Abstract
Skeletal mass is maintained by a balance between formation and resorption, cell proliferation and apoptosis. In vitro, glucocorticoids (GCs) decrease extracellular signal-regulated kinases (ERK) activation by mitogens, thus inhibiting osteoblast proliferation. Both ERK activity and proliferation are restored by co-treatment with the protein tyrosine phosphatase inhibitor, vanadate. Since ERK signalling may also be anti-apoptotic, we explored the effects of vanadate on GC-induced apoptosis in vitro and in vivo. Apoptosis in MBA-15.4 pre-osteoblasts increased from 6 h and remained up to eightfold higher through 6 days of 10−6 M dexamethasone (Dex) treatment. Co-incubation with 10−7 M vanadate markedly reduced apoptosis at all time points. Vanadate also prevented GC-induced poly-ADP-ribose polymerase cleavage. We assessed the transcriptional profiles of seven anti-apoptotic proteins (Bcl-2, Bcl-XL, inhibitors of apoptosis protein-1 (IAP-1), IAP-2, X-linked IAP (XIAP), Fas-associated death-domain-like IL-1β-converting enzyme-inhibitory protein (FLIPLong) and FLIPShort) in osteoblasts subjected to various stimuli using real-time quantitative PCR. Although these anti-apoptotic genes responded to different mitogenic conditions, Dex failed to repress their expression, and in fact significantly up-regulated Bcl-XL, IAP-2 and XIAP. Dex may therefore induce apoptosis by up-regulating pro-apoptotic gene expression. We have previously demonstrated that rats treated with GC develop low formation osteoporosis (bone histomorphometry and DEXA) and skeletal fragility (breaking strength) that were largely prevented by co-treatment with vanadate. We report here that vertebrae from rats treated with 3·5 mg/kg per day methylprednisolone for 9 weeks showed increased incidence of terminal deoxynucleotidyl transferase-mediated biotin-dUTP nick end-labelling-positive apoptotic osteocytes, which was reduced by vanadate co-treatment. We conclude that vanadate prevents GC-induced apoptosis of pre-osteoblasts in vitro and osteocytes in vivo, and this may contribute to its bone-sparing effects in vivo.
Introduction
Bone loss and fractures resulting from glucocorticoid (GC) therapy is the most prevalent form of secondary osteoporosis (Van Staa et al. 2000, Angeli et al. 2006). Skeletal health and repair depends on replenishment of ageing cells from precursor stem cells, efficient function of mature cells and an adequate lifespan, all of which are compromised by clinical doses of GCs. Although GC treatment causes rapid bone loss via transiently increased resorption, loss of bone in response to GC therapy is primarily due to impaired osteoblastic bone formation (Hulley et al. 1998, Manolagas 2000, Canalis & Delany 2002).
GCs induce apoptosis in both bone and cartilage, causing excessive and premature loss of osteoblast precursors (Weinstein et al. 1998, Zalavras et al. 2003), osteocytes (Weinstein et al. 1998), articular (Van Offel et al. 2002) and growth plate chondrocytes (Silvestrini et al. 2000, Mehls et al. 2001). Although cell proliferation and differentiation are also negatively affected, this premature cell death may contribute significantly to vertebral fractures, osteonecrosis of the femoral head, stunting of long bone growth and thinning of articular cartilage. The loss of osteocytes might be particularly important in terms of bone structure because this mechanosensor is important in the repair of bone microdamage. However, the mechanism of GC-induced apoptotic cell death is not known. It has been reported to depend on classical GC:GC receptor interactions, receptor dimerization and regulation of gene expression (Bortner & Cidlowski 2002).
Apoptosis can be triggered by multiple pathways, including the classical extrinsic (death receptor activated) and intrinsic (mitochondrially mediated) pathways. Common survival strategies are deployed by most cell types (for recent reviews, see Bortner & Cidlowski 2002, Zalavras et al. 2003) and the principal approach is prevention of caspase activation. Caspase 8 is activated following death receptor clustering or integrin dysfunction and this is prevented by decoy proteins, the Fas-associated death-domain-like IL-1β-converting enzyme-inhibitory proteins (FLIPs). Caspase 9 is activated downstream of mitochondrial permeability transition (ψT), which is mediated by cytochrome release and regulated by pro- and anti-apoptotic Bcl-2 family members (Adams & Cory 1998, Kelekar & Thompson 1998, Creagh & Martin 2003). Activation of caspase 9 is prevented by a balance being maintained between prosurvival Bcl-2 and Bcl-XL and pro-death Bad, Bid, Bim, Bax, Bak and others. Caspases 8 and 9 are initiator caspases that in turn activate downstream executioner caspases, such as 3, 6 and 7. Osteoblasts and osteocytes undergoing apoptosis in response to GCs display typical features, such as activation of caspase 3 and DNA laddering (Chua et al. 2003, Liu et al. 2004). Executioner caspases, such as caspase 3, are blocked by a family of inhibitors of apoptosis proteins (IAPs), many of which are ubiquitin ligases and target the caspases for proteasomal degradation (Deveraux & Reed 1999, Hu & Yang 2003).
The intracellular signal transducers extracellular signal-regulated kinases (ERK), PKB/Akt, PKA and p90RSK are not only important regulators of cell proliferation but also cell survival and are thought to be involved in protection against apoptosis (Plotkin & Bellido 2001, Tran et al. 2001, Bortner & Cidlowski 2002, Wang et al. 2002, Dan et al. 2004, Ley et al. 2004). ERK is implicated in the regulation of all three major anti-apoptotic families, but particularly the FLIPs. It has also been reported that FLIP activates the NF-κB as well as the ERK (mitogen-activated protein kinase, MAPK) signalling pathways that have been shown to override apoptotic signalling from Fas, tumour necrosis factor and TNF-related apoptosis-inducing ligand (TRAIL) death receptors (Kataoka et al. 2000, Tran et al. 2001). The role of FLIP in cell survival is admittedly complex, with reports implicating phosphorylated FLIP in both pro- and anti-apoptotic signalling (Higuchi et al. 2003, Yang et al. 2003). Moreover, in immune cells, the expression of a number of anti-apoptotic genes such as Bcl-2 has been shown to be up-regulated (Galon et al. 2002), while many pro-apoptotic genes were down-regulated by GCs (Galon et al. 2002). Since the mechanism of GC-induced apoptosis in skeletal cells remains poorly defined, a more effective approach might be to investigate mechanisms of protection against GC-induced apoptosis. This may provide additional insight into the mechanism(s) of death, as well as a rational basis for the development of useful bone active drugs.
We find that GCs mediate their inhibitory effects on osteoblast proliferation in vitro via up-regulation of the vanadate-sensitive dual-specificity phosphatase, MKP-1, which profoundly represses the ERK mitogenic signalling pathway (Engelbrecht et al. 2003). Vanadate, a non-specific but broad-spectrum protein tyrosine phosphatase (PTP) inhibitor, reverses this negative effect of GCs both in vitro and in vivo (Hulley et al. 1998, 2002). Given the role of ERK in both cell proliferation and survival, we hypothesized that GC-induced inactivation of ERK might also affect apoptosis of osteoblasts, and therefore set out to explore the effect of vanadate on GC-treated osteoblasts in vitro and osteocytes in vivo.
Materials and Methods
Materials
TUNEL was performed using the In Situ Cell Death Detection kit with metal-enhanced diaminobenzidine (DAB) stain (Roche). Caspase-cleaved poly-ADP-ribose polymerase (PARP) polyclonal antibody was from Promega. Dexamethasone (Dex) was dissolved in ethanol as a 10−2 M stock. U0126 (Promega) was dissolved in dimethyl sulfoxide (DMSO) to a stock concentration of 10 mM. If inhibitors were added to cells, control cultures were treated with equivalent amounts of ethanol and/or DMSO. Foetal calf serum (FCS) was from Delta Bioproducts (Johannesburg, Republic of South Africa). All other chemicals including tissue culture media, Dex and sodium orthovanadate were purchased from Sigma.
