Abstract
New neurons are continually produced in the adult mammalian brain from progenitor cells located in specific brain regions, including the subgranular zone (SGZ) of the dentate gyrus of the hippocampus. We hypothesized that neurogenesis occurs in the canine brain and is reduced with age. We examined neurogenesis in the hippocampus of 5 young and 5 aged animals using doublecortin (DCX) and bromodeoxyuridine (BrdU) immunostaining. The total unilateral number of new neurons in the canine SGZ and granule cell layer (GCL) was estimated using stereological techniques based upon unbiased principles of systematic uniformly random sampling. Animals received 25 mg/kg of BrdU once a day for 5 days and were euthanized 9 days after the last injection. We found evidence of neurogenesis in the canine brain and that cell genesis and neurogenesis are greatly reduced in the SGZ/GCL of aged animals compared to young. We further tested the hypothesis that an antioxidant fortified food or behavioral enrichment would improve neurogenesis in the aged canine brain and neurogenesis may correlate with cognitive function. Aged animals were treated for 2.8 years and tissue was available for 6 that received the antioxidant food, 5 that received the enrichment and 6 receiving both treatments. There were no significant differences in the absolute number of DCX or DCX-BrdU neurons or BrdU nuclei between the treatment groups compared to control animals. The number of DCX-positive neurons and double labeled DCX-BrdU-positive neurons, but not BrdU-positive nuclei alone, significantly correlated with performance on several cognitive tasks including spatial memory and discrimination learning. These results suggest that new neurons in the aged canine dentate gyrus may participate in modulating cognitive functions.
Keywords: Canine, brain, aging, hippocampus, neurogenesis, antioxidants, enrichment
INTRODUCTION
Neurogenesis in the postnatal adult mammalian brain has been reported in rats (Kuhn, Dickinson-Anson & Gage, 1996), mice (Kempermann, Kuhn & Gage, 1997), tree shrews (Gould, McEwen, Tanapat, Galea & Fuchs, 1997), guinea pigs (Altman & Das, 1967), rabbits (Gueneau, Privat, Drouet & Court, 1982), cats (Wyss & Sripanidkulchai, 1985), monkeys (Gould, Tanapat, McEwen, Flugge & Fuchs, 1998; Gould, Reeves, Fallah, Tanapat, Gross & Fuchs, 1999c; Rakic & Nowakowski, 1981) and humans (Erikson, Perfilieva, Bjork-Eriksson, Alborn, Nordborg, Peterson & Gage, 1998). The hippocampal formation continually produces new neurons in the dentate gyrus subgranular zone (SGZ) in adult mammals. These new neurons display passive membrane properties, action potentials and functional synaptic inputs similar to those found in mature dentate granule cells (van Praag, Schinder, Christie, Toni, Palmer & Gage, 2002). In hippocampal neurogenesis, although many of the new neurons die shortly after they are born, the surviving neurons migrate into the molecular layer and send axons to the CA3 region to form mossy fibers and project dendrites to the outer molecular layer (Hastings & Gould, 1999). These new cells start to receive synaptic inputs from the cortex to become functionally integrated in existing circuits (Jessberger & Kempermann, 2003; van Praag et al., 2002). The addition of new neurons is one way in which the brain can modify its own functional circuitry in response to challenges.
Neurogenesis is a dynamic process that can be modulated by various exogenous stimuli. Enriched environments (Kempermann et al., 1997), running wheel exercise (van Praag, Kempermann & Gage, 1999), hippocampal dependent learning (Gould, Beylin, Tanapat, Reeves & Shors, 1999b), and dietary restriction (Lee, Duan, Long, Ingram & Mattson, 2000; Lee, Seroogy, & Mattson, 2002) increase neurogenesis in the adult hippocampus. Other stimuli reduce hippocampal neurogenesis including stress (Gould et al., 1997; Gould et al., 1998) and social isolation (Lu, Bao, Chen, Xia, Fan, Zhang, Pei & Ma, 2003). Reduced levels of neurogenesis are also observed with advanced age in rats (Kuhn et al., 1996; McDonald & Wojtowicz, 2005), mice (Kempermann, Kuhn & Gage, 1998) and monkeys (Gould et al., 1999c). The aging brain, however, retains neuroplasticity and several environmental stimuli can ameliorate the age-related decline in neurogenesis including physical exercise (Kronenberg, Bick-Sander, Bunk, Wolf, Ehninger & Kempermann, 2006) and environmental enrichment (Kuhn et al., 1996).
The modulation of neurogenesis can be achieved through two dissociable pathways, progenitor cell proliferation or the survival of the newly generated cells. Olson, Eadie, Ernst and Christie (2006) reported that environmental enrichment has a survival promoting effect on newly generated cells while physical exercise leads to an increase in progenitor cell proliferation. Nutritional manipulations can also affect neurogenesis through both pathways. Vitamin E (alpha-tocopherol) has antioxidant properties and supplementation in rats increases the survival of newborn cells in the dentate gyrus but inhibits cell proliferation (Cecchini, Ciaroni, Ferri, Ambrogini, Cuppini, Santi & Del Grande, 2003; Cuppini, Ciaroni, Cecchini, Ambrogini, Ferri, Cuppini, Ninfali & Del Grande, 2002).
Although the functions of hippocampal neurogenesis are not fully understood, several studies have reported that hippocampal neurogenesis may be involved in specific types of hippocampal-dependent learning and memory (Bruel-Jungerman, Laroche & Rampon, 2005; Drapeau, Mayo, Aurousseau, Le Moal, Piazza & Abrous 2003; Kemperman & Gage, 2002; Gould et al., 1999b; Shors, Miesegaes, Beylin, Zhao, Rydel & Gould, 2001; Shors, Townsend, Zhao, Kozorovitskiy & Gould, 2002). Some authors postulate that it is specifically the survival of new neurons that is linked to cognitive function and not progenitor proliferation (Bizon & Gallagher, 2003; Merrill, Karim, Darraq, Chiba & Tuszynski, 2003; Wati, Kudo, Qiao, Kuroki & Kanba, 2006).
The present study had three goals. First, we wanted to establish that neurogenesis occurs in the canine hippocampus and determine whether changes occur with age. Second, we examined whether hippocampal neurogenesis in the aged canine brain could be modified by two interventions, an antioxidant fortified food and behavioral enrichment. Third, we wanted to determine whether there is an association between the extent of hippocampal neurogenesis and cognitive performance in the aged canine.
