Abstract
Three IS5 elements were mapped in overlapping chromosomal segments on a series of F-prime plasmids by restriction analysis and hybridization. IS5A was located clockwise of proA near 6 min, IS5B was located clockwise of purE near 12 min, and IS5C was tentatively located near 14 min on the Escherichia coli K-12 map. The physical structures of nine type II F-prime plasmids that contain chromosomal DNA from this region indicated that these plasmids were excised from the chromosome by recombination between pairs of IS5 elements.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Roth J. Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between rRNA (rrn) cistrons. Proc Natl Acad Sci U S A. 1981 May;78(5):3113–3117. doi: 10.1073/pnas.78.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anilionis A., Ostapchuk P., Riley M. Identification of a second cryptic lambdoid prophage locus in the E. coli K12 chromosome. Mol Gen Genet. 1980;180(2):479–481. doi: 10.1007/BF00425865. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Curtiss R., 3rd Transposition derivatives of an Hfr strain of Escherichia coli K-12. Genetics. 1967 Jul;56(3):503–525. doi: 10.1093/genetics/56.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Fiandt M., Hass K. K., Twose P. A., Szybalski W. Deletions and insertions in the immunity region of coliphage lambda: revised measurement of the promoter-startpoint distance. Virology. 1974 Dec;62(2):458–471. doi: 10.1016/0042-6822(74)90407-3. [DOI] [PubMed] [Google Scholar]
- Broda P., Meacock P. Isolation and characterisation of Hfr strains from a recombination-deficient strain of Escherichia coli. Mol Gen Genet. 1971;113(2):166–173. doi: 10.1007/BF00333190. [DOI] [PubMed] [Google Scholar]
- Cullum J., Broda P. Chromosome transfer and Hfr formation by F in rec+ and recA strains of Escherichia coli K12. Plasmid. 1979 Jul;2(3):358–365. doi: 10.1016/0147-619x(79)90019-2. [DOI] [PubMed] [Google Scholar]
