Abstract
Fusion of transport vesicles with their target organelles involves specific membrane proteins, SNAREs, which form tight complexes bridging the membranes to be fused. Evidence from yeast and mammals indicates that Sec1 family proteins act as regulators of membrane fusion by binding to the target membrane SNAREs. In experiments with purified proteins, we now made the observation that the ER to Golgi core SNARE fusion complex could be assembled on syntaxin Sed5p tightly bound to the Sec1-related Sly1p. Sly1p also bound to preassembled SNARE complexes in vitro and was found to be part of a vesicular/target membrane SNARE complex immunoprecipitated from yeast cell lysates. This is in marked contrast to the exocytic SNARE assembly in neuronal cells where high affinity binding of N-Sec1/Munc-18 to syntaxin 1A precluded core SNARE fusion complex formation. We also found that the kinetics of SNARE complex formation in vitro with either Sly1p-bound or free Sed5p was not significantly different. Importantly, several presumably nonphysiological SNARE complexes easily generated with Sed5p did not form when the syntaxin was first bound to Sly1p. This indicates for the first time that a Sec1 family member contributes to the specificity of SNARE complex assembly.
Keywords: membrane fusion; Sec1 family; Sly1 protein; SNARE complex; syntaxin
Introduction
The prelude to and the execution of membrane fusion in exo- and endocytosis require sets of stage-specific core proteins, which are highly conserved from yeast to man (for review see Bennet and Scheller, 1993; Jahn and Südhof, 1999). Among them are donor and target membrane receptors, SNAREs (Söllner et al., 1993), SNARE-binding Sec1 family proteins (Hata et al., 1993; Grabowski and Gallwitz, 1997; Carr et al., 1999; Misura et al., 2000), and small GTPases of the Ypt/Rab family, which appear to act as regulators during fusion complex assembly (for review see Lazar et al., 1997; Novick and Zerial, 1997; Schimmöller et al., 1998).
SNAREs are type II membrane proteins with the membrane-spanning domain at or close to their COOH-terminal ends. Adjacent to this, they contain a conserved coiled-coil region, also termed the SNARE motif, which during SNARE pairing adopts an α-helical fold. In a fully assembled core fusion complex, four such domains donated by three or four individual SNAREs form a rigid four-helix bundle (Poirier et al., 1998; Sutton et al., 1998; Antonin et al., 2002). Instead of subdividing the membrane receptors into vesicular SNAREs (v-SNAREs) and target membrane SNAREs (t-SNAREs) (Söllner et al., 1993), it was proposed recently to classify them as Q- and R-SNAREs, depending on whether a glutamine or an arginine, two highly conserved amino acids residues, occupy the center position of the ionic layer of the four-helix bundle. It was also proposed that core fusion complexes are generally made up of three Q-SNAREs and one R-SNARE. (Fasshauer et al., 1998). Although SNARE pairing in solution is rather promiscuous (Fasshauer et al., 1999; Yang et al., 1999), yeast SNAREs inserted into liposomes were shown to interact in a remarkably specific way and to lead to membrane fusion, although at low rate (McNew et al., 2000).
It is unclear presently how within living cells SNAREs are activated, how the correct trans-SNARE interactions are established, and how membrane fusion efficiency is regulated. However, it is likely that Sec1 family proteins contribute to these events, and yeast genetic data regarding vesicular transport in exo- and endocytosis argue in favor of a positive rather than a negative regulatory function of these proteins (Novick and Scheckman, 1979; Ossig et al., 1991; Grote et al., 2000; Sato et al., 2000; Bryant and James, 2001). In mammals, deletion of the neuron-specific N-Sec1/Munc18-1 protein, which binds the target membrane SNARE syntaxin 1A with high affinity (Pevsner et al., 1994), results in a complete inhibition of neurotransmitter secretion from synaptic vesicles (Verhage et al., 2000). Since binding of N-Sec1 to syntaxin 1A in solution prevents the interaction with the synaptosome-associated SNARE SNAP-25 and the vesicular SNARE synaptobrevin, N-Sec1 has been proposed to be involved in the regulation of SNARE fusion complex assembly without being able to bind to preassembled complexes (Yang et al., 2000). Although the yeast syntaxin 1A homologue Sso1p like syntaxin 1A (Dulubova et al., 1999) adopts a closed conformation (Munson et al., 2000), yeast Sec1p does not form a complex with Sso1p in solution. Instead, Sec1p was shown to bind to preassembled exocytic SNARE complexes consisting of Sso1p, Snc1p, and Sec9p (Carr et al., 1999), and it was suggested that it positively influences the membrane fusion rate in vivo (Grote et al., 2000).
Because of the strikingly different interactions of core components of the exocytic machinery in yeast and in mammalian neurons, we inquired into the binding properties of the yeast Sly1 and Sed5 proteins with respect to SNARE complex assembly. Sly1p (Dascher et al., 1991; Ossig et al., 1991) and Sed5p (Hardwick and Pelham, 1992) are the functional counterparts of N-Sec1 and syntaxin 1A and are essential for ER to Golgi vesicular transport. N-Sec1 and Sly1p share the same high affinity binding to their corresponding syntaxins (Pevsner et al., 1994; Grabowski and Gallwitz, 1997). In spite of this, we found that in contrast to N-Sec1–associated syntaxin 1A Sly1p-bound Sed5p allows the efficient formation of the core SNARE complex with the v-SNAREs Bet1p, Bos1p, and Sec22p and that Sly1p can associate with the preassembled ER to Golgi core fusion complex. Importantly, Sed5p bound to Sly1p does not allow the assembly of certain SNARE complexes with other v-SNARE combinations that are, however, generated efficiently in the absence of Sly1p.
Results
The t-SNARE Sed5p but not the v-SNAREs Bet1p, Bos1p, or Sec22p bind to Sly1p in vitro
We have shown previously (Grabowski and Gallwitz, 1997) that the yeast Golgi syntaxin Sed5p binds to the Sec1 family member Sly1p with the same nanomolar affinity as the neuronal plasma membrane-localized syntaxin 1A to N-Sec1/Munc18 (Pevsner et al., 1994). N-Sec1 binding requires syntaxin 1A to be in a closed conformation, and this bimolecular complex does not allow the assembly with other SNAREs to form the core fusion complex (Yang et al., 2000). Therefore, we sought to investigate the Sly1p-SNARE binding properties in more detail with the goal to come closer to an understanding of Sly1 protein function in ER to Golgi transport.
To begin with, in vitro binding studies were performed with a glutathione S-transferase (GST)*–Sly1 fusion protein and NH2-terminally His6-tagged t-/v-SNAREs Sed5p, Bos1p, Sec22p, and Bet1p, each lacking the COOH-terminal membrane anchor and all being produced in Escherichia coli and purified by affinity chromatography (Fig. 1 A). Glutathione agarose beads with bound GST–Sly1p were incubated at 4°C for 2 h with the individual SNAREs, and after extensive washing with binding buffer proteins bound to the beads were separated by SDS-PAGE. As expected, the syntaxin Sed5p bound efficiently to Sly1p, whereas none of the v-SNAREs exhibited binding to the Sec1 family member in this assay (Fig. 1 B).
Figure 1.