Cell culture
MBA-15.4 mouse bone marrow stromal cells were a kind gift from Prof. S Wientroub, Tel Aviv University, Israel. They express osteoblastic markers such as alkaline phosphatase and collagen type I, but very low levels of parathyroid hormone receptors, in vitro and can be induced to produce bone in vivo (Benayahu et al. 1989, Fried et al. 1993). MBA-15.4 cells were grown in bicarbonate-buffered Dulbecco's modified Eagle's medium (DMEM) with 10% (v/v) heat-inactivated FCS, 100 U/ml penicillin and 100 μg/ml streptomycin. For experiments, 70% confluent cells were lifted with a cell scraper and seeded in 24-well plates on cover glasses (for detection of apoptosis) or 100-mm culture dishes (all other experiments). Medium was changed to DMEM with 1% FCS (v/v) for 24 h to serum starve and synchronize the cell cycle of the cells prior to 20% FCS (v/v) stimulation. The MEK inhibitor, U0126, was added 30 min before 20% FCS (v/v) stimulation to a final concentration of 10 μM in experiments where the effect of MAPK inhibition on gene expression was ascertained. In cell culture experiments involving Dex treatment, cells were cultured to at least 50% confluence in medium with 10% FCS (v/v) prior to the addition of Dex, dissolved in ethanol, to a final concentration of 1 μM. Cells treated with Dex for <3 days were treated once and for 6 days were treated on day 1 and again on day 3.
In vivo study
The treatment protocol for the in vivo rat study was approved by the Ethics Committee and the Animal Research Committee, University of Stellenbosch and complies with the FRAME 1999 guidelines. This study has been described in detail in Hulley et al. (2002) and vertebral bones from this study were preserved for the current apoptosis investigation. In brief, the study was carried out on 3·5- to 4-month-old female Sprague–Dawley rats weighing on average 260 g at the beginning of the experiment. They were maintained on a 12-h light/darkness cycle with free access to food (Rat and Mouse Breeder Feed, Animal Specialities, Pty, Ltd, Klapmuts, Cape Town, South Africa) and water. Diet was assessed to ensure adequate intake of calcium and vitamin D for bone growth (18 g/kg Ca2+, 8 g/kg phosphorus, 1000 IU/kg vitamin D). Treatment groups (n=10) over 9 weeks were as follows: C, sham-injected once daily, 5 days/week; S, 3·5 mg/kg per day methylprednisolone (Solu-medrol) injected subcutaneously; V, 0·5 mg/ml sodium orthovanadate continuously in drinking water plus sham injection and SV, methylprednisolone plus vanadate. Sodium orthovanadate was dissolved at 0·5 mg/ml in sterile, deionized water and corrected to pH 6·0–7·0 with ascorbic acid. All other rat groups received the same amount of ascorbic acid in their drinking water, corrected to neutral pH with sodium hydroxide. Rats in the vanadate and steroid plus vanadate treatment groups received only vanadate-supplemented water at all times throughout the 9-week study. Vanadate intake was assessed by determining serum vanadium levels at 9 weeks by inductively coupled plasma-mass spectrometry (Hulley et al. 2002). Water intake was not measured for the different groups but was sufficient for the rats to be weight matched to their control groups after 9 weeks. Rats were weighed twice or thrice per week during the study and showed a slight but steady weight gain throughout. After killing the rats with pentobarbital, the vertebrae were removed and stored in 70% ethanol at 4 °C. One of the lumbar vertebrae (L5) from each of five rats per treatment group was removed, fixed in modified Millonig's solution (100 ml 37% (v/v) formaldehyde solution, 900 ml distilled water, 18·6 g NaH2PO4, 4·2 g NaOH, 5 g sucrose; pH 7·4 at room temperature) for 24 h, and then decalcified using serial changes of 10% EDTA (w/v), pH 7·0 at 4 °C over 2 weeks, embedded in wax and sectioned at 5 μm. Matching sections from the vertebral bodies (embedded disc side down) were assessed for apoptotic osteocytes using the Terminal deoxynucleotidyl transferase-mediated biotin-dUTP nick end-labelling (TUNEL) method.
Apoptosis assay
TUNEL was used to detect apoptotic cells in vitro and in rat sections, according to the manufacturer's instructions. Cell death was determined by counting, blinded to the treatment groups, the number of cells containing nuclei with apoptotic morphology that were also stained dark brown with DAB. Cells grown on cover glasses were fixed with freshly prepared, ice-cold 4% paraformaldehyde (w/v) in PBS, pH 7·4, for 30 min at room temperature. Cells were pretreated by blocking endogenous peroxidases and were permeabilized in 0·2% Triton-X (v/v) on ice for 2 min, prior to TUNEL staining according to the manufacturer's protocol for adherent cells, cell smears and cytospin preparation, but with TUNEL reaction overnight at 4 °C, followed by metal DAB-enhanced peroxidase detection. Positive controls were prepared by pretreating cells with DNase according to the manufacturer's instructions prior to TUNEL staining, and terminal transferase enzyme was omitted from negative controls. Sections cut from decalcified paraffin-embedded specimens of rat vertebrae were dewaxed. They were then treated for 10 min with 3% H2O2 (v/v) in methanol to inactivate endogenous peroxidase and processed according to the manufacturer's method for difficult sections, incorporating microwave pretreatment in citrate buffer, overnight TUNEL labelling at 4 °C, followed by metal DAB-enhanced peroxidase detection. Both cultured cells and treated sections were counterstained with 1% methyl green.
Counting of apoptotic cells
Apoptotic cells were counted by a single researcher (M M C), blinded to the study groups. TUNEL staining can lead to overestimation or underestimation of the frequency of apoptosis but should not lead to an inaccurate relative assessment between treatment groups. Using standard bright field microscopy, cells with both dark brown nuclear stain and apoptotic morphology were interpreted as positive. Mitotic pairs sometimes stained positively and were excluded. On cover glasses ±120 fields were counted. In the rat sections, osteocytes were identified inside cortical lacunae and ±25 fields were examined in each section from five rats per group at 250× magnification.
Caspase activity
Apoptosis in vitro was confirmed in these cells by the detection of a caspase-cleaved substrate, using an antibody that only detects the large 89 kDa caspase-cleaved fragment of PARP. PARP fragments stained dark brown, using metal DAB-enhanced peroxidase detection, and were counted as above. Specificity of the antibody was controlled by detection of a single ∼90 kDa band on western blot of lysates from osteoblasts undergoing mass apoptosis following treatment with 40 μg/ml cycloheximide. Immunostaining on cover glasses was also carried out using secondary antibody only and on a serum-deprived, heavily apoptotic population as a positive control.
Western blotting
Cells were treated for 24 h with 40 μg/ml cycloheximide, 1 μM Dex, 0·1 μM sodium orthovanadate, 10 μm U0126 or combinations of these. Cell lysates were prepared as previously described (Hulley et al. 1998) and equal protein samples subjected to SDS-PAGE electrophoresis, followed by transfer to Polyvinylidene fluoride (PVDF) membrane, blocking in 3% fat-free milk and incubation with anti-cleaved PARP antibody at 4 °C overnight. Detection was performed using horse radish peroxidase-conjugated secondary antibody and enhanced chemiluminescence reagents from Amersham.
Real-time quantitative PCR (rtqPCR)
Total RNA extraction from MBA-15.4 osteoblasts, reverse transcription of DNase-treated RNA and rtqPCR were performed as described previously (Engelbrecht et al. 2003). Briefly, gene transcript levels of the GC-insensitive house keeping gene, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the anti-apoptotic mouse genes Bcl-2, Bcl-XL, IAP-1, IAP-2, X-linked IAP (XIAP), FLIPLong and FLIPShort were quantified by rtqPCR on a LightCycler (Roche Diagnostic Systems). Hot start PCR was performed according to the manufacturer's instructions, with a final concentration of 3–5 mM MgCl2 and 0·5 μM primer (LightCycler – FastStart DNA Master SYBR Green I, Roche). The various gene transcript levels of the anti-apoptotic genes in treated samples were quantified and compared with the respective transcript levels in untreated controls using the relative standard curve method (Applied Biosystems, division of Perkin–Elmer, Foster City, CA, USA). A total of three independent experimental repeats were performed, and repeat assessments of cDNA from each experiment were also made.
