Abstract
The evolution of complex signals may be favoured by hidden preferences or pre-existing sensory biases. Females of two species of grey treefrogs (Hyla chrysoscelis and Hyla versicolor) were tested with combinations of a conspecific advertisement call and acoustic appendages. Appendages consisted of aggressive calls and segments of advertisement calls from conspecific males and males of three other species and bursts of filtered noise. When a wide variety of these acoustic appendages followed the advertisement call, the resulting compound signal was often more attractive than the same advertisement call alone. When the same appendages led advertisement calls, however, the compound signal was never more attractive and sometimes less attractive. The order effect was especially strong in tests of H. versicolor in which advertisement-call duration was decreased. These results cannot be explained by a general pre-existing bias for extra stimulation per se. Rather, order and other effects may constrain the evolution and subsequent modification of complex and extravagant signals, examples of which have been reported for a wide range of taxa.
Keywords: complex signals, temporal order, hidden preferences, pre-existing sensory biases
1. Introduction
Monotonously repeated signals consisting of a single kind of element are typical of animal communication systems, but complex signals, with two or more distinctive elements occurring in close temporal proximity (less than 0.5 s), occur in a wide range of taxa (Bradbury & Vehrencamp 1998; Gerhardt & Huber 2002). A first step in understanding the evolution of complex signals is to identify the factors that increase the effectiveness of compound signals with two different elements relative to a single-element signal. Are there, for example, characteristics of novel elements that make a compound call more attractive to prospective mates than a single established element alone? Or is any novel element that increases sensory stimulation per se likely to have this effect?
These questions arise from predictions that some novel signals will always be more attractive to females than already established signals and hence have the potential to spread rapidly throughout a population. The hidden preference hypothesis, derived from neural-net studies of visual pattern recognition, posits that novel signals will be preferred if their properties better match the recognition system than those of established signals (Arak & Enquist 1993). The pre-existing sensory bias hypothesis, based on studies showing that acoustic appendages (Ryan & Rand 1993) or visual ornaments (Basolo 1996) enhanced the attractiveness of simpler conspecific signals, emphasizes that additional elements increase the attractiveness of novel signals mainly by increasing the quantity of sensory stimulation. Indeed, as proposed by Ryan & Keddy-Hector (1992), all manner of preferences for more intense, longer and more rapidly repeated signals might reflect pre-existing sensory biases, even if evidence about the order of evolution of preferences and signals is equivocal or lacking (Endler & Basolo 1998). In the context of traditional sexual selection theory, complex signals are one class of extravagant signals, which may be maintained and elaborated by coevolutionary processes arising from the mutual selective pressures exerted by signallers and receivers (Andersson 1994). Despite the great interest in the evolution of extravagant signals, relatively little is known about the characteristics of receiver biases (receiver psychology; Guilford & Dawkins 1991) that ultimately determine which kinds of novel signals are likely to become established (Arnqvist 2006).
We addressed questions regarding female preferences for novel compound signals in playback experiments with two species of grey treefrog in which males produce only single-element advertisement calls consisting of uniform trills. We found that adding a variety of acoustic appendages may result in compound signals that are more attractive than advertisement calls alone. The enhanced attraction of compound calls was, however, conditional on the temporal order and the relative durations of the established call and the acoustic appendage. In more attractive compound calls, the appendage always followed the advertisement call whereas when the same appendage led the advertisement call, the resulting compound signal was never more attractive and frequently less attractive than the advertisement call alone. In tests of Hyla versicolor, the temporal-order effect was accentuated when advertisement-call duration was reduced. These results indicate that extra sensory stimulation per se is inadequate to favour the evolution of complex signals. The temporal-order and relative-duration effects may be two of many factors that constrain the origin and subsequent elaboration of complex signals used in mate choice and other forms of intraspecific communication.