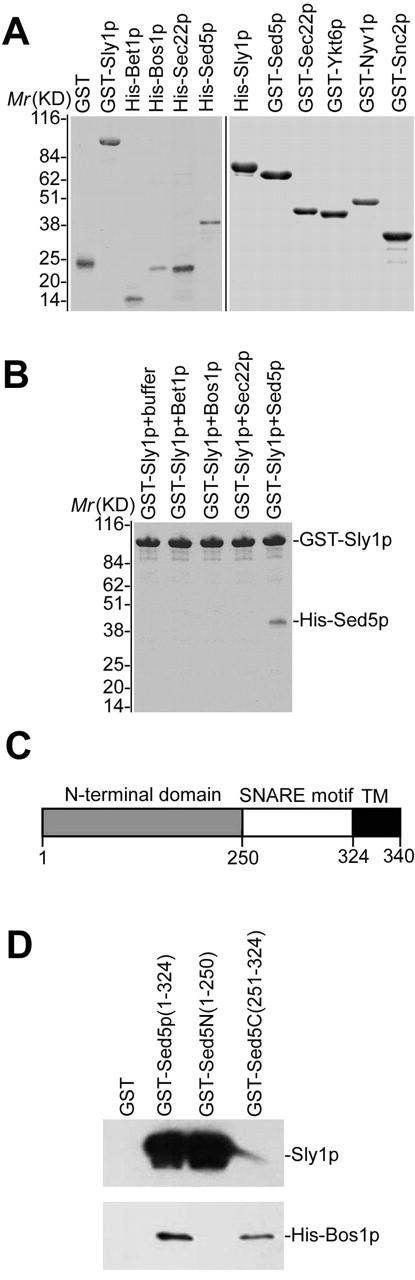
Characterization of Sly1p–Sed5p interaction. (A) Coomassie blue–stained gels showing purified GST fusion and His-tagged proteins used in various experiments. (B) Of the yeast ER to Golgi SNAREs, only Sed5p binds Sly1p. GST–Sly1p (1 μM) was incubated with individual His-tagged SNAREs lacking their membrane anchors. Proteins bound to extensively washed glutathione agarose beads were separated by SDS-PAGE and stained with Coomassie blue. (C) Schematic representation of Sed5p domain structure. (D) 0.5 μM of purified GST, GST–Sed5p (entire cytosolic region), GST–Sed5N (NH2-terminal domain), or GST–Sed5C (SNARE motif) was incubated in 100 μl buffer with Sly1p (1.0 μM) cleaved previously from purified GST–Sly1p or with His6-Bos1p (1.0 μM) lacking the transmembrane (TM). Protein complexes retained on glutathione agarose beads were separated by SDS-PAGE and identified by immunoblotting with affinity purified antibodies against Sly1p and Bos1p.
The brain plasma membrane syntaxin 1A requires the NH2-terminal variable region for high affinity binding to N-Sec1 (Kee et al., 1995). In contrast, Vam3p, the yeast t-SNARE essential for homotypic vacuole fusion (Nichols et al., 1997; Wada et al., 1997; Seals et al., 2000), appears to bind its cognate Sec1 family member Vps33p via the SNARE motif region (Dulubova et al., 2001). In a previous report, Sly1 protein binding was assigned to the NH2-terminal 78 amino acids of Sed5p (Kosodo et al., 1998). As in this study in which GST–Sly1 or MBP-Sly1 fusions were probed for binding with GST–Sed5 fusions, we performed an affinity study with untagged soluble Sly1p that was incubated with agarose bead-bound GST fusions of either the NH2-terminal domain or the SNARE motif region of Sed5p (Fig. 1 C). In accordance with the results of Kosodo et al. (1998), Sly1p bound efficiently only to the NH2-terminal region of Sed5p, whereas the v-SNARE Bos1p (Sacher et al., 1997) bound exclusively to the SNARE motif (Fig. 1 D).
Efficient SNARE complex formation in vitro on Sly1p-bound Sed5p
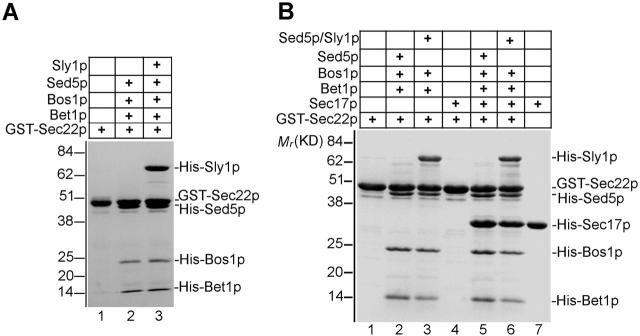
Since a bimolecular complex of Sly1p and Sed5p could be easily formed on beads, we addressed the question of whether in this complex the syntaxin Sed5p was able to associate with cognate v-SNAREs, Bos1p, Sec22p, and Bet1p. Preformed GST–Sly1p–Sed5p complex was incubated at 4°C for 17–22 h with an excess of His6-tagged v-SNAREs, the latter at equimolar ratio. As shown in Fig. 2 A, lane 2, extensively washed beads retained, in addition to GST–Sly1p and Sed5p, all three v-SNAREs at a stoichiometry of 1.0:0.7:0.8. Binding of the v-SNAREs to GST alone was not observed (Fig. 2 A, lane 1). To explore the significance of different v-SNAREs in the process of fusion complex formation in vitro, the GST–Sly1p–Sed5p subcomplex on agarose beads was incubated with each of the three v-SNAREs separately or with two of them in different combinations. Whereas a single v-SNARE did not efficiently bind to Sly1p-bound Sed5p, only Bet1p in combination with either Bos1p or Sec22p formed an apparent stoichiometric complex with Sed5p bound to Sly1p (unpublished data). These results underline the critical role of the v-SNAREs Bet1p in fusion complex formation with the t-SNARE Sed5p (Stone et al., 1997; Parlati et al., 2000), and importantly, they demonstrate that only these trimeric SNARE complexes (among other possible ones) could form with the syntaxin Sed5p tightly bound to Sly1p.
Figure 2.
Core SNARE complexes are generated on Sly1p-bound syntaxin Sed5p. (A) GST–Sly1p (15 μg) and His-tagged Sed5p (15 μg) were incubated at 4°C for 3 h, and the GST–Sly1p–Sed5p subcomplex was bound to glutathione agarose beads. Beads either bound with GST (5 μg, lane 1) or with the dimeric complex (lane 2) were incubated for 20 h with soluble v-SNAREs Bos1p (15 μg), Sec22p (15 μg), and Bet1p (5 μg), and proteins bound to beads characterized by SDS-PAGE, Coomassie blue staining, and densitometry. (B) Sly1p and Sed5p form a 1:1 complex. GST–Sed5p (8 μg) was incubated with 0, 1, 10, 20, 50, and 100 μg of His10-Sly1p at 4°C for 3 h. The GST–Sed5p–Sly1p complexes formed (lanes 1–6) were isolated by glutathione agarose beads and analyzed as in A. The stoichiometry of Sly1p to Sed5p in the complexes formed with excess of Sly1p (lanes 5 and 6) was on average 1:0.85 as determined by densitometry. (C) GST–Sed5p (10 μg, lane 2) and the heterodimeric complex formed from GST–Sed5p (10 μg) and His10-Sly1p (50 μg) bound to beads were incubated at 4°C for 18 h with Bos1p (15 μg), Sec22p (15 μg), and Bet1p (5 μg). The complexes formed were analyzed as in A. The stoichiometry of the Sed5p–Bos1p–Sec22p–Bet1p in the complex was 1:1.3:1.3:1.5 (lane 2).
To further analyze the molar ratio of individual components within the SNARE complex assembled in vitro, a fixed amount of GST–Sed5p (Fig. 1 A) was incubated with increasing amounts of NH2-terminally His10-tagged Sly1p. The bimolecular complexes were purified on glutathione agarose beads. As can be seen in Fig. 2 B; even with an eightfold excess of Sly1p over Sed5p, a complex of ∼1:1 stoichiometry was formed. Assembly of the fusion complex was then performed with the cognate v-SNAREs, and the molar ratios of Sly1p, Sed5p, Bos1p, Sec22p, and Bet1p in the purified complex were determined by densitometry (Fig. 2 C, lane 3). Assuming that equal amounts of Bos1p and Sec22p (that did not clearly separate in gels) were in the complex, a stoichiometry of 1.2:1.0:1.4:1.4:1.3 was determined.
This result indicated that in sharp contrast to what has been found with mammalian syntaxin 1A bound to N-Sec1 (Yang et al., 2000) a core fusion complex can be formed in vitro on Sed5p bound to the Sec1 family member Sly1p. This might indicate that Sly1p associates with Sed5p being in an open conformation.