The following primer pairs were used: mouse GAPDH (accession no. NM_008084) forward: 5′-ATTGTCAGCAATGCATCCTG-3′, reverse: 5′-ATGGACTGTGGTCATGAGCC-3′; mouse Bcl-2 (accession no. M16506) forward: 5′-ACTTCTCTCGTCGCTACCGT-3′, reverse: 5′-CTGTTGACGCTCTCCACACA-3′; mouse Bcl-XL (accession no. L35049) forward: 5′-CGTAGACAAGGAGATGCAGG-3′, reverse: 5′-TCAGGAACCAGCGGTTGAAG-3′; mouse IAP-1 (accession no. U88908) forward: 5′-AAGTGTGTATGGACCGAGAG-3′, reverse: 5′-GG- ACCATTAGTCTTGTTCAG-3′; mouse IAP-2 (accession no. U88909) forward: 5′-TGTGTATGGACAGAGAGGTT-3′, reverse: 5′-CAGCTTCTGATGTCCAACAA-3′; mouse XIAP (accession no. NM_009688) forward: 5′-ATACGGAGGATGAGTCAAGT-3′, reverse: 5′-GGTTGAACGTAATGACGGTG-3′; mouse FLIPLong (accession no. U97076) forward: 5′-CTGTGTCTGCCGAGGTCATT-3′, reverse: 5′-AACCGCCTCACTCTGTAGAG-3′ and mouse FLIPshort (accession no. XM_124038) forward-1: 5′-GCCT-GAAGAACATCCACAGA-3′, reverse-1: 5′-TCTCTGAGACTGGTTCATGC-3′, forward-2: 5′-CTGAACAGAGACTCCTTAGA-3′, reverse-2: 5′-AGGAGGCTGAAG- CAAGAGGA-3′. As a negative control for DNA contamination, purified mRNA was used as template for PCR with all of the above-mentioned primer pairs. No PCR products were detected. To further eliminate possible variation in rtqPCR due to the presence of residual contaminating DNA, the primer pairs were designed to span intron/exon boundaries where possible. The identities of all PCR products were confirmed by sequencing.
Statistical analysis
Results are given as the mean±s.e.m. or ±s.d. as indicated. Data were analysed using GraphPad Prism v 2.01 (GraphPad Software Inc., San Diego, CA, USA) by one-way ANOVA with Dunnett's post hoc test or Tukey's post hoc test for multi-group comparisons. Differences were considered statistically significant at P<0·05.
Results
Dex induces apoptosis in osteoblasts in vitro
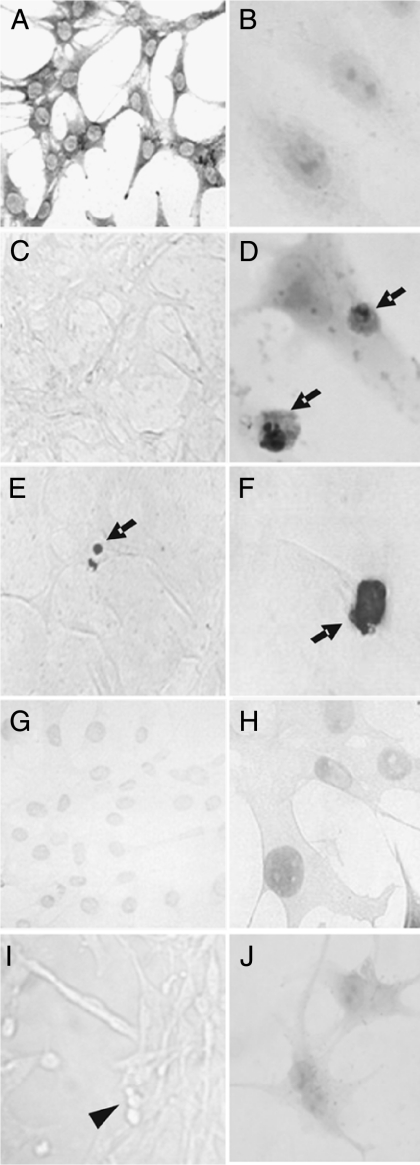
Treatment of MBA-15.4 osteoblasts (Fig. 1) with high-dose Dex induced typical apoptotic features, including cell shrinkage, caspase cleavage of PARP (Fig. 1D) and DNA fragmentation (Fig. 1E and F). Only MBA-15.4 osteoblasts with both dark brown stain and apoptotic morphology (cell shrinkage, condensed chromatin, formation of apoptotic bodies) were interpreted as positive.
Figure 1.
Morphology of apoptotic MBA-15.4 osteoblasts. Healthy MBA-15.4 osteoblasts (A–C) were treated with dexamethasone (D–F and J) and assessed by immunocytochemistry for cleaved PARP (B and D) and by TUNEL (C, E–H). Normal morphology is shown using methylene blue stain (A). Apoptotic cells were identified on the basis of both positive staining and typical morphological changes of the cells (arrows; D–F). TUNEL staining was controlled using cover glasses pretreated with DNase to create breaks in the DNA of healthy cells (G and H), or as a negative control, highly apoptotic serum-starved cells with TUNEL but no terminal transferase enzyme (I). This produced a lighter stain than true apoptosis and no change in morphology (compare F with H), or no staining but apoptotic morphology with condensed, rounded cells (arrowhead; I). Secondary antibody only control (cPARP protocol) is shown for cells treated with dexamethasone (J).
Prevention of GC-induced apoptosis by vanadate in vitro
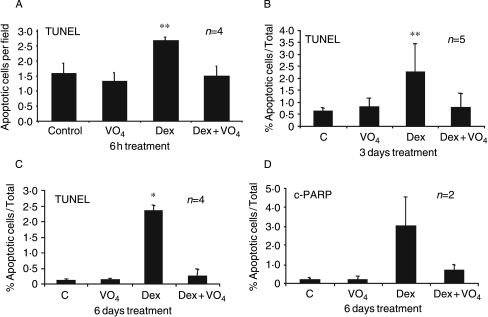
MBA-15.4 murine osteoblasts were treated with doses of Dex ranging from 1 nM to 10 μM (results not shown), for periods of 6 h to 6 days. A maximum increase in apoptosis, at all time points tested, was achieved with 0·1 μM and 1 μM Dex. Basal apoptosis, as detected by TUNEL, is low (below 0·5%) in this cell line. This rose to a maximum of 2·5% with Dex treatment and this level of apoptosis was maintained throughout 6 days of treatment. We have previously reported that the tyrosine phosphatase inhibitor, sodium orthovanadate, prevents Dex-induced inhibition of ERK activity and dysregulation of osteoblast proliferation in rats, and strongly protected against GC-osteoporosis (Hulley et al. 1998, 2002, Engelbrecht et al. 2003). Since ERK is also a prosurvival kinase, we tested the effect of concurrent vanadate treatment on GC-induced apoptosis of osteoblasts and found effective protection at 6 h (Fig. 2A), 3 days (Fig. 2B) and 6 days (Fig. 2C).
Figure 2.
Effect of dexamethasone and vanadate on apoptosis in MBA-15.4 osteoblasts. MBA-15.4 osteoblasts, grown in 10% FCS, were treated for 6 h, 3 days and 6 days with 10−6 M Dex and/or co-treated with 10−7 M vanadate (VO4). Dex was added every 3 days and vanadate daily. Apoptotic cells were identified on the basis of morphological changes and either TUNEL staining (A–C) or cleaved PARP immunocytochemistry (D). Total cell counts were not performed in the first set of experiments (A), so these data are expressed as apoptotic cells per field. Results (mean±s.d.) are the average of at least four independent experimental repeats, except where otherwise stated. *P<0·05, **P<0·01.
Dex induces cleavage of PARP in osteoblasts
To confirm these results using a different method, we performed immunocytochemistry (ICC) with an antibody detecting the apoptosis-specific 89-kDa C-terminal caspase cleavage product of PARP. PARP cleavage, detected by ICC, was almost absent in control cultures but was readily detected in 3% of cells following 6 days of Dex treatment (Fig. 2D), in agreement with TUNEL data (Fig. 2C). Once again, vanadate co-treatment strongly reduced the number of apoptotic cells, indicating protection against both caspase activation and DNA fragmentation.