We used two markers, doublecortin (DCX) and bromodeoxyuridine (BrdU), that have previously been shown to reflect levels of neurogenesis and the presence of newly born cells respectively (Couillard-Despres, Winner, Schaubeck, Aigner, Vroemen, Weidner, Bogdahn, Winkler, Kuhn & Aigner, 2005; Rao & Shetty, 2004). DCX is a 40 kDa microtubule-associated protein expressed exclusively in neurons that has been used as a marker of neurogenesis in the adult dentate gyrus of mice (Jin, Sun, Xie, Batteur, Mao, Smelick, Logvinova & Greenberg, 2003), rats (Brown, Couillard-Despres, Cooper-Kuhn, Winkler, Aigner & Kuhn, 2003; Rao, Hattiangady, Abdel-Rahman, Stanley & Shetty, 2005; Rao, Hattiangady & Shetty, 2006), monkeys (Tonchev, Yamashima, Zhao, Okano & Okano, 2003), and humans (Jin, Peel, Mao, Xie, Cottrell, Henshell & Greenberg, 2004). BrdU, a thymidine analogue incorporated into newly synthesized DNA, dates the birth of newly generated cells and marks all cell types including neurons, glia and endothelial cells. DCX can colabel with BrdU, which indicates that the neurons were born at the time when BrdU was injected and available for incorporation. This does not imply any special function but simply confirms that the DCX immunopositive neurons were born when BrdU was available. Thus, BrdU labeled cells represent cells born at the time of BrdU administration and reflect only a subpopulation of the new cells produced (Cameron & McKay, 2001). DCX immunopositive neurons without BrdU labeling represent neurons that were born prior to, in between or following the last injection of BrdU prior to euthanasia, when BrdU is not available for incorporation (Brown et al., 2003). Thus, our primary measure of neurogenesis was DCX alone and secondarily used the subset of DCX-BrdU double labeled neurons to confirm that the birth of these neurons coincided with BrdU availability. BrdU alone was used as a general marker of cell proliferation.
MATERIALS AND METHODS
Aging Study Animals
The brains from 5 aged (13.0 – 15.0 years old) and 5 young (3.4 - 4.5 yrs old) Beagles from the Lovelace Respiratory Research Institute (Albuquerque, NM) breeding colony were used to examine age-related differences in the number of new neurons in the hippocampus.
Treatment Study Animals
After 2.8 years of antioxidant fortified food and/or behavioral enrichment, tissue was available for 6 Beagles (11.0 – 15.0 years old) that received the antioxidant fortified food, 5 Beagles (12.2 – 14.4 years old) that received the behavioral enrichment, 6 Beagles (10.7 – 14.8 years old) receiving both treatments and 5 Beagles (13.0 – 15.0 years old) that did not receive either treatment. The 5 animals that did not receive either treatment were the same ones used for the age comparisons.
Animals in the treatment study have been described in several publications ((Milgram, Head, Muggenburg, Holowachuk, Murphey, Estrada, Ikeda-Douglas, Zicker & Cotman, 2002a; Milgram, Head, Zicker, Ikeda-Douglas, Murphey, Muggenburg, Siwak, Tapp & Cotman, 2005; Milgram, Head, Zicker, Ikeda-Douglas, Murphey, Muggenburg, Siwak, Tapp, Lowry & Cotman, 2004; Milgram, Zicker, Head, Muggenburg, Murphey, Ikeda-Douglas & Cotman, 2002b; Siwak, Tapp, Head, Zicker, Murphey, Muggenburg, Ikeda-Douglas, Cotman & Milgram, 2005) as part of a longitudinal study on aging and the effects of chronic treatment with behavioral enrichment and/or an antioxidant fortified food on cognitive function. The treatment study animals were cognitively assessed over the course of the study, which provided behavioral correlates to assess the functional significance of cell counts.
Antioxidant Fortified Food
The control and antioxidant fortified foods were formulated to meet the nutrient profile for the American Association of Feed Control Officials recommendations for adult dogs (AAFCO, 1999). Control and test foods were identical in composition, other than inclusion of a broad-based antioxidant and mitochondrial cofactor supplementation to the test food. The control and fortified foods had the following differences in formulation on an as fed basis respectively: dl-alpha-tocopherol acetate, (120 ppm vs approximately 1000 ppm), l-carnitine (< 20 ppm vs 250-300 ppm), dl-alpha-lipoic acid (<20 ppm vs approximaetly 120 ppm), ascorbic acid as Stay-C (< 30 ppm vs approximately 80-100 ppm), and 1% inclusions of each of the following (1 to 1 exchange for corn): spinach flakes, tomato pomace, grape pomace, carrot granules and citrus pulp. The food was produced by an extrusion process and was fed for no more than 6 months before a new lot was milled.
Behavioral enrichment
The behavioral enrichment protocol consisted of social enrichment, by housing animals in pairs, environmental enrichment, by providing play toys on a weekly rotation, physical enrichment, by providing two 20-minute outdoor walks per week, and cognitive enrichment, through continuous cognitive testing. The cognitive enrichment consisted of a landmark discrimination task within 2 weeks of starting treatment (Milgram, Adams, Callahan, Head, Mackay, Thirlwell & Cotman, 1999; Milgram et al., 2002a), an oddity discrimination task after 6 months of treatment (Milgram et al., 2002b), and size concept learning after 1.5 years of treatment (Siwak et al., 2005; Tapp, Siwak, Head, Cotman, Murphey, Muggenburg, Ikeda-Douglas & Milgram, 2004). All animals, regardless of treatment group were evaluated on a test of spatial memory annually; at baseline, 1 and 2 years after the start of treatment (Chan, Nippak, Murphey, Ikeda-Douglas, Muggenburg, Head, Cotman & Milgram, 2002), size discrimination and reversal learning at the 1 year timepoint (Milgram et al., 2004, 2005; Tapp, Siwak, Estrada, Head, Muggenburg, Cotman & Milgram, 2003), and black/white discrimination and reversal at the 2 year timepoint (Fox, 1971; Milgram et al., 2005). Animals were euthanized at the end of the black/white discrimination and reversal learning task.
