Abstract
Eph kinases and their ephrin ligands are widely expressed in epithelial cells in vitro and in vivo. Our results show that activation of endogenous EphA kinases in Madin-Darby canine kidney (MDCK) cells negatively regulates hepatocyte growth factor/scatter factor (HGF)–induced branching morphogenesis in collagen gel. Cotreatment with HGF and ephrin-A1 reduced sprouting of cell protrusions, an early step in branching morphogenesis. Moreover, addition of ephrin-A1 after HGF stimulation resulted in collapse and retraction of preexisting cell protrusions. In a newly developed assay that simulates the localized interactions between Ephs and ephrins in vivo, immobilized ephrin-A1 suppressed HGF-induced MDCK cell scattering. Ephrin-A1 inhibited basal ERK1/2 mitogen-activated protein kinase activity; however, the ephrin-A1 effect on cell protrusion was independent of the mitogen-activated protein kinase pathway. Ephrin-A1 suppressed HGF-induced activation of Rac1 and p21-activated kinase, whereas RhoA activation was retained, leading to the preservation of stress fibers. Moreover, dominant-negative RhoA or inhibitor of Rho-associated kinase (Y27632) substantially negated the inhibitory effects of ephrin-A1. These data suggest that interfering with c-Met signaling to Rho GTPases represents a major mechanism by which EphA kinase activation inhibits HGF-induced MDCK branching morphogenesis.
Keywords: EphA2; ephrin-A1; Rho GTPases; kidney; MAPK
Introduction
Epithelial branching morphogenesis is a fundamental developmental process that gives rise to many epithelial organs including the kidney, lung, prostate, and salivary and mammary gland. Although the exact process varies depending on tissues and species, a conserved series of events can be discerned. They include formation of an organ anlage, primary bud formation, initiation of branching morphogenesis, branch outgrowth, reiteration of branching process, and differentiation of organospecific proximal and distal structures (Affolter et al., 2003). At the subcellular level, the initiation of branching morphogenesis is characterized by the extension of cell protrusions from the basolateral side of epithelial bud, a step that is conserved during evolution. For example, Drosophila tracheal branching morphogenesis involves the extension of cell processes at the tip of invading epithelial bud (Sutherland et al., 1996; Ribeiro et al., 2002). Similarly, in mouse kidney development, membrane protrusions occur on the tip of a ureteric bud (UB) that is invading into metanephric mesenchyme, an early step during kidney organogenesis (Davies et al., 1995; Fisher et al., 2001; Piscione and Rosenblum, 2002).
Cytokines and their receptors are among the critical regulators of branching morphogenesis. Hepatocyte growth factor/scatter factor (HGF), a mesenchymally derived factor, is a potent mitogen, motogen, and morphogen, and functions in virtually every tissue of the body through a receptor tyrosine kinase (RTK) c-Met (Boros and Miller, 1995; Brinkmann et al., 1995). In vitro, HGF/c-Met signaling induces branching morphogenesis of several types of epithelial cells grown in a three-dimensional matrix (Montesano et al., 1991a; Weidner et al., 1993; Soriano et al., 1995; Pohl et al., 2000), which simulates some aspects of in vivo epithelial morphogenesis process. HGF-induced branching morphogenesis of MDCK epithelial cells in collagen gels is characterized by initial extension of fine membrane processes that take place within hours of treatment with HGF, and which are then further elaborated into cell strands and tubular structures (Montesano et al., 1991b).
Morphologically, the invasive growth of the membrane protrusions is analogous to the extension of neurites after neurotrophic factor stimulation of neurons. One of the neurites polarizes to become the axon, whose growth cone navigates to predetermined innervation sites under the guidance of both attractive and repulsive factors. These guidance cues stimulate a number of signaling pathways, many of which converge on the Rho family small GTPases (Luo, 2000; da Silva and Dotti, 2002; Etienne-Manneville and Hall, 2002). As epithelial cell branching morphogenesis involves similar cellular processes, it has been suggested that Rho GTPases also contribute to epithelial morphogenesis (Lubarsky and Krasnow, 2003). However, the experimental evidence is still lacking.
With 16 members, Eph RTKs represent the largest family of vertebrate RTKs. Ligands for Eph kinases, called ephrins, are membrane anchored through either a glycosylphosphatidylinositol lipid moiety (ephrin-A) or a transmembrane domain (ephrin-B; Eph Nomenclature Committee, 1997; Wilkinson, 2001). Thus, Eph–ephrin interactions mediate cell–cell contact signaling. Unique to Eph kinase–ephrin interactions, both receptors and ligands can transmit signals to the cell interior. In the nervous system, Ephs and ephrins are known to be involved in axon guidance, neural crest cell migration, compartment boundary formation (Flanagan and Vanderhaeghen, 1998; Holder and Klein, 1999; Wilkinson, 2000), and synapse formation (Dalva et al., 2000). Eph receptors and their ligands also play important roles in vascular development (Adams and Klein, 2000; Cheng et al., 2002).
In addition to nervous and vascular systems, Eph kinases and ephrins are widely expressed in other cell types in vitro and in vivo (Tuzi and Gullick, 1994). For example, EphA1 and EphA2 kinases are highly expressed in numerous epithelial tissues (Lindberg and Hunter, 1990; Coulthard et al., 2001). However, the role of Eph–ephrin interactions in epithelial organogenesis remains unclear. Here, we report that EphA activation regulates epithelial branching morphogenesis. Stimulation of endogenous EphA kinases in MDCK cells by ephrin-A1 inhibited HGF-induced sprouting of cell protrusions and subsequent branching morphogenesis in collagen gel. Moreover, addition of ephrin-A1–Fc after HGF treatment caused collapse and retraction of preexisting membrane protrusions. Cellular and biochemical evidence shows that EphA kinases negatively regulate HGF-induced epithelial branching morphogenesis by differentially regulating Rho family small GTPases. Finally, one of the EphA kinases, EphA2, is preferentially expressed on UB epithelial cells that are actively undergoing branching morphogenesis, suggesting that Eph–ephrin interactions may also regulate epithelial organogenesis in vivo.
Results
Stimulation of MDCK cells with ephrin-A1 antagonizes HGF-induced branching morphogenesis
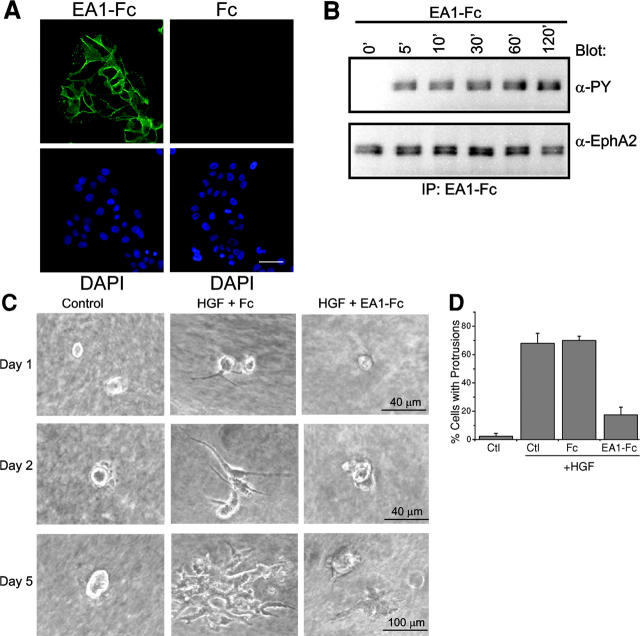
MDCK cells are derived from the canine renal tubule and are commonly used as an in vitro model system to study epithelial branching morphogenesis (Montesano et al., 1991b; Pohl et al., 2000). To determine expression of EphA kinases, immunofluorescence analysis was performed using recombinant ephrin-A1 dimerized by fusion to human IgG1 heavy chain (Fc). In this and all following experiments, we used the previously characterized MDCK clone 8 cells (Nelson and Veshnock, 1986; referred to as MDCK cells hereafter). Staining with ephrin-A1–Fc, which binds to most EphA kinases (Wilkinson, 2001), showed that MDCK cells highly expressed EphA receptors at cell–cell contact regions (Fig. 1 A). In contrast, no staining was observed with Fc control. Fig. 1 B shows that EphA kinases in MDCK cells can be rapidly activated by its ligand ephrin-A1, which was indicated by the increased tyrosine phosphorylation of the receptors.
Figure 1.