GC but not vanadate treatment regulates the expression of prosurvival genes in MBA-15.4 osteoblasts
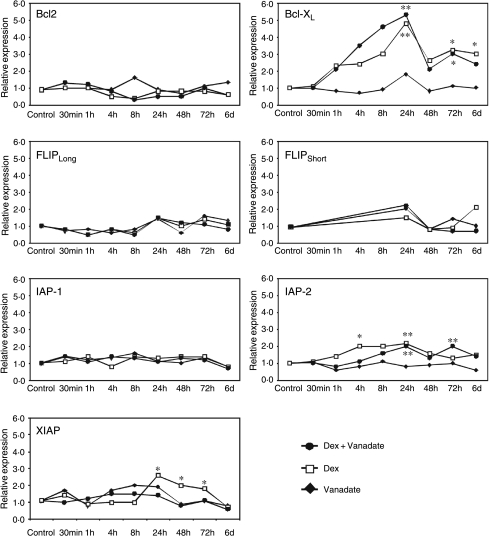
Since 6 days of Dex treatment consistently produced the highest level of osteoblast apoptosis, we tested the effect of Dex, alone and in combination with vanadate, on expression of the panel of prosurvival genes at multiple time points over 6 days (Fig. 3). Unexpectedly, Dex did not down-regulate expression of any prosurvival genes and in fact up-regulated several. The expression of Bcl-XL was increased approximately sevenfold within 24 h of Dex treatment and this remained approximately fivefold up-regulated throughout 6 days of treatment. IAP-2 and XIAP transcription was reproducibly doubled, with IAP-2 showing up-regulation as early as 4 h after Dex treatment before tapering off after 24 h. XIAP transcription levels were doubled at 24 h but remained elevated for 3 days. The transcript levels of anti-apoptotic proteins Bcl-2, IAP-1, FLIPLong and FLIPShort were not detectably altered by Dex treatment. Interestingly, treatment of cells with vanadate alone produced no changes in expression of these prosurvival genes at any time point. Co-treatment of osteoblastic cells with both Dex and vanadate produced no significant alteration in the expression patterns observed with Dex alone, indicating that Dex and vanadate in this context are acting via separate pathways.
Figure 3.
The anti-apoptotic genes Bcl-XL, XIAP and IAP-2 are up-regulated by dexamethasone in osteoblasts. Real-time quantitative PCR was used to quantify the expression of seven anti-apoptotic genes in response to treatment with Dex alone (open squares), vanadate alone (closed diamonds) or Dex and vanadate in combination (closed circles) in the MBA-15.4 mouse osteoblastic cell line. Osteoblasts were treated with 1 μM Dex and/or 0·1 μM vanadate for 30 min to 6 days while growing in 10% FCS. Levels of the anti-apoptotic genes and the dexamethasone-insensitive housekeeper, GAPDH, in treated samples were quantified and compared to the respective transcript levels in untreated controls using the relative standard curve method. A total of three independent experimental repeats were performed, and repeat assessments of cDNA from each experiment were also made. *P<0·05, **P<0·01.
Mitogen withdrawal induces osteoblast apoptosis and regulates prosurvival gene expression
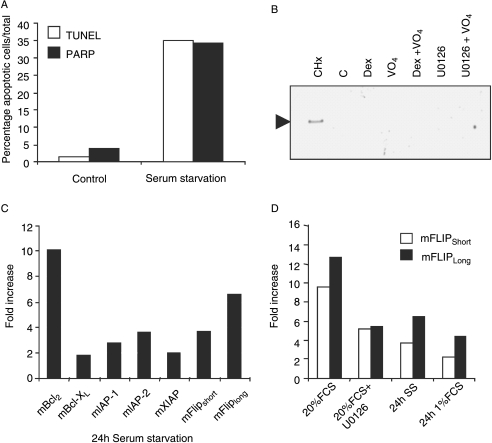
Given the unexpected up-regulation of prosurvival genes by Dex, and since vanadate appeared to promote survival via the mitogenic ERK and PKB pathways, we set out to identify prosurvival signalling molecules in osteoblasts that are highly regulated by changing mitogenic conditions. MBA-15.4 osteoblasts were subjected to serum withdrawal for 24 h in order to deplete mitogenic growth factors. This caused a 30-fold increase in apoptosis, detected with both TUNEL and caspase-cleaved PARP antibody (Fig. 4A). Specificity of this antibody was confirmed by western blot using a strong pro-apoptotic stimulus, 40 μg/ml cycloheximide, but please note that we were unable to detect the low levels of apoptosis seen with Dex, alone or in combination with inhibitors (Fig. 4B). Real-time quantitative PCR showed that serum withdrawal-induced apoptosis was associated with a strong, transcriptional up-regulation of a panel of prosurvival molecules (Fig. 4C). However, in contrast to Dex treatment, Bcl-2 expression was approximately tenfold increased, whereas Bcl-XL was unchanged. FLIPLong and FLIPShort were increased approximately seven- and fourfold respectively and IAP-1 and IAP-2 both increased approximately threefold.
Figure 4.
Serum deprivation induces apoptosis in MBA-15.4 osteoblasts and regulates prosurvival genes. Osteoblasts were cultured until 70% confluence in standard medium with 10% FCS and then medium was replaced with either serum-free medium for 24 h (A, C and D), medium containing 1% serum (v/v) (C), medium containing 10% FCS (v/v) with or without 40 μg/ml cycloheximide (CHx), 10 μM Dex, 0·1 μM vanadate, 10 μM MEK inhibitor, U0126 or combinations as indicated (B), or medium containing 20% FCS (v/v) with or without 10 μM MEK inhibitor, U0126 (D). Cells grown on cover glasses were assessed by immunocytochemistry for cleaved PARP or TUNEL and the number of apoptotic and total cells counted (A). Specificity of the cleaved PARP antibody was controlled by western blot detection of a single ∼90 kDa band in lysates of cells undergoing mass apoptosis following cycloheximide treatment (B). Real-time quantitative PCR was performed on mRNA extracted from cells grown on 10-cm dishes to compare expression of a panel of prosurvival genes following serum starvation (C). A more detailed rtqPCR analysis of the responses of FLIPLong (open bars) and FLIPShort (filled bars) to serum stimulation (20% FCS), treatment with the MEK inhibitor, U0126, for 30 min prior to 20% FCS (v/v) stimulation, total (0%) and partial (1%) reduction in serum was performed (D). Real-time analysis was performed in duplicate and is expressed relative to untreated controls and normalized to GAPDH. Each figure represents the average of two independent experimental repeats.
Both FLIP isoforms have previously been shown to be regulated by ERK activity (Wang et al. 2002). To investigate this in osteoblasts, the expression levels of FLIPLong and FLIPShort were quantified in MBA-15.4 cells mitogenically stimulated with 20% FCS (following 24 h of reduced serum culture to synchronize cell cycle and mitogenic response). FLIPLong and FLIPShort transcript levels were both approximately tenfold up-regulated in response to mitogen stimulation (Fig. 4D). This increase was halved for both FLIPLong and FLIPShort when cells were co-treated with the MEK inhibitor, U0126. This inhibitor was used at 10 μM, a concentration validated in this cell line not only to inhibit ERK phosphorylation but also to inhibit serum-stimulated osteoblast proliferation (Engelbrecht et al. 2003). Therefore, the FLIPs are highly mitogen-sensitive in osteoblasts, responding very strongly to mitogenic stimulation and more weakly to mitogen withdrawal. Up-regulation by serum stimulation is at least partially MEK–ERK dependent in osteoblasts.