2. Material and methods
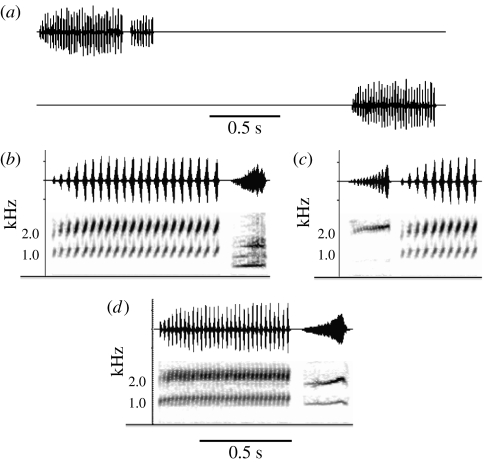
Selective phonotaxis of females of two species of closely related treefrogs (Hyla chrysoscelis and H. versicolor) was tested in two alternative forced-choice playbacks of single-element conspecific signals and novel compound signals. In both species, advertisement calls consist of uniform trills, and females prefer long calls to short calls, even when the total acoustic stimulation is equalized by reducing the call rate of the long alternative (Klump & Gerhardt 1987; Gerhardt et al. 1995). Aggressive calls, which are typically emitted only during male–male encounters, are significantly shorter and have different spectral and temporal properties (figure 1; Gerhardt 2001). Combinations of advertisement and aggressive calls are rarely heard, and the temporal order and the duration of silent gaps between the elements of such signals are highly variable (H.C. Gerhardt 2006, unpublished data).
Figure 1.
Oscillograms and sonograms of representative acoustic stimuli: (a) H. chrysoscelis control stimulus showing timing relationship with the alternative (same advertisement call; oscillogram only); (b) normal-duration advertisement call of H. versicolor and following aggressive call of H. arenicolor; (c) aggressive call of H. avivoca leading short-duration advertisement call of H. versicolor; and (d) normal-duration call of H. chrysoscelis and following aggressive call of H. versicolor.
(a) Playback experiments: acoustic stimuli
We made compound calls by appending to advertisement calls of H. chrysoscelis and H. versicolor the aggressive calls and advertisement-call segments of these and two other species: the closely related bird-voiced treefrog (Hyla avivoca) and the canyon treefrog (Hyla arenicolor), a species in a sister clade (Gerhardt 2001; see examples in figure 1). These species also produce advertisement calls consisting of trills, and the spectral properties of both advertisement and aggressive calls overlap to some extent with those of the grey treefrogs, thus assuring their audibility to females. Historically, females of each species of grey treefrog would have been frequently exposed to the advertisement and aggressive calls of the other species owing to the large areas of overlap in their ranges of distribution. There are areas of partial overlap between the ranges of grey treefrogs and that of H. avivoca, and no overlap with that of H. arenicolor. Thus, the signals of the last species as well as the burst of filtered noise (see below) represent completely novel appendages. No complex calls exist in the repertoires of any of these species.
Advertisement and aggressive calls were originally recorded with high-quality recorders (Nagra IV analog; Tascam DA-P1 DAT or Marantz PMD671 solid-state) and directional microphones (Sennheiser ME80; 415); single calls (one per male) were extracted and edited from digitized (44.1 kHz, 16 bit, wave) sound files. Duration of appendages ranged from 115 to 275 ms; advertisement-call segments contained a whole number of pulses. We also used an appendage consisting of a 200 ms burst of filtered white noise (spectral structure similar to conspecific calls) in tests of H. versicolor.
In the first set of experiments, there were two exemplars of the conspecific advertisement call and each of the appendages (except the noise burst used to test females of H. versicolor only). The two advertisement-call exemplars of H. versicolor were edited to create versions with different pulse numbers (10 or 21) and hence duration (370 or 850 ms; figure 1b,c). These durations are representative of values at the short end of the distribution and a somewhat longer-than-average call (by less than 1 s.d.), respectively (Gerhardt et al. 1995). The advertisement-call exemplars of H. chrysoscelis had 31 (duration of 536 ms) and 36 pulses (duration of 640 ms; figure 1d). The duration of the longer call was close to the mean in Missouri populations from which we collected females for testing (H.C. Gerhardt, unpublished data). In a second set of experiments, we used only one set of advertisement-call exemplars (shown in figure 1) and 10 exemplars of each type of aggressive call. This decision was based on the fact that the two advertisement-call exemplars were equivalent in attractiveness, and that aggressive calls were highly variable in structure.
In all compound calls, a silent interval of 50 ms was inserted between the end of the first element and the beginning of the second element. Compound calls with added segments of conspecific advertisement calls served as controls for the effect of the increased duration per se. Owing to the silent interval, control signals were approximately equivalent to a longer advertisement call with one missing pulse and one interpulse interval in H. versicolor, and three missing pulses and two interpulse intervals in H. chrysoscelis. Peak-to-peak amplitudes of aggressive, noise and control appendages were approximately 70–85% of that of the advertisement calls.