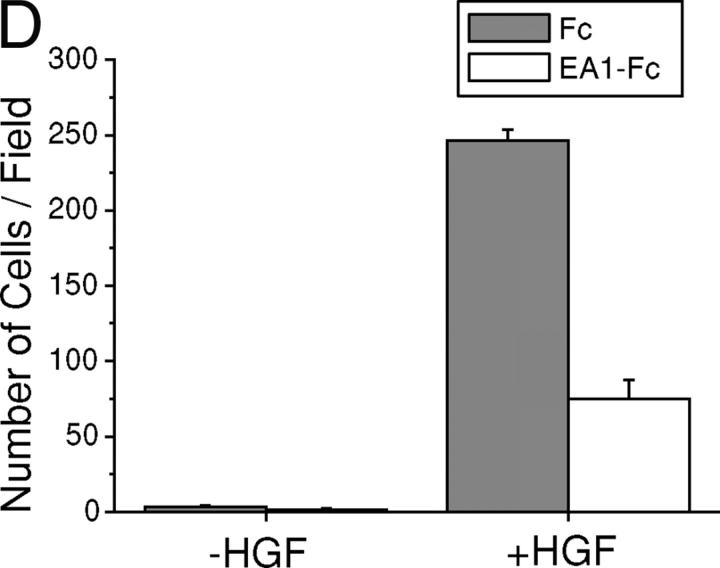
Activation of endogenous EphA kinases by ephrin-A1 inhibits HGF-induced MDCK cell protrusions and branching morphogenesis. (A) Expression of EphA kinases in MDCK cells. Cells were incubated with ephrin-A1–Fc (EA1-Fc) or Fc for 1 h at 4°C. The ligand-bound receptors were detected using FITC-conjugated goat anti–human Fc. Bar, 20 μm. (B) Induction of tyrosine phosphorylation of EphA by ephrin-A1–Fc stimulation. Lysates from cells stimulated with ephrin-A1–Fc for the indicated times were precipitated with ephrin-A1–Fc and analyzed by immunoblot using anti-phosphotyrosine and anti-EphA2. (C) Cotreatment with ephrin-A1 inhibits HGF-induced branching morphogenesis. MDCK cells were cultured in collagen gel and stimulated with 20 ng/ml HGF in the presence of 1 μg/ml ephrin-A1–Fc or Fc as described in Materials and methods. Images were collected at the indicated times on a digital camera mounted on an inverted microscope. (D) Quantitation of cell protrusions. Cells with membrane processes longer than the diameters of cell bodies after 24 h of incubation were counted. Values represent means from six randomly selected fields, ± SD.
To test the effect of EphA activation on branching morphogenesis, MDCK cells were seeded in collagen gel in the presence of ephrin-A1–Fc, HGF, or both. As reported previously, in control medium, MDCK cells in collagen matrix formed cysts (Wang et al., 1994; Fig. 1 C). A complex morphogenetic response was induced by HGF (Montesano et al., 1991b; Santos and Nigam, 1993). Outgrowth of cell protrusions took place during overnight stimulation with HGF. 2 d later, cells began to organize into strands with thicker cell protrusions invading deeper into the surrounding gel. By d 5, these tubular structures were further elaborated and elongated to form an organized tubular branching tree (Fig. 1 C). Addition of ephrin-A1–Fc dramatically attenuated extension of cell protrusions, leading to retarded tubule formation. A quantitative analysis was presented in Fig. 1 D; a two- to threefold reduction of HGF-induced membrane protrusion was consistently observed with ephrin-A1–Fc, but not Fc control.
EphA ligation causes collapse and retraction of preexisting cell protrusions
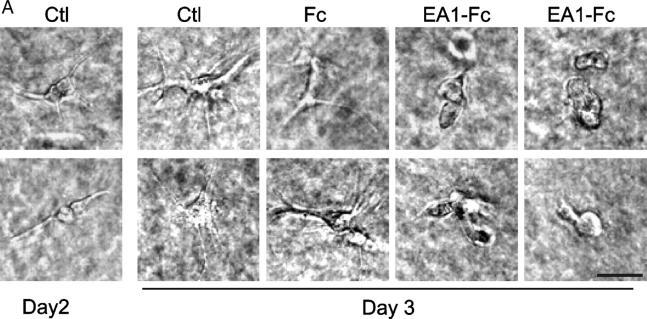
The results from the previous paragraph demonstrate that EphA activation can significantly attenuate the sprouting of new cell protrusions induced by HGF. Next, we examined how ephrin-A1 stimulation affected preexisting processes. MDCK cells were seeded in collagen gel and stimulated with HGF for 24 h to induce cell protrusions. Ephrin-A1–Fc (or Fc alone) was then added together with fresh HGF. Cells were examined 24 h later. Fig. 2 shows the addition of ephrin-A1–Fc caused collapse and retraction of the preexisting membrane processes as well as prevention of new outgrowth. Therefore, when given at the same time as HGF, ephrin-A1–Fc reduced initiation of cell protrusions; when given after HGF stimulation, ephrin-A1–Fc caused collapse and retraction of preexisting cell outgrowth. These results revealed a novel cross talk mechanism between EphA and c-Met in regulating epithelial morphogenesis.
Figure 2.
Ephrin-A1 causes collapse of preexisting cell protrusions induced by HGF in MDCK cells. (A) Membrane protrusions were induced by 20 ng/ml HGF in collagen gel on d 1. After 24 h of HGF treatment (d 2), ephrin-A1–Fc was added to a final concentration of 1.5 μg/ml to the growth medium containing fresh HGF, and the cells were cultured for an additional 24 h (d 3). Branching morphogenesis response was recorded on d 2 and d 3. Bar, 40 μm. (B) Quantitative analysis of data in A. Cell protrusions longer than the diameters of cell bodies were counted. Values represent means from six randomly selected fields, ± SD.
Ephrin-A1 inhibits HGF-induced MDCK cell scattering and directional cell migration
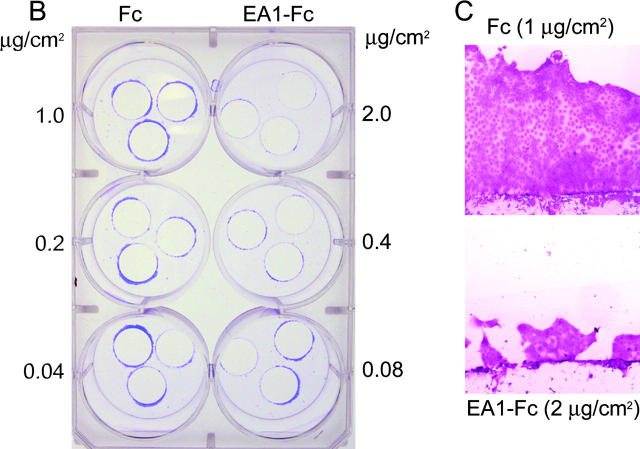
A prerequisite for branching morphogenesis is the ability for cells to scatter. We reasoned that Eph kinases are likely to be activated in vivo when the leading edge of a migrating cell, but not its trailing cell body, comes in contact with an ephrin-presenting cell. To simulate this localized contact, we devised a scattering onto immobilized ligand (SOIL) assay (Fig. 3 A). In this assay, MDCK cells were cultured to near confluency on coverslips, and were transferred onto dishes that were precoated with different densities of Fc or ephrin-A1–Fc. The cells were then induced to scatter off the edge of coverslips onto the coated surface by treatment with HGF, allowing localized engagement between EphAs at the leading edge of the scattered cell and the immobilized ephrin-A1 on the plate. In the absence of HGF, MDCK cells grew as a compact monolayer on the coverslips during the course of the experiments, and rarely scattered onto the coated dishes (unpublished data). In the presence of HGF, MDCK cells readily scattered off the edge of the coverslips and onto the uncoated or Fc-coated surface. In contrast, immobilized ephrin-A1–Fc significantly inhibited MDCK scattering in a density-dependent manner (Fig. 3, B and C).
Figure 3.