Vanadate has anti-apoptotic effects in vivo
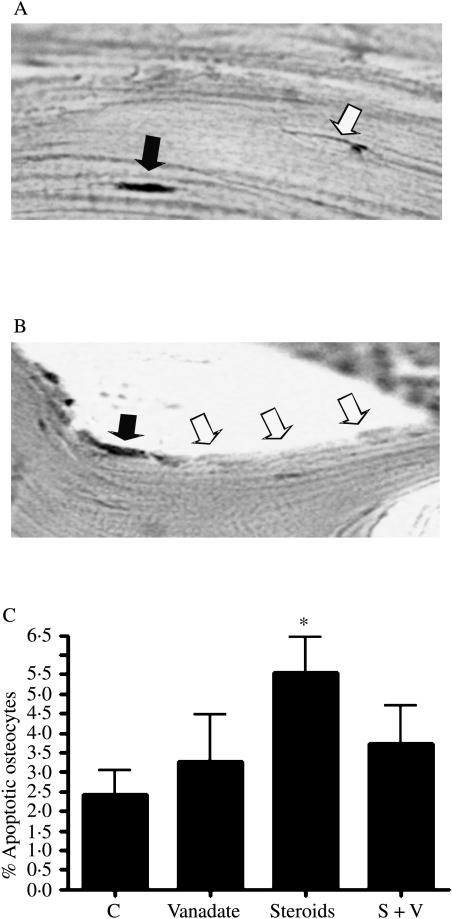
To assess the effect of vanadate on GC-induced apoptosis in vivo, we examined the effect of vanadate in a rat model of GC-induced osteoporosis. Rats treated with high-dose GC (3·5 mg/kg per day methylprednisolone for 9 weeks) develop osteoporosis which, as shown by our group (Hulley et al. 2002) and others (Ortoft et al. 1995, Ortoft & Oxlund 1996), is characterized by a severe inhibition of new bone formation and a decrease in BMD, bone size and bone strength. Co-treatment with vanadate largely prevented these GC effects (Hulley et al. 2002). Precise identification of osteoblasts is difficult in histological sections unless double-staining protocols are used and we therefore analysed apoptosis in osteocytes which occupy lacunae. Employing TUNEL, GC-induced apoptosis was detectable in both osteocytes (Fig. 5A) and bone-lining cells (Fig. 5B). Quantification revealed between two- and fivefold increases in the number of apoptotic osteocytes (Fig. 5C; P<0·05 versus control). Rats co-treated with vanadate were indistinguishable from untreated controls, indicating protection of osteocytes from GC-induced apoptosis over a period of 9 weeks (Fig. 5C).
Figure 5.
The in vivo effect of vanadate on osteocyte apoptosis in rats treated for 9 weeks with glucocorticoid. Rats received either daily sham injections, injections of 3·5 mg/kg per day methylprednisolone, sodium orthovanadate at 0·5 mg/ml continuously in their drinking water or a combination of vanadate and steroid injections (S+V) (as described in Materials and Methods). L5 vertebrae were decalcifed, wax-embedded and matching sections were TUNEL stained. TUNEL-positive osteocytes were identified inside cortical lacunae (A) and apoptotic bone-lining cells also noted (B). High-dose GC treatment induced a significant increase in apoptotic osteocytes (C). Incidence in rats treated with vanadate alone or in combination with GC was indistinguishable from controls. Sections were analysed from five animals in each group by a single investigator (MMC) blinded to the treatments. Data are expressed relative to total osteocyte number ±s.d. *P<0·05.
Discussion
GC-induced osteoporosis is characterized by decreased bone formation and reduced osteoblast numbers, the latter resulting from a combination of impaired recruitment and proliferation of immature osteoblasts, transdifferentiation of osteoblasts to adipocytes and accelerated osteoblast apoptosis (Manolagas 2000, Canalis & Delany 2002). In addition to induction of apoptosis in primary osteoblast cultures, GCs have also been shown to increase apoptosis in several cell lines including MLO-Y4 osteocytic cells (Ahuja et al. 2003), MC3T3-E1, OCT-1 and C3H10T1/2 osteoblastic cells (Chen et al. 2002, Ahuja et al. 2003, Liu et al. 2004). GCs have, however, also been shown to protect primary osteoblasts and osteoblastic cell lines (MC3T3-E1, MG63) from apoptosis, depending on culture conditions (Zalavras et al. 2003). Up-regulation of the anti-apoptotic proteins Bcl-2 or Bcl-XL by GCs has been reported in MC3T3-E1 osteoblastic cells (Chua et al. 2003) and lymphocytes (Galon et al. 2002), and this may provide a mechanism for GC resistance to apoptosis in some transformed cell lines. Cell lines like MBA-15.4 and MLO-Y4 can, however, be reliably induced to respond to GCs with increased apoptosis and provide useful models for preliminary studies of the primary tissue.
The present study confirms previous reports of GC-induced apoptosis, demonstrating that high-dose (100 nM and 10 μM) Dex induces apoptosis of MBA-15.4 mouse osteoblasts within 6 h of treatment. The number of apoptotic osteoblasts detected by TUNEL stain (DNA fragmentation) increased from very low basal levels to 2–3% after 6 h of GC treatment and remained at this level throughout 6 days of continuous exposure. Similar increases in cell death were seen using immunocytochemical detection of the 89 kDa caspase-cleaved product of PARP (poly ADP-ribose polymerase). The total number of dead cells following Dex treatment did not exceed 10%, although higher levels (30%) could be induced by 24-h serum deprivation. This low level of GC-induced apoptosis correlates well with levels of apoptosis in vivo in patients with steroid osteoporosis, reported as 5% osteocytes (Weinstein et al. 1998). Moreover, employing TUNEL staining on histological sections from the vertebrae of rats treated with 3·5 mg/kg per day methylprednisolone for 9 weeks, we found similar increases in osteocyte apoptosis. The rat model of steroid-induced osteoporosis has been well documented, although rats have been reported to be relatively resistant to corticosteroid-induced bone damage (Binz et al. 1994, King et al. 1996, Shen et al. 1997). However, in several in vivo studies including our own (Hulley et al. 2002), marked inhibition of bone formation (Goulding & Gold 1986, 1988, Jowell et al. 1987, Unoki 1995, Ortoft & Oxlund 1996, Shen et al. 1997, Ortoft et al. 1999), decrease in BMD (Lindgren & DeLuca 1983, Goulding & Gold 1986, Ortoft & Oxlund 1996) and cortical strength (Ortoft et al. 1995, 1999, Ortoft & Oxlund 1996) have been reported, as well as impaired growth in juvenile rats (Ortoft et al. 1998).
Although osteoblast/osteocyte apoptosis is a well-established feature of GC-induced osteoporosis, the molecular mechanisms that underlie this process remain poorly understood. Proteins comprising the MAPK family constitute important mediators of signal transduction processes that serve to coordinate cellular response to a variety of extracellular stimuli. Of the three major mammalian MAPK subfamilies described, ERK, cJun-N-terminal kinases (JNK) and p38 kinases, the ERK pathway has been shown to play a major role in regulating cell growth and differentiation. The role of MAPK signalling pathways in regulating apoptosis during conditions of stress has also been widely investigated. Many (Tran et al. 2001, Bortner & Cidlowski 2002, Ley et al. 2004), but not all (Wang et al. 2000, Zhuang & Schnellmann 2006), such studies have supported the general view that activation of the ERK pathway mediates survival signals which counteract pro-apoptotic effects associated with JNK and p38 activation. Pharmacological inhibition of ERK activation (e.g. employing the MEK inhibitor U0126) has been shown to enhance chemotherapy-induced apoptosis of the lung, ovarian and breast carcinoma cell lines (MacKeigan et al. 2000, McDaid & Horwitz 2001). In skeletal tissue, ERK has been shown to mediate some of the protective anti-apoptotic effects of bisphosphonates (Plotkin et al. 1999, Plotkin & Bellido 2001, Van Offel et al. 2002), calcitonin (Plotkin et al. 1999), activated transforming growth factor-β (Karsdal et al. 2002), (non-steroidal anti-inflammatory drugs NSAIDs; Yoon et al. 2003) and others. Furthermore, we have previously shown that ERK activity is profoundly impaired by high-dose GC, mediated in part by rapid and sustained up-regulation of MKP-1 (Hulley et al. 1998, Engelbrecht et al. 2003). This vanadate-sensitive dual-specificity phosphatase is an immediate early gene product, normally involved in negative feedback inhibition of the MAP kinases (Slack et al. 2001, Lasa et al. 2002). Taken together, these observations suggest that repression of the ERK pathway by GCs might play a role in GC-induced apoptosis of skeletal cells.