Sly1p binds to preassembled SNARE complex
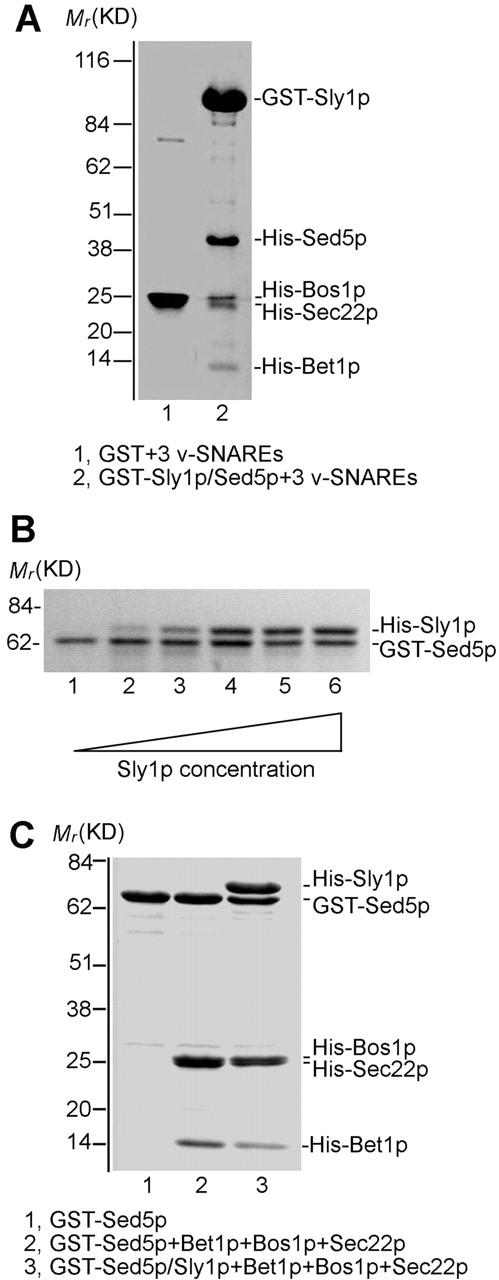
One might argue that once a SNARE complex has formed in vitro or in vivo Sed5p would be in a conformation unable to associate with Sly1p. This in fact is the case for the neuronal syntaxin 1A, which after assembled into a SNARE complex does not bind N-Sec1 (Yang et al., 2000). Therefore, we generated a SNARE fusion complex, this time with GST–Sec22p and the His6-tagged SNARES Sed5p, Bos1p, and Bet1p. After extensive washing, the complexes on glutathione beads were incubated with His10-tagged Sly1p. As shown in Fig. 3 A, a complex of the four SNARE proteins could be formed in solution to which Sly1p did bind in stoichiometric amounts.
Figure 3.
Preassembled core SNARE complexes bind Sly1p and Sec17p. (A) A complex formed in solution with GST–Sec22p (5 μg), Sed5p (10 μg), Bet1p (5 μg), and Bos1p (15 μg) was purified on glutathione agarose beads (lane 2) and challenged with purified His10-Sly1p (50 μg) in 100 μl reaction at 4°C for 3 h (lane 3). Proteins bound to beads were separated by SDS-PAGE and stained with Coomassie blue. The stoichiometry of Sly1p–Sed5p–Bos1p–Sec22p–Bet1p in the isolated complex was 1:1.2:0.8:1.4:1.3 according to densitometric analysis of Coomassie blue–stained gels. (B) Purified complexes of GST–Sec22p, Sed5p, Bos1p, and Bet1p without (lane 2) or with Sly1p (lane 3), prepared as above, were incubated with His6-Sec17p (20 μg) at 4°C for 3 h, and complexes on beads were characterized by SDS-PAGE and densitometry. The Sed5p to Sec17p stoichiometry in Sly1p-free and Sly1p-containing complexes was 1:3.3 and 1:2.7, respectively.
Here again the Sec1 binding properties of the ER to Golgi and the neuronal exocytic SNARE complexes differ, but the former appear to resemble the yeast exocytic SNARE complex, which by immunoprecipitation experiments has been shown to associate with Sec1p in cellular extracts (Carr et al., 1999).
Sec17p binds to Sly1p-containing SNARE complexes
α-SNAP and its yeast homologue Sec17p have been shown to bind to neuronal and exocytic SNARE complexes, respectively, that were generated from recombinant proteins (Söllner et al., 1993; Rossi et al., 1997). We felt it to be of interest to investigate whether Sec17p could also associate with ER to Golgi SNARE complexes to which stoichiometric amounts of Sly1p were already bound.
Using GST–Sec22p as a tool for SNARE complex isolation on glutathione beads, complexes formed with Bos1p, Bet1p, and either free or Sly1p-bound Sed5p interacted equally efficiently with purified Sec17p. Densitometric analysis revealed that the multimeric complexes (Fig. 3 B) contained an approximately threefold higher molar concentration of Sec17p compared with the individual SNAREs. Importantly, binding of Sec17p was independent of whether Sly1p was part of the preassembled SNARE complex or not.
Evidence for a Sly1p-bound SNARE complex in vivo
To complement the in vitro studies and to demonstrate that ER to Golgi SNARE complexes bound to Sly1p are also present in vivo, two different experimental approaches were followed. First, a yeast strain was constructed with the essential chromosomal SLY1 gene deleted and replaced by a vector-contained gene expressing a protein A–Sly1 fusion protein. This strain grew like wild-type yeast, showing that the Sly1 fusion protein was functionally active. A cleared cell lysate of this strain was incubated with IgG-Sepharose to capture the Sly1 fusion protein and proteins bound to it. After extensive washing of the affinity beads, bound proteins were separated by SDS-PAGE and subjected to immunoblotting with specific polyclonal antibodies. Under conditions where the protein A–Sly1 fusion protein of the cell extract was entirely bound to the beads, fractions of the total cellular SNAREs Sed5p, Bos1p, Sec22p, and Bet1p were copurified with Sly1p (Fig. 4 A). Except for Sed5p, of which a significant part copurified with the Sly1 fusion protein, only small fractions of the SNAREs were found in the immunocomplex. Quantification of band intensities with the help of a LumiImager revealed that ∼74% of total Sed5p but <1% of the cognate v-SNAREs coprecipitated with Sly1p. This would be consistent with the reasonable assumption that in growing cells only limited fractions of the SNAREs are in quaternary complexes (and bound to Sly1p) at any given time. However, consistent with a previous report (Søgaard et al., 1994) a significant percentage of the cellular Sed5 protein appears to be always associated with Sly1p. Small amounts of the exocytic v-SNAREs Snc1/2p but no vacuolar v-SNARE Nyv1p were detected to coprecipitate with Sly1p.
Figure 4.
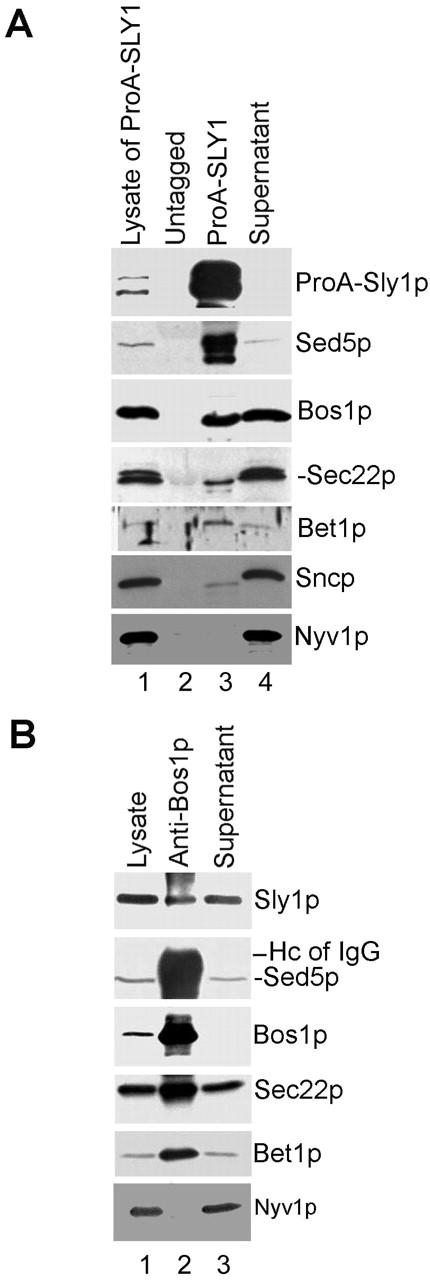
Sly1p is a component of the core SNARE fusion complex in vivo. (A) Cleared detergent lysates (1.2 mg of protein) from wild-type yeast strain MSUC1A (lane 2, untagged) and from yeast strain RPY137 expressing a protein A–Sly1 fusion protein (lane 3, ProA-SLY1) were incubated with IgG-Sepharose beads at 4°C overnight. Proteins on beads (lanes 2 and 3) were separated by SDS-PAGE along with a fraction of either the original lysate (lane 1; representing 3.2% of protein applied to beads) or the protein not bound to beads (lane 4; representing 4% of protein applied to beads) and subjected to immunoblot analysis with anti-SNARE and anti-Sly1p antibodies. Note that ProA-Sly1p and Sed5p were partially degraded. (B) A cleared detergent lysate of yeast strain MSUC1A was subjected to immunoprecipitation with affinity purified anti-Bos1p antibody. The immunoprecipitate (lane 2) along with a fraction of the original lysate (lane 1) and the nonprecipitated protein (lane 3) representing 3 and 3.25% of the material used for immunoprecipitation, respectively, were subjected to immunoblot analysis with antibodies to proteins indicated to the right. Sed5p is somewhat obscured by the presence of the IgG heavy chain.