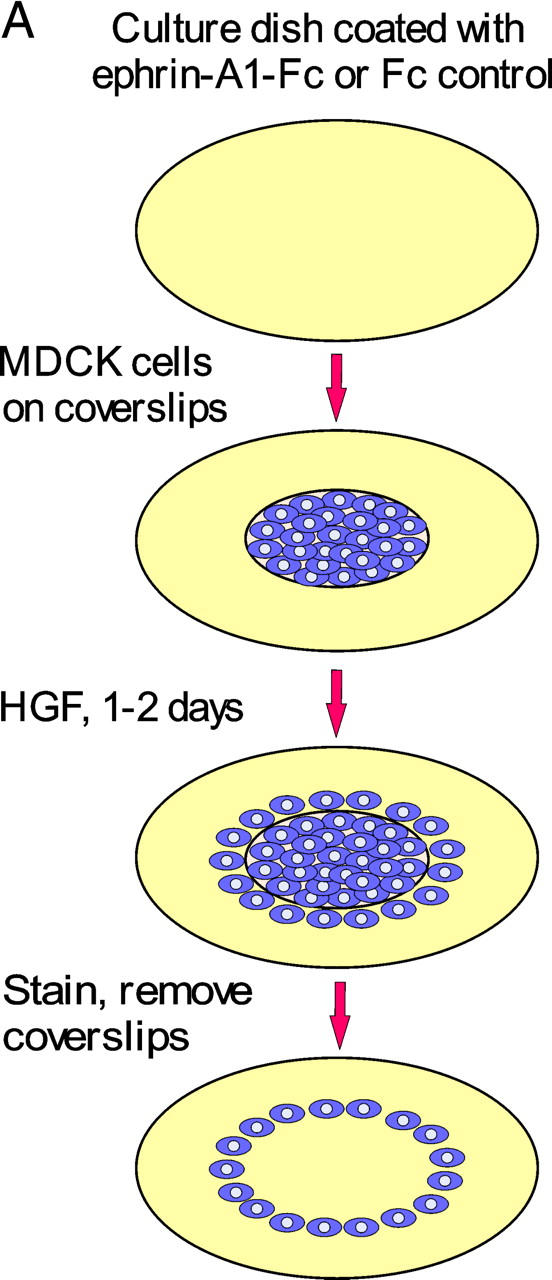
Ephrin-A1 inhibits HGF-induced MDCK cell scattering and directional cell migration. (A) Schematic illustration of SOIL assay. MDCK cells were cultured to reach near confluence on coverslips in 24-well plates and were transferred onto 6-well dishes precoated with either Fc or ephrin-A1–Fc. After 24–48 h of stimulation with 20 ng/ml HGF, cells were fixed and stained with crystal violet. After removing coverslips, the rings of cells that had scattered off the coverslips were photographed. (B) Immobilized ephrin-A1–Fc (but not Fc) inhibits HGF-induced MDCK cell scattering in a density-dependent manner. (C) High resolution image shows that coating with 2 μg/cm2 ephrin-A1–Fc significantly inhibited HGF-induced cell scattering compared with Fc control. (D) Ephrin-A1 inhibits HGF-induced directional cell migration. 100 ng FN was dried on the underside of Transwell filter, and both sides of the Transwell were then coated with10 μg/ml collagen. MDCK cells were added to the upper chamber of the Transwell and allowed to migrate toward the lower chamber containing 20 ng/ml HGF and 1 μg/ml ephrin-A1–Fc or Fc overnight. Cells migrating through the filter were counted from six high power fields. Values represent means ± SD.
Cell migration in vivo is frequently directional in response to a gradient of soluble chemotactic or insoluble contact-attractant factors (Lauffenburger and Horwitz, 1996). The SOIL assay described in the previous paragraph measures the effects of EphA kinase activation on chemokinetic movement of cells when HGF is presented uniformly. To investigate how EphA activation can modulate directional cell motility, a modified Boyden chamber cell migration assay was used to present HGF in a gradient to induce directional cell migration. Fig. 3 D shows that in the absence of HGF, MDCK cells exhibited little migration. Addition of HGF to the lower chamber induced vigorous directional cell migration, which was potently inhibited by the inclusion of ephrin-A1–Fc in the lower chamber (Fig. 3 D). Thus, both random scattering and directional migration of MDCK cells induced by HGF were inhibited by ephrin-A1.
Inhibition of ERK1/2 MAPK by EphA activation is not responsible for suppression of HGF-induced membrane protrusions
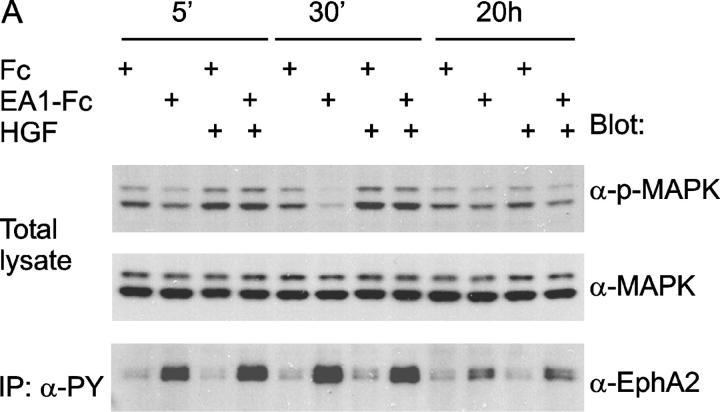
Next, we investigated molecular mechanisms underlying how ephrin-A1 inhibits HGF-induced sprouting of new cell protrusions and causes the collapse of preexisting processes. Co-immunoprecipitation assays did not detect physical association between c-Met and EphA kinases, nor was there cross tyrosine phosphorylation between the kinases (unpublished data), indicating that the interactions may take place further downstream from receptor activation. HGF-induced breakdown of epithelial cell–cell junctions and cell migration are known to require MEK activation (Royal and Park, 1995; Potempa and Ridley, 1998), and activation of ERK1 and ERK2 MAPK is an essential component of HGF-mediated renal epithelial morphogenesis in vitro (Karihaloo et al., 2001). Previously, we have shown that stimulation of endogenous EphA kinases with ephrin-A1 potently inhibits the Ras-MAPK signaling cascade in several different cell types, including epithelial cells (Miao et al., 2001). Examination of MAPK activity from ephrin-A1–stimulated MDCK cells revealed sustained suppression of basal ERK1/2 MAPK activation (Fig. 4 A).
Figure 4.
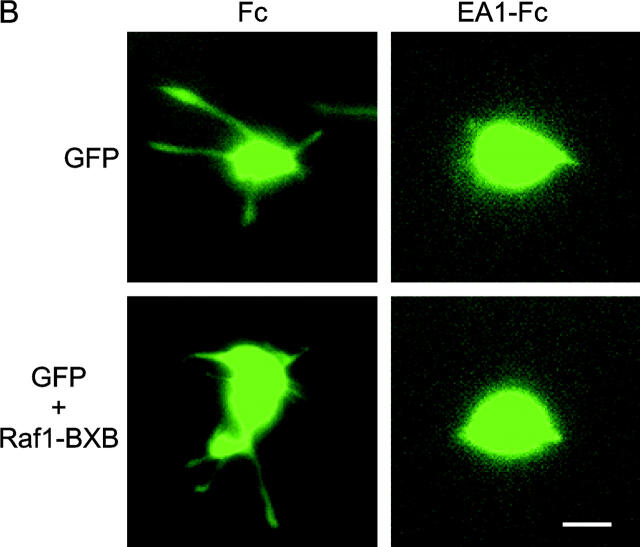
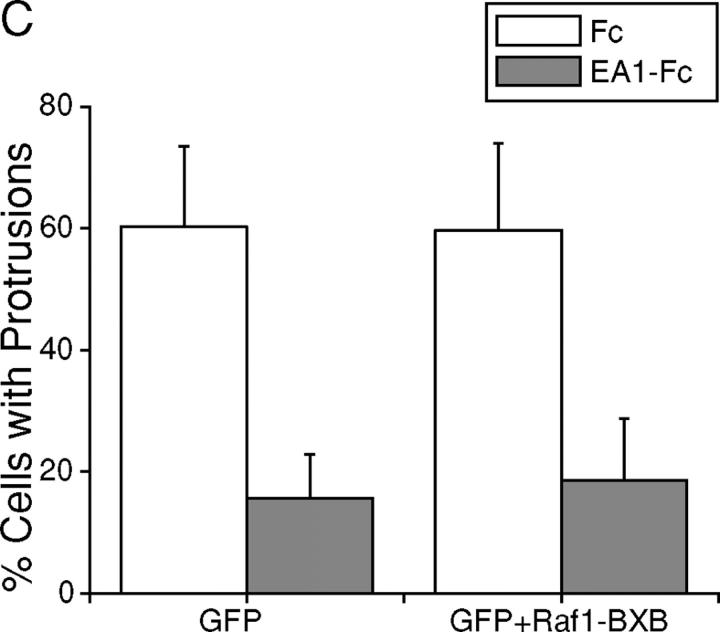
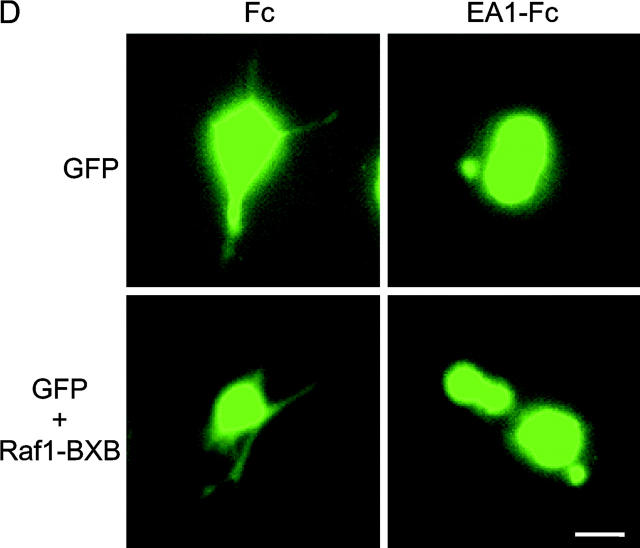
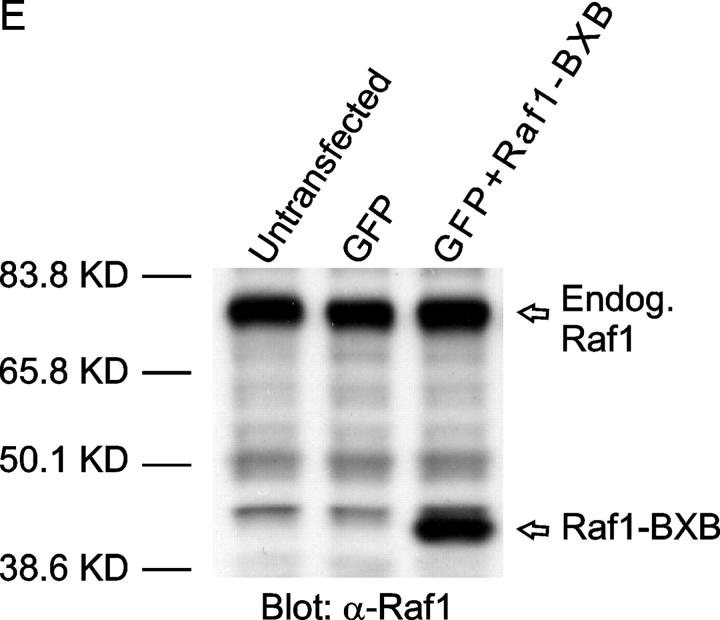