(b) Playback experiments: procedure
Alternative stimuli were output (44 kHz sampling rate and 16 bit resolution) in an alternating fashion at a rate of one call per 4 s from a personal computer using CoolEdit Pro/Audition v. 5.1 software. After digital-to-analogue conversion (Edirol), the signals were amplified (Nagra DSM amplifiers) and broadcast through two Analog-Digital Systems 200 loudspeakers separated by 2 m and located in a semi-anechoic chamber described by Gerhardt (1994). One speaker emitted a conspecific advertisement call alone and the other, the same advertisement call to which a single appendage was added. Advertisement-call amplitude was equalized (Larsen Davis sound level meter 800B) at 85 dB SPL (re: 20 μPa; fast root mean square) at the female release point midway between the speakers. Order of presentation of alternatives and the speakers playing them back were randomly predetermined. Phonotaxis was observed remotely under infrared illumination with a closed-circuit video system. Females were collected in Boone County, Missouri (USA; H. versicolor) and Phelps County, Missouri (H. chrysoscelis; exact localities in Gerhardt 2005). After at least 30 min of acclimation to the test temperature of 20°C, females were placed in a circular acoustically transparent cage. After three repetitions of each alternative stimulus, the top of the cage was removed remotely. A choice was recorded when a female approached to within 10 cm of one of the speakers. Single-speaker experiments were also performed with females of H. versicolor in order to determine whether the acoustic appendages alone were attractive (Bush et al. 2002; details are presented in the electronic supplementary material).
(c) Design and statistical analysis
In the first set of experiments conducted during 2003–2006, we observed responses from 195 females of H. versicolor and 114 females of H. chrysoscelis. We recorded 32 responses per test. Each female contributed one response per test, but responded in a variable number of different tests with a time-out of at least 5 min between the tests (see Gerhardt et al. (2000) for evidence for a lack of carry-over effects). Thus, no global statistical comparisons were made. We considered a preference to be statistically significant if at least 66% (21 out of 32) of the females chose the compound call or if fewer than 34% (11 out of 32) chose the compound call (p=0.039, two-tailed binomial test). These criteria translate to 95% credible limits of 50% or more if most females chose the compound call and up to 50% if most females chose the advertisement call alone.
Even though there was little indication that the two different aggressive-call exemplars affected female preferences in the tests just described, we designed a second set of experiments that allowed us to test globally for effects of species (H. versicolor and H. chrysoscelis), aggressive-call type (four species), exemplar (10 of each aggressive-call type), position (leading or following) and advertisement-call duration (average or short; H. versicolor only). Each of the 100 females (70 H. versicolor and 30 H. chrysoscelis) was tested with eight pairs of alternatives in one of the three series of tests; the sequence of tests within each series was randomized with respect to aggressive-call type, exemplar and position. The basic generalized linear model for each test series (group) was a randomized complete block in which the individual frog served as the complete block. Since there were no significant effects of exemplar version, the tests were arranged as a 4×2 factorial. When groups were compared, the generalized linear model was a split-plot design: the main plot contained the effect of group and the subplot, the effects of position, appendage type and all possible interactions of group, appendage type and position. Effects were assessed in terms of the average odds and the differences between tests, in terms of the odds ratio. All models were computed using the GENMOD procedure in SAS (Statistical Analysis System, Institute, v. 11.1, Cary, NC). We used the 50% limits of the 95% credible intervals as described above as a criterion for a significant preference. We assert, however, that the statistical and biological significance of our results is based on overall patterns of preference rather than the statistical significance of the results of individual tests.
