Abstract
Peptides that target tissue for an apoptotic death have potential as therapeutics in a variety of disease conditions. The class of peptides described herein enters the cell through a specific receptor-mediated interaction. Once inside the cell, the peptide migrates toward the mitochondria, where the membrane barrier is disrupted. These experiments demonstrate that upon treatment with these short peptides large unilamellar vesicles are not lysed, a graded mode of leakage is observed and the transient pores formed by these peptides are large enough to release entrapped cytochrome c from the vesicles.
Keywords: Apoptosis, vesicles, leakage, cytochrome c, cell penetrating peptides
1. Introduction
Peptide ligands that interact with cell surface receptors and induce apoptosis can be valuable lead compounds into the design of targeted therapy for a variety of disease conditions. Recent studies report high-throughput screening for peptide-induced apoptosis via mimicry of the TRAIL:tumor necrosis (TNF) factor interaction[1] and other cell surface receptors [2,3]. The class of peptides described herein, hunter killer peptides (HKPs) use receptor interactions as a vehicle for intracellular delivery and have been reported for targeting angiogenic tumor vasculature[4], white adipose tissue[5], and arthritic tissue[6]. These pro-apoptotic peptides contain a variable amino terminal homing sequence, which mediates interactions with cell surface receptors, and a common positively charged carboxyl terminal apoptosis sequence motif that is connected through a glycinyl glycine linker (Figure 1). Once inside the cell, the peptide initiates apoptosis through a membrane perturbing interaction with the mitochondria [4]. The apoptotic sequence is comprised of D-amino acids to increase the in vivo stability. NMR derived structures in the presence of lipid micelles have been reported[7,8] for two peptides that target angiogenic tumor cells for apoptosis but the mechanism of their interaction with lipid bilayers is unknown.
Figure 1.

Sequences for HKP1, HKPao and Mastoparan. The amino acids of the pro-apoptotic sequence of HKP1 and HKPao have D-stereochemical configuration and are shown in lower case letters.
Cell penetrating peptides (CPPs) are another class of cationic peptides that include penetratin, tat, and transportan, which cross the cell membrane. Several mechanisms of intracellular transport have been proposed for these peptides[9,10]. CPPs have been modified for delivery of cargo including peptides [11] and nucleic acids[12-14] to intracellular targets; however, cellular uptake and toxicity have been shown to be cargo dependent [15-17]. Hunter-Killer peptides (HKPs) have some similarity to CPPs, but transport into cells is targeted by the receptor-specific homing sequence. HKPs have demonstrated approximately ten-fold selective toxicity for targeted cells over cells not expressing the receptor [5] and selective reduction in body mass in white adipose tissue [4,5].
The potential therapeutic activity of HKPs is based upon their ability to induce apoptosis. Release of cytochrome c into the cytoplasm stimulates assembly of the apoptosome , subsequent activation of caspase-9[18,19] and apoptosis. In this work, we report experiments that characterize interactions between HKPs with two different homing sequences and large phospholipid unilamellar vesicles (LUVs). These experiments show that when vesicles are treated with peptide, the vesicles are not lysed and that transient pores with defined size are formed. The ability of these peptides to induce efflux of cytochrome c is investigated as well as the impact of swapping homing sequences. Relative insensitivity to the homing sequence will suggest the potential for wider application of these peptides for potential therapeutic treatment of disease.
2. Materials and Methods
2.1 Materials
Egg phosphatidylglycerol (PG) and egg phosphatidylcholine (PC), were purchased from Avanti Polar Lipids (Alabaster, AL). Fitc-Dextrans of 4 kD (FD4), 10 kD (FD10), 20 kD (FD20), 40 kD (FD40), 70 kD (FD70) size, bee venom mastoparan and bovine heart cytochrome c were purchased Sigma-Aldrich (St. Louis, MO). Immunoblotting was done on Protran® BA83, 0.2 μm membrane (VWR, Cat. No. 89026-732) using a Minifold II System (Whatman, Schleicher & Schuell). The membrane was probed with a 1:1000 dilution of mouse anti-cytochrome c monoclonal antibody (Clone 2B5.F8) purchased from Assay Designs Inc.