The non-selective PTP inhibitor, vanadate, protected MBA-15.4 osteoblasts against apoptosis, induced by 6-h, 3-day and 6-day treatment with high-dose Dex. Vanadate alone had no effect on apoptosis. In rats, a threefold increase in osteocyte apoptosis induced by high-dose GC exposure for 9 weeks was largely abrogated by co-treatment with vanadate. While these studies supported a role for the ERK pathway in GC-apoptosis, the primary site of vanadate's protective action remained unclear. ERK could be directly inactivated by the vanadate-sensitive phosphates, MKP-1, as alluded to previously (Hulley et al. 1998, Engelbrecht et al. 2003), and MKP-1-induced dephosphorylation of ERK has been shown to be essential for nitric oxide-induced apoptosis of certain cells (Pervin et al. 2003). Alternatively, ERK could be indirectly protected by vanadate since it is downstream of kinase cascades that are primarily activated by tyrosine phosphorylation. Vanadate-sensitive PTPs like PTP-1B inactivate ERK and Akt/PK B indirectly by dephosphorylating upstream growth factor receptors and insulin receptor substrates, thereby preventing tyrosine-dependent docking of molecules like shc, grb2 or PI3-kinase (Neel & Tonks 1997, Elchebly et al. 1999, Galic et al. 2005). Like ERK, PKB has been shown to phosphorylate and target pro-apoptotic molecules for proteasomal destruction (Qi et al. 2006). PKB also phosphorylates and stabilizes anti-apoptotic proteins like XIAP (Dan et al. 2004).
In order to explore the possible downstream effectors of GC-induced apoptosis further, we employed rtqPCR to examine the expression of the major families of prosurvival genes (Bcl-2, Bcl-XL, IAP-1, IAP-2, XIAP, FLIPLong and FLIPShort) in osteoblasts subjected to high-dose Dex. Contrary to expectations, Dex did not repress expression of any of the candidate protective genes. Instead, Dex up-regulated the expression of Bcl-XL, IAP-2 and XIAP, while the transcript levels of the anti-apoptotic proteins Bcl-2, IAP-1, FLIPLong and FLIPShort were unaltered by Dex treatment. GCs cause death of osteoblasts, so the up-regulation of prosurvival genes by Dex is difficult to explain, but may indicate a compensatory, survival strategy that cells deploy when exposed to high-dose Dex. Vanadate alone produced no changes in expression of these prosurvival genes. This is not unexpected since vanadate also had no effect on the rate of apoptosis. Moreover, we have previously reported that vanadate alone does not significantly alter ERK levels in MBA-15.4 cells, although it effectively protects ERK against GC-induced inactivation (Hulley et al. 1998, Engelbrecht et al. 2003). Vanadate also did not significantly alter the up-regulation of genes induced by Dex, suggesting that this is not an ERK-mediated process.
Given the role of ERK in both cell proliferation and survival, we also explored the effects of mitogen withdrawal and mitogen stimulation on osteoblast prosurvival gene expression. Subjecting MBA-15.4 osteoblasts to mitogen withdrawal by culturing cells in the absence of serum for 24 h, markedly (sevenfold) stimulated apoptosis. Analogous to, although different from the observed effects of Dex exposure, mitogen withdrawal also did not decrease expression of any prosurvival genes, and instead resulted in increased transcription of the anti-apoptotic proteins Bcl-2, IAP-1, IAP-2, FLIPLong and FLIPShort. Interestingly, expression of FLIPLong and FLIPShort was stimulated with 20% FCS and this increase was halved when cells were treated with the MEK inhibitor U0126, for 30 min prior to mitogen stimulation, suggesting a regulatory role for MEK–ERK in FLIP gene expression in osteoblasts. Taken together, these results do not support a role for GC-induced down-regulation of prosurvival genes. Instead, GC-apoptosis appears more likely to result from direct or indirect effects on pro-apoptotic molecules, including their phosphorylation and proteasomal destruction – a process that can be mediated by MEK–ERK (Tran et al. 2001, Ley et al. 2004).
We report here that vanadate is strongly anti-apoptotic in vivo, protecting rat vertebral osteocytes from GC-induced apoptosis over a period of 9 weeks. The mechanism of action of vanadate in vivo is unclear. It has been described in several animal models (Heyliger et al. 1985, Meyerovitch et al. 1987, Brichard et al. 1988) and also in diabetic patients (Cohen et al. 1995) to act as an insulin mimic via its inhibition of PTPs in the insulin signalling pathway (Zinker et al. 2002). Vanadate enhances and sustains action of PI3-kinase and PKB/Akt, with anti-apoptotic effects in some cell types (Gerling et al. 2004). An alternative mechanism of action could be the inhibition by vanadate of several ATPases, including both plasma and endoplasmic reticulum membrane Ca-ATPases and plasma membrane NaK-ATPases (Ortiz et al. 1984, Garrahan & Rega 1988), although there are no reports of this having anti-apoptotic effects. In contrast, long-term disruption of membrane pumps usually induces apoptosis (Zhou et al. 1998). Vanadate also inhibits the multi-drug transporter protein, P-glycoprotein (Urbatsch et al. 1995, Taguchi et al. 1997), responsible for exporting toxic chemicals from cells and is known to disrupt the transport functions of most ATP-binding casette proteins (Terasaka et al. 2003). However, it seems unlikely that blocking a protective clearance mechanism would be protective over a period of 9 weeks, and in support of this, P-glycoprotein knockout mice are symptom free but hypersensitive to environmental toxins (Schinkel et al. 1994).
To conclude, vanadate protects MBA-15.4 mouse osteoblasts in vitro and rat osteocytes in vivo from apoptosis induced by high-dose GC. This protective mechanism may involve both ERK and PI3-Kinase pathways but does not involve transcriptional up-regulation of any major anti-apoptotic proteins in osteoblasts. Dex does not down-regulate transcription of these prosurvival proteins and in fact up-regulates several. GC and vanadate are therefore more likely to converge on the transcription of pro-apoptotic proteins and/or the post-transcriptional regulation of either pro- or anti-apoptotic mediators.