BrdU Treatment
Prior to the end of the study and after 2.8 years of treatment, animals were given 5 daily injections of 25 mg/kg of BrdU i.v. Animals were euthanized 9 days after the last injection (14 days after the first injection; personal communication with F. H. Gage).
Tissue Preparation
While under anesthesia (5% isoflurane), animals were ex-sanguinated by cardiac puncture and within 15 minutes the brain was removed from the skull. The brain was sectioned midsagitally, with the entire left hemisphere being immediately placed in 4% paraformaldehyde for 48-72 hours at 4°C then transferred to phosphate buffered saline with 0.05% sodium azide at 4°C for long term storage. The left hemisphere was sent to NeuroScience Associates for sectioning. NeuroScience Associates treated individual canine hemispheres with 20% glycerol and 2% dimethylsulfoxide to prevent freezeartifacts and subsequently embedded two hemispheres (i.e. two animals) per block in a gelatin matrix using MultiBrain TechnologyTM (NeuroScience Associates, Knoxville, TN; www.neuroscienceassociates.com/multibrain.htm). After curing in formaldehyde, the block of embedded hemispheres was rapidly frozen by immersion in isopentane chilled to -70°C with crushed dry ice and mounted on a freezing stage of an AO 860 sliding microtome. The MultiBrainTM block was sectioned coronally at 40μm. All sections cut (none were discarded) were collected sequentially into a 4x6 array of containers which were filled with Antigen Preserve solution (50% PBS pH 7.0, 50% Ethylene glycol, 1% Polyvinyl Pyrrolidone) for sections to be immunohistochemically stained. At the completion of sectioning, each container held a serial set of one-of-every-24th section (or one section every 960μm). Approximately 1400 sections were generated per hemisphere. One container of free-floating sections was randomly selected per animal and all sections in that container that included the hippocampus were collected for immunohistochemical staining.
Immunohistochemistry
Standard immunohistochemical methods were used and have been published elsewhere (Cummings, Head, Afagh, Milgram & Cotman, 1996; Head, Callahan, Muggenburg, Cotman & Milgram, 1998). 40 μm thick free-floating sections were first pretreated with formamide (50%/2xSSC for 2 hours at 65°C) to denature DNA wrapped around histones to reveal the BrdU epitopes. The tissue was then incubated in anti-BrdU antibody (Vector Laboratories Inc, Burlingame, California 1:100) overnight at room temperature. In order to identify new neuron phenotypes of BrdU positive nuclei, the tissue was subsequently incubated in anti-goat-doublecortin (DCX) at 1:500 (50:50 C-18 and N-19 antibodies; Santa Cruz Biotechnology Inc, Santa Cruz, California) overnight at room temperature. Bound secondary biotinylated anti-mouse or anti-goat IgG (Vector Laboratories, Burlingame, CA) antibody was detected using ABC peroxidase kits. BrdU immunostaining was visualized using a DAB substrate kit and DCX with an SG Blue substrate kit, all from Vector Laboratories (Burlingame, CA). Free-floating sections were mounted onto gelatin coated slides, dehydrated through ethanols and coverslipped with DePeX mounting medium. Control experiments where primary or secondary antibody was omitted resulted in negative staining. To reduce variability across immunohistochemistry experiments, batches of 3 sections from each animal were stained simultaneously. Penetration of the antibody was complete through the section since labeled cells were detectable in deeper layers within a section. There were no significant differences in post-processing tissue thickness between the groups of animals in this tissue set (Siwak-Tapp, Head, Muggenburg, Milgram & Cotman, 2006).
Stereological Evaluation
Total unilateral BrdU-positive nuclei, DCX-positive new neurons and DCX-BrdU double labeled neurons were counted in the SGZ and GCL of the dentate gyrus of the hippocampus. We used DCX as a marker of new neurons because studies in rats show that peak levels of DCX-BrdU double labeling occurs 7 days after BrdU administration while mature neuronal markers do not appear until 28 days after BrdU injection (McDonald & Wojtowicz, 2005). Our protocol assessed cell numbers 9 days after the last BrdU injection. Counting was performed using an Olympus BX50 microscope (Olympus, Tokyo, Japan) equipped with a motorized stage (Prior Scientific Inc., Rockland, MA) connected to a digital microcator (Heidenhain, Germany), and a video camera (JVC, Japan) connected to a computer with the CAST-GRID software package (Olympus, Copenhagen, Denmark).
Individual estimates of the total unilateral neuron number (N) for each region were calculated according to the following formula: N = ∑Q− × 1/ssf × 1/asf × 1/tsf where ∑Q− is the sum of counted neurons, ssf is the section sampling fraction, asf is the area sampling fraction, and tsf is the thickness sampling fraction. The thickness of the section was used as the height of the disector box making the tsf equal to 1. Optical fractionator rules typically require the use of guard zones at the upper and lower surfaces of the section. In the current study, we used a modified optical fractionator technique in which we did not use guard zones making the height of the counting frame equal to the section thickness. Several authors have used full section thickness and indicate that undercounting may occur (Czeh, Simon, van der Hart, Schmelting, Hesselink & Fuchs, 2005; Hatton & von Bartheld, 1999; Ngwenya, Peters & Rosene, 2005). This counting procedure may have yielded results that were a slight undercount because of the inability to compensate for lost caps or cells damaged at the inclusion surface but all groups would be equally affected (Lister, Blatt, DeBassio, Kemper, Tonkiss, Galler & Rosene, 2005; Siwak-Tapp et al., 2006). The top plane was used as the exclusion plane. Due to the low numbers of both BrdU nuclei and DCX neurons, the entire SGZ and GCL were used as the counting frame making the asf = 1. The ssf was 1/24. Estimates were based on counting BrdU-positive nuclei or DCX-positive cell bodies as they came into focus.
Data Analysis
To detect age differences in neurogenesis, a multivariate analysis of variance (MANOVA) was performed with age as a between subject variable and DCX, BrdU, DCX-BrdU numbers and the proportion of BrdU nuclei expressing DCX as the dependent measures. For the treatment study, a 2-way MANOVA was performed with food and enrichment as the between subject factors and DCX, BrdU, DCX-BrdU numbers and the proportion of BrdU nuclei expressing DCX as the dependent measures. The alpha level was .05. Pearson product correlations were used to examine the relationship between the counts and cognitive data available from the study. Due to the high number of correlations performed we considered only p< .025 to be more conservative.