2.2 Creation of large unilamellar vesicles
LUVs were made by drying down phospholipids from a chloroform solution and vacuum dried overnight. The lipids were suspended with 70 mM calcein, 75 mg/ml FITC-Dextrans (FD) or 20 mg/ml cytochrome c in 20 mM Hepes buffer pH 7.5 to a phospholipid concentration of 35 mM. Suspensions were vortexed and placed in a water bath at 45°C for 5 min and repeated until the phospholipids were suspended. Five freeze/thaw cycles in N2 (l) were performed. The sample was placed in an extruder containing 2 stacked membranes (100 nm) and then passed 11 times. The vesicles were passed over a 60 ml Sephacryl S-300 or Sephracyl S-100 (GE Healthcare) column to separate the vesicles from dye, Fitc-Dextrans or cytochrome c. Total phospholipid concentration was assayed by a modified phosphate assay [20]. Vesicles eluted from gel filtration generally contained approximately 1 mM phospholipid.
2.3 Vesicle leakage studies
All fluorescence measurements were carried out on a Jasco FP6600 spectrofluorometer equipped with Pelletier temperature controller at 25°C. Leakage experiments with calcein and Fitc-dextran entrapped vesicles were performed as described previously[8,21]. Baseline fluorescence (IO) was measured on samples containing 20 mM Hepes buffer and 30uL of the collected vesicles diluted to 3 mL. Peptide was then added and another fluorescence measurement (IP) was taken. Total leakage (IT) was measured after addition of 5% triton X-100 to a final concentration of 0.01%. Percent leakage was calculated by the equation: %L= (IP - IO) / (IT-IO) × 100. Phospholipid assays were carried out after leakage experiments [20]. Leakage assays were carried out within 24 hours of creation of the vesicles.
2.4 Mode of leakage
Calcein entrapped LUVs with 70:30 PC:PG molar ratio were created and separated from the free dye as described above. The bee venom peptide, mastoparan was used as a positive control. Details of these experiments and graded mode of leakage by mastoparan X and magainin have previously been reported [22,23]. These vesicles were diluted ten-fold in 20 mM Hepes, pH 7.0. Mastoparan or HKP1 was added to a concentration of 4 μM to vesicles at a peptide:lipid ratio of 0.04 to induce leakage. For a negative control, buffer was added in place of peptide. From the peptide treated samples and the control, 300 μl was removed for a ten-fold dilution upon which leakage was quantified via fluorescence measurements. Triton X-100 was added to determine 100% leakage. The quench factor (It/Io) was determined for the vesicles prior to leakage. The remaining leakage sample was loaded onto gel filtration column to separate the free dye formed from leakage from the vesicles. The vesicle eluent was collected and It/Io was remeasured.
2.5 Comparison of hydrodynamic radius of fitc-dextrans and cytochrome c
The stokes radius of the fitc-dextrans are reported by the manufacturer (Sigma), and the stokes radius of cytochrome c is reported [24]. Because dextrans are linear and cytochrome c is globular, we carried out experiments that would provide relative hydrodynamic size of FD4, FD10, FD20 and cytochrome c under the identical conditions using gel filtration chromatography. The column (1.5 cm diameter) was packed with Sephacryl-S100 resin (GE Health Sciences) with a bed volume of 55 ml and equilibrated with three column volumes of 50 mM Tris, 150 mM NaCl pH 7.5 (column buffer). Fitc-Dextrans (FD4, FD10 and FD20) were loaded in samples containing 15 mg in 500 μl of column buffer. Cytochrome c samples containing 10 mg/ml in 500 μl of column buffer were loaded onto the column. The column flow rate was 0.9 ml/min for all samples. Elution was monitored by absorbance at 280 nm.
2.6 Detection and analysis of cytochrome c leakage from LUVs
Cytochrome c loaded vesicles were treated with HKP1 to final concentrations of 4.6 μM, 11.5 μM, 23 μM and 46 μM. Untreated vesicle samples were used as the controls to verify stability of the vesicles. Total leakage of cytochrome c was demonstrated by addition of triton X-100 to a final concentration of 1.1%. At the higher concentrations required for these experiments, the vesicle suspensions became turbid with treatment of peptide, suggesting fusion of vesicles. In order to avoid interference with gel filtration, HKP1 treated vesicles were centrifuged at 13000 × g for 30 seconds at room temperature prior to column separation to sediment fused vesicles. Vesicles and free cytochrome c in the supernatant were separated by gel filtration. Fractions were collected and immunoblotted with cytochrome c antibody. The vesicle pellets were resuspended in 300 mM NaCl, 20 mM HEPES, pH 7.5, monitored for relative turbidity by OD 280 nm and transferred to a nitrocellulose membrane, which was probed with a monoclonal anti-cytochrome c antibody followed by alkaline phosphatase detection to verify retention of cytochrome c in the fused vesicles.