EphA kinases negatively regulate HGF-induced cell protrusions through an ERK1/2 MAPK-independent pathway. (A) EphA activation inhibits basal ERK1/2 MAPK activities in MDCK cells. Cells were stimulated with 1 μg/ml Fc or ephrin-A1–Fc in the absence or presence of 20 ng/ml HGF for the indicated times and lysed. Immunoprecipitates and total cell lysate were analyzed for the indicated proteins by immunoblot. (B) Dominant-active Raf1 (BXB) does not block the inhibitory effect of ephrin-A1 on branching morphogenesis. MDCK cells transiently transfected with plasmids encoding Raf1-BXB and/or GFP were seeded in collagen gel in optical tissue culture 96-well plates. 24 h later, GFP-positive cells were recorded. Bar, 10 μm. (C) Quantitative analysis of branching morphogenesis results in B. Values represent means from at least eight randomly selected fields ± SD. (D) Dominant-active Raf1 (BXB) does not prevent ephrin-A1–induced collapse of membrane protrusions. Transiently transfected MDCK cells were stimulated with HGF for 24 h; ephrin-A1–Fc was then added to medium replenished with HGF. Branching morphogenesis was recorded 24 h later. Bar, 10 μm. (E) The expression of transfected Raf1-BXB was detected by immunoblot with antibody against Raf1.
HGF treatment stimulates MAPK activity, particularly at early time points. In cells co-stimulated with ephrin-A1–Fc and HGF, MAPK activity was stimulated to a similar degree as that observed after HGF alone, with only a minor inhibitory effect at 30-min and 20-h time points. These results indicate that the mild suppression of ERK1/2 MAPK activity by ephrin-A1 treatment may not be responsible for the inhibition of HGF-stimulated cell protrusion.
To further investigate the role of MAPK in this process, MDCK cells were transfected with dominant-active Raf1 (BXB) together with a GFP expression vector as a tracer, and subject to collagen gel assay. If suppression of MAPK contributes to the inhibition of branching morphogenesis by EphA activation, BXB would keep ERK1/2 constitutively active, bypass MAPK inhibition by ephrin-A1, and negate the inhibitory effects of ephrin-A1 on cell protrusions. As shown in Fig. 4 (B and C), cells transfected with GFP alone or cotransfected with GFP and BXB both exhibited typical sprouting of cell protrusions in response to HGF; both were equally inhibited by ephrin-A1. Similarly, ephrin-A1 caused the retraction of preexisting cell protrusions in both transfectants (Fig. 4 D). Together, these data suggest that EphA negatively regulates the cell protrusion step of branching morphogenesis via Raf/MAPK-independent mechanisms.
EphA activation enhances cortical cytoskeleton and stress fiber assembly, and inhibits the loss of actin stress fibers caused by HGF
During cell migration and morphogenesis, the actin cytoskeleton undergoes extensive reorganization. Rho family small GTPases are key regulators of actin cytoskeletal reorganization. We wondered whether Rho GTPases represent a point of convergence for the cross talk between EphA and c-Met RTKs. To this end, we first analyzed the actin cytoskeletal changes after short- and long-term treatment with ephrin-A1–Fc, HGF, or both. Immunofluorescence analysis revealed that the effects of HGF on actin stress fiber assembly were biphasic. Early after treatment, HGF promotes stress fiber assembly in the inner cells of an MDCK colony (Fig. 5) and disassembly of cortical cytoskeletons in periphery cells. The latter was correlated with extension of lamellipodia. Treatment for 2 h or longer resulted in the loss of actin stress fibers. In contrast, ephrin-A1–Fc induced persistent cortical actin bundles along the edge of peripheral cells. In the presence of HGF, ephrin-A1 significantly reduced the initial loss of cortical cytoskeleton in peripheral cells, and preserved significant levels of stress fibers even after overnight cotreatment. Cortical actin is a key structure for stabilizing the cell membrane; breaking the constraints of the cortical cytoskeleton has been suggested as a critical step in the sprouting of neurites (da Silva and Dotti, 2002), and is also likely to be a critical step in the formation of membrane protrusions during epithelial branching morphogenesis (Fig. 5). In sum, EphA activation could prevent the loss of cortical cytoskeleton and stress fibers induced by the activated c-Met.
Figure 5.
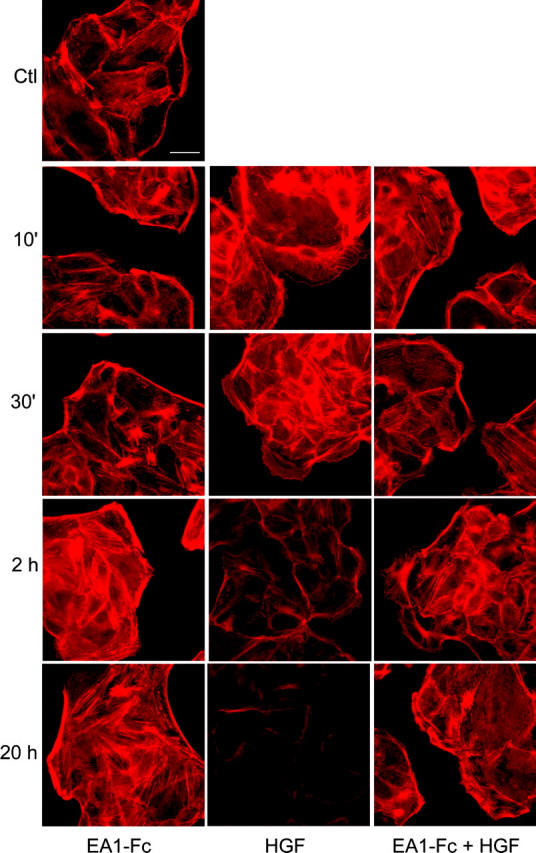
Ephrin-A1 and HGF cause distinct actin cytoskeletal changes in MDCK cells. Cells were stimulated with ephrin-A1–Fc or HGF or both for the indicated times. The actin cytoskeleton was stained using Texas red–conjugated phalloidin. Images were taken at a constant exposure time. Bar, 20 μm.