RESULTS
Importantly, we showed that neurogenesis occurs in the adult canine brain. Figure 1 shows the immunolabeling in the SGZ/GCL of the hippocampus in a young (A,C) and aged (B,D) animal. Only a subset of DCX-positive neurons contains BrdU (arrow). The confocal images (E,F,G) demonstrate co-localization of DCX and BrdU in the same new neurons.
Figure 1.
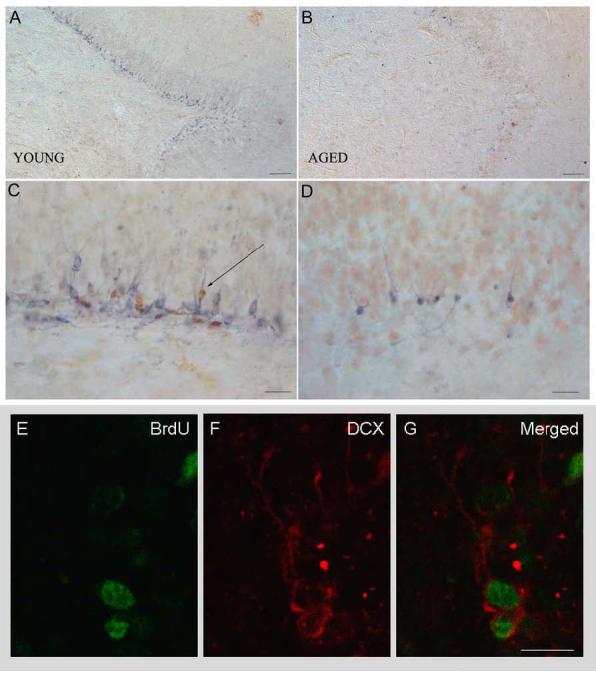
DCX and BrdU immunohistochemical staining in the SGZ and GCL of the hippocampus in a young (A,C) and aged (B,D) Beagle. Only a subset of DCX-positive neurons colabeled with BrdU (arrow). Scale in A and B is 100 μm and scale in C and D is 20 μm. Confocal image showing colocalization. Scale in E, F and G is 5 μm.
Aging Study
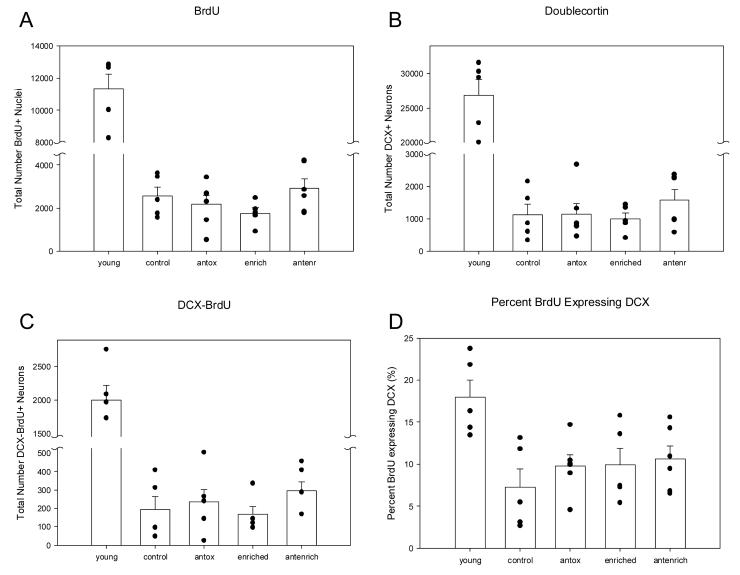
Table 1 and Figure 2 show that there are significant age-related decreases in cell genesis and neurogenesis in the canine SGZ/GCL. The number of BrdU-positive nuclei, F(1, 9) = 74.00, p = .000 026 (Fig 2A), DCX-immunoreactive neurons F(1,9) = 125.66, p = .000 004 (Fig 2B), and double labeled DCX-BrdU positive neurons, F(1,9) = 60.65, p = .000 053 (Fig 2C) were lower in aged animals compared to young, consistent with findings from other animal models. The proportion of BrdU cells expressing DCX was significantly higher in young adults compared to old animals, F(1,9) = 12.64, p = .007 (Fig 2D).
Table 1.
Estimated individual unilateral new neuron (DCX and DCX-BrdU) and new cell (BrdU) numbers in the SGZ and GCL of the hippocampus in young and aged Beagles.
| Age | DCX | BrdU | DCX-BrdU | %DCX-BrdU/BrdU | Sections |
|---|---|---|---|---|---|
| 3.4 | 30,288 | 10,032 | 1,440 | 14.35 | 10 |
| 4.1 | 20,040 | 8,280 | 1,968 | 23.77 | 11 |
| 4.2 | 22,848 | 12,840 | 1,728 | 13.46 | 12 |
| 4.4 | 29,400 | 12,792 | 2,088 | 16.32 | 11 |
| 4.5 | 31,560 | 12,648 | 2,760 | 21.82 | 11 |
| Mean | 26,827* | 11,318* | 1,997* | 17.94* | 11 |
| SD | 5,071 | 2,070 | 493 | 4.60 | |
| 13.0 | 600 | 1,752 | 96 | 5.48 | 9 |
| 13.6 | 2,160 | 3,456 | 408 | 11.81 | 13 |
| 14.2 | 1,632 | 3,624 | 96 | 2.65 | 10 |
| 14.5 | 336 | 1,560 | 48 | 3.08 | 12 |
| 15.0 | 864 | 2,376 | 312 | 13.13 | 9 |
| Mean | 1,118 | 2,554 | 192 | 7.23 | 10.6 |
| SD | 757 | 952 | 158 | 4.93 | |
| Old as a percentage of young: | |||||
| 4.17% | 22.56% | 9.62% | |||
young significantly greater than aged animals p<0.05
SD = standard deviation
Figure 2.
Graphs plotting the numbers of (A) BrdU-positive nuclei, (B), DCX-positive new neurons, (C) double labeled DCX-BrdU-positive neurons, and (D) the proportion of BrdU cells expressing DCX in young, aged and treated-aged canines. Young animals had significantly higher number of cells and proportions compared to aged animals (p < .05). Only the proportion of BrdU cells expressing DCX (D) was higher in the treatment groups compared to age-matched controls even though this did not achieve statistical significance. Control = aged untreated animals; antox = aged animals receiving the antioxidant fortified food; enriched = aged animals receiving behavioral enrichment; antenr = aged animals receiving both the antioxidant food and behavioral enrichment. Errors bars = SEM.