3. Results
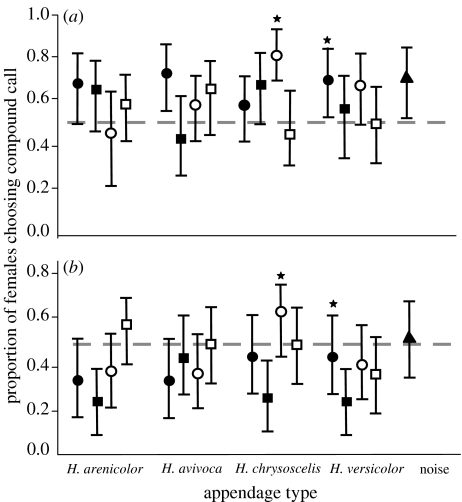
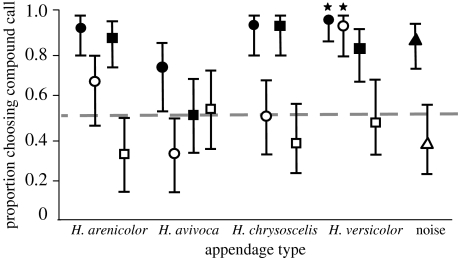
In single-speaker tests of H. versicolor, no appendage by itself reliably attracted females (details are provided in the electronic supplementary material). The results of the first set of two-speaker tests of H. versicolor and H. chrysoscelis are summarized in figures 2 and 3. When appendages followed an advertisement call of average duration, the combinations were either as attractive as or more attractive than the advertisement call alone (figure 2a). When appendages led an advertisement call of average duration, the combinations were never more attractive and sometimes less attractive than the advertisement call alone (figure 2b). When the advertisement call was shorter, the order effect was accentuated (figure 3). In other words, all compound calls with following appendages were strongly preferred to the short advertisement call alone, whereas, aside from the control stimulus, the same appendages in the leading position did not result in a more attractive compound call. Two compound calls were significantly less attractive than the advertisement call alone.
Figure 2.
Choices of female grey treefrogs in tests of compound calls versus normal-duration advertisement calls alone. (a) Proportions of females (n=32 females per test) of H. versicolor and H. chrysoscelis choosing compound calls in tests in which appendages followed advertisement calls. Filled symbols, H. versicolor females; open symbols, H. chrysoscelis females; circles, advertisement-call appendages; squares, aggressive-call appendages; triangles, noise-burst appendage; stars, control compound calls (see text). (b) Results of tests in which the appendage led the advertisement call. The 95% exact confidence limits were computed using the F-distribution method employed in SAS v. 11.1, and hence are not always symmetrical around the observed proportion. We interpret them as Bayesian credible intervals (uniform prior distribution).
Figure 3.
Proportions of females of H. versicolor (n=32 females per test) choosing compound calls with appendages added to an advertisement call of shorter-than-average duration. Filled symbols, appendage in following position; open symbols, appendage in leading position; circle, advertisement-call appendage; square, aggressive-call appendage; triangle, noise-burst appendage; star, control test with conspecific advertisement-call segment.
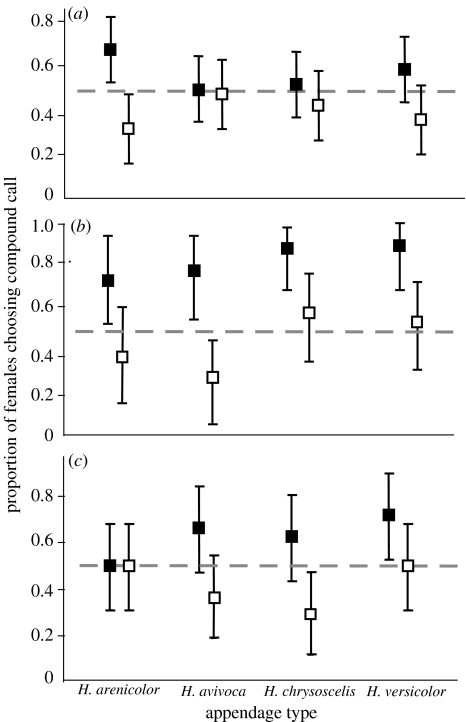
The results of the randomized block tests with the four types of aggressive calls are summarized in figure 4. Formal analyses (split-plot) comparing the choices of two different sets of H. versicolor females, tested using advertisement calls of normal and short duration (figures 4a,b), confirmed significant effects of appendage position (Χ12=18.75, p<0.0001), advertisement-call duration (Χ12=9.92, p<0.002) and the interaction of these two variables (Χ12=7.66, p<0.006). Comparisons of the data from tests of H. versicolor and H. chrysoscelis using advertisement calls of typical duration (figures 4a,c) showed a significant effect of appendage position (Χ12=9.84, p<0.002), but no significant difference between the two species (Χ12=0.56, p=0.456). The p-values for the effect of appendage type and exemplar in nearly all comparisons (within and between groups) were of the order of 0.1 or greater.