Ephrin-A1 preserves RhoA and suppresses Rac1 activation by HGF
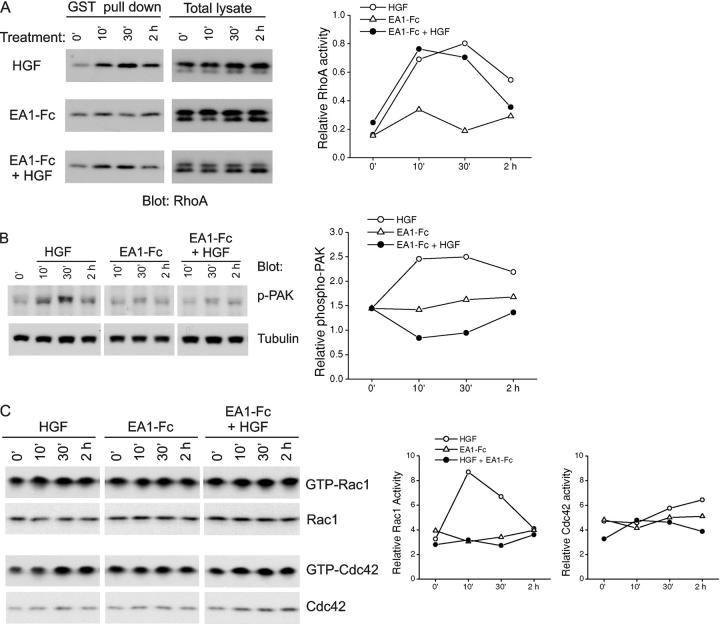
RhoA is a key regulator of actin stress fibers (Hall, 1998). Using a GST-Rhotekin pull-down assay, we consistently observed a moderate increase in RhoA activities upon ephrin-A1 stimulation (Fig. 6 A). Surprisingly, despite its ability to induce dissolution of stress fibers, HGF also persistently stimulated the RhoA activity, even to a greater degree than ephrin-A1. In cells treated with both HGF and ephrin-A1, RhoA activation level and kinetics were similar to HGF treatment alone. Therefore, RhoA activation alone could not account for the effects of ephrin-A1.
Figure 6.
Ephrin-A1 differentially regulates HGF-induced activation of Rho GTPases in MDCK cells. (A) Ephrin-A1 has no significant influence on HGF-induced RhoA activity. Active RhoA was precipitated using GST-Rhotekin. The GST pull-down materials were analyzed by immunoblot with anti-RhoA. Quantitative analysis is shown in the right panel. (B) Ephrin-A1 suppresses the activation of PAK by HGF. Total cell lysates were analyzed for the activation of PAK by immunoblot using a previously described phospho-PAK antibody (Shamah et al., 2001). (C) Rac1 (but not Cdc42) activation by HGF is inhibited by ephrin-A1. Active Rac1/Cdc42 was precipitated using GST-PAK-CD. The precipitates were analyzed by immunoblot with anti-Rac1 or anti-Cdc42. Representative results from three or more independent experiments are shown.
Recent evidence shows that it is the balance between RhoA and Rac1 activities that determines biological processes controlled by Rho GTPases (Shamah et al., 2001; Etienne-Manneville and Hall, 2002). Therefore, we next measured the activation status of p21-activated kinase (PAK), a downstream effector of Rac1 and Cdc42. Immunoblot with a previously characterized antibody (Shamah et al., 2001) revealed a dramatic increase in PAK phosphorylation in cells treated with HGF (Fig. 6 B). In contrast, no significant effect was observed with ephrin-A1–Fc treatment. Importantly, the HGF-induced PAK activation was suppressed in the presence of ephrin-A1 (Fig. 6 B). Consistent with the PAK activation status, HGF treatment stimulated Rac1 GTP-loading and cotreatment with ephrin-A1–Fc prevented Rac1 activation (Fig. 6 C). Cdc42 activity was moderately stimulated by HGF treatment alone, the pattern of which was not significantly altered by ephrin-A1 cotreatment (Fig. 6 C). Together, these data suggest that, although both HGF and ephrin-A1 activate RhoA, they exert different effects on Rac1. By suppressing the stimulatory effects of HGF on Rac1 while maintaining RhoA activity, ephrin-A1 tilted the balance among different GTPase activities in favor of RhoA. The relative increase in RhoA activity may be responsible for the significant preservation of actin stress fibers in cells treated with both HGF and ephrin-A1 compared with those treated with HGF alone (Fig. 5).
Inhibition of RhoA-dependent signaling attenuates the negative regulation of branching morphogenesis by EphA kinases
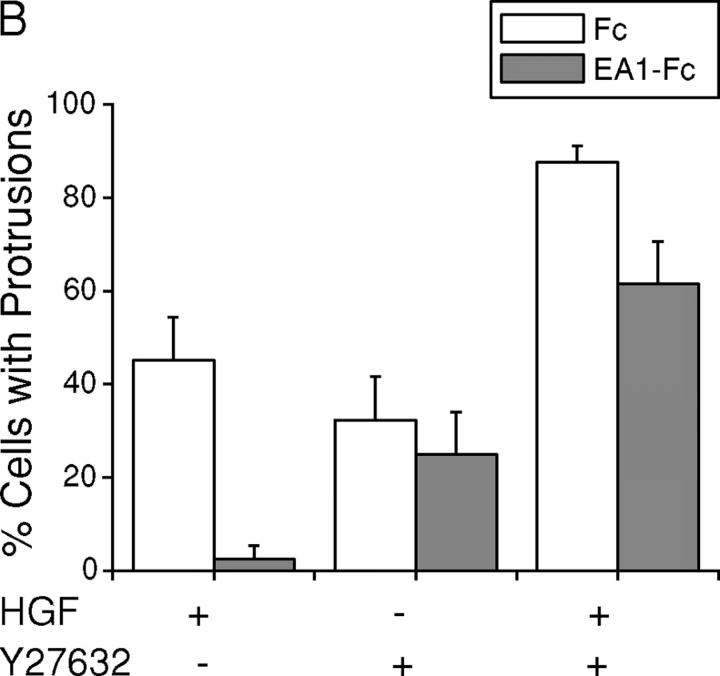
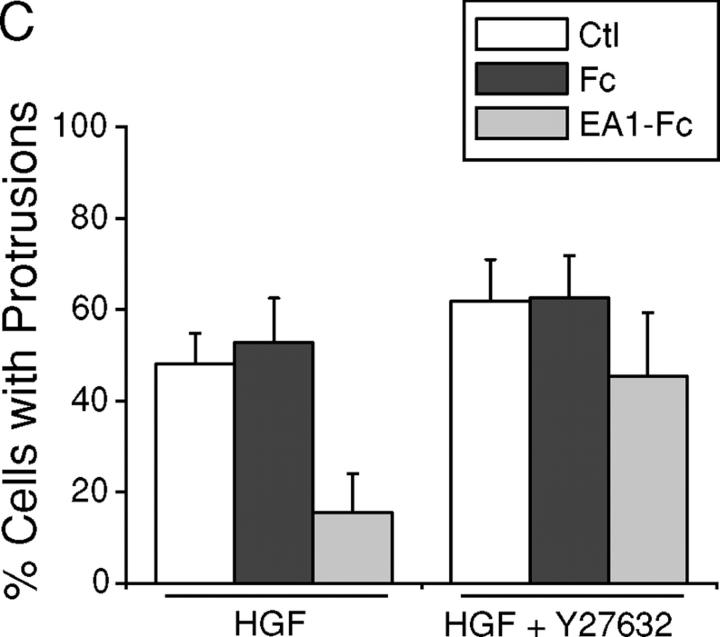
To directly assess the functional significance of relative increase in Rho activity in the regulation of branching morphogenesis, we tested the effects of Rho-associated kinase (ROCK) inhibitor Y27632 and dominant-negative RhoA (19N). Strikingly, Y27632 by itself induced outgrowth of cell processes in collagen gels, albeit to a less extent than HGF treatment, suggesting that basal activity of ROCK is important for stabilizing the epithelial cell membrane and preventing sprouting of membrane processes (Fig. 7, A and B). Cotreatment with Y27632 and HGF resulted in precocious branching morphogenesis, far exceeding HGF treatment alone (Fig. 7, A and B). Moreover, in the presence of Y27632, HGF-induced cell protrusions became resistant to the inhibitory effect of ephrin-A1 (Fig. 7, A and B). Similarly, Y27632 largely prevented the collapse of preexisting cell protrusions by ephrin-A1 (Fig. 7 C), indicating that EphA kinases require Rho/ROCK-mediated signaling to induce the collapsive response.
Figure 7.