Treatment Study
Once we established that neurogenesis occurs in the aged canine brain, we then examined the potential to modify neurogenesis in the aged brain and whether neurogenesis correlated with cognitive function. First, we looked for differences between the discrete treatment groups to determine whether the food or enrichment paradigms were able to modify neurogenesis in the aged canine brain compared to aged controls. Second, we examined the relationship of the neuron and nuclei counts with cognitive performance to test the hypothesis that neurogenesis may be related to cognition as suggested by some rodent studies.
Group Differences
In the aged dogs of the treatment study, there were no statistically significant differences in the enrichment condition for total number of BrdU-positive nuclei, F(1, 21) = .007, p = .932, DCX-immunoreactive neurons, F(1, 21) = .238, p = .632, or DCX-BrdU labeled neurons, F(1, 21) = .095, p = .761, (Fig 2A-C). No statistically significant differences were obtained with the antioxidant fortified food condition for total number of BrdU-positive nuclei, F(1, 21) = .923, p = .349, DCX-immunoreactive neurons, F(1, 21) = .936, p = .346, or DCX-BrdU labeled neurons, F(1, 21) = 2.173, p = .158. These data indicate that the behavioral enrichment and antioxidant fortification did not have a clear measurable effect on the number of new nuclei or new neurons. All three treatment groups tended to have a higher proportion of BrdU nuclei express the neuronal marker DCX but this did not achieve statistical significance, F(1,21) = .678, p = .577 (Fig 2D).
Cognition: Annual Measures for All Animals
We then examined the data for a potential relationship between new cell counts and individual scores on cognitive tasks administered during the ∼3 years of the study prior to euthanasia. Although it is unlikely that the new cells were present during the administration of the cognitive tasks, it may suggest that animals with higher rates of or potential for neurogenesis are the ones that perform superiorly on cognitive tasks. We hypothesized that there would be a link between the amount of neurogenesis and cognition.
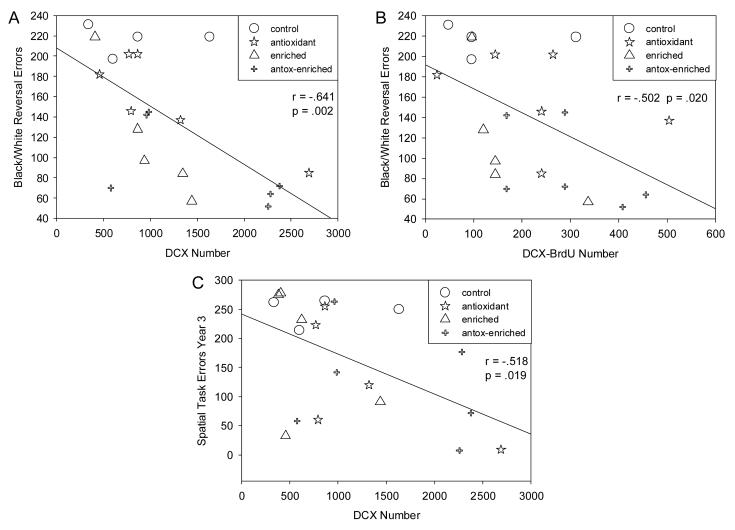
Table 2 shows the correlations between cell counts and errors scores for the baseline and treatment periods of the study. The table shows that more tasks significantly correlate with the counts as the treatment period progresses. The correlations begin to appear after the treatments were started and no significant correlations appeared with the baseline data. More significant correlations appear in the second year of the treatment period than the first year. The correlations are also only for neuron counts, DCX-positive neurons or double labeled DCX-BrdU-positive neurons, and not for BrdU-positive nuclei alone. The correlations are all negative indicating that error scores increase as new neuron numbers decrease. We see a consistent effect despite the strength of the association.
Table 2.
Correlations between cell counts in the SGZ/GCL of the aged canine hippocampus and error scores on the annual measures of cognitive function.
| Timepoint | Task | DCX | DCX-BrdU | BrdU | |||
|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | ||
| BASELINE | Object discrimination | .022 | .924 | −.280 | .207 | .134 | .553 |
| Object discrimination reversal | −.205 | .359 | −.320 | .146 | .028 | .903 | |
| Spatial memory | −.131 | .572 | −.389 | .082 | −.124 | .591 | |
| YEAR 1 | Spatial memory year 1 | −.710 | <.0001 | −.331 | .143 | −.318 | .160 |
| Size discrimination | −.398 | .067 | −.465 | .029 | −.399 | .066 | |
| Size discrimination reversal | −.255 | .253 | −.487 | .021 | −.337 | .125 | |
| YEAR 2 | Spatial memory year 2 | −.518 | .019 | −.438 | .054 | −.358 | .121 |
| Black/white discrimination | −.440 | .046 | −.403 | .070 | −.257 | .261 | |
| Black/white discrimination reversal | −.641 | .002 | −.502 | .020 | −.344 | .127 | |
Figure 3 graphs the association between DCX (Fig 3A) and DCX-BrdU (Fig 3B) labeling with a black/white reversal learning task, the task administered closest to the time of death. Figure 3C also shows the relationship between DCX and a hippocampal dependent spatial memory task administered immediately prior to the black/white task.
Figure 3.
Higher numbers of new neurons in the SGZ and GCL of the aged canine hippocampus correlated with lower error scores on the black-white discrimination reversal learning task (A, B) and the spatial memory task (C).
Cognition: Enrichment Measures for a Subset of Animals
Behaviorally enriched animals were provided with additional cognitive tasks and several significant correlations also appeared when only the animals given the enrichment tasks were considered (Table 3). We see consistent effects even when a smaller number of subjects was considered. Only DCX or DCX-BrdU labeled cells showed significant correlations. All enrichment tasks were conducted after the start of treatment.
Table 3.