3. Results
3.1 Graded mode of leakage
Initial experiments (Table 1) indicated the HKP1 undergoes graded leakage and that the vesicles remain intact after treatment with the peptide. The experiment was performed in parallel with mastoparan (Mst), a pore forming peptide with a graded mode of leakage that we have observed previously. Graded mode of leakage [23] has been reported for Mastoparan X. The relative fluorescence (It/Io) is not expected to increase for the control sample, but may be the result of incomplete separation of vesicles from free calcein in the initial purification of vesicles, which are at 10 fold higher concentration than subsequent separations. The quench factor(It/Io) was plotted as a function of peptide concentration and leakage. Figure 2 shows that calcein self-quenching decreases as a function of HKP1 concentration until 7 μM HKP1, where quenching ceases because 100 % leakage has occurred. The quench factor is linear with leakage, demonstrating that the vesicles lose a portion of their contents when they undergo leakage (Figure 2B). This graded mode of leakage is estimated to have an upper limit of 5 ms for the lifetime of the pore, through which the contents diffuse[25].
Table 1.
Determination that homing pro-apoptotic peptides induce graded mode of leakage.
| HKP1 | Mst | Control | |
|---|---|---|---|
| It/Io before | 4.4 | 4.4 | 4.4 |
| L | 63% | 69% | 0% |
| It/Io after | 2.3 | 2.3 | 5.7 |
Figure 2.
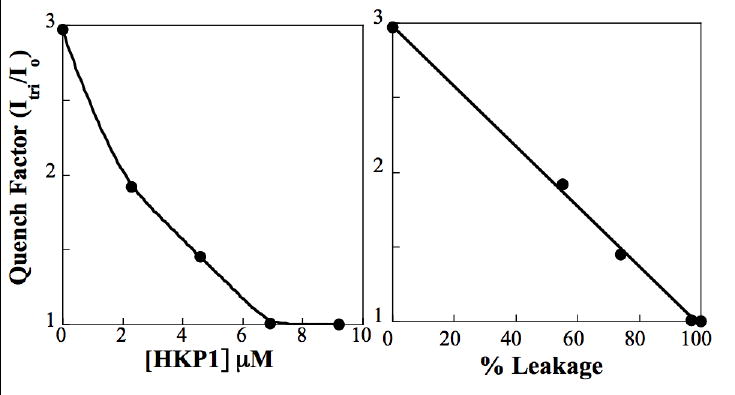
A plot of It/Io against peptide concentration (A) and leakage (B). Decreasing It/Io with leakage indicates partial leakage of vesicle contents, and therefore survival of vesicles to treatment with peptide.
3.2 Hydrodynamic radius of molecules released from vesicles by HKPs
HKP1 and HKPao can induce leakage of fluorescent dextrans from vesicles that have a larger hydrodyanmic radius than cytochrome c (Figure 3A). Similar size dependence profiles suggest that the size of the transient pores formed by both these peptides are the same. Fitc-dextrans have self-quenching properties[26-28], and fluorescence intensity increases as the local concentration of dye decreases with leakage from vesicles. FD4 and FD10 both leak from vesicles with high efficiency under the same peptide:phospholipid ratios that have been previously reported with calcein-entrapped vesicles[8]. The size of the pore appears to become limiting for molecules with size between FD10 and FD20, which have a stokes radius of 2.3 nm and 3.3 nm [29]. Minimal leakage occurs for dyes larger than FD20. Gel filtration chromatography verified the relative hydrodynamic radii of FD10, FD4 and cytochrome c (Figure 3B), demonstrating that cytochrome c is of intermediate size between the 1.4 nm and 2.3 nm reported for FD4 and FD10.
Figure 3.

