The Dot/Icm system secretes DotA protein into culture supernatant during growth of Legionella in liquid broth (Nagai and Roy, 2001). The DotA protein contains seven hydrophobic transmembrane helices, and electron micrographs show that secreted DotA protein is part of a doughnut-shaped oligomer that one could envision functioning as a membrane channel (Roy and Isberg, 1997; Nagai and Roy, 2001). All mutants of Legionella that are unable to secrete DotA are defective in forming pores in host cellular membranes (Coers et al., 2000). There are proteins with amino acid similarity to DotA found in other type IV secretion systems (Christie and Vogel, 2000). These properties make DotA an attractive candidate for being a component of a pore or mating channel-like structure that is inserted into the host cell during Legionella infection. However, it cannot be stated for certain that DotA is a pore-forming molecule because initial attempts to isolate DotA protein from host cellular membranes have been unsuccessful.
The icmS and icmW genes encode interacting proteins that Legionella require for transport of their phagosomes (Coers et al., 2000). Unlike most of the other dot/icm products, proteins encoded by icmS and icmW are not required for DotA secretion (Nagai and Roy, 2001). The IcmS and IcmW proteins have properties that suggest they are functioning as chaperones that assist in the secretion of a distinct class of substrates by the Dot/Icm apparatus (Wattiau et al., 1996). Namely, they are both small proteins (<20 kD) with an acidic isoelectric point that are not themselves secreted by Legionella (Zuckman et al., 1999; Coers et al., 2000). Importantly, phagosomes containing icmS or icmW mutants of Legionella fuse with late endosomes and lysosomes, indicating that the putative secreted substrates that are guided by these chaperones are essential for blocking endocytic maturation (Fig. 1, step B).
The IcmR protein is another chaperone required for Dot/Icm secretion system function (Coers et al., 2000; Dumenil and Isberg, 2001). Approximately half of the phagosomes containing icmR mutants of Legionella manage to evade immediate endocytic maturation after macrophage uptake; however, these phagosomes do not support robust replication of Legionella and are transported to lysosomes eventually (Coers et al., 2000). Thus, the phagosome transport phenotype of an icmR mutant is quite different than that of icmS or icmW mutants, suggesting that formation of a replicative vacuole by Legionella requires evasion of endocytic maturation and a second event that requires at least one additional protein that the Dot/Icm secretion system injects into host cells (Fig. 1, step C).
Host vesicles associate rapidly with Legionella phagosomes
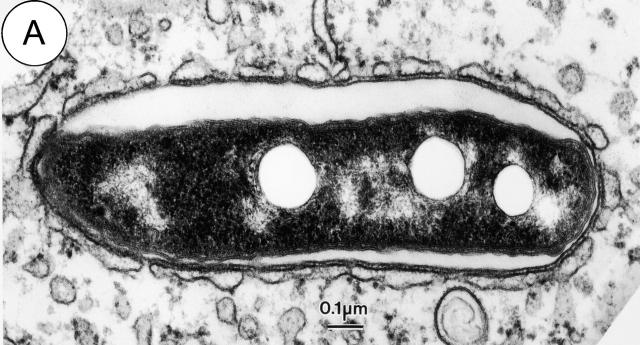
Marcus Horwitz made the initial observation in 1983 that young phagosomes containing Legionella are surrounded by smooth vesicles (Horwitz, 1983a). Recently, this phenomenon was examined more closely (Tilney et al., 2001). It was found that shortly after uptake host vesicles come in contact with Legionella phagosomes. As host vesicles make contact with phagosomes containing Legionella they begin to flatten along the surface of the phagosome membrane (Fig. 2 A). Periodic threads that bridge the space between the phagosome and host vesicle membranes are clearly visible by EM, suggesting that a receptor-mediated “zippering” event may be driving this intimate interaction.
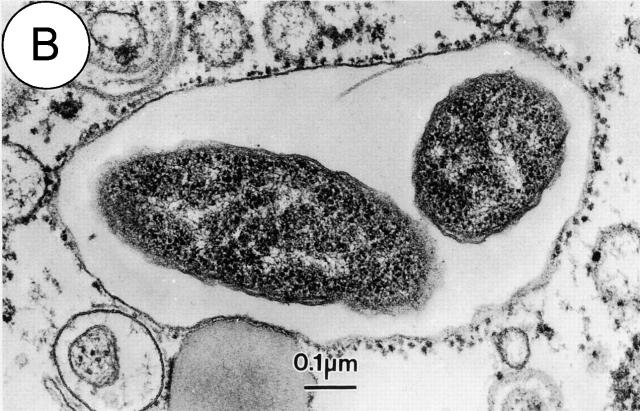
Figure 2.