In a second set of experiments, immunoprecipitations from cleared cell lysates were performed with a polyclonal antibody directed against Bos1p. Under conditions where Bos1p was completely precipitated, fractions of total Sed5p, Bet1p, Sec22p, and, importantly, of Sly1p were coprecipitated. In contrast, the vacuolar v-SNARE Nyv1p was not detected in the anti-Bos1p immunoprecipitate (Fig. 4 B).
Sly1p does not stabilize Sed5p in vivo or core SNARE complexes generated in vitro
In a recent study, it was reported that the yeast Sec1 family member Vps45p stabilizes the endocytic t-SNARE Tlg2p by preventing its proteasomal degradation (Bryant and James, 2001). Since Sly1p to a large extent is complexed with Sed5p in living cells, we sought to investigate whether Sed5p would be prone to degradation in cells depleted of Sly1p.
A haploid strain (GSF4) with the chromosomal SLY1 gene under transcriptional control of the GAL10 promoter and its isogenic wild-type strain were grown in galactose and then placed into glucose-containing medium to block Sly1p production in GSF4 cells. The mutant cells stopped multiplying around 12–14 h after medium shift. Lysates from GSF4 cells after different times after shutdown of SLY1 gene transcription and those from parallely grown wild-type cells were prepared and subjected to SDS-PAGE and immunoblot analysis. As shown in Fig. 5 A, the Sly1p level fell under that of wildtype cells ∼10 h after shift to glucose medium, and after further 10 h Sly1p was hardly detectable with the antibody used. According to densitometric measurement of the intensity of immunoblot signals, no significant change of the cellular levels of either Sed5p or Bos1p could be observed over the time course followed (see legend to Fig. 5).
Figure 5.
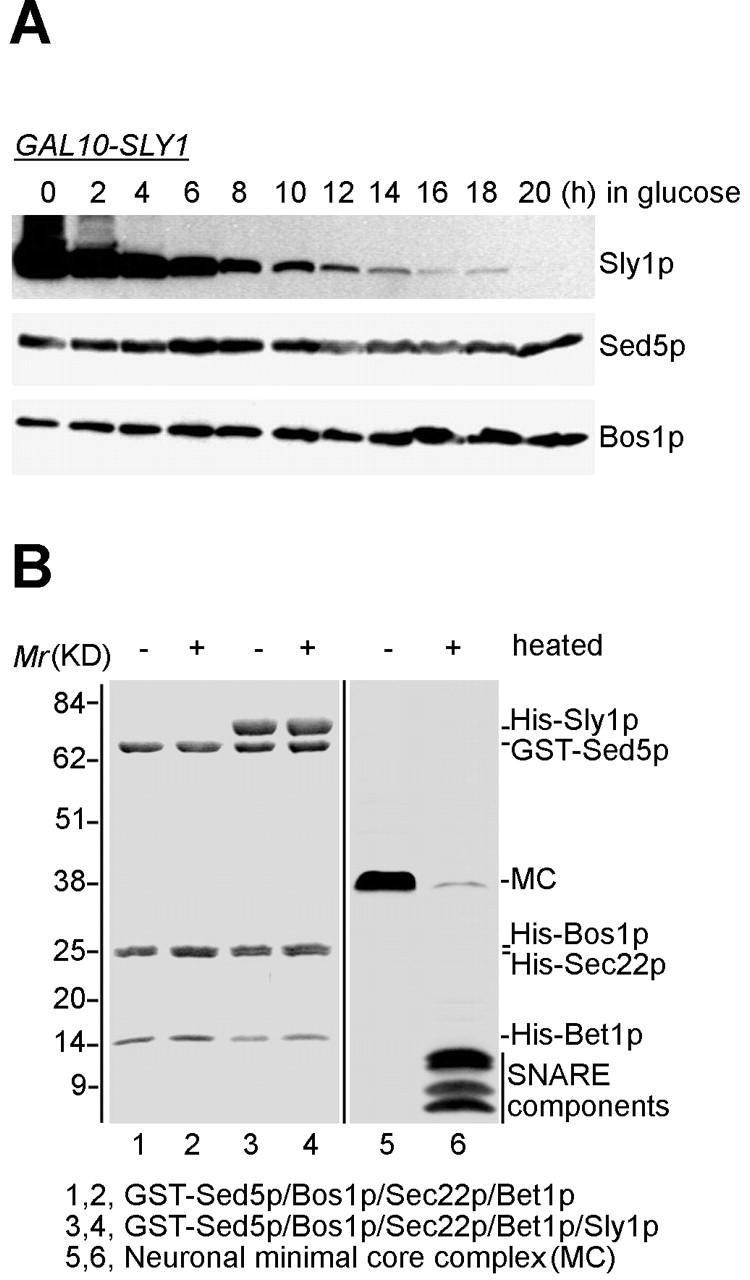
Sly1p does not affect stability of Sed5p in vivo or of Sed5p-containing ER to Golgi SNARE complexes generated in vitro. (A) GSF4 (GAL10-SLY1) was grown in galactose-containing medium and then transferred to glucose medium to switch off transcription of the SLY1 gene. At the indicated times, aliquots of the cultures were removed, and the same amount of cells from each sample were lysed with NaOH and subjected to immunoblot analysis with affinity purified antibodies against Sly1p, Sed5p, and Bos1p. Densitometric data analysis revealed that cells at 20 h in glucose contained ∼2.4% of Sly1p compared with the level of wild-type cells, which corresponds to the 10-h time point. The Sed5p level (set at 1.0 at the 0-h time point) was calculated to be 1.0, 1.1, 1.5, 1.3, 1.2, 0.8, 1.0, 0.8, 1.0, and 1.3 for the 2–20-h time points. (B) SNARE complexes generated without (lanes 1 and 2) or with Sly1p (lanes 3 and 4) and purified on glutathione beads as described in the legend to Fig. 2 C were subjected to treatment with sample buffer containing 2% SDS at room temperature (−) or 95°C (+) for 5 min. Proteins were separated on a 16% SDS-polyacrylamide gel and visualized by Coomassie blue staining. As a control, the in vitro assembled neuronal minimal SNARE complex (lanes 5 and 6) was treated and processed in the same way. Note the SDS resistance of this complex (lane 5).
Neuronal core SNARE complexes assembled in vitro are resistant to SDS at room temperature and can be disassembled by heating only. In contrast, yeast SNARE complexes generated from recombinant proteins appear not to be resistant to SDS (Tsui et al., 2001). Therefore, we sought to examine whether Sly1p bound to in vitro–generated ER to Golgi SNARE complexes would gain resistance to SDS. As shown in Fig. 5 B, SNARE complexes exposed to SDS-containing buffer for only 5 min at room temperature readily disassembled regardless of whether Sly1p was bound or not. As expected, a ternary neuronal SNARE complex (Pabst et al., 2000) proved SDS resistant at room temperature under identical conditions.
These results suggest that in living cells, the lifetime or the production of Sed5p is not dependent on Sly1p and that Sly1p does not confer SDS resistance to core SNARE complexes generated in vitro.
Sly1p does not influence the kinetics of SNARE complex assembly in vitro
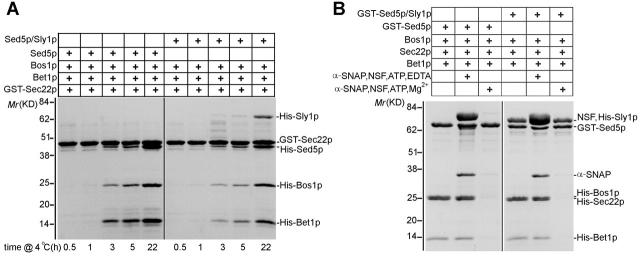
The assembly of SNARE complexes in vitro is a slow process. This allowed us to easily follow the kinetics of their formation and to answer the question whether Sly1p might facilitate or slow down SNARE complex assembly. To GST–Sec22p on agarose beads, Bos1p, Bet1p, and either free Sed5p or Sly1p-bound Sed5p were added, and incubations at 4°C were allowed to proceed for up to 22 h. Complexes of the four SNAREs could be detected after ∼3 h of incubation. About 20 h were needed for maximal complex formation. Although there was some variability in the extent of SNARE assembly in different experiments, no clear difference in the kinetics of complex formation was observed depending on whether Sly1p was present or not. A representative experiment (Fig. 6 A) shows that the formation of both types of complexes started to be seen at the same time point (3 h) and that Sed5p alone and the Sed5p–Sly1p heterodimer were incorporated into SNARE complexes with comparable kinetics. This was monitored by the densitometric assessment of Sec22p to Sed5p stoichiometry at different time points, which amounted to 1:0.3 and 1:0.3 at 3 h, 1:0.5 and 1:0.44 at 5 h, and 1:1.1 and 1:0.99 at 22 h in the absence and presence of Sly1p, respectively.