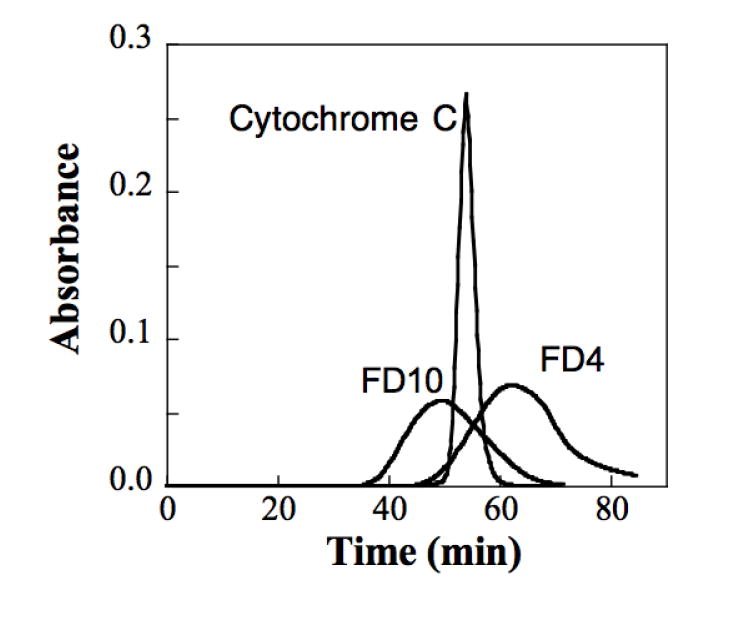
(A) For the determination of size of molecules that can leak from vesicles with treatment of peptide, efflux of entrapped FD dyes of 4 kD, 10 kD, 20 kD and 70 kD size from 70:30 molar ratio PC:PG vesicles was monitored. Efflux of the fitc-dextrans was detected via loss of fluorescence self-quenching[26] at 520 nm for 5 minutes. Excitation was at 490 nm with excitation and emission slit widths set to 1 nm. The assay concentration of peptide and phospholipid were 150 nM and 14 +/- 3 μM, respectively for all experiments. The stokes radius of cytochrome c (1.7 nm)[24] is represented by the dotted line. (B) Elution of FD4 , FD10 and cytochrome c from gel filtration chromatography demonstrates relative hydrodynamic radius under identical conditions.
3.3 HKP1 treatment promotes cytochrome c leakage from LUVs
Treatment of PC:PG vesicles with HKP1 initiated release of entrapped cytochrome c (Figure 4) in a concentration dependent fashion at peptide:lipid ratios ranging from 0.007 to 0.07, in the same range as the fitc-dextran experiments described earlier. Additionally, at the millimolar concentrations of phospholipid required for these experiments, an increase in sample turbidity was observed upon addition of peptide, suggesting vesicle fusion or aggregation is also induced by HKP1. Chromatographic separation of the vesicles (11 ml elution volume) from released cytochrome c (33 ml) is shown in Figure 4A-D as a function of HKP1 concentration. Little change in the elution profile was observed relative to untreated vesicles in the presence of 4.6 μM (data not shown). At 11.5 μM of HKP1, the vesicle peak area is 50% of the untreated sample (Fig. 4B) accompanied by a small increase in absorbance at the cytochrome c peak area. Sample turbidity is observed at this HKP1 concentration and higher, probably due to vesicle aggregation. Increasing the HKP-1 concentration of the vesicles to 23 μM and 46 μM results in a decrease in the vesicle elution profile and further increase in the peak area of released cytochrome c (Fig. 4C and D). Precipitation of cytochrome c can be excluded as the source of turbidity because the similar results were obtained with vesicles with entrapped buffer lacking cytochrome c and treatment of free cytochrome c with peptide did not result in precipitation. At 46 μM HKP1 approximately 60% of the cytochrome c is released, suggesting that a significant portion is retained in the fused vesicles. Immunoblotting of the resuspended pellet verified the presence of cytochrome c. An alternate explanation for the turbidity is that under the conditions required for these experiments, the vesicles are lysed but the retention of the approximately 40% cytochrome c in the fused vesicles suggests that this is not the case.
Figure 4.


Leakage of cytochrome c from vesicles was evaluated by gel filtration. The profiles are identified as (A), untreated vesicles (B), 11.5 μM HKP-1 (C), 23 μM HKP1 and (D), 46 μM HKP1. Vesicles elute at 11 ml and released cytochrome c elutes at 33 ml. (E) Relative peak area of the vesicle (open circles) and cytochrome c (solid squares) peaks were plotted as a function of HKP1 concentration. The increase in turbidity (solid circles) was monitored by light scattering at 280 nm of the resuspended pellet.