- Daniels D. L., de Wet J. R., Blattner F. R. New map of bacteriophage lambda DNA. J Virol. 1980 Jan;33(1):390–400. doi: 10.1128/jvi.33.1.390-400.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonier R. C., Hadley R. G., Hu M. Enumeration and identification of IS3 elements in Escherichia coli strains. J Bacteriol. 1979 Mar;137(3):1421–1424. doi: 10.1128/jb.137.3.1421-1424.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonier R. C., Oh G. R., Hu M. Further mapping of IS2 and IS3 in the lac-purE region of the Escherichia coli K-12 genome: structure of the F-prime ORF203. J Bacteriol. 1977 Feb;129(2):1129–1140. doi: 10.1128/jb.129.2.1129-1140.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S K, Adelberg E A. The Occurrence of a Genetic Transposition in a Strain of Escherichia Coli. Genetics. 1962 May;47(5):577–585. doi: 10.1093/genetics/47.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler J. A., van Bree M. P. The nucleotide sequence and protein-coding capability of the transposable element IS5. Gene. 1981 Aug;14(3):155–163. doi: 10.1016/0378-1119(81)90111-6. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Bennett P. M., Higginson S., Richmond M. H. Regional preference of insertion of Tn501 and Tn802 into RP1 and its derivatives. Mol Gen Genet. 1978 Nov 9;166(3):313–320. doi: 10.1007/BF00267624. [DOI] [PubMed] [Google Scholar]
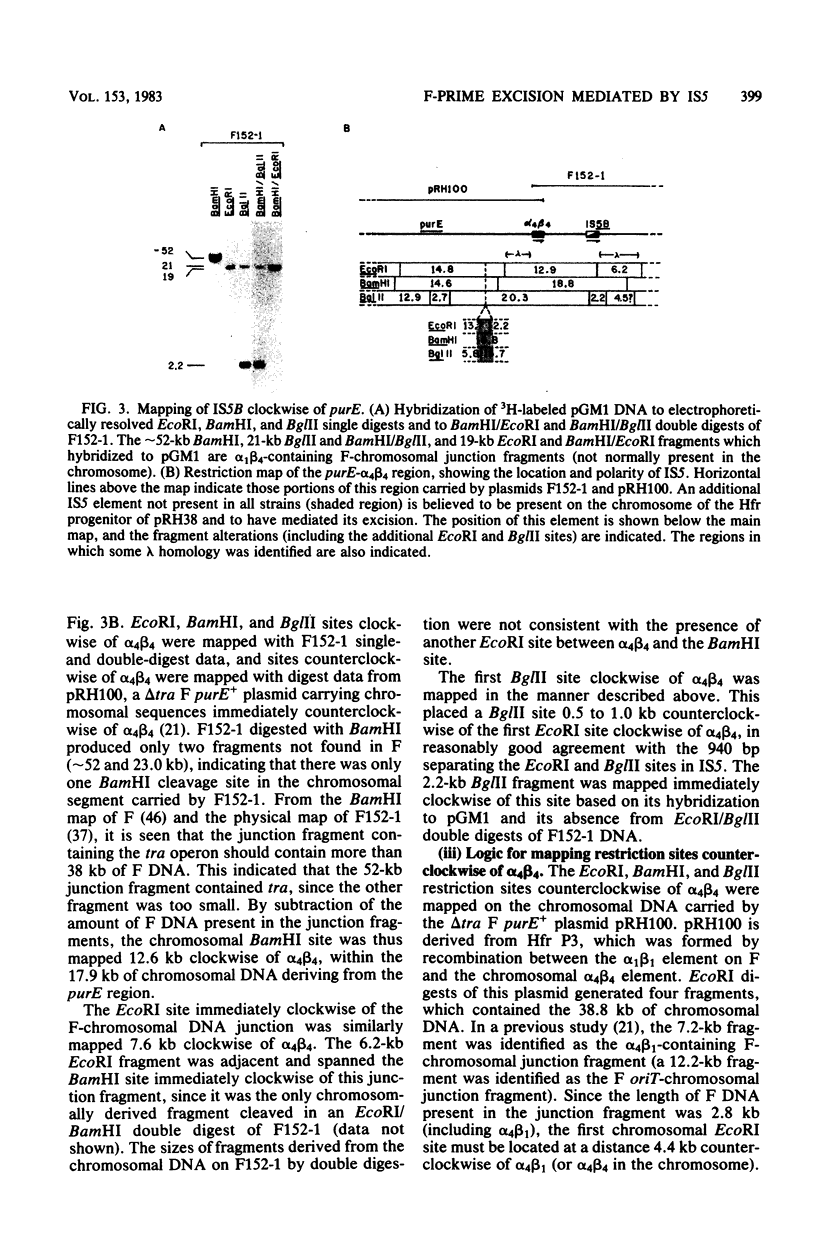
- Guyer M. S., Davidson N., Clark A. J. Heteroduplex analysis of tra delta f' plasmids and the mechanism of their formation. J Bacteriol. 1977 Sep;131(3):970–980. doi: 10.1128/jb.131.3.970-980.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. G., Deonier R. C. Specificity in formation of type II F' plasmids. J Bacteriol. 1979 Sep;139(3):961–976. doi: 10.1128/jb.139.3.961-976.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. G., Deonier R. C. Specificity in the formation of delta tra F-prime plasmids. J Bacteriol. 1980 Aug;143(2):680–692. doi: 10.1128/jb.143.2.680-692.