Acknowledgements
We would like to thank Prof. Rob Weinstein, Little Rock, Arkansas for his helpful advice regarding TUNEL staining of in vivo material. This study was funded by the Wellcome Trust CRIG 064335 (F S Hough, J M Burrin, P A Hulley), the Arthritis Research Campaign, UK, arc16343 (P A Hulley), the South African Medical Research Council (P A Hulley, F S Hough) and the South African National Research Foundation GUN 2047295 (P A Hulley). The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Ahuja SS, Zhao S, Bellido T, Plotkin LI, Jimenez F, Bonewald LF. CD40 ligand blocks apoptosis induced by tumor necrosis factor alpha, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology. 2003;144:1761–1769. doi: 10.1210/en.2002-221136. [DOI] [PubMed] [Google Scholar]
- Angeli A, Guglielmi G, Dovio A, Capelli G, de Feo D, Giannini S, Giorgino R, Moro L, Giustina A. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39:253–259. doi: 10.1016/j.bone.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Benayahu D, Kletter Y, Zipori D, Wientroub S. Bone marrow-derived stromal cell line expressing osteoblastic phenotype in vitro and osteogenic capacity in vivo. Journal of Cell Physiology. 1989;140:1–7. doi: 10.1002/jcp.1041400102. [DOI] [PubMed] [Google Scholar]
- Binz K, Schmid C, Bouillon R, Froesch ER, Jurgensen K, Hunziker EB. Interactions of insulin-like growth factor I with dexamethasone on trabecular bone density and mineral metabolism in rats. European Journal of Endocrinology. 1994;130:387–393. doi: 10.1530/eje.0.1300387. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Cellular mechanisms for the repression of apoptosis. Annual Review of Pharmacology and Toxicology. 2002;42:259–281. doi: 10.1146/annurev.pharmtox.42.083101.143836. [DOI] [PubMed] [Google Scholar]
- Brichard SM, Okitolonda W, Henquin JC. Long term improvement of glucose homeostasis by vanadate treatment in diabetic rats. Endocrinology. 1988;123:2048–2053. doi: 10.1210/endo-123-4-2048. [DOI] [PubMed] [Google Scholar]
- Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Annals of the New York Academy of Science. 2002;966:73–81. doi: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- Chen HL, Demiralp B, Schneider A, Koh AJ, Silve C, Wang CY, McCauley LK. Parathyroid hormone and parathyroid hormone-related protein exert both pro- and anti-apoptotic effects in mesenchymal cells. Journal of Biological Chemistry. 2002;277:19374–19381. doi: 10.1074/jbc.M108913200. [DOI] [PubMed] [Google Scholar]
- Chua CC, Chua BH, Chen Z, Landy C, Hamdy RC. Dexamethasone induces caspase activation in murine osteoblastic MC3T3-E1 cells. Biochemica et Biophysica Acta. 2003;1642:79–85. doi: 10.1016/s0167-4889(03)00100-9. [DOI] [PubMed] [Google Scholar]
- Cohen N, Halberstam M, Shlimovich P, Chang CJ, Shamoon H, Rossetti L. Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in patients with non-insulin-dependent diabetes mellitus. Journal of Clinical Invesigation. 1995;95:2501–2509. doi: 10.1172/JCI117951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creagh EM, Martin SJ. Cell stress-associated caspase activation: intrinsically complex? Science's STKE . 2003;175:11. doi: 10.1126/stke.2003.175.pe11. [DOI] [PubMed] [Google Scholar]
- Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) Journal of Biological Chemistry. 2004;279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Reed JC. IAP, family proteins – suppressors of apoptosis. Genes and Development. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Engelbrecht Y, de Wet H, Horsch K, Langeveldt CR, Hough FS, Hulley PA. Glucocorticoids induce rapid up-regulation of mitogen-activated protein kinase phosphatase-1 and dephosphorylation of extracellular signal-regulated kinase and impair proliferation in human and mouse osteoblast cell lines. Endocrinology. 2003;144:412–422. doi: 10.1210/en.2002-220769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried A, Benayahu D, Wientroub S. Marrow stroma-derived osteogenic clonal cell lines: putative stages in osteoblastic differentiation. Journal of Cell Physiology. 1993;155:472–482. doi: 10.1002/jcp.1041550306. [DOI] [PubMed] [Google Scholar]
- Galic S, Hauser C, Kahn BB, Haj FG, Neel BG, Tonks NK, Tiganis T. Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Molecular and Cellular Biology. 2005;25:819–829. doi: 10.1128/MCB.25.2.819-829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O'Shea JJ, Chrousos GP, Bornstein SR. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB Journal. 2002;16:61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- Garrahan PJ, Rega AF. Comparison between plasma membrane Ca2+ and Na,K-ATPases: short review. Brazilian Journal of Medical and Biological Research. 1988;21:1261–1267. [PubMed] [Google Scholar]
- Gerling N, Culmsee C, Klumpp S, Krieglstein J. The tyrosine phosphatase inhibitor orthovanadate mimics NGF-induced neuroprotective signaling in rat hippocampal neurons. Neurochemistry International. 2004;44:505–520. doi: 10.1016/j.neuint.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Goulding A, Gold E. Effects of dietary sodium chloride loading on parathyroid function, 1,25-dihydroxyvitamin D, calcium balance, and bone metabolism in female rats during chronic prednisolone administration. Endocrinology. 1986;119:2148–2154. doi: 10.1210/endo-119-5-2148. [DOI] [PubMed] [Google Scholar]
- Goulding A, Gold E. Effects of chronic prednisolone treatment on bone resorption and bone composition in intact and ovariectomized rats and in ovariectomized rats receiving beta-estradiol. Endocrinology. 1988;122:482–487. doi: 10.1210/endo-122-2-482. [DOI] [PubMed] [Google Scholar]
- Heyliger CE, Tahiliani AG, McNeill JH. Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science. 1985;227:1474–1477. doi: 10.1126/science.3156405. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Yoon JH, Grambihler A, Werneburg N, Bronk SF, Gores GJ. Bile acids stimulate cFLIP phosphorylation enhancing TRAIL-mediated apoptosis. Journal of Biological Chemistry. 2003;278:454–461. doi: 10.1074/jbc.M209387200. [DOI] [PubMed] [Google Scholar]
- Hu S, Yang X. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. Journal of Biological Chemistry. 2003;278:10055–10060. doi: 10.1074/jbc.M207197200. [DOI] [PubMed] [Google Scholar]
- Hulley PA, Gordon F, Hough FS. Inhibition of mitogen-activated protein kinase activity and proliferation of an early osteoblast cell line (MBA 15.4) by dexamethasone: role of protein phosphatases. Endocrinology. 1998;139:2423–2431. doi: 10.1210/endo.139.5.6020. [DOI] [PubMed] [Google Scholar]
- Hulley PA, Conradie MM, Langeveldt CR, Hough FS. Glucocorticoid-induced osteoporosis in the rat is prevented by the tyrosine phosphatase inhibitor, sodium orthovanadate. Bone. 2002;31:220–229. doi: 10.1016/s8756-3282(02)00807-4. [DOI] [PubMed] [Google Scholar]
- Jowell PS, Epstein S, Fallon MD, Reinhardt TA, Ismail F. 1,25-Dihydroxyvitamin D3 modulates glucocorticoid-induced alteration in serum bone Gla protein and bone histomorphometry. Endocrinology. 1987;120:531–536. doi: 10.1210/endo-120-2-531. [DOI] [PubMed] [Google Scholar]
- Karsdal MA, Larsen L, Engsig MT, Lou H, Ferreras M, Lochter A, Delaisse JM, Foged NT. Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. Journal of Biological Chemistry. 2002;277:44061–44067. doi: 10.1074/jbc.M207205200. [DOI] [PubMed] [Google Scholar]
- Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M, et al. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Current Biology. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends in Cell Biology. 1998;8:324–330. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- King CS, Weir EC, Gundberg CW, Fox J, Insogna KL. Effects of continuous glucocorticoid infusion on bone metabolism in the rat. Calcified Tissue International. 1996;59:184–191. doi: 10.1007/s002239900107. [DOI] [PubMed] [Google Scholar]
- Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Molecular and Cellular Biology. 2002;22:7802–7811. doi: 10.1128/MCB.22.22.7802-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R, Ewings K.E, Hadfield K, Howes E, Balmanno K, Cook S.J. Extracellular signal-regulated kinases 1/2 are serum-stimulated ‘Bim(EL) kinases’ that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. Journal of Biological Chemistry. 2004;279:8837–8847. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- Lindgren JU, DeLuca HF. Oral 1,25(OH)2D3: an effective prophylactic treatment for glucocorticoid osteopenia in rats. Calcified Tissue International. 1983;35:107–110. doi: 10.1007/BF02405014. [DOI] [PubMed] [Google Scholar]