Figure 6.
Sly1p does not influence the kinetics of assembly or the NSF-catalyzed disassembly of ER to Golgi SNARE complexes. (A) Samples of GST–Sec22p (5 μg) bound to glutathione agarose beads were incubated at 4°C for the indicated times with His-tagged Bos1p (15 μg), Bet1p (5 μg), and either free Sed5p (10 μg) or preformed Sed5p–Sly1p complex. Reactions were stopped with 5 vol of ice-cold buffer C, and beads were washed four times. Proteins bound to beads were characterized on Coomassie blue–stained gels. (B) SNARE complexes assembled with GST–Sed5p or with the heterodimeric GST–Sed5p–Sly1p complex were purified on glutathione agarose as described in the legend to Fig. 2 C. Complexes on beads were mixed with equal molar concentration of NSF and α-SNAP, 2 mM MgCl2, 2.5 mM ATP in 20 mM Tris, pH 7.4, 100 mM NaCl, and 1 mM DTT for 20 min at 25°C. The reaction was stopped by dilution with ice-cold reaction buffer. As a control, the ATPase activity of NSF was inhibited by replacing MgCl2 with 10 mM EDTA. GST–Sed5p and its associated proteins on glutathione agarose beads were analyzed by SDS-PAGE and Coomassie blue staining.
From this we conclude that the speed of SNARE complex formation from recombinant proteins is not significantly influenced by Sly1p.
Sly1p does not protect core SNARE complexes from disassembly by NSF/α-SNAP
The simultaneous binding of Sly1p and Sec17p to in vitro–generated ER to Golgi SNARE complexes (Fig. 3 B) allowed us to investigate whether the Sec1 family protein affects the disassembly of core complexes. To address this question, SNARE complexes were formed with either GST–Sed5p or Sly1p-bound GST–Sed5p and the remaining three SNAREs. The complexes, purified on and bound to glutathione agarose beads, were then treated for 20 or 40 min with a 1:1 mix of recombinant rat NSF and α-SNAP in the presence of either Mg-ATP or EDTA. As can be seen in Fig. 6 B, α-SNAP and NSF bound to both SNARE complexes under conditions where ATP hydrolysis was inhibited. Importantly, Sly1p did not protect the core complexes from disassembly when NSF was active. At the shortest reaction time tested (20 min), the SNARE complexes were completely disassembled without affecting the Sed5p–Sly1p interaction.
Sly1p contributes to the specificity of SNARE complex formation
It has been shown previously that SNAREs are promiscuous with respect to their integration into complexes assembled in vitro and that mammalian syntaxin-containing cognate and noncognate SNARE complexes have similar biochemical and biophysical characteristics (Fasshauer et al., 1999; Yang et al., 1999). Since in contrast to N-Sec1–bound syntaxin Sly1p-bound Sed5p can be assembled into a core fusion complex, we inquired into the possibility that Sly1p might interfere with the generation of noncognate SNARE complexes.
First, we tried to replace one of the ER to Golgi v-SNAREs by the exocytic v-SNARE Snc2p. Of the three cognate v-SNAREs, only Bet1p was absolutely needed for complex formation with free Sed5p (Fig. 7 A). The combination of Snc2p–Bos1p–Bet1p allowed tetrameric SNARE complexes to be formed with free and Sly1p-bound Sed5p. Interestingly, the combination of v-SNARES Snc2p, Sec22p, and Bet1p efficiently assembled into a tetrameric complex with free Sed5p but not Sly1p-bound Sed5p (Fig. 7 A). In similar experiments, SNARE complex assembly was also probed with the Golgi v-SNARE Ykt6p and the vacuolar v-SNARE Nyv1p. Of all three possible combinations, only Nyv1p–Sec22p–Bet1p (Fig. 7 B) or Ykt6p–Sec22p–Bet1p (Fig. 7 C) readily formed tetrameric complexes with free Sed5p. Importantly, none of these complexes was generated when Sed5p was first bound to Sly1p.
Figure 7.
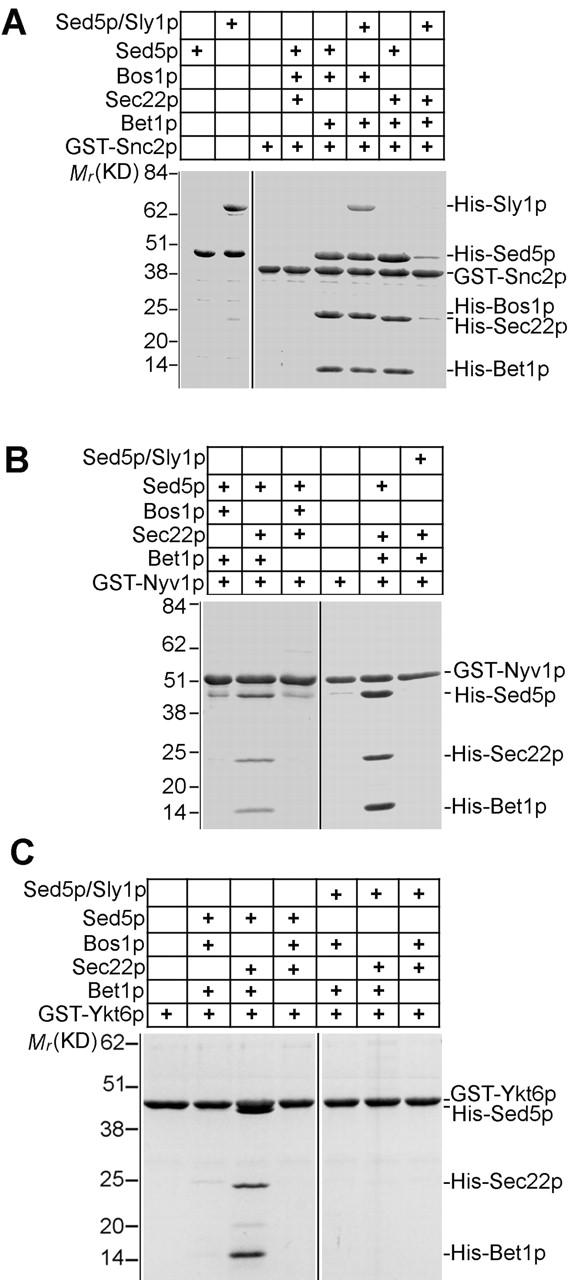
Sly1p prevents assembly of nonphysiological SNARE complexes. (A) SNARE complexes were formed with GST–Snc2p (5 μg) and His-tagged Sed5p (10 μg) or Sed5p–Sly1p heterodimer and different combinations of two of the ER to Golgi v-SNAREs Bos1p (15 μg), Sec22p (15 μg), and Bet1p (5 μg) and purified on beads. Protein in complexes were characterized in stained gels. In B and C, complex formation was investigated as in A but with either GST–Nyv1p (8 μg) or GST–Ykt6p (8 μg) instead of GST–Snc2p.
These results indicate that at least in vitro the Sec1 family member Sly1p can prevent the formation of noncognate tetrameric SNARE complexes.