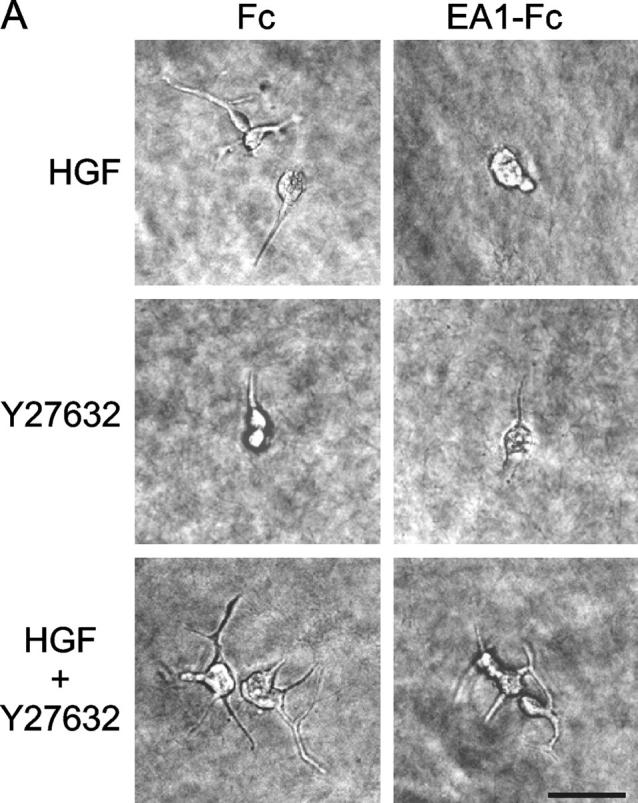
Inhibition of ROCK enhances HGF-induced cell protrusions and attenuates the inhibitory effect of ephrin-A1. (A) Y27632 significantly attenuates ephrin-A1–induced inhibition of branching morphogenesis. MDCK branching morphogenesis assay was performed in the absence or presence of 10 μM Y27632. Cell images were taken 24 h later. Bar, 40 μm. (B) Quantitation of branching morphogenesis results in A. Values represent means from six randomly selected fields ± SD. (C) Y27632 inhibits ephrin-A1–induced collapse of preexisting membrane processes. Cell protrusions were induced in collagen gel by HGF in the presence of Y27632 for 24 h. Ephrin-A1–Fc was then added to the growth medium containing fresh HGF and Y27632. Branching morphogenesis response was recorded. Values represent means from six randomly selected fields ± SD.
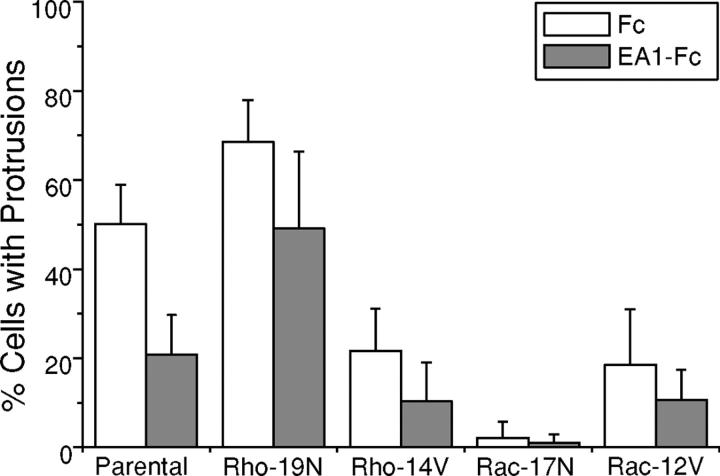
To further confirm these observations, we used the previously characterized MDCK cells that inducibly express dominant-negative RhoA (19N), dominant-active RhoA(14V), dominant-negative Rac1 (17N), or dominant-active Rac1 (12V; Jou and Nelson, 1998). Consistent with the effects of Y27632, enhanced branching morphogenesis was observed in MDCK cells inducibly expressing dominant-negative RhoA (19N). Moreover, the inhibitory effect of ephrin-A1–Fc was substantially negated by expression of RhoA-19N mutant (Fig. 8). By contrast, the inducible expression of dominant-active RhoA (14V) significantly inhibited the branching morphogenesis.
Figure 8.
Dominant-negative RhoA substantially abolishes the inhibitory effect of ephrin-A1 on cell protrusions. Expression of dominant-negative and dominant-active RhoA and Rac1 in MDCK cells was induced by removing tetracycline from the culture medium for 2 d. Branching morphogenesis assays were conducted as described in Materials and methods. After a 24-h stimulation with HGF in the presence of ephrin-A1–Fc or Fc, cells with membrane processes longer than the diameters of cell bodies were counted. Values represent means from six randomly selected fields, ± SD.
C-Met activation by HGF led to robust Rac1 signaling in MDCK cells, which was largely negated by ephrin-A1 cotreatment. If Rac1 signaling plays a significant role in HGF-induced branching morphogenesis, dominant-negative Rac1 (17N) is expected to inhibit the process. This indeed turns out to be the case, as induced expression of dominant-negative Rac1 (17N) completely abolished sprouting of cell protrusions (Fig. 8). Intriguingly, dominant-active Rac1 (12V) also caused a decreased branching morphogenesis, instead of the predicted stimulatory effects (Fig. 8). Such unexpected effects of dominant-active Rac1 have also been reported in other systems (Etienne-Manneville and Hall, 2001). One explanation suggested is that GTP–GDP exchange may be required for proper Rac1 function. Alternatively, Rac1 activation may be subject to tight spatial control; in which case global activation can lead to unintended targets and consequences.
In sum, these data suggest that tilting the balance in favor of RhoA activation represents a key mechanism by which EphA kinase activation inhibits cell extension, the initiation step of branching morphogenesis. However, it should be noted that differential regulation of Rho GTPase activities is unlikely to be the only mechanism through which EphA activation inhibits the membrane protrusion step of branching morphogenesis. In the presence of Y27632 or dominant-negative RhoA (19N), ephrin-A1 treatment could still inhibit ∼20% of the morphogenetic effects of HGF. Other yet unidentified mechanisms may also play a minor role.
One of the EphA kinases, EphA2, is selectively expressed on a UB that is undergoing branching morphogenesis
In addition to MDCK cells, we also found potent inhibition of branching morphogenesis of mouse murine inner medullary-collecting duct cells in three-dimensional collagen gel (unpublished data). Both cell types are widely used as in vitro models of renal branching morphogenesis (Pohl et al., 2000). Next, we investigated potential in vivo relevance of our findings during early fetal kidney development, which is characterized by repeated branching of a UB upon reciprocal induction by metanephric mesenchyme (Saxen, 1987; Vainio and Lin, 2002). To this end, we examined expression of EphA2 in embryonic kidneys using the previously described EphA2-lacZ knockout mice (line KST085; Mitchell et al., 2001). Immunoblot revealed that fetal kidneys express multiple EphA kinases, including EphA1, EphA2, EphA3, and EphA4 (unpublished data), which may account for the lack of renal phenotypes in the EphA2-null mice. Nevertheless, the β-gal reporter gene expression in KST085 EphA2-lacZ mice enables us to precisely localize the expression of endogenous EphA2. E12.5 kidneys were dissected from heterozygous embryos, cultured overnight to allow attachment, photographed (Fig. 9, A and C), and stained for β-gal activity (Fig. 9 B). EphA2 was selectively expressed in UB cells (Fig. 9 B) that were marked by cytokeratin expression (Fig. 9 D). Very low or undetectable expression was observed in metanephric mesenchyme that was marked by the expression of Pax-2 (compare Fig. 9 B with Fig. 9 D). EphA2 was also not detected in tubular epithelial cells of developing nephrons derived from metanephric mesenchyme after longer organ culture (unpublished data). The preferential expression of EphA2 in UB epithelial cells undergoing active branching morphogenesis suggests that EphA2 is positioned to play a potential regulatory role in renal organogenesis.
Figure 9.
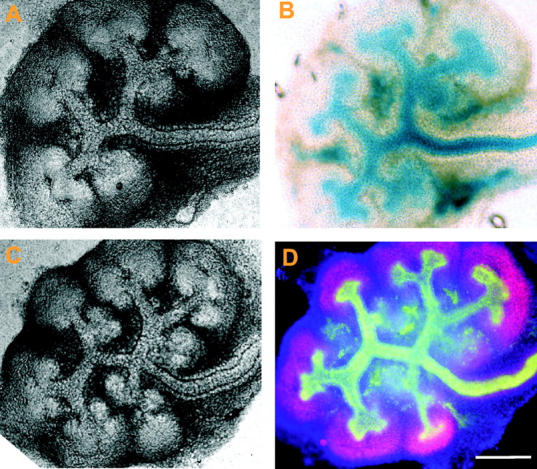
EphA2 is selectively expressed on UBs of embryonic kidneys. E12.5 kidneys were dissected from the heterozygous EphA2-lacZ knockout embryos. They were cultured overnight, photographed (A and C) and processed for X-gal staining (B). Alternatively, kidneys were fixed with methanol and stained with anti-cytokeratin (green) and anti-Pax-2 (red) to mark UB epithelial cells and metanephric mesenchyme, respectively. DAPI (blue) was used to stain all nuclei (D). Bar, 100 μm.