Figure 4.
Proportions of females of H. versicolor and H. chrysoscelis choosing compound calls in two-speaker choice tests in a randomized block design using aggressive-call appendages. Filled symbols, appendages in the following position; open symbols, appendages in the leading position. Error bars are 95% credible (confidence) intervals. (a) Proportions of females of H. versicolor (n=50) choosing compound calls with appendages added to a normal-duration advertisement call. (b) Proportions of females of H. versicolor (n=20) choosing compound calls with appendages added to a shorter-than-average advertisement call. (c) Proportions of females of H. chrysoscelis (n=30) choosing compound calls with appendages added to a normal-duration advertisement call.
In table 1, we present a meta-analysis that summarizes the order effect from all test results presented in figures 2–4 (excluding tests with control stimuli) using a more liberal criterion of 60% or more for a positive effect of an appendage and up to 40% for a negative effect of an appendage. A log-likelihood test of these results was highly significant (Χ22=51.4, p<0.0001).
Table 1.
Effect of appendage position on attractiveness of compound call. (Numbers are tests (excluding controls) shown in figures 2–4 in which a compound call was more attractive (positive, 60% or more choice of compound call), equally attractive (neutral, less than 60% and more than 40% choice of compound call) or less attractive (negative, up to 40% choice of compound call) when compared with the advertisement call alone.)
| position | positive | neutral | negative |
|---|---|---|---|
| following | 23 | 13 | 0 |
| leading | 1 | 15 | 19 |
4. Discussion
Since male grey treefrogs and closely related species produce only simple calls, the greater attractiveness of some experimentally generated compound signals relative to the established signal alone is consistent with the pre-existing bias and hidden preference hypotheses (Arak & Enquist 1993; Ryan & Rand 1993). Recent empirical research and theoretical models suggest that the origin of male traits by sensory exploitation has been widespread (Arnqvist 2006). Traditional sexual selection theory also predicts that once established, females are likely to favour more extravagant signals than males usually produce (Andersson 1994). The fact that a wide variety of appendage types, including sounds that females of these species have never experienced in their evolutionary history, had such a positive effect is also consistent with the idea that the quality of the extra stimulation may be secondary to its quantity (e.g. Ryan & Keddy-Hector 1992).
The most significant result of this study, which is not an expectation of either pre-existing bias or sexual selection models, is that the relative attractiveness of a compound call was best predicted by the temporal order of the established signal and the acoustic appendage. The very same appendage often had a positive effect on the relative attractiveness of a compound signal in the following position and a negative effect in the leading position. The temporal-order effect was stronger in tests with H. versicolor when the duration of the established element was reduced to a lower-than-average value, but compound signals with appendages in the leading position were still no more attractive than short advertisement calls alone.
Well-known psychological phenomena, such as forward masking (Yost & Nielson 1985), can be ruled out as a proximate mechanism for the observed order effect because the silent interval was always the same. Thus, if forward masking had been operating, adding appendages in the following position could not have resulted in more attractive combinations. Perhaps first hearing an attractive signal predisposes an animal to further excitatory stimulation by a new following element, whereas hearing the same elements in the opposite order interferes with the normal processing of the established element. This explanation is supported by the results of a previous test of H. versicolor, in which females preferred a synthetic advertisement call in which four attractive pulses led four unattractive (time-reversed) pulses to a call with the same four-pulse segments in the opposite order (Gerhardt & Schul 1999). The same explanation may apply to the processing of complex signals in other animals. In cotton-topped tamarins (Sanguinus oedipus), species-typical elements lead other elements that provide additional information about the sender within the combination long call (Miller et al. 2005). In white-crowned sparrows (Zonotrichia leucophrys), heterospecific songs may be learned by nestlings if they follow a species-typical introductory whistle (Soha & Marler 2000).
Additional experimental and comparative studies of species with simple signals can assess the generality of our results. This research has the potential to discover other factors that may enhance the attractiveness of compound and more complex signals relative to simple ones. Such factors include the relative amplitude of the two elements, the duration of the silent gap between elements and the number of elements.