4. Discussion
HKPs are synthetic peptides designed to target specific tissue for programmed cell death for therapeutic purposes. A common pro-apoptotic peptide sequence is used and homing sequences are swapped to mediate specific cell surface receptor interactions. In these experiments, we have shown that HKPs are sufficient to cause the release of cytochrome c from phospholipid vesicles. Two peptides with different homing sequences form similar size pores, suggesting a common mechanism and adaptability of these peptides to a variety of tissues. The vesicles retain interior contents after treatment with peptide, and are therefore not lysed. Fusion or aggregation of vesicles may occur. Future experiments will examine the ability of these peptides to fuse vesicles.
Acknowledgments
The work was supported by NIH R15 grant GM068431-02A1.
Abbreviations
- FD4
fitc-dextran 4 kD
- FD10
fitc-dextran 10 kD
- FD20
fitc-dextran 20 kD
- FD40
fitc-dextran 40 kD
- FD70
fitc-dextran 70 kD
- fitc
fluorescein isothiocyanate
- HKP
hunter-killer peptide
- LUVs
large unilamellar vesicles
- Mst
mastoparan
- PC
phosphatidylcholine
- PG
phosphatidylglycerol
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Okochi M, Nakanishi M, Kato R, Kobayashi T, Honda H. High-throughput screening of cell death inducible short peptides from TNF-related apoptosis-inducing ligand sequence. FEBS Lett. 2006;580:885–9. doi: 10.1016/j.febslet.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Bussolati B, Grange C, Tei L, Deregibus MC, Ercolani M, Aime S, Camussi G. Targeting of human renal tumor-derived endothelial cells with peptides obtained by phage display. J Mol Med. 2007;85:897–906. doi: 10.1007/s00109-007-0184-3. [DOI] [PubMed] [Google Scholar]
- 3.Kalie E, Jaitin DA, Abramovich R, Schreiber G. An interferon alpha2 mutant optimized by phage display for IFNAR1 binding confers specifically enhanced antitumor activities. J Biol Chem. 2007;282:11602–11. doi: 10.1074/jbc.M610115200. [DOI] [PubMed] [Google Scholar]
- 4.Ellerby HM, et al. Anti-cancer Activity of Targeted Pro-Apoptotic Peptides. Nature Medicine. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 5.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–32. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 6.Gerlag DM, Borges E, Tak PP, Ellerby HM, Bredesen DE, Pasqualini R, Ruoslahti E, Firestein GS. Suppression of Murine Collagen-Induced Arthritis by Targeted Apoptosis of Synovial Neovasculature. Arthritis Research. 2001;3:357–361. doi: 10.1186/ar327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plesniak LA, Parducho JI, Ziebart A, Geierstanger BH, Whiles JA, Melacini G, Jennings PA. Orientation and helical conformation of a tissue-specific hunter-killer peptide in micelles. Protein Sci. 2004;13:1988–96. doi: 10.1110/ps.04853204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandoval CM, et al. Structural evaluation of a novel pro-apoptotic peptide coupled to CNGRC tumor homing sequence by NMR. Chemical Biology & Drug Design. 2006;67:417–24. doi: 10.1111/j.1747-0285.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 9.Palm C, Jayamanne M, Kjellander M, Hallbrink M. Peptide degradation is a critical determinant for cell-penetrating peptide uptake. Biochim Biophys Acta. 2007;1768:1769–76. doi: 10.1016/j.bbamem.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Thoren PE, Persson D, Isakson P, Goksor M, Onfelt A, Norden B. Uptake of analogs of penetratin, Tat(48-60) and oligoarginine in live cells. Biochem Biophys Res Commun. 2003;307:100–7. doi: 10.1016/s0006-291x(03)01135-5. [DOI] [PubMed] [Google Scholar]