Correlations between cell counts in the SGZ/GCL of the aged canine hippocampus and error scores on cognitive tasks administered to the behaviorally enriched animals only.
| Timepoint | Task | DCX | DCX-BrdU | BrdU | n | |||
|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | |||
| BASELINE | Landmark discrimination 0 | −.515 | .105 | −.526 | .096 | −.414 | .205 | 11 |
| Landmark discrimination 1 | −.549 | .080 | −.500 | .118 | −.500 | .117 | 11 | |
| YEAR 1 | Oddity discrimination 1 | −.660 | .027 | −.373 | .259 | −.214 | .527 | 11 |
| Oddity discrimination 2 | −.642 | .033 | −.641 | .034 | −.449 | .166 | 11 | |
| Oddity discrimination 3 | −.352 | .319 | −.505 | .137 | −.468 | .173 | 10 | |
| Oddity discrimination 4 | −.200 | .579 | −.329 | .353 | −.381 | .278 | 10 | |
| YEAR 2 | Size concept blocks | −.044 | .911 | .057 | .884 | −.368 | .329 | 9 |
| Size concept balls | −.813 | .008 | −.665 | .051 | −.464 | .208 | 9 | |
| Size concept bottles | −.702 | .052 | −.532 | .175 | −.538 | .169 | 8 | |
DISCUSSION
Neurogenesis, reflected by either the number of cells positive for DCX or by the number of double labeled DCX-BrdU cells, decreases in the aged canine hippocampus. Neurogenesis also appears to be related to cognitive function in the aged canine since lower error scores correlated with higher new neuron numbers.
Aging
Neurogenesis and cell genesis decrease with age in a canine model of brain aging. Aged animals had fewer DCX-positive neurons and BrdU-positive nuclei than young adults. Overall neurogenesis is reduced substantially by 90-96% in the aged canine even though the number of granule layer neurons does not change with age (Siwak-Tapp et al., 2006). DCX counts include neurons that are born prior to, in between and after the BrdU injections while the DCX-BrdU counts only consider neurons that were born during the 5-day period of BrdU injections, not counting any born in between the BrdU injections that were not able to incorporate BrdU. Both DCX and DCX-BrdU colabeling of new neurons indicate neurogenesis is greatly reduced in the aged canine. This is consistent with work published in other mammals including rats (Kuhn et al., 1996; McDonald & Wojtowicz 2005), mice (Kempermann et al., 1998) and monkeys (Gould et al., 1999c). In aged rats, Kuhn et al. (1996) found that neurogenesis is reduced to 12% of the young adult level and McDonald and Wojtowicz (2005) also reported a 94% reduction in neurogenesis, even though the overall number of granule cells is preserved (Rapp & Gallagher, 1996).
The reduction in neurogenesis with age may be due to a decrease in neural precursor proliferation or a decrease in new neuron survival (Kuhn et al., 1996) or a combination of both factors. Our data indicate that there is a decrease in progenitor cell proliferation in the aged canine since BrdU labeled cells in the aged animals were only 23% of the level in young adults. We also found that a smaller proportion of BrdU-positive progenitor cells expressed the new neuron marker DCX (18% in young compared to 7% in old) suggesting that decreased new neuron survival contributed to the age-related decrease in neurogenesis as well.
We see a similar drop in neurogenesis with age as is reported in rodents suggesting that a similar mechanism may be disrupting neurogenesis in both species. A unique feature of the canine model of brain aging is the natural deposition of beta-amyloid and beta-amyloid itself may interfere with neurogenesis (Haughey, Nath, Chan, Borchard, Rao & Mattson, 2002). Since rodents, however, do not naturally deposit beta-amyloid and the decline in neurogenesis is similar to the canine, beta-amyloid is not likely the main mechanism or initiating factor contributing to the age-related decrease in neurogenesis.
Other factors that show changes with age may be affecting neurogenesis. For example, reactive gliosis may lead to reduced neurogenesis. Astrocytes display increasing activation with age with increases in expression of glial fibrillary acidic protein and vimentin, which are both intermediate filament proteins (Rozovsky, Wei, Morgan & Finch, 2005). Old mice lacking both glial fibrillary acidic protein and vimentin have increased post-traumatic synaptogenesis and higher rates of neurogenesis (Larsson, Wilhemsson, Pekna & Pekny, 2004; Wilhelmsson, Li, Pekna, Berthold, Blom, Eliasson, Renner, Bushong, Elissman, Morgan & Pekny, 2004). Additionally, the production of new neurons in the dentate gyrus depends on multiple factors including cell death in the GCL as a stimulatory factor (Gould & Tanapat, 1997), hormones such as glucocorticoids (Gould et al., 1998), growth factors (Lichtenwalner, Forbes, Bennett, Lynch, Sonntag & Riddle, 2001), and neurotransmitters (Gould, 1999a). Changes in any of these factors could contribute to the decrease in neurogenesis with age.
Antioxidants and Enrichment
We next examined the effects of behavioral enrichment and an antioxidant fortified food on neurogenesis in the aged canine brain. Our previous work shows that the behavioral enrichment and antioxidant fortification produce a significant improvement in cognitive function with the combined treatment being additive (Milgram et al., 2002a, 2002b, 2004, 2005; Siwak et al., 2005). Thus, we hypothesized that treated animals might show some restoration of age-related decreases in neurogenesis. Our data indicate that the behavioral enrichment and antioxidant fortification do not have a clear measurable effect on the absolute number of new nuclei or new neurons in the aged canine brain. All 3 treatment groups, however, tended to have a higher proportion of BrdU nuclei expressing the new neuron marker DCX, although not statistically significant, suggesting that new neuron survival may be promoted by both treatment paradigms.
Cognitive Function
In support of this possibility we report several significant correlations between cognitive scores and new neuron estimates. All of the correlations with the error scores were negative indicating that aged animals with higher numbers of new neurons were the ones that performed better (fewer errors) on the cognitive tasks during the study. The correlations were only significant or approached significance for DCX and DCX-BrdU counts indicating that it is neurogenesis specifically that is involved in cognitive function, not the progenitor cell population marked by BrdU alone, which could include glia or endothelial cells. Several other authors have reported correlations of neurogenesis with cognitive ability when the survival of new neurons is considered and not progenitor proliferation (Bizon & Gallagher, 2003; Merrill et al., 2003; Wati et al., 2006).