The Legionella-containing phagosome is converted from a vacuole surrounded by host vesicles to a replicative organelle that resembles the ER. Electron micrographs of phagosomes containing Legionella show host vesicles intimately attached to the surface of phagosomes 15 min after bacterial internalization (A). A portion of a replicative organelle identified 19 h after infection shows that the cytoplasmic surface of the vacuole membrane is at this time covered with ribosomes (B).
Host vesicles begin to associate with the basal surface of phagosomes containing Legionella within 5 min of uptake, and >90% of the phagosome surface is covered by host vesicles within 15 min (Fig. 1, step D). Initially, the host vesicles have a lipid bilayer that is noticeably thinner than that of the plasma membrane-derived phagosome harboring Legionella. However, the membrane surrounding wild-type Legionella rapidly thins and becomes the same thickness as the bilayer of the host vesicles that have become attached. This membrane conversion event suggests that the interaction with host vesicles results in a flux of either lipids or proteins from the phagosome membrane surrounding Legionella.
After 4 h, as Horwitz reported initially, there are fewer host vesicles associated with vacuoles containing Legionella. Instead, there are ribosomes attached to the exposed regions of the vacuole membrane where host vesicles were found earlier (Fig. 2 B). Thus, at this time Legionella have established residence in an ER-like organelle.
Host vesicles do not attach to phagosomes containing most dot/icm mutants of Legionella, indicating that host vesicle attachment requires a factor secreted by the Dot/Icm apparatus. Interestingly, phagosomes containing icmS or icmW mutants of Legionella still recruit host vesicles and their phagosomal membrane thins. The fact that these phagosomes fuse with late endosomes and lysosomes indicates that the ability of Legionella to evade immediate endocytic maturation cannot be explained simply by attached host vesicles remodeling these phagosomes or constructing a barricade that prevents endocytic maturation.
In support of the hypothesis that evasion of lysosome fusion by Legionella occurs independently of host vesicle recruitment, it was found that phagosomes containing icmR mutants that have evaded endocytic maturation remain naked, and due to the absence of host vesicles attached to their surface, the phagosomal membrane does not thin. These data indicate that the protein(s) injected into host cells that mediate evasion of endocytic maturation are distinct from those that recruit host vesicles. Thus, the delay in endocytic maturation observed for phagosomes containing Legionella is an event that buys precious time necessary for these bacteria to remodel their phagosome by a slower process that involves subverting the transport of host vesicles.
Legionella inject a protein into host cells that activates ADP ribosylation factor
Ribosomes can be seen on many of the host vesicles attached to phagosomes containing Legionella. These vesicles also stain positive for protein disulfide isomerase (unpublished data), which is a soluble protein that resides primarily in the ER lumen but can also be found in vesicles that cycle between the ER and Golgi (Ferrari and Soling, 1999). Thus, the vesicles being recruited to phagosomes containing Legionella are ER derived.
In addition to ER vesicles, a recent study found that the host protein ADP ribosylation factor (ARF)*1 is recruited to phagosomes containing Legionella (Nagai et al., 2002). ARF proteins are highly conserved small GTP-binding proteins that regulate several membrane transport processes in eukaryotic cells (Donaldson and Jackson, 2000). ARF1 stimulates the formation of COPI-coated vesicles, which are essential for vesicular transport of cargo proteins between the ER and Golgi (Barlowe, 2000). Although a role for ARF1 in the transport of endocytic vesicles has been described (Gu and Gruenberg, 2000), phagosomes containing dot/icm mutants of Legionella do not stain positive for ARF1 as they are transported through the endocytic pathway. Thus, ARF1 is observed only on Legionella phagosomes in transit to the ER, and recruitment of ARF1 requires the Dot/Icm secretion system.
From these data it was predicted that wild-type Legionella may encode an ARF1-interacting protein that is injected into macrophages by the Dot/Icm secretion system. This hypothesis was correct. Analysis of the Legionella genome revealed a protein that has a 200 amino acid region homologous to the catalytic domain found in all eukaryotic proteins that function as guanine nucleotide exchange factors (GEFs) for ARF (Nagai et al., 2002). This conserved catalytic region is known as the Sec7 homology domain (Jackson and Casanova, 2000). Like eukaryotic proteins that contain Sec7 homology domains, this Legionella protein activates ARF in vitro by stimulating the exchange of GDP for GTP.