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and alpha-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968 Oct;53(3):363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Grafstrom R. H., Harnish B. W., Hillman B. S. Tandem duplications resulting from recombination between ribosomal RNA genes in Escherichia coli. J Mol Biol. 1977 Nov 5;116(3):407–428. doi: 10.1016/0022-2836(77)90077-8. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Harnish B. W. Transposition of a chromosomal segment bounded by redundant rRNA genes into other rRNA genes in Escherichia coli. J Bacteriol. 1982 Feb;149(2):449–457. doi: 10.1128/jb.149.2.449-457.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Deonier R. C. Comparison of IS1, IS2 and IS3 copy number in Escherichia coli strains K-12, B and C. Gene. 1981 Dec;16(1-3):161–170. doi: 10.1016/0378-1119(81)90072-x. [DOI] [PubMed] [Google Scholar]
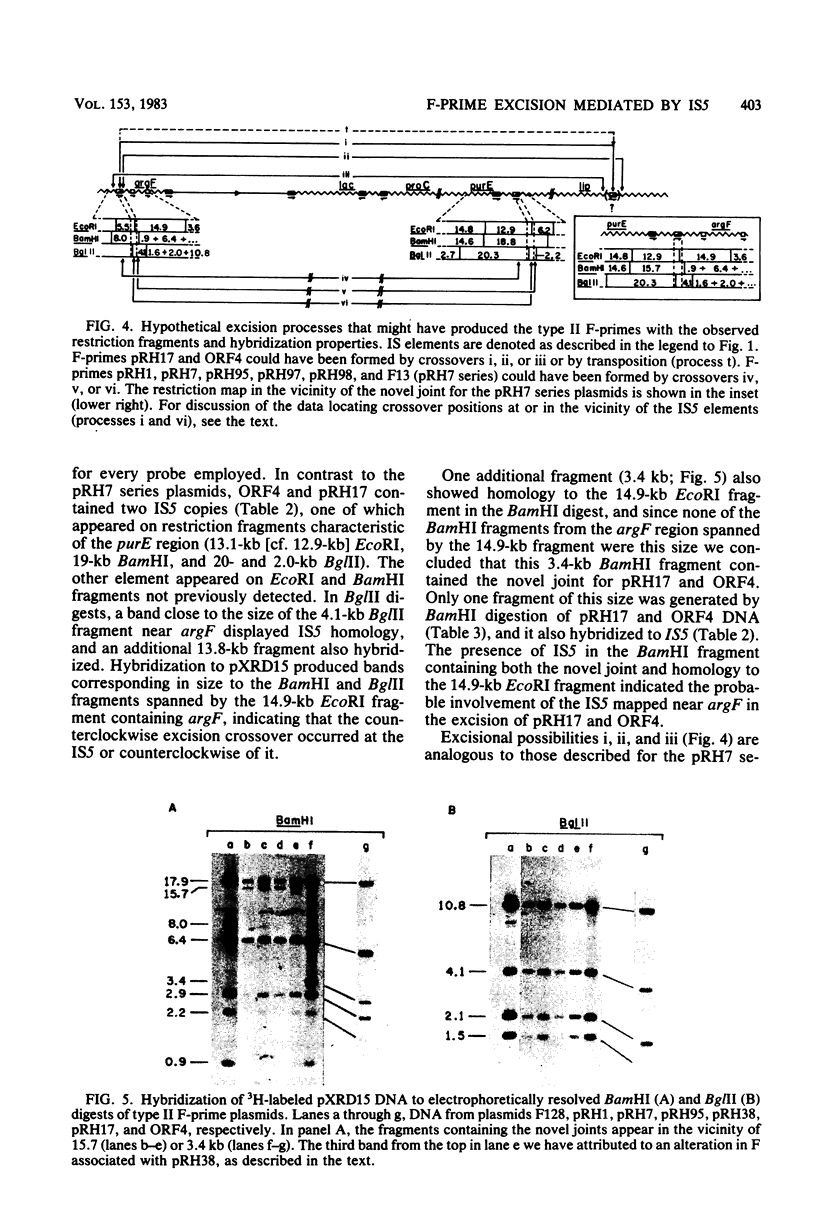
- Hu M., Deonier R. C. Mapping of IS1 elements flanking the argF gene region on the Escherichia coli K-12 chromosome. Mol Gen Genet. 1981;181(2):222–229. doi: 10.1007/BF00268430. [DOI] [PubMed] [Google Scholar]
- Hu S., Ohtsubo E., Davidson N. Electron microscopic heteroduplex studies of sequence relations among plasmids of Escherichia coli: structure of F13 and related F-primes. J Bacteriol. 1975 May;122(2):749–763. doi: 10.1128/jb.122.2.749-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaer R., Starlinger P. IS4 at its chromosomal site in E. coli K12. Mol Gen Genet. 1980;178(2):285–291. doi: 10.1007/BF00270474. [DOI] [PubMed] [Google Scholar]
- Kretschmer P. J., Cohen S. N. Selected translocation of plasmid genes: frequency and regional specificity of translocation of the Tn3 element. J Bacteriol. 1977 May;130(2):888–899. doi: 10.1128/jb.130.2.888-899.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B., Porter D. D. Modes of gene transfer and recombination in bacteria. Annu Rev Genet. 1978;12:249–287. doi: 10.1146/annurev.ge.12.120178.001341. [DOI] [PubMed] [Google Scholar]
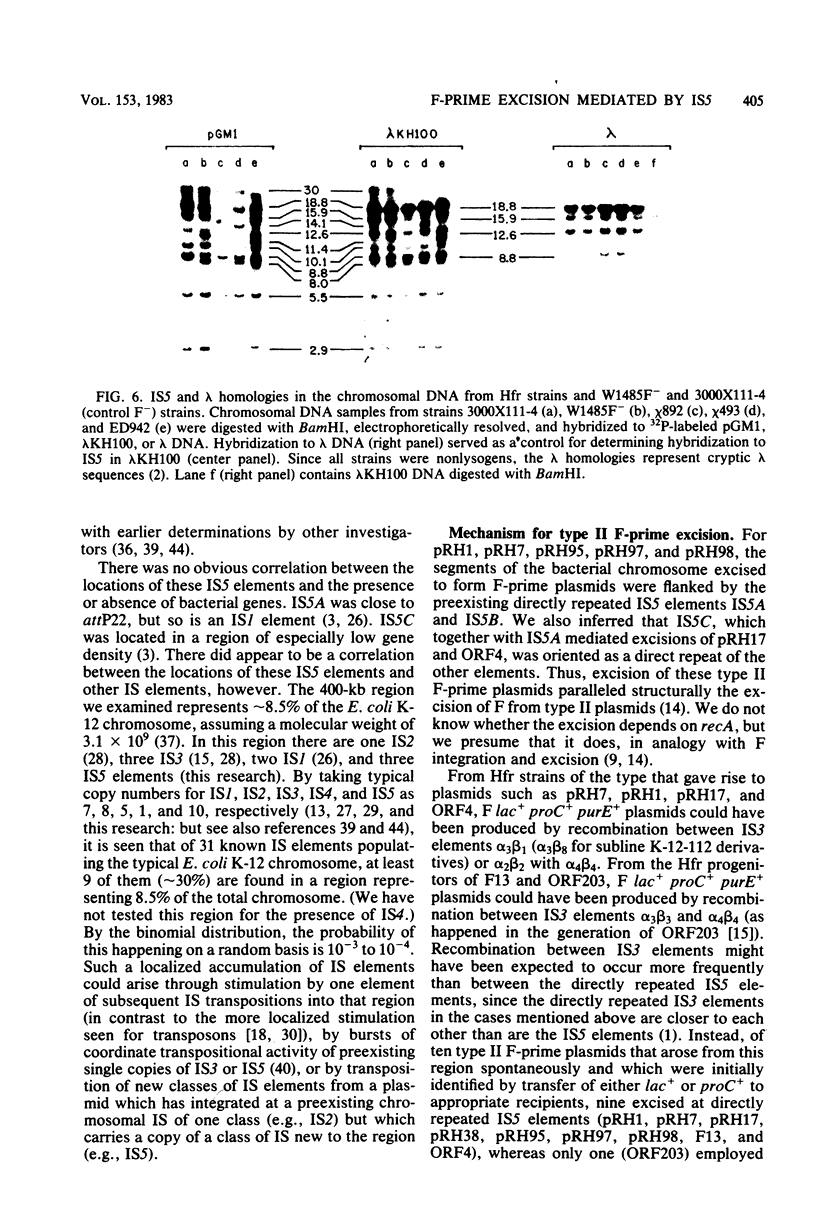