Discussion
Eph kinases and ephrins are widely distributed in epithelial cells in vitro and in vivo. However, their role in epithelial cell biology remains incompletely understood. Using the well-characterized MDCK cell model system, we show here for the first time that activation of endogenous EphA kinases negatively regulates HGF-induced epithelial branching morphogenesis in three-dimensional collagen matrix. Cotreatment with HGF and ephrin-A1 reduced the sprouting of cell protrusions, an early step in branching morphogenesis. Ephrin-A1 stimulation after HGF treatment induced collapse and retraction of preexisting protrusions. We further demonstrate that a tilt in the balance between RhoA and Rac1 activities in favor of RhoA, but not the inhibition of ERK1/2 MAPK, plays an important role in regulating HGF-induced branching morphogenesis by EphA kinases. Finally, one of the EphA kinases, EphA2, is selectively expressed on a UB epithelium that is undergoing active branching morphogenesis, suggesting the in vivo relevance of these findings.
Epithelial branching morphogenesis is a fundamental biological process underlying the development of many vital organs such as kidney, lung, breast, and prostate. In renal development, the process is initiated by the invasion of UB epithelial cells into metanephric mesenchyme in response to the glial-derived neurotrophic factor (GDNF). GDNF is secreted by metanephric mesenchyme and acts on Ret receptor on UB cells. The initial induction is followed by repetitive migration, proliferation, and branching of UBs that eventually form the collecting system, and differentiation of metanephric mesenchyme that generate filtering nephrons (Saxen, 1987; Piscione and Rosenblum, 2002; Vainio and Lin, 2002). Microscopically, an early event is the extension of membrane processes from UB epithelial cells facing the inductive mesenchyme (Davies et al., 1995; Tepass et al., 2000). Epithelial cells of kidney origin have been used to investigate cellular processes and the underlying molecular mechanisms of branching morphogenesis. Here, we found that activation of endogenous EphA kinases in MDCK cells inhibited HGF-induced extension of new cell protrusions, and caused collapse of existing ones.
The in vivo relevance of our findings remains to be determined, although our preliminary analyses reveal that one of the EphA kinases (EphA2) is selectively expressed in UB epithelial cells of embryonic kidneys. Because ephrins are membrane anchored, Eph–ephrin interaction and signaling necessitate cell–cell contact. Epithelial organogenesis involves migration and reorganization of epithelial cells in precise spatial patterns, which will encounter different types of cell–cell contacts including epithelial–epithelial and epithelial–stromal interactions. The in vitro results here show that EphA activation inhibited MDCK scattering and directional cell migration in two-dimensional assays, and caused collapse of membrane processes in three-dimensional collagen gels. Conceivably, such negative guidance mechanisms can also operate in vivo, and may help determine the direction and space of branching morphogenesis.
HGF-induced branching morphogenesis is initiated by the binding to its receptor c-Met, which triggers autophosphorylation of the receptor and the recruitment of various proteins; many of these proteins are capable of activating the Ras-MAPK signaling pathway (Furge et al., 2000). Our previous data demonstrate that endogenous EphA kinases mediate down-regulation of Ras-MAPK signaling in several different cell types (Miao et al., 2001). Here, we show that activation of endogenous EphA kinases can also inhibit the basal ERK1/2 MAPK activity in MDCK cells. However, the inhibitory effect of ephrin-A1–Fc on MAPK was not strong enough to override the stimulatory effect of HGF on MAPK. Moreover, in MDCK cells transiently transfected with dominant-active Raf1, ephrin-A1–Fc still inhibited branching morphogenesis. These data suggest that EphA kinases negatively regulate epithelial branching morphogenesis via Raf/MAPK-independent mechanisms; other mechanisms that are more sensitive to regulation by EphA kinases are involved in regulating epithelial morphogenesis.
The Rho family small GTPases control actin cytoskeleton and cell shape. Rac1 activation leads to localized actin polymerization at the cell periphery, resulting in the formation of lamellipodia, and activation of Cdc42 stimulates the formation of filopodia (Ridley et al., 1992; Kozma et al., 1995; Nobes and Hall, 1995). RhoA acts via its downstream effector ROCK, stimulates myosin light chain phosphorylation, and regulates cell contractility. ROCK together with another downstream effecter of Rho, Dia, induces stress fiber formation (Kaibuchi et al., 1999; Ridley, 1999). Branching morphogenesis entails highly coordinated membrane process formation, cell shape change, and cell reorganization, much of which involves dynamic regulation of actin cytoskeleton. For this reason, Rho GTPases have been suspected as key regulators of branching morphogenesis. Our results support this notion, and demonstrate that the balance between different members of Rho GTPases critically regulates epithelial morphogenesis. First, Y27632 treatment alone was sufficient to induce extension of membrane processes, and could potently synergize with HGF. By inhibiting RhoA signaling to ROCK, Y27632 is expected to tilt the balance in favor of Rac1. Second, supporting an important role of Rac1 signaling in epithelial morphogenesis, HGF potently stimulates Rac1 signaling, and dominant-negative Rac1 blocked its morphogenetic effects. Third, ephrin-A1 attenuated the morphogenetic effects of HGF by suppressing Rac1 signaling while leaving RhoA in the active state. When RhoA activity was prevented either by Y27632 or dominant-negative RhoA (19N), ephrin-A1 lost most of its inhibitory effects on morphogenesis. Thus, epithelial branching morphogenesis is promoted either by a relative decrease in RhoA activity or by a relative increase in Rac1 activity.
Eph kinase regulation of Rho GTPases has been reported in the repulsive guidance of axonal growth cones and dendritic spine morphogenesis (Wahl et al., 2000; Ethell et al., 2001; Penzes et al., 2001; Shamah et al., 2001; Irie and Yamaguchi, 2002). Stimulation of ganglion axons with ephrin-A5 caused activation of RhoA and induced growth cone collapse (Wahl et al., 2000). Using yeast two-hybrid screening, Shamah et al. (2001) found that EphA kinases directly associate with a guanine nucleotide exchange factor called ephexin. Moreover, ephexin mediates RhoA activation and Rac1/Cdc42 inactivation upon EphA kinase ligation, leading to the collapse of axonal growth cones (Shamah et al., 2001). Morphologically, HGF-induced formation of membrane protrusions in MDCK cells is analogous to sprouting of neurites from neurons upon neurotrophic factor stimulation (da Silva and Dotti, 2002). After neuronal polarization, one of the neurites becomes an axon. Here, we found that similar to repulsive guidance of axons, EphA activation negatively regulated epithelial branching morphogenesis. Furthermore, ephrin-A1 was able to suppress HGF-induced activation of Rac1 signaling while simultaneously maintaining RhoA activation. Because ephexin is preferentially expressed in the nervous system, further investigation is necessary to identify the EphA effector proteins that mediate suppression of Rac1 activation by HGF in MDCK cells.
Cell dissociation is an early phase of HGF-induced formation of branched tubular structures. Altered cell–cell and cell–matrix adhesion can regulate this phenotype change, for instance from a dispersed pattern to an aggregated one, or vice versa. We have observed that ephrin-A1 binding to EphA kinases resulted in a decreased basal as well as HGF-stimulated MDCK cell adhesion to fibronectin, consistent with what we have reported earlier in PC-3 cells (Miao et al., 2000). The weakened cell–matrix adhesion was associated with the acquisition of a more compact morphology of MDCK cell colonies, possibly reflecting enhanced cell–cell adhesion (unpublished data).
In summary, the report here describes a novel function of EphA kinases in regulating epithelial branching morphogenesis in the well-characterized MDCK model systems. Moreover, our data demonstrate that Rho GTPases are the converging point of cell signaling by EphA and c-Met RTKs that negatively and positively regulate branching morphogenesis, respectively. A relative increase in RhoA activity is largely responsible for the effects of EphA activation in preserving actin stress fibers and inhibiting branching morphogenesis. Further investigation is required to assess the various roles of EphAs and ephrin-As in orchestrating diverse environmental cues that in concert control epithelial organogenesis in vitro and in vivo.
Materials and methods
Cell culture
MDCK clone 8 (Nelson and Veshnock, 1986) and murine inner medullary-collecting duct cells were grown in DME supplemented with 10% FBS and 100 U/ml penicillin-streptomycin. MDCK cells inducibly expressing RhoA(14V), RhoA(19N), Rac1(12V), and Rac1(17N) were gifts of Dr. W. James Nelson (Stanford University School of Medicine, Stanford, CA). Induction of gene expression was performed as described previously (Jou and Nelson, 1998).