It is possible that the cognitive testing itself led to higher rates of neurogenesis (Gould et al., 1999b). The treatments may have led to improved learning through other mechanisms and as a result of the improved learning there was an increase in neurogenesis. There is still much controversy in the literature surrounding this question. Our data simply support the suggestion that learning and memory are related to neurogenesis and provide no information about which event occurs first. Although, one argument against learning causing the increase in neurogenesis is that the antioxidant treated animals received the same cognitive testing as the controls but still showed a slightly higher proportion of BrdU cells expressing DCX and a stronger association between cognitive score and neurogenesis. This suggests that the treatments might be directly contributing to an increase in neurogenesis rather than just simply through learning-mediated plasticity mechanisms.
Significant or near significant correlations between the extent of neurogenesis and cognitive test scores only appeared after the treatments were initiated. Correlations between measures of neurogenesis with baseline performance were not significant suggesting that this difference is related to the treatments since it was not present prior to treatment. Neurogenesis may be one mechanism to increase resistance to the development of age-related cognitive problems. Although it is unlikely that the new neurons counted were present during the administration of the cognitive tasks, it suggests that animals with higher rates of or potential for neurogenesis perform superiorly on cognitive tasks.
Technical Considerations
The decreased number of BrdU nuclei observed in the aged dogs could be affected by differences in the concentrations of BrdU entering the brains of young and aged dogs since our injections were peripheral and a non-saturating dose of BrdU was used. Studies in embryonic and adult rats found that a higher systemic dose of BrdU is needed to optimally label S-phase cells in the adult brain compared to the embryonic brain (Cameron & McKay, 2001). This is attributed to the development of the blood-brain barrier, which limits entry of BrdU into the brain when injected systemically (Cameron & McKay, 2001). Additional mechanisms may be relevant with advancing age. BrdU entry into the brain depends upon several factors including nucleoside transport mechanisms, blood flow to the brain or other pharmacokinetic variables, which could be affected by age (Cameron & McKay, 2001). Younger, healthier dogs may have increased blood flow to the brain making BrdU more available (Tapp et al., 2005). The integrity of the blood-brain barrier, however, deteriorates with age in dogs (Morita et al., 2005), which suggests that BrdU may also enter the aged brain more easily. Thus, although our data suggest that progenitor cell proliferation is reduced with age, other considerations related to the concentrations of BrdU entering the brain may affect this result.
Additionally, treatments that affect the blood-brain barrier or blood flow to the brain could also alter BrdU availability in the brain (Cameron & McKay, 2001). We have not examined whether the antioxidant fortified food or behavioral enrichment paradigms used in the present study had an effect on blood-brain barrier integrity or flow of blood to the brain or other factors affecting BrdU entry into the brain. All animals in the treatment study were similar in age and received the same dose of BrdU so the relationship between treatment and number of BrdU nuclei should be constant. We did not find significant differences in the number of BrdU nuclei between the treatment groups compared to control animals.
Conclusions
The aged canine brain shows a 90-96% decrease in hippocampal neurogenesis which could be a result of a decrease in progenitor cell proliferation or a decrease in new neuron survival. An antioxidant fortified food and a program of behavioral enrichment may affect the survival of new neurons in the aged canine brain. Neurogenesis in the aged canine brain is associated with cognitive performance and our data suggest that new neurons in the dentate gyrus may participate in modulating cognitive function.
Acknowledgements
This research was supported by the National Institute on Aging (NIA AG12694, AG17066), and by the United States Army Medical Research and Material Command under Contract No. DAMD17-98-1-8622. The views, opinions, and/or findings contained in this report are those of the authors and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation. Additional support was provided by the Canadian Institutes of Health Research Institute of Aging Fellowship awarded to CTS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AAFCO: American Association of Feed Control Officials Commercial Feed Annual Report. 1999 [Google Scholar]
- Altman J, Das GD. Postnatal neurogenesis in the guinea-pig. Nature. 1967;214(5093):1098–1101. doi: 10.1038/2141098a0. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. European Journal of Neuroscience. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. Journal of Comparative Neurology. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. European Journal of Neuroscience. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. Journal of Comparative Neurology. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cecchini T, Ciaroni S, Ferri P, Ambrogini P, Cuppini R, Santi S, Del Grande P. α-Tocopherol, an exogenous factor of adult hippocampal neurogenesis regulation. Journal of Neuroscience Research. 2003;73:447–455. doi: 10.1002/jnr.10690. [DOI] [PubMed] [Google Scholar]
- Chan AD, Nippak PM, Murphey H, Ikeda-Douglas CJ, Muggenburg B, Head E, Cotman CW, Milgram NW. Visuospatial impairments in aged canines (Canis familiaris): the role of cognitive-behavioral flexibility. Behavioural Neuroscience. 2002;116(3):443–54. [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn H-G, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. European Journal of Neuroscience. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Head E, Afagh AJ, Milgram NW, Cotman CW. Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiology of Learning and Memory. 1996;66(1):11–23. doi: 10.1006/nlme.1996.0039. [DOI] [PubMed] [Google Scholar]
- Cuppini R, Ciaroni S, Cecchini T, Ambrogini P, Ferri P, Cuppini C, Ninfali P, Del Grande P. Tocopherols enhance neurogenesis in dentate gyrus of adult rats. International Journal for Vitamin and Nutrition Research. 2002;72(3):170–176. doi: 10.1024/0300-9831.72.3.170. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, van der Hart MGC, Schmelting B, Hesselink MB, Fuchs E. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology. 2005;30:67–79. doi: 10.1038/sj.npp.1300581. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza P-V, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proceedings of the National Academy of Science USA. 2003;100(24):14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson PS, Perfilieva E, Bjork-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fox MW. Integrative development of brain and behavior in the dog. The University of Chicago Press; Chicago: 1971. [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LAM, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. Journal of Neuroscience. 1997;17(7):2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neural progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80(2):427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proceedings of the National Academy of Science USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999a;11:S46–S51. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience. 1999b;2(3):260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves A, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult old world primates. Proceedings of the National Academy of Science USA. 1999c;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueneau G, Privat A, Drouet J, Court L. Subgranular zone of the dentate gyrus of young rabbits as a secondary matrix. A high resolution autoradiographic study. Developmental Neuroscience. 1982;5(4):345–358. doi: 10.1159/000112694. [DOI] [PubMed] [Google Scholar]