- Liu Y, Porta A, Peng X, Gengaro K, Cunningham EB, Li H, Dominguez LA, Bellido T, Christakos S. Prevention of glucocorticoid-induced apoptosis in osteocytes and osteoblasts by calbindin-D28k. Journal of Bone and Mineral Research. 2004;19:479–490. doi: 10.1359/JBMR.0301242. [DOI] [PubMed] [Google Scholar]
- MacKeigan JP, Collins TS, Ting JP. MEK inhibition enhances paclitaxel-induced tumor apoptosis. Journal of Biological Chemistry. 2000;275:38953–38956. doi: 10.1074/jbc.C000684200. [DOI] [PubMed] [Google Scholar]
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocrine Reviews. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- McDaid HM, Horwitz SB. Selective potentiation of paclitaxel (taxol)-induced cell death by mitogen-activated protein kinase kinase inhibition in human cancer cell lines. Molecular Pharmacology. 2001;60:290–301. doi: 10.1124/mol.60.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehls O, Himmele R, Homme M, Kiepe D, Klaus G. The interaction of glucocorticoids with the growth hormone-insulin-like growth factor axis and its effects on growth plate chondrocytes and bone cells. Journal of Pediatric Endocrinology and Metabolism. 2001;14(suppl 6):1475–1482. [PubMed] [Google Scholar]
- Meyerovitch J, Farfel Z, Sack J, Shechter Y. Oral administration of vanadate normalizes blood glucose levels in streptozotocin-treated rats. Characterization and mode of action. Journal of Biological Chemistry. 1987;262:6658–6662. [PubMed] [Google Scholar]
- Neel BG, Tonks NK. Protein tyrosine phosphatases in signal transduction. Current Opinion in Cell Biology. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- Van Offel JF, Schuerwegh AJ, Bridts CH, Stevens WJ, De Clerck LS. Effect of bisphosphonates on viability, proliferation, and dexamethasone-induced apoptosis of articular chondrocytes. Annals of the Rheumatic Diseases. 2002;61:925–928. doi: 10.1136/ard.61.10.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A, Garcia-Carmona F, Garcia-Canovas F, Gomez-Fernandez JC. A kinetic study of the interaction of vanadate with the Ca2++ Mg2+-dependent ATPase from sarcoplasmic reticulum. Biochemistry Journal. 1984;221:213–222. doi: 10.1042/bj2210213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortoft G, Oxlund H. Qualitative alterations of cortical bone in female rats after long-term administration of growth hormone and glucocorticoid. Bone. 1996;18:581–590. doi: 10.1016/8756-3282(96)00077-4. [DOI] [PubMed] [Google Scholar]
- Ortoft G, Bruel A, Andreassen TT, Oxlund H. Growth hormone is not able to counteract osteopenia of rat cortical bone induced by glucocorticoid with protracted effect. Bone. 1995;17:543–548. doi: 10.1016/8756-3282(95)00386-x. [DOI] [PubMed] [Google Scholar]
- Ortoft G, Gronbaek H, Oxlund H. Growth hormone administration can improve growth in glucocorticoid-injected rats without affecting the lymphocytopenic effect of the glucocorticoid. Growth Hormone and IGF Research. 1998;8:251–264. doi: 10.1016/s1096-6374(98)80118-4. [DOI] [PubMed] [Google Scholar]
- Ortoft G, Andreassen TT, Oxlund H. Growth hormone increases cortical and cancellous bone mass in young growing rats with glucocorticoid-induced osteopenia. Journal of Bone and Mineral Research. 1999;14:710–721. doi: 10.1359/jbmr.1999.14.5.710. [DOI] [PubMed] [Google Scholar]
- Pervin S, Singh R, Freije WA, Chaudhuri G. MKP-1-induced dephosphorylation of extracellular signal-regulated kinase is essential for triggering nitric oxide-induced apoptosis in human breast cancer cell lines: implications in breast cancer. Cancer Research. 2003;63:8853–8860. [PubMed] [Google Scholar]
- Plotkin LI, Bellido T. Bisphosphonate-induced, hemichannel-mediated, anti-apoptosis through the Src/ERK pathway: a gap junction-independent action of connexin43. Cell Communication and Adhesion. 2001;8:377–382. doi: 10.3109/15419060109080757. [DOI] [PubMed] [Google Scholar]
- Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. Journal of Clinical Investigation. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. Journal of Biological Chemistry. 2006;281:813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood–brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Shen V, Birchman R, Liang XG, Wu DD, Lindsay R, Dempster DW. Prednisolone alone, or in combination with estrogen or dietary calcium deficiency or immobilization, inhibits bone formation but does not induce bone loss in mature rats. Bone. 1997;21:345–351. doi: 10.1016/s8756-3282(97)00153-1. [DOI] [PubMed] [Google Scholar]
- Silvestrini G, Ballanti P, Patacchioli FR, Mocetti P, Di Grezia R, Wedard BM, Angelucci L, Bonucci E. Evaluation of apoptosis and the glucocorticoid receptor in the cartilage growth plate and metaphyseal bone cells of rats after high-dose treatment with corticosterone. Bone. 2000;26:33–42. doi: 10.1016/s8756-3282(99)00245-8. [DOI] [PubMed] [Google Scholar]
- Slack DN, Seternes OM, Gabrielsen M, Keyse SM. Distinct binding determinants for ERK2/p38alpha and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. Journal of Biological Chemistry. 2001;276:16491–16500. doi: 10.1074/jbc.M010966200. [DOI] [PubMed] [Google Scholar]
- Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. Journal of Bone and Mineral Research. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- Taguchi Y, Yoshida A, Takada Y, Komano T, Ueda K. Anti-cancer drugs and glutathione stimulate vanadate-induced trapping of nucleotide in multidrug resistance-associated protein (MRP) FEBS Letters. 1997;401:11–14. doi: 10.1016/s0014-5793(96)01421-4. [DOI] [PubMed] [Google Scholar]
- Terasaka K, Shitan N, Sato F, Maniwa F, Ueda K, Yazaki K. Application of vanadate-induced nucleotide trapping to plant cells for detection of ABC proteins. Plant and Cell Physiology. 2003;44:198–200. doi: 10.1093/pcp/pcg019. [DOI] [PubMed] [Google Scholar]
- Tran SE, Holmstrom TH, Ahonen M, Kahari VM, Eriksson JE. MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. Journal of Biological Chemistry. 2001;276:16484–16490. doi: 10.1074/jbc.M010384200. [DOI] [PubMed] [Google Scholar]
- Unoki E. Effect of human PTH on steroid-induced osteopenia: a histomorphometric study of decalcified and undecalcified trabecular bone sections in rat. Nippon Sanka Fujinka Gakkai Zasshi. 1995;69:1064–1075. [PubMed] [Google Scholar]
- Urbatsch IL, Sankaran B, Weber J, Senior AE. P-glycoprotein, is stably inhibited by vanadate-induced trapping of nucleotide at a single catalytic site. Journal of Biological Chemistry. 1995;270:19383–19390. doi: 10.1074/jbc.270.33.19383. [DOI] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. Journal of Biological Chemistry. 2000;275:39435–39443. doi: 10.1074/jbc.M004583200. [DOI] [PubMed] [Google Scholar]
- Wang W, Prince CZ, Mou Y, Pollman MJ. Notch3 signaling in vascular smooth muscle cells induces c-FLIP expression via ERK/MAPK activation. Resistance to Fas ligand-induced apoptosis. Journal of Biological Chemistry. 2002;277:21723–21729. doi: 10.1074/jbc.M202224200. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. Journal of Clinical Investigation. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BF, Xiao C, Roa WH, Krammer PH, Hao C. Calcium/calmodulin-dependent protein kinase II regulation of c-FLIP expression and phosphorylation in modulation of Fas-mediated signaling in malignant glioma cells. Journal of Biological Chemistry. 2003;278:7043–7050. doi: 10.1074/jbc.M211278200. [DOI] [PubMed] [Google Scholar]
- Yoon JB, Kim SJ, Hwang SG, Chang S, Kang SS, Chun JS. Non-steroidal anti-inflammatory drugs inhibit nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes independent of cyclooxygenase activity. Journal of Biological Chemistry. 2003;278:15319–15325. doi: 10.1074/jbc.M212520200. [DOI] [PubMed] [Google Scholar]
- Zalavras C, Shah S, Birnbaum MJ, Frenkel B. Role of apoptosis in glucocorticoid-induced osteoporosis and osteonecrosis. Critical Reviews in Eukaryotic Gene Expression. 2003;13:221–235. [PubMed] [Google Scholar]
- Zhou YP, Teng D, Dralyuk F, Ostrega D, Roe MW, Philipson L, Polonsky KS. Apoptosis in insulin-secreting cells. Evidence for the role of intracellular Ca2+ stores and arachidonic acid metabolism. Journal of Clinical Investigation. 1998;101:1623–1632. doi: 10.1172/JCI1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. Journal of Pharmacological and Experimental Therapeutics. 2006;319:991–997. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]
- Zinker BA, Rondinone CM, Trevillyan JM, Gum RJ, Clampit JE, Waring JF, Xie N, Wilcox D, Jacobson P, Frost L, et al. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. PNAS. 2002;99:11357–11362. doi: 10.1073/pnas.142298199. [DOI] [PMC free article] [PubMed] [Google Scholar]