In species that produce compound signals, we might expect some conservation of the ‘rules’ that favoured their evolution. For example, a temporal-order effect, albeit much weaker than that in grey treefrogs, was also found in túngara frogs (Physalaemus pustulosus): preferences for combinations of whine (the normal advertisement call) and chucks (signals produced most often in aggregations of males) were stronger when the chuck was in its usual following position than in the leading position (Ryan & Rand 1999; Wilczynski et al. 1999). Changing the order of the elements of the complex calls of the grasshopper Chorthippus dorsatus decreased signal attractiveness to females (Stumpner & Helversen 1992). A general rule regarding the conservation of temporal ordering is also consistent with the rarity or absence of language-like syntax in non-human animals (Hauser et al. 2002). Even in the handful of species in which the reordering of acoustic elements of complex calls seems to follow some grammar-like rules, the evidence that receivers perceive the resulting combinations as new messages in the same way as humans remains limited and controversial (e.g. Freeberg & Lucas 2002; Hauser et al. 2002; Crockford & Boesch 2005; Gentner et al. 2006). We anticipate that our results will stimulate studies of complex signal preferences in other kinds of animals and other signal modalities in order to discover whether temporal order and other constraints on the evolution and elaboration of complex signals are widespread.
Acknowledgments
This work was supported by grants to HCG from the NSF and PHS. We thank S. Bisges, A. Bockhorst, D. Dittmer, A. Evers, M. Tucker, T. Cook, M. Hellman, G. Höbel, C. McConkey and C. Tegtmeyer for help in collecting and testing female frogs, G. Höbel for acoustic analyses of the aggressive appendages, R. Cocroft, M. Hauser, G. Höbel, C. Murphy, R. Rodriquez, M. Ryan, J. Schul and W. Wilczynski for comments on a previous version of the manuscript, and M. Ellersieck for help with the statistical analysis.
This research adhered to the Association for the Study of Animal Behaviour/Animal Behavior Society Guidelines for the Use of Animals in Research, the legal requirements of the country in which the work was carried out and all institutional guidelines (University of Missouri ACUC protocol no. 1910).
Supplementary Material
methods, results and interpretation
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Arak A, Enquist M. Hidden preferences and the evolution of signals. Phil. Trans. R. Soc. B. 1993;340:207–213. doi:10.1098/rstb.1993.0059 [Google Scholar]
- Arnqvist G. Sensory exploitation and sexual conflict. Phil. Trans. R. Soc. B. 2006;361:375–386. doi: 10.1098/rstb.2005.1790. doi:10.1098/rstb.2005.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basolo A.L. Phylogenetic evidence for the role of a preexisting bias in sexual selection. Proc. R. Soc. B. 1996;259:307–311. doi: 10.1098/rspb.1995.0045. doi:10.1098/rspb.1995.0045 [DOI] [PubMed] [Google Scholar]
- Bradbury J.W, Vehrencamp S.L. Sinauer Associates; Sunderland, MA: 1998. Principles of animal communication. [Google Scholar]
- Bush S.L, Gerhardt H.C, Schul J. Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Anim. Behav. 2002;63:7–14. doi:10.1006/anbe.2001.1880 [Google Scholar]
- Crockford C, Boesch C. Call combinations in wild chimpanzees. Behaviour. 2005;142:397–421. doi:10.1163/1568539054012047 [Google Scholar]
- Endler J.A, Basolo A.L. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. doi:10.1016/S0169-5347(98)01471-2 [DOI] [PubMed] [Google Scholar]
- Freeberg T.D, Lucas J.R. Receivers respond differently to chick-a-dee calls varying in note composition in Carolina chickadees, Poecile carolinensis. Anim. Behav. 2002;63:837–845. doi:10.1006/anbe.2001.1981 [Google Scholar]