This Legionella ARF–GEF was called RalF because it was found to be required for the recruitment of ARF to the Legionella phagosome. As predicted, the RalF protein is injected into macrophages by a process requiring the Dot/Icm secretion system. Even though Legionella ralF mutants are transported in phagosomes that do not stain positive for ARF1, these phagosomes still evade fusion with lysosomes and mature into vacuoles that support intracellular replication. Thus, the RalF protein is not essential for transport of Legionella to the ER.
So why would Legionella inject an exchange factor for ARF into eukaryotic hosts during infection? It is likely that the function of RalF is to stimulate normal host processes that Legionella subvert during biogenesis of an ER vacuole. Accordingly, RalF may play a role in the creation or transport of ER vesicles that associate with Legionella phagosomes shortly after uptake. The reason RalF function is not required by Legionella during infection of host cells cultured in the laboratory may be because ER vesicles that transport cargo to the Golgi are created constitutively in healthy cells growing in nutritionally rich medium. However, in nature Legionella is likely to encounter protozoan hosts that are conserving energy and are less active metabolically. Under these conditions, host ARF–GEFs are likely down-regulated, reducing vesicular transport between the ER and Golgi. By injecting their own ARF exchange factor during infection, Legionella may be able to stimulate the creation of the ER–Golgi transport vesicles these bacteria require to remodel their phagosomes.
Future directions
In conclusion, these data provide evidence that in addition to RalF Legionella must be injecting additional proteins into macrophages that bind ER vesicles and promote phagosome remodeling. One possibility is that these undiscovered bacterial proteins function similar to SNARE proteins that mediate tethering and fusion of ER vesicles with downstream secretory organelles such as the ER Golgi intermediate compartment or the cis-Golgi (Guo et al., 2000). Curiously, the studies in which ER vesicles were found attached to Legionella phagosomes also showed ER vesicles attached to the plasma membrane of uninfected macrophages by periodic threads similar to those seen at the ER–Legionella phagosome interface (Tilney et al., 2001). Perhaps what these data are telling us is that Legionella are subverting a host pathway to the ER that is not well understood or appreciated—a road less traveled. There have been recent studies suggesting that ER membrane may contribute to the formation of vacuoles formed during phagocytosis (Garin et al., 2001; Muller-Taubenberger et al., 2001). Thus, investigations on Legionella phagosome transport could provide important details on a process by which plasma membrane-derived vesicles can be transported to the ER. Regardless of the mechanism, one thing is certain: determining the function of proteins injected by the Dot/Icm secretion system will further our knowledge on normal vesicular transport pathways that lead to the ER.
Footnotes
Abbreviations used in this paper: ARF, ADP ribosylation factor; GEF, guanine nucleotide exchange factor.
References
- Anderson, T.D., and N.F. Cheville. 1986. Ultrastructural morphometric analysis of Brucella abortus-infected trophoblasts in experimental placentitis. Bacterial replication occurs in rough endoplasmic reticulum. Am. J. Pathol. 124:226–237. [PMC free article] [PubMed] [Google Scholar]
- Barlowe, C. 2000. Traffic COPs of the early secretory pathway. Traffic. 1:371–377. [DOI] [PubMed] [Google Scholar]
- Berger, K.H., J.J. Merriam, and R.R. Isberg. 1994. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol. Microbiol. 14:809–822. [DOI] [PubMed] [Google Scholar]
- Christie, P.J. 2001. Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol. Microbiol. 40:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie, P.J., and J.P. Vogel. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, D.L., and M.A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coers, J., C. Monahan, and C.R. Roy. 1999. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat. Cell Biol. 1:451–453. [DOI] [PubMed] [Google Scholar]
- Coers, J., J.C. Kagan, M. Matthews, H. Nagai, D.M. Zuckman, and C.R. Roy. 2000. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol. Microbiol. 38:719–736. [DOI] [PubMed] [Google Scholar]
- Donaldson, J.G., and C.L. Jackson. 2000. Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol. 12:475–482. [DOI] [PubMed] [Google Scholar]