- Mullins J. I., Casey J. W., Nicolson M. O., Davidson N. Sequence organization of feline leukemia virus DNA in infected cells. Nucleic Acids Res. 1980 Aug 11;8(15):3287–3305. doi: 10.1093/nar/8.15.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. Inactivation of the Serratia marcescens gene for the lipoprotein in Escherichia coli by insertion sequences, IS1 and IS5; sequence analysis of junction points. Mol Gen Genet. 1981;183(1):107–114. doi: 10.1007/BF00270147. [DOI] [PubMed] [Google Scholar]
- Ohtsubo E., Hsu M. T. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli: structure of F100, F152, and F8 and mapping of the Escherichia coli chromosomal region fep-supE-gal-attlambda-uvrB. J Bacteriol. 1978 Jun;134(3):778–794. doi: 10.1128/jb.134.3.778-794.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak B., Lusky M., Hable M. Expression of two proteins from overlapping and oppositely oriented genes on transposable DNA insertion element IS5. Nature. 1982 May 13;297(5862):124–128. doi: 10.1038/297124a0. [DOI] [PubMed] [Google Scholar]
- Read H. A., Das Sarma S., Jaskunas S. R. Fate of donor insertion sequence IS1 during transposition. Proc Natl Acad Sci U S A. 1980 May;77(5):2514–2518. doi: 10.1073/pnas.77.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Scaife J. Episomes. Annu Rev Microbiol. 1967;21:601–638. doi: 10.1146/annurev.mi.21.100167.003125. [DOI] [PubMed] [Google Scholar]
- Schoner B., Kahn M. The nucleotide sequence of IS5 from Escherichia coli. Gene. 1981 Aug;14(3):165–174. doi: 10.1016/0378-1119(81)90112-8. [DOI] [PubMed] [Google Scholar]
- Schoner B., Schoner R. G. Distribution of IS5 in bacteria. Gene. 1981 Dec;16(1-3):347–352. doi: 10.1016/0378-1119(81)90093-7. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Construction and BamHL analysis of chimeric plasmids containing EcoRL DNA fragments of the F sex factor. Plasmid. 1978 Feb;1(2):174–186. doi: 10.1016/0147-619x(78)90037-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thompson R., Hughes S. G., Broda P. Plasmid identification using specific endonucleases. Mol Gen Genet. 1974;133(2):141–149. doi: 10.1007/BF00264835. [DOI] [PubMed] [Google Scholar]