Discussion
In the present study, we have used soluble ER to Golgi SNAREs for the assembly into specific complexes to address the question as to whether the Sec1 family member Sly1p, which binds with high affinity to the syntaxin Sed5p, affects SNARE complex formation or specificity. Surprisingly and in sharp contrast to the neuronal N-Sec1 and its associated syntaxin 1A, the Sed5p–Sly1p dimer proved as efficient as the free t-SNARE to assemble into equimolar complexes with its cognate v-SNAREs Bos1p, Sec22p, and Bet1p without affecting significantly the kinetics of complex formation or their NSF-catalyzed disassembly. Sly1p did also bind to preassembled tetrameric SNARE complexes in vitro, and it could be precipitated with Sed5p, Bos1p, Sec22p, and Bet1p from yeast cell lysates. These results suggest that like the yeast exocytic fusion complexes (Carr et al., 1999) but unlike the neuronal ones (Yang et al., 2000) SNARE complexes of ER to Golgi transport are associated with its specific Sec1 family protein in vivo. From different studies, it now emerges that the interaction of Sec1 proteins with their cognate SNAREs (binding to NH2- and/or COOH-terminal region or to open or closed conformation of syntaxins) and/or with SNARE fusion complexes (formation of SNARE complexes on Sec1-bound syntaxins, association with preassembled SNARE complexes) is distinct for different family members. The results of our study also disfavor the often generalized view that Sec1 family proteins are negative regulators of trans-SNARE pairing, and they are at odds with the conclusion of previously published work (Lupashin and Waters, 1997) that Sly1p in vivo has to be released from Sed5p as a prerequisite for SNARE-mediated fusion of ER-derived vesicles with the cis-Golgi compartment.
In a previous in vitro study, a remarkable specificity of fusion-competent yeast SNARE complexes was found when SNARE proteins were fixed in lipid bilayers, although some complexes remained unexplained (McNew et al., 2000; Parlati et al., 2000). It was argued that analyzing specificity of complex assembly with soluble SNAREs lacking the COOH-terminal transmembrane domain might be less meaningful. However, the pairings of soluble SNAREs that we have observed in solution also are not simply random. For effective complex assembly, not every of the three ER to Golgi v-SNAREs could be replaced by nonsyntaxin SNAREs acting in different pathways. For example, the plasma membrane v-SNARE Snc2p, which in vivo is interchangeable with Snc1p, could replace Sec22p or Bos1p but not Bet1p. Ykt6p, which in vivo appears to act in Golgi transport and homotypic vacuole fusion (McNew et al., 1997; Ungermann et al., 1999), and the vacuolar v-SNARE Nyv1p could only replace Bos1p but not Sec22p or Bet1p. Interestingly, Snc2p in combination with Bos1p and Bet1p could readily assemble into SNARE complexes regardless of whether the syntaxin Sed5p was free or bound to Sly1p. Since Snc1/2p could also be recovered in immunoprecipitates from cell extracts with anti-Sly1p and anti-Bos1p antibodies, we take these findings as an indication that functional ER to Golgi SNARE fusion complexes in vivo can integrate Snc2p instead of Sec22p into the four-helix bundle. A similar conclusion was reached by Grote and Novick (1999).
However, presumably nonphysiological tetrameric complexes could be efficiently formed in vitro when Snc2p, Ykt6p, or Nyv1p, all R-SNAREs like Sec22p, replaced Bos1p. Complex assembly with two R-SNAREs was unexpected, since it has been proposed (Fasshauer et al., 1998) and backed in part by mutational analysis (Katz and Brennwald, 2000; Ossig et al., 2000) that functional SNARE complexes could accommodate only three glutamines and one arginine in the central layer of the four-helix bundle. We cannot exclude the possibility that some of these complexes form in vitro because the SNAREs used lack their membrane anchors. However, SNARE combinations that led to apparent nonphysiological core complexes in our study (Nyv1p replacing Bos1p, for example) were not investigated in the study with SNAREs embedded into lipid vesicles (McNew et al., 2000), precluding a direct comparison of the two investigations. Importantly, the presumably nonphysiological SNARE complexes could not be generated when Sed5p was prebound with Sly1p. This indicates that Sly1p tightly bound to the NH2-terminal helical region of syntaxin Sed5p could potentially interfere with the assembly of nonphysiological SNARE complexes and contribute to the specificity of SNARE fusion complexes in vivo. A chaperone-like function of Sec1 family members in preventing nonproductive SNARE complexes has indeed been discussed to explain phenotypes of certain yeast SNARE mutants (Katz and Brennwald, 2000). Preliminary experiments aimed at identifying nonphysiological SNARE complexes in yeast cells depleted of Sly1p did not give clear results. This might be explained by very small fractions of such complexes due to the fact that even 20 h after the switch-off of SLY1 gene expression a low level of Sly1p (∼2.4% of wild-type level) (Fig. 5) still persists in mutant cells, which at this stage ceased multiplication and efficient protein transport activity (Ossig et al., 1991).
The first experimental evidence for a possible role of a Sec1 protein in adding to the specificity of SNARE complex formation is furnished by the results we have reported here. On the other hand, a general role for Sec1 family proteins to regulate the stability of their cognate syntaxins, as recently proposed (Bryant and James, 2001), could be excluded for Sly1p whose depletion from growing cells, as shown here, did not affect the cellular level of the syntaxin Sed5p to which it binds.
Materials and methods
Yeast strains and growth conditions
Yeast strains used in this study were MSUC1A3D (MATa/α ura3/ura3 leu2/leu2 his3/his3 trp1/trp1 LYS2/lys2 ade8/ADE8), MSUC1A (MATa ura3 leu2 his3 trp1 ade8) and its isogenic strain RPY137 (MATa sly1: KanMX4 ura3 leu2 his3 trp1 ade8 pRS416-ProA-SLY1), SEY6210 (MATa ura3-52 leu2-3 his3-Δ200 trp1-Δ901 lys2-Δ801 suc2-Δ9) and its isogenic strain GSF4 (MATa LEU2-GAL10-SLY1 ura3-52 leu2-3 his3-Δ200 trp1-Δ901 lys2-Δ801 suc2-Δ9). RPY137 was constructed using techniques described previously (Peng et al., 2000). Yeast was grown in standard YPD or in selective media with the necessary additions.
Plasmid construction
All plasmids were propagated in the E. coli strain DH5α. The coding sequences of Sly1p (amino acids 1–666), Sed5N (amino acids 1–250), Sed5C (amino acids 251–324), the whole cytosolic region of Sed5p (amino acids 1–324), and Ykt6p (amino acids 1–195) were amplified by PCR from Saccharomyces cerevisiae genomic DNA and inserted into pGEX-TT, a vector for NH2-terminal GST fusion, to create pGEX-SLY1 (GST–Sly1p), pGEX-SED5N (GST–Sed5N), pGEX-SED5C (GST–Sed5C), pGEX-SED5 (GST–Sed5p), and pGEX-YKT6 (GST–Ykt6p), respectively. Full-length Sly1p (NH2-terminally His10-tagged) was expressed from pET19b-SLY1. We expressed His6-Sec22p (amino acids 1–188) and GST–Sec22p from pQE30-SEC22 and pGEX-SEC22, respectively. They carry the SEC22 gene lacking the sequence encoding the COOH-terminal transmembrane domain. Expression of His6-Sed5p was described previously (Peng et al., 1999). His6-Bet1p and His6-Bos1p were expressed from plasmids pET14b-BET1 (SFNB358) and pET11d-BOS1 (SFNB358) described previously (Stone et al., 1997). Plasmids expressing the His6-Sec17p (full length), GST–Nyv1p (full length), and GST–Snc2p (amino acids 1–93) were provided by A. Mayer (Max-Planck-Gesellschatt, Tübingen, Germany), T. Söllner (Memorial Sloan Kettering Cancer Center, New York, NY), and D. Banfield (The Hong Kong University of Science and Technology, Hong Kong, China), respectively. Plasmid pRS416-ProA carrying two copies of IgG-binding motif of protein A was a gift of M. Peterson (University of California at San Diego, San Diego, CA) and was used to construct pRS416-ProA-SLY1, which expresses the protein A fusion of Sly1p (ProA-Sly1p).
Antibodies
A polyclonal antibody recognizing Snc1p and Snc2p was provided by P. Brennwald (Cornell University, New York, NY), and anti-Nyv1p antibody was from A. Mayer. Polyclonal anti-Bos1p and anti-Bet1 antibodies were affinity purified with GST–Bos1p and GST–Bet1p from antisera produced in rabbits. Antibodies against Sly1p and Sed5p were described previously (Peng et al., 2000).