- Gentner T.Q, Fenn K.M, Margoliash D, Nusbaum H.C. Recursive syntactic pattern learning by songbirds. Nature. 2006;440:1204–1207. doi: 10.1038/nature04675. doi:10.1038/nature04675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H.C. Reproductive character displacement of female mate choice in the gray treefrog, Hyla chrysoscelis. Anim. Behav. 1994;47:959–969. doi:10.1006/anbe.1994.1127 [Google Scholar]
- Gerhardt H.C. Acoustic communication in two groups of closely related treefrogs. Adv. Study Behav. 2001;30:99–167. doi:10.1016/S0065-3454(01)80006-1 [Google Scholar]
- Gerhardt H.C. Advertisement-call preferences in diploid-tetraploid treefrogs (Hyla chrysoscelis and Hyla versicolor): implications for mate choice and the evolution of communication systems. Evolution. 2005;59:395–408. doi:10.1554/04-471 [PubMed] [Google Scholar]
- Gerhardt H.C, Huber F. University of Chicago Press; Chicago, IL: 2002. Acoustic communication in insects and anurans: common problems and diverse solutions. [Google Scholar]
- Gerhardt H.C, Schul J. A quantitative analysis of behavioral selectivity for pulse rise-time in the gray treefrog, Hyla versicolor. J. Comp. Physiol. A. 1999;185:33–40. doi: 10.1007/s003590050363. doi:10.1007/s003590050363 [DOI] [PubMed] [Google Scholar]
- Gerhardt H.C, Dyson M.L, Tanner S.D. Dynamic properties of the advertisement calls of gray treefrogs: patterns of variability and female choice. Behav. Ecol. 1995;7:7–18. doi:10.1093/beheco/7.1.7 [Google Scholar]
- Gerhardt H.C, Tanner S.D, Corrigan C.M, Walton H.C. Female preference functions based on call duration in the gray treefrog (Hyla versicolor) Behav. Ecol. 2000;11:663–669. doi:10.1093/beheco/11.6.663 [Google Scholar]
- Guilford T, Dawkins M.S. Receiver psychology and the evolution of animal signals. Anim. Behav. 1991;42:1–14. doi:10.1016/S0003-3472(05)80600-1 [Google Scholar]
- Hauser M.D, Chomsky N, Fitch W.T. The faculty of language: what is it, who has it, and how did it evolve? Science. 2002;298:1569–1579. doi: 10.1126/science.298.5598.1569. doi:10.1126/science.298.5598.1569 [DOI] [PubMed] [Google Scholar]
- Klump G.M, Gerhardt H.C. Use of non-arbitrary acoustic criteria in mate choice by female gray treefrogs. Nature. 1987;350:286–288. doi:10.1038/326286a0 [Google Scholar]
- Miller C.T, Iguina C.G, Hauser M.D. Processing vocal signals for recognition during anitiphonal calling in tamarins. Anim. Behav. 2005;69:1387–1398. doi:10.1016/j.anbehav.2004.08.021 [Google Scholar]
- Ryan M.J, Keddy-Hector A. Directional patterns of female mate choice and the role of sensory biases. Am. Nat. 1992;139:S4–S35. doi:10.1086/285303 [Google Scholar]
- Ryan M.J, Rand A.S. Sexual selection and signal evolution: the ghost of biases past. Phil. Trans. R. Soc. B. 1993;340:187–195. doi:10.1098/rstb.1993.0057 [Google Scholar]
- Ryan M.J, Rand A.S. Phylogenetic inference and the evolution of communication in túngara frogs. In: Hauser M.D, Konishi M, editors. The design of animal communication. MIT Press; Cambridge, MA: 1999. pp. 535–557. [Google Scholar]
- Soha J.A, Marler P. A species-specific cue for selective song learning in the white-crowned sparrow. Anim. Behav. 2000;60:297–306. doi: 10.1006/anbe.2000.1499. doi:10.1006/anbe.2000.1499 [DOI] [PubMed] [Google Scholar]
- Stumpner A, Helversen O.v. Recognition of the two-element song in the grasshopper Chorthippus dorsatus (Orthoptera: Gomphocerinae) J. Comp. Physiol. A. 1992;178:227–233. [Google Scholar]
- Wilczynski W, Rand A.S, Ryan M.J. Female preferences for temporal order of call components in the tungara frog: a Bayesian analysis. Anim. Behav. 1999;58:841–851. doi: 10.1006/anbe.1999.1208. doi:10.1006/anbe.1999.1208 [DOI] [PubMed] [Google Scholar]
- Yost W.A, Nielson D.W. 2nd edn. Holt Rinehardt and Winston; New York, NY: 1985. Fundamentals of hearing: an introduction. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
methods, results and interpretation