- Dumenil, G., and R.R. Isberg. 2001. The Legionella pneumophila IcmR protein exhibits chaperone activity for IcmQ by preventing its participation in high-molecular-weight complexes. Mol. Microbiol. 40:1113–1127. [DOI] [PubMed] [Google Scholar]
- Ferrari, D.M., and H.D. Soling. 1999. The protein disulphide-isomerase family: unraveling a string of folds. Biochem. J. 339:1–10. [PMC free article] [PubMed] [Google Scholar]
- Fraser, D.W., T.R. Tsai, W. Orenstin, W.E. Parken, H.J. Beechan, R.G. Sharrar, J. Harris, G.F. Mallison, S.M. Martin, J.E. McDade, et al. 1977. Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297:1189–1197. [DOI] [PubMed] [Google Scholar]
- Garin, J., R. Diez, S. Kieffer, J.F. Dermine, S. Duclos, E. Gagnon, R. Sadoul, C. Rondeau, and M. Desjardins. 2001. The phagosome proteome: insight into phagosome functions. J. Cell Biol. 152:165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, F., and J. Gruenberg. 2000. ARF1 regulates pH-dependent COP functions in the early endocytic pathway. J. Biol. Chem. 275:8154–8160. [DOI] [PubMed] [Google Scholar]
- Guo, W., M. Sacher, J. Barrowman, S. Ferro-Novick, and P. Novick. 2000. Protein complexes in transport vesicle targeting. Trends Cell Biol. 10:251–255. [DOI] [PubMed] [Google Scholar]
- Horwitz, M.A. 1983. a. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, M.A. 1983. b. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome lysosome fusion in human monocytes. J. Exp. Med. 158:2108–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, M.A., and F.R. Maxfield. 1984. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J. Cell Biol. 99:1936–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, M.A., and S.C. Silverstein. 1980. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J. Clin. Invest. 66:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, C.L., and J.E. Casanova. 2000. Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 10:60–67. [DOI] [PubMed] [Google Scholar]
- Katz, S.M., and S. Hashemi. 1982. Electron microscopic examination of the inflammatory response to Legionella pneumophila in guinea pigs. Lab. Invest. 46:24–32. [PubMed] [Google Scholar]
- Kirby, J.E., J.P. Vogel, H.L. Andrews, and R.R. Isberg. 1998. Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol. 27:323–336. [DOI] [PubMed] [Google Scholar]
- McDade, J.E., C.C. Shepard, D.W. Fraser, T.R. Tsai, M.A. Redus, and W.R. Dowdle. 1977. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory diseases. N. Engl. J. Med. 297:1197–1203. [DOI] [PubMed] [Google Scholar]
- Muller-Taubenberger, A., A.N. Lupas, H. Li, M. Ecke, E. Simmeth, and G. Gerisch. 2001. Calreticulin and calnexin in the endoplasmic reticulum are important for phagocytosis. EMBO J. 20:6772–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, H., and C.R. Roy. 2001. The DotA protein from Legionella pneumophila is secreted by a novel process that requires the Dot/Icm transporter. EMBO J. 20:5962–5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, H., J.C. Kagan, X. Zhu, R.A. Kahn, and C.R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 295:679–682. [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerda, J., S. Meresse, R.G. Parton, G. van der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J.P. Gorvel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, C.R., and R.I. Isberg. 1997. Topology of Legionella pneumophila DotA: an inner membrane protein required for replication in macrophages. Infect. Immun. 65:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, C.R., K. Berger, and R.R. Isberg. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663–674. [DOI] [PubMed] [Google Scholar]
- Segal, G., M. Purcell, and H.A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA. 95:1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill-Koszycki, S., and M.S. Swanson. 2000. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 192:1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, M.S., and R.R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L.G., O.S. Harb, P.S. Connelly, C.G. Robinson, and C.R. Roy. 2001. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J. Cell Sci. 114:4637–4650. [DOI] [PubMed] [Google Scholar]
- Vogel, J.P., H.L. Andrews, S.K. Wong, and R.R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 279:873–876. [DOI] [PubMed] [Google Scholar]
- Wattiau, P., S. Woestyn, and G.R. Cornelis. 1996. Customized secretion chaperones in pathogenic bacteria. Mol. Microbiol. 20:255–262. [DOI] [PubMed] [Google Scholar]
- Zuckman, D.M., J.B. Hung, and C.R. Roy. 1999. Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Mol. Microbiol. 32:990–1001. [DOI] [PubMed] [Google Scholar]