Protein expression and purification
Plasmids used for protein expression were transformed into E. coli strain C41. Transformed cells were grown at 37°C to an absorbance of 1.0 at 600 nm and induced for recombinant protein expression with 1 mM IPTG for 4 h at 37°C or for 6 h at 30°C (GST–Sly1p). Cells from one liter cultures were collected by centrifugation and lysed by sonication in 10 ml of buffer A (25 mM Tris-HCl, pH 7.5, 400 mM KCl, 10% glycerol, 4% Triton X-100, 5 mM β-mercaptoethanol, 1 mM PMSF). Lysates were clarified by centrifugation and incubated with 1 ml of 50% glutathione-agarose (Amersham Pharmacia Biotech) or Ni2+-NTA agarose slurry (QIAGEN). The binding was conducted at 4°C for 60 min before the mixture was loaded onto a minicolumn. Affinity matrices were then washed with 50 ml of buffer B (25 mM Tris-HCl, pH 7.5, 500 mM KCl, 10% glycerol, 1% Triton X-100, 2 mM β-mercaptoethanol, 1 mM PMSF). The bound proteins were eluted with buffer C (which is buffer B without PMSF and with 150 mM instead 500 mM KCl) containing either 10 mM reduced glutathione (for GST fusion proteins) or 250 mM imidazole (for His-tagged proteins). In case the GST moiety was removed by thrombin, the column was treated with elution buffer containing 0.015 U/μl of thrombin (Sigma-Aldrich) at 4°C overnight.
In vitro binding assay
In vitro binding assays were performed essentially as described (Peng et al., 1999). Briefly, purified GST or GST fusion proteins reconjugated onto glutathione agarose beads were incubated at 4°C in buffer C for the indicated time period with purified proteins with or without His6 tag. Proteins on the extensively washed beads were dissolved by boiling in SDS buffer, separated by SDS-PAGE, and visualized by Coomassie blue staining or immunoblotting using specific antibodies.
Assembly of Sed5p–Sly1p and SNARE complexes
The Sed5p–Sly1p complex was formed by mixing GST–Sly1p conjugated on glutathione beads and His6-Sed5p of ∼1:3 molecular ratio (Sly1p to Sed5p) or by His-tagged Sed5p and Sly1p of equal molar amounts. Complex formation proceeded for 90–180 min at 4°C on a rotation wheel. The complexes on the beads (GST–Sly1p–His-Sed5p) or in solution (His-Sly1p–His-Sed5p) were then incubated with cognate and noncognate v-SNAREs. Complex formation with single or multiple v-SNAREs was conducted at 4°C for 17–22 h in 100 μl buffer C (see above). After extensive washing with buffer C, bound proteins were analyzed by SDS-PAGE and Coomassie blue staining. For saturation experiments, GST–Sed5p was first bound to glutathione agarose beads for 90 min, the beads were then washed and incubated with His-Sly1p. Although the concentration of GST–Sed5p on beads was kept constant at ∼1.0 μM, the concentration of His10-Sly1p was varied between 0.1 and 10 μM. The binding reactions were performed at 4°C for 3 h. For Sly1p and Sec17p binding experiments, SNARE complexes were preassembled and isolated by binding to glutathione agarose beads through GST–Sec22p. Beads were washed in buffer C and used for incubation with excess of His10-Sly1p or His6-Sec17p at 4°C for 3 h. The samples were processed by SDS-PAGE followed by Coomassie blue staining. A minimal neuronal SNARE complex was formed as described (Pabst et al., 2000).
The molecular ratios of individual proteins in complexes were quantitated by scanning the Coomassie blue–stained gels (on Linoscan). Images were processed by Photoshop 3.0®, and the signal intensities of protein bands were analyzed by a program of Advanced Image Data Analyzer (AIDA, version 2.11; Raytest Isotopenmessgeraete GmbH). In gels where His6-tagged Sec22p and Bos1p did not separate, the value for signal intensity was divided by two to arrive at the approximate stoichiometries of the two proteins.
Immunoprecipitation and Western blot analysis
For immunoprecipitation experiments, cells of strain MSUC1A were grown at 25°C in YPD to an optical density of 0.5–1.0 at 600 nm. Cells (20–25 U at OD600) were collected at 4°C and washed with ice-cold wash buffer (20 mM Tris-HCl, pH 7.5, 20 mM NaN3, 20 mM NaF) to deplete the cells of ATP. In a 2-ml tube, cells were suspended in 1 ml of ice-cold IP buffer (50 mM Hepes, pH 7.5, 150 mM KCl, 1 mM DTT, 1 mM EDTA, 0.5% NP-40) in the presence of protease inhibitors and lysed with 1.7 g of glass beads in a minibeadbeater for 4 min at full speed. Lysates were centrifuged at 500 g for 5 min to separate the cell extract from glass beads and unbroken cells. The supernatant was centrifuged again at 16,000 g for 30 min at 4°C and transferred to a new tube to determine protein concentration with a Bio-Rad Laboratories protein assay kit using mouse IgG (Pierce Chemical Co.) as standard. The samples (lysate) were adjusted to 4 mg/ml of total protein with IP buffer. To remove proteins unspecifically adhering to protein A–Sepharose, lysates were precleared with protein A–Sepharose beads at 4°C for 2–3 h with rocking. For immunoprecipitation, 1 ml of the precleared lysate was incubated at 4°C overnight with affinity purified anti-Bos1p antibody. Beads were washed five times with 1 ml of IP buffer. Proteins were eluted from beads by boiling in SDS buffer, separated by SDS-PAGE, and visualized by immunoblotting with specific antibodies. For lysates containing ProA-Sly1p, samples were treated essentially the same except that they were precleared with Sepharose CL-4B (Amersham Pharmacia Biotech) followed by ProA-Sly1p binding to IgG-Sepharose fast flow (Amersham Pharmacia Biotech). Serial dilutions of lysate were probed on blots with anti-Sed5p and v-SNARE antibodies to quantify the relative amounts of these proteins in immunoprecipitates through calculation of band intensities with a LumiImager (Boehringer).
Acknowledgments
We thank Susan Ferro-Novick, Andreas Mayer, Thomas Söllner, Patrick Brennwald, David Banfield, and Michael R. Peterson for providing plasmids and antibodies. We are indebted to Reinhard Jahn, Wolfram Antonin, Dirk Fasshauer, and Michael Wolde for discussions and suggestions, Stefan Pabst for providing NSF and α-SNAP, Xiaoping Yang and Uwe Andag for purified GST–Sed5p, Ursula Welscher-Altschäffel and Peter Mienkus for excellent technical assistance, and Ingrid Balshüsemann and Christa Niemann for secretarial help.
This work was supported by the Max Planck Society and by grants to D. Gallwitz from the Deutsche Forschungsgemeinschaft, Fonds der Chemischen Industrie, and the Human Frontier Science Program.
Footnotes
Abbreviations used in this paper: GST, glutathione S-transferase; t-SNARE, target membrane SNARE; v-SNARE, vesicular SNARE.