Antibodies and reagents
Ephrin-A1–Fc was produced as described previously (Davis et al., 1994; Pandey et al., 1994). Rabbit polyclonal anti-EphA2 antibody, anti-ERK and anti-RhoA, mouse monoclonal anti-phosphotyrosine (PY99), and anti-p-ERK and anti-Raf1 were from Santa Cruz Biotechnology, Inc. Polyclonal anti-p-PAK was provided by Dr. Michael E. Greenberg (Harvard Medical School, Boston, MA) (Shamah et al., 2001). Mouse monoclonal anti-Rac1 and anti-Cdc42 were purchased from BD Biosciences. Human Fc fragment and FITC-conjugated goat anti–human Fc were purchased from Jackson ImmunoResearch Laboratories. Texas red–conjugated phalloidin was obtained from Molecular Probes, Inc. Recombinant human HGF was purchased from R&D Systems. Y27632 was obtained from Calbiochem.
Branching morphogenesis assays in three-dimensional collagen gels
For each ml of the gel, 800 μl 2.5 mg/ml type I collagen (BD Biosciences) was mixed with 100 μl 10× DME/F12 (GIBCO BRL), 10 μl 1 M Hepes, pH 7.4, and 5 μl 1 M Na2HCO3. 0.1 N NaOH was used to neutralize the pH. Cells were detached using trypsin-EDTA and suspended at a concentration of 2–5 × 104 cells /ml in collagen gel, after the volume has been adjusted to 1 ml. 100-μl aliquots of the cell suspension were dispensed in 96-well plates and allowed to gel for 30 min at 37°C before adding medium. After adjustment of growth in collagen gel, 20 ng/ml recombinant human HGF or 1 μg/ml ephrin-A1–Fc was added as indicated. The medium was changed every day. Branching morphogenesis was recorded using an inverted microscope (model DM IRE2; Leica) equipped with a digital camera (CoolSNAP cf; Photometrics).
Immunofluorescence
Cells plated on coverslips were chilled down on ice and then incubated with 2 μg/ml ephrin-A1–Fc or Fc for 1 h. At the end of the incubation, cells were washed with ice-cold PBS and fixed with 3.7% PFA on ice for 20 min. Cells were then blocked with 2% goat serum and 3% BSA in PBS for 1 h at RT followed by incubation with FITC-conjugated goat anti–human Fc for 30 min. For actin cytoskeleton analysis, Texas red–conjugated phalloidin was used. After the wash cycles, the coverslips were mounted on slides using vector-shield mounting medium containing DAPI.
SOIL assay
Ephrin-A1–Fc or Fc was coated on 6-well cluster culture plates at the indicated densities in PBS overnight at 4°C and washed with PBS. MDCK cells were detached with trypsin-EDTA and plated on coverslips in 24-well plates. When cells reached near confluence, coverslips were transferred cell-face-up onto the coated or uncoated 6-well dishes containing 1.5 ml control medium or medium supplemented with 20 ng/ml HGF. Coverslips were gently pressed down with pipette tips. Up to three coverslips were placed in each well. After 24–48 h of culture, cells were fixed with 3.7% PFA for 20 min and stained with crystal violet. Coverslips were removed, and the rings of cells that had scattered onto the immobilized Fc or ephrin-A1–Fc were photographed.
Cell migration assay
The Transwell (Costar) was coated by spotting 100 ng FN in 10 μl PBS onto the underside of the filter membrane, and allowing to air-dry. Both sides of the Transwell were then coated with 10 μg/ml collagen overnight. MDCK cells were detached with trypsin-EDTA and washed three times with serum-free medium and suspended in a concentration of 106 cells/ml in serum-free medium containing 0.1% BSA. Cell suspension in 100 μl was added to the upper chamber of the Transwell. The same medium containing 20 ng/ml HGF and 1 μg/ml ephrin-A1–Fc or Fc was added to the lower chamber. After overnight incubation at 37°C, cells on both sides were fixed and stained with crystal violet. Cells retained on the upper side were gently wiped off with Q-tips®. Cells migrating through the filter were counted from six high power fields.
Transient transfection
About 40% confluent MDCK cells were transfected with plasmid encoding Raf1-BXB together with that encoding GFP (3:1) using LipofectAMINE™ Plus transfection reagents (GIBCO BRL). 24 h after transfection, cells were detached with trypsin-EDTA and processed for branching morphogenesis assay as described above.
GST pull-down assay
After stimulation, subconfluent MDCK cells were washed once with ice-cold PBS and lysed with a buffer containing 50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 100 mM NaCl, 10% glycerol, 5 mM MgCl2, phosphatase inhibitor cocktail (Sigma-Aldrich), and protease inhibitors on ice for 5 min. The lysate was clarified at 14,000 rpm at 4°C for 5 min. Clarified cell lysate was incubated with GST-Rhotekin– or GST-PAK-CD–coupled beads (Ren et al., 1999) at 4°C for 30 min. After washing three times with lysis buffer, the beads were suspended in SDS-PAGE sample buffer.
Stimulation, immunoprecipitation, and immunoblot
Cells were stimulated with 1 μg/ml ephrin-A1–Fc in the absence or presence of 20 ng/ml HGF for the indicated times, and then were processed for immunoprecipitation and immunoblot as described previously (Miao et al., 2000). For quantitative analysis, band densities were measured using Scion Image software from the National Institutes of Health (NIH; Bethesda, MD).
Kidney organ culture and staining
EphA2 knockout mice (line KST085) were described previously (Mitchell et al., 2001). Heterozygous E12.5 kidneys were dissected and immediately grown at the air–liquid interface on 0.45-μm Transwells at 37°C, 100% humidity, and 5% CO2, in serum-free Ideal Explant Media consisting of DME/F12 (Hepes and l-glutamine) with 45 mM NaHCO3, penicillin-streptomycin, 1× ITS (insulin-transferring selenium; GIBCO BRL), 2 nM T3, 70 nM PGE2, and 100 nM trans-retinoic acid (Sigma-Aldrich).
For X-gal staining, after overnight culture kidneys were fixed for 30 min in a fixative (60 mM Na2HPO4, 40 mM NaH2PO4, 1% formaldehyde, 0.2% glutaldehyde, and 0.02% NP-40 for 30 min), washed with PBS twice, and stained overnight at 30°C with X-gal in a staining solution (60 mM Na2HPO4, 40 mM NaH2PO4, 2 mM MgCl2, 1 mM K3Fe(CN)3, 1 mM K4Fe(CN)6, and 0.1% X-gal).
Staining with antibodies against Pax-2 and cytokeratin was essentially performed as described previously, with modifications (Cho et al., 1998). In brief, cultured kidneys were fixed with 100% methanol for 15 min, washed with PBS/0.1% Tween 20 (PBST), and blocked for 3 h in blocking solution (BS; 2% BSA and 1% goat serum in PBST). The kidneys were incubated with 3 μg/ml rabbit anti-Pax-2 (Zymed Laboratories) and 10 μg/ml mouse anti-pan-cytokeratin (Sigma-Aldrich) in BS for 2 h at 37°C, washed overnight, and then incubated with FITC-conjugated anti-mouse and Red-X–conjugated anti–rabbit antibodies in BS for 2 h at 37°C. After washing with PBST over 3 h, a drop of DAPI-containing mounting solution (Vector Laboratories) was added, and the images of whole kidney were collected on a digital camera.
Acknowledgments
We thank Dr. Michael E. Greenberg for anti-phospho-PAK antibody, and Dr. W. James Nelson for MDCK cells inducibly expressing mutant Rho GTPases. We are grateful to Dr. Martha Konieczkonski for advice in the GST-Rhotekin pull-down assay and to Nancy Liu for technological assistance in fusion protein preparation.
This work is supported by grants to B. Wang from Department of the Army (DAMD17-99-1-9019), the NIH (CA96533, CA92259), and to L.G. Cantley by the NIH (DK54911).
Abbreviations used in this paper: HGF, hepatocyte growth factor/scatter factor; PAK, p21-activated kinase; ROCK, Rho-associated kinase; RTK, receptor tyrosine kinases; SOIL, scattering onto immobilized ligands; UB, ureteric bud.
References
- Adams, R.H., and R. Klein. 2000. Eph receptors and ephrin ligands. Essential mediators of vascular development. Trends Cardiovasc. Med. 10:183–188. [DOI] [PubMed] [Google Scholar]
- Affolter, M., S. Bellusci, N. Itoh, B. Shilo, J.P. Thiery, and Z. Werb. 2003. Tube or not tube. Remodeling epithelial tissues by branching morphogenesis. Dev. Cell. 4:11–18. [DOI] [PubMed] [Google Scholar]
- Boros, P., and C.M. Miller. 1995. Hepatocyte growth factor: a multifunctional cytokine. Lancet. 345:293–295. [DOI] [PubMed] [Google Scholar]
- Brinkmann, V., H. Foroutan, M. Sachs, K.M. Weidner, and W. Birchmeier. 1995. Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J. Cell Biol. 131:1573