- Hatton WJ, von Bartheld CS. Analysis of cell death in the trochlear nucleus of the chick embryo: calibration of the optical disector counting method reveals systematic bias. Journal of Comparative Neurology. 1999;409:169–86. [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. Journal of Comparative Neurology. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid β-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. Journal of Neurochemistry. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiology of Aging. 1998;19(5):415–425. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. European Journal of Neuroscience. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of age mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer's disease. Proceedings of the National Academy of Science USA. 2004;101(1):343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. Journal of Neuroscience. 1998;18(9):3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemperman G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. European Journal of Neuroscience. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiology of Aging. 2006;27(10):1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. Journal of Neuroscience. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A, Wilhelmsson U, Pekna M, Pekny M. Increased cell proliferation and neurogenesis in the hippocampal dentate gyrus of old GFAP-/-Vim-/-mice. Neurochemical Research. 2004;29(11):2069–2073. doi: 10.1007/s11064-004-6880-2. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. Journal of Molecular Neuroscience. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. Journal of Neurochemistry. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-1 ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107(4):603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lister JP, Blatt GJ, DeBassio WA, Kemper TL, Tonkiss J, Galler JR, Rosene DL. Effect of prenatal protein malnutrition on numbers of neurons in the principal cell layers of the adult rat hippocampal formation. Hippocampus. 2005;15:393–403. doi: 10.1002/hipo.20065. [DOI] [PubMed] [Google Scholar]
- Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, Pei G, Ma L. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Experimental Neurology. 2003;183:600–609. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- McDonald HY, Wojtowicz J,M. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neuroscience Letters. 2005;385:70–75. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Karim R, Darraq M, Chiba AA, Tuszynski MH. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. Journal of Comparative Neurology. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Adams B, Callahan H, Head E, Mackay B, Thirlwell C, Cotman CW. Landmark discrimination learning in the dog. Learning and Memory. 1999;6(1):54–61. [PMC free article] [PubMed] [Google Scholar]
- Milgram NW, Head E, Muggenburg B, Holowachuk D, Murphey H, Estrada J, Ikeda-Douglas CJ, Zicker SC, Cotman CW. Landmark discrimination learning in the dog: effects of age, an antioxidant fortified food, and cognitive strategy. Neuroscience and Biobehavioral Reviews. 2002a;26(6):679–695. doi: 10.1016/s0149-7634(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Zicker SC, Head E, Muggenburg BA, Murphey H, Ikeda-Douglas CJ, Cotman CW. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiology of Aging. 2002b;23(5):737–745. doi: 10.1016/s0197-4580(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Zicker SC, Ikeda-Douglas C, Murphey H, Muggenburg BA, Siwak CT, Tapp PD, Lowry SR, Cotman CW. Long-term treatment with antioxidants and a program of behavioral enrichment reduces age-dependent impairment in discrimination and reversal learning in beagle dogs. Experimental Gerontology. 2004;39(5):753–765. doi: 10.1016/j.exger.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Head E, Zicker SC, Ikeda-Douglas CJ, Murphey H, Muggenburg B, Siwak C, Tapp D, Cotman CW. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiology of Aging. 2005;26(1):77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Morita T, Mizutani Y, Sawada M, Shimada A. Immunohistochemical and ultrastructural findings related to the blood-brain barrier in the blood vessels of the cerebral white matter in aged dogs. Journal of Comparative Pathology. 2005;133:14–22. doi: 10.1016/j.jcpa.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Ngwenya LB, Peters A, Rosene DL. Light and electron microscopic immunohistochemical detection of bromodeoxyuridine-labeled cells in the brain: different fixation and processing protocols. Journal of Histochemistry and Cytochemistry. 2005;53(7):821–832. doi: 10.1369/jhc.4A6605.2005. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Rakic P, Nowakowski RS. The time of origin of neurons in the hippocampal region of the rhesus monkey. Journal of Comparative Neurology. 1981;196(1):99–128. doi: 10.1002/cne.901960109. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. European Journal of Neuroscience. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. European Journal of Neuroscience. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5(6):545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proceedings of the National Academy of Science USA. 1996;93(18):9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovsky I, Wei M, Morgan TE, Finch CE. Reversible ageimpairments in neurite outgrowth by manipulations of astrocytic GFAP. Neurobiology of Aging. 2005;26(5):705–715. doi: 10.1016/j.neurobiolaging.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwak CT, Tapp PD, Head E, Zicker SC, Murphey HL, Muggenburg BA, Ikeda-Douglas CJ, Cotman CW, Milgram NW. Chronic antioxidant and mitochondrial cofactor administration improves discrimination learning in aged but not young dogs. Progress in Neuropsychopharmacology and Biological Psychiatry. 2005;29(3):461–469. doi: 10.1016/j.pnpbp.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Region specific neuron loss in the aged canine hippocampus is reduced by enrichment. Neurobiology of Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.09.018. epub. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, Milgram NW. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learning and Memory. 2003;10(1):64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Head E, Cotman CW, Murphey H, Muggenburg BA, Ikeda-Douglas C, Milgram NW. Concept abstraction in the aging dog: development of a protocol using successive discrimination and size concept tasks. Behavioural Brain Research. 2004;153(1):199–210. doi: 10.1016/j.bbr.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Tapp PD, Chu Y, Araujo JA, Chiou JY, Head E, Milgram NW, Su M-Y. Effects of scopolamine challenge on regional cerebral blood volume. A pharmacological model to validate the use of contrast enhanced magnetic resonance imaging to assess cerebral blood volume in a canine model of aging. Progress in Neuropsychopharmacology & Biological Psychiatry. 2005;29(3):399–406. doi: 10.1016/j.pnpbp.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Tonchev AB, Yamashima T, Zhao L, Okano HJ, Okano H. Proliferation of neural and neuronal progenitors after global brain ischemia in young adult macaque monkeys. Molecular and Cellular Neuroscience. 2003;23:292–301. doi: 10.1016/s1044-7431(03)00058-7. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wati H, Kudo K, Qiao C, Kuroki T, Kanba S. A descreased survival of proliferated cells in the hippocampus is associated with a decline in spatial memory in aged rats. Neuroscience Letters. 2006;399:171–174. doi: 10.1016/j.neulet.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson U, Li L, Pekna M, Berthold C-H, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. Journal of Neuroscience. 2004;24(21):5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM, Sripanidkulchai B. An autoradiographic analysis of the time of origin of neurons in the hypothalamus of the cat. Brain Research. 1985;353(1):89–98. doi: 10.1016/0165-3806(85)90026-4. [DOI] [PubMed] [Google Scholar]