References
- Antonin, W., D. Fasshauer, S. Becker, R. Jahn, and T.R. Schneider. 2002. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat. Struct. Biol. 9:107–111. [DOI] [PubMed] [Google Scholar]
- Bennet, M.K., and R.H. Scheller. 1993. The molecular machinery for secretion is conserved from yeast to neurons. Proc. Natl. Acad. Sci. USA. 90:2559–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, N.J., and D.E. James. 2001. Vps45p stabilizes the syntaxin homologue Tlg2p and positively regulates SNARE complex formation. EMBO J. 20:3380–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, C.M., E. Grote, M. Munson, F.M. Hughson, and P. Novick. 1999. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol. 146:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher, C., R. Ossig, D. Gallwitz, and H.D. Schmitt. 1991. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol. Cell. Biol. 11:872–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova, I., S. Sugita, S. Hill, M. Hosaka, I. Fernandez, T.C. Südhof, and J. Rizo. 1999. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 18:4372–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova, I., T. Yamaguchi, Y. Wang, T.C. Südhof, and J. Rizo. 2001. Vam3p structure reveals conserved and divergent properties of syntaxins. Nat. Struct. Biol. 8:258–264. [DOI] [PubMed] [Google Scholar]
- Fasshauer, D., R.B. Sutton, A.T. Brünger, and R. Jahn. 1998. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. USA. 95:15781–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer, D., W. Antonin, M. Margittai, S. Pabst, and R. Jahn. 1999. Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J. Biol. Chem. 274:15440–15446. [DOI] [PubMed] [Google Scholar]
- Grabowski, R., and D. Gallwitz. 1997. High-affinity binding of the yeast cis-Golgi t-SNARE, Sed5p, to wild-type and mutant Sly1p, a modulator of transport vesicle docking. FEBS Lett. 411:169–172. [DOI] [PubMed] [Google Scholar]
- Grote, E., and P. Novick. 1999. Promiscuity in Rab-SNARE interactions. Mol. Biol. Cell. 10:4149–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote, E., C.M. Carr, and P. Novick. 2000. Ordering the final events in yeast exocytosis. J. Cell Biol. 151:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick, K.G., and H.R. Pelham. 1992. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J. Cell Biol. 119:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata, Y., C.A. Slaughter, and T.C. Südhof. 1993. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 366:347–351. [DOI] [PubMed] [Google Scholar]
- Jahn, R., and T.C. Südhof. 1999. Membrane fusion and exocytosis. Annu. Rev. Biochem. 68:863–911. [DOI] [PubMed] [Google Scholar]
- Katz, L., and P. Brennwald. 2000. Testing the 3Q:1R “rule”: mutational analysis of the ionic “zero” layer in the yeast exocytic SNARE complex reveals no requirement for arginine. Mol. Biol. Cell. 11:3849–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee, Y., R.C. Lin, S.C. Hsu, and R.H. Scheller. 1995. Distinct domains of syntaxin are required for synaptic vesicle fusion complex formation and dissociation. Neuron. 14:991–998. [DOI] [PubMed] [Google Scholar]
- Kosodo, Y., Y. Noda, and K. Yoda. 1998. Protein-protein interactions of the yeast Golgi t-SNARE Sed5 protein distinct from its neural plasma membrane cognate syntaxin 1. Biochem. Biophys. Res. Commun. 250:212–216. [DOI] [PubMed] [Google Scholar]
- Lazar, T., M. Götte, and D. Gallwitz. 1997. Vesicular transport: how many Ypt/Rab-GTPases make a eukaryotic cell? Trends Biochem. Sci. 22:468–472. [DOI] [PubMed] [Google Scholar]
- Lupashin, V.V., and M.G. Waters. 1997. t-SNARE activation through transient interaction with a rab-like guanosine triphosphatase. Science. 276:1255–1258. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., M. Sogaard, N.M. Lampen, S. Machida, R.R. Ye, L. Lacomis, P. Tempst, J.E. Rothman, and T.H. Sollner. 1997. Ykt6p, a prenylated SNARE essential for endoplasmic reticulum-Golgi transport. J. Biol. Chem. 272:17776–17783. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., F. Parlati, R. Fukuda, R.J. Johnston, K. Paz, F. Paumet, T.H. Söllner, and J.E. Rothman. 2000. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 407:153–159. [DOI] [PubMed] [Google Scholar]
- Misura, K.M., R.H. Scheller, and W.I. Weis. 2000. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 404:355–362. [DOI] [PubMed] [Google Scholar]
- Munson, M., X. Chen, A.E. Cocina, S.M. Schultz, and F.M. Hughson. 2000. Interactions within the yeast t-SNARE Sso1p that control SNARE complex. Nat. Struct. Biol. 7:894–902. [DOI] [PubMed] [Google Scholar]
- Nichols, B.J., C. Ungermann, H.R. Pelham, W.T. Wickner, and A. Haas. 1997. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 387:199–202. [DOI] [PubMed] [Google Scholar]
- Novick, P., and R. Scheckman. 1979. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 76:1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick, P., and M. Zerial. 1997. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 9:496–504. [DOI] [PubMed] [Google Scholar]
- Ossig, R., C. Dascher, H.H. Trepte, H.D. Schmitt, and D. Gallwitz. 1991. The yeast SLY gene products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol. Cell. Biol. 11:2980–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossig, R., H.D. Schmitt, B. de Groot, D. Riedel, S. Keranen, H. Ronne, H. Grubmuller, and R. Jahn. 2000. Exocytosis requires asymmetry in the central layer of the SNARE complex. EMBO J. 19:6000–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst, S., J.W. Hazzard, W. Antonin, T.C. Südhof, R. Jahn, J. Rizo, and D. Fasshauer. 2000. Selective interaction of complexin with the neuronal SNARE complex. Determination of the binding regions. J. Biol. Chem. 275:19808–19818. [DOI] [PubMed] [Google Scholar]
- Parlati, F., J.A. McNew, R. Fukuda, R. Miller, T.H. Söllner, and J.E. Rothman. 2000. Topological restriction of SNARE-dependent membrane fusion. Nature. 407:194–198. [DOI] [PubMed] [Google Scholar]
- Peng, R., R. Grabowski, and D. Gallwitz. 1999. Specific interaction of the yeast cis-Golgi syntaxin Sed5p and the coat protein complex II component Sec24p of endoplasmic reticulum-derived transport vesicles. Proc. Natl. Acad. Sci. USA. 96:3751–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, R., A. De Antoni, and D. Gallwitz. 2000. Evidence for overlapping and distinct functions in protein transport of coat protein Sec24p family members. J. Biol. Chem. 275:11521–11528. [DOI] [PubMed] [Google Scholar]
- Pevsner, J., S.C. Hsu, and R.H. Scheller. 1994. n-Sec1: a neural-specific syntaxin-binding protein. Proc. Natl. Acad. Sci. USA. 91:1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier, M.A., W. Xiao, J.C. Macosko, C. Chan, Y.R. Shin, and M.K. Bennett. 1998. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat. Struct. Biol. 5:765–769. [DOI] [PubMed] [Google Scholar]
- Rossi, G., A. Salminen, L.M. Rice, A.T. Brunger, and P. Brennwald. 1997. Analysis of a yeast SNARE complex reveals remarkable similarity to the neuronal SNARE complex and a novel function for the C terminus of the SNAP-25 homolog, Sec9. J. Biol. Chem. 272:16610–16617. [DOI] [PubMed] [Google Scholar]
- Sacher, M., S. Stone, and S. Ferro-Novick. 1997. The synaptobrevin-related domains of Bos1p and Sec22p bind to the syntaxin-like region of Sed5p. J. Biol. Chem. 272:17134–17138. [DOI] [PubMed] [Google Scholar]
- Sato, T.K., P. Rehling, M.R. Peterson, and S.D. Emr. 2000. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell. 6:661–671. [DOI] [PubMed] [Google Scholar]
- Schimmöller, F., I. Simon, and S.R. Pfeffer. 1998. Rab GTPases, directors of vesicle docking. J. Biol. Chem. 273:22161–22164. [DOI] [PubMed] [Google Scholar]
- Seals, D.F., G. Eitzen, N. Margolis, W.T. Wickner, and A. Price. 2000. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl. Acad. Sci. USA. 97:9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard, M., K. Tani, R.R. Ye, S. Geromanos, P. Tempst, T. Kirchhausen, J.E. Rothman, and T. Söllner. 1994. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 78:937–948. [DOI] [PubMed] [Google Scholar]
- Söllner, T., S.W. Whiteheart, M. Brunner, H. Erdjument-Bromage, S. Geromanos, P. Tempst, and J.E. Rothman. 1993. SNAP receptors implicated in vesicle targeting and fusion. Nature. 362:318–324. [DOI] [PubMed] [Google Scholar]
- Stone, S., M. Sacher, Y. Mao, C. Carr, P. Lyons, A.M. Quinn, and S. Ferro-Novick. 1997. Bet1p activates the v-SNARE Bos1p. Mol. Biol. Cell. 8:1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, R.B., D. Fasshauer, R. Jahn, and A.T. Brünger. 1998. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 395:347–353. [DOI] [PubMed] [Google Scholar]
- Tsui, M.M., W.C. Tai, and D.K. Banfield. 2001. Selective formation of Sed5p-containing snare complexes is mediated by combinatorial binding interactions. Mol. Biol. Cell. 12:521–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann, C., G.F. von Mollard, O.N. Jensen, N. Margolis, T.H. Stevens, and W. Wickner. 1999. Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J. Cell Biol. 145:1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage, M., A.S. Maia, J.J. Plomp, A.B. Brussaard, J.H. Heeroma, H. Vermeer, R.F. Toonen, R.E. Hammer, T.K. van den Berg, M. Missler, et al. 2000. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 287:864–869. [DOI] [PubMed] [Google Scholar]
- Wada, Y., N. Nakamura, Y. Ohsumi, and A. Hirata. 1997. Vam3p, a new member of syntaxin related protein, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae. J. Cell Sci. 110:1299–1306. [DOI] [PubMed] [Google Scholar]
- Yang, B., L. Gonzalez, Jr., R. Prekeris, M. Steegmaier, R.J. Advani, and R.H. Scheller. 1999. SNARE interactions are not selective. Implications for membrane fusion specificity. J. Biol. Chem. 274:5649–5653. [DOI] [PubMed] [Google Scholar]
- Yang, B., M. Steegmaier, L. Gonzalez, Jr., and R.H. Scheller. 2000. nSec1 binds a closed conformation of syntaxin1A. J. Cell Biol. 148:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]