- 11.Pero SC, Shukla GS, Cookson MM, Flemer S, Jr, Krag DN. Combination treatment with Grb7 peptide and Doxorubicin or Trastuzumab (Herceptin) results in cooperative cell growth inhibition in breast cancer cells. Br J Cancer. 2007;96:1520–5. doi: 10.1038/sj.bjc.6603732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abes R, Arzumanov AA, Moulton HM, Abes S, Ivanova GD, Iversen PL, Gait MJ, Lebleu B. Cell-penetrating-peptide-based delivery of oligonucleotides: an overview. Biochem Soc Trans. 2007;35:775–9. doi: 10.1042/BST0350775. [DOI] [PubMed] [Google Scholar]
- 13.Marshall NB, Oda SK, London CA, Moulton HM, Iversen PL, Kerkvliet NI, Mourich DV. Arginine-rich cell-penetrating peptides facilitate delivery of antisense oligomers into murine leukocytes and alter pre-mRNA splicing. J Immunol Methods. 2007 doi: 10.1016/j.jim.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Moschos SA, Williams AE, Lindsay MA. Cell-penetrating-peptide-mediated siRNA lung delivery. Biochem Soc Trans. 2007;35:807–10. doi: 10.1042/BST0350807. [DOI] [PubMed] [Google Scholar]
- 15.El-Andaloussi S, Jarver P, Johansson HJ, Langel U. Cargo dependent cytotoxicity and delivery efficacy of cell-penetrating peptides: a comparative study. Biochem J. 2007 doi: 10.1042/BJ20070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller S, Bothe M, Bienert M, Dathe M, Blume A. A simple fluorescence-spectroscopic membrane translocation assay. Chembiochem. 2007;8:546–52. doi: 10.1002/cbic.200600553. [DOI] [PubMed] [Google Scholar]
- 17.Esbjorner EK, Lincoln P, Norden B. Counterion-mediated membrane penetration: cationic cell-penetrating peptides overcome Born energy barrier by ion-pairing with phospholipids. Biochim Biophys Acta. 2007;1768:1550–8. doi: 10.1016/j.bbamem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26:390–7. doi: 10.1016/s0968-0004(01)01844-8. [DOI] [PubMed] [Google Scholar]
- 19.Bouchier-Hayes L, Lartigue L, Newmeyer DD. Mitochondria: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2640–7. doi: 10.1172/JCI26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen PS, Toribara TY, Warner H. Microdetermination of Phosphorus. Analytical Chemistry. 1956;28:1756. [Google Scholar]
- 21.Medina ML, Chapman BS, Bolender JP, Plesniak LA. Transient Vesicle Leakage Initiated by a Synthetic Peptide Derived from the Death Domain of Neurotrophin Receptor, p75NTR. Journal of Peptide Research. 2002;59:149–158. doi: 10.1034/j.1399-3011.2002.1o971.x. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzaki K, Murase O, Tokuda H, Funakoshi S, Fujii N, Miyahima K. Orientational and Aggregational States of Magainin 2 in Phospholipid Bilayers. Biochemistry. 1994;33:3342–3349. doi: 10.1021/bi00177a027. [DOI] [PubMed] [Google Scholar]
- 23.Matsuzaki K, Yoneyama S, Murase O, Miyajima K. Transbilayer Transport of Ions and Lipids Coupled with Mastoparan X Translocation. Biochemistry. 1996;35:8450–8456. doi: 10.1021/bi960342a. [DOI] [PubMed] [Google Scholar]
- 24.Ackers GK. Molecular exclusion and restricted diffusion processes in molecular-sieve chromatography. biochemistry. 1964;3:723–30. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- 25.Rex S, Schwarz G. Quantitative Studies on the Melittin-Induced Leakage Mechanism of Lipid Vesicles. Biochemistry. 1998;37:2336–45. doi: 10.1021/bi971009p. [DOI] [PubMed] [Google Scholar]
- 26.Rex S. Pore formation induced by the peptide melittin in different lipid vesicle membranes. Biophys Chem. 1996;58:75–85. doi: 10.1016/0301-4622(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 27.Stutzin A. A fluorescence assay for monitoring and analyzing fusion biological membrane vesicles in vitro. FEBS Lett. 1986;197:274–80. doi: 10.1016/0014-5793(86)80341-6. [DOI] [PubMed] [Google Scholar]
- 28.Stutzin A, Cabantchik ZI, Lelkes PI, Pollard HB. Synexin-mediated fusion of bovine chromaffin granule ghosts. Effect of pH Biochim Biophys Acta. 1987;905:205–12. doi: 10.1016/0005-2736(87)90024-1. [DOI] [PubMed] [Google Scholar]
- 29.Sigma, editor. Sigma. Product Information Fluorescein Isothiocyanate-Dextran. 1997. [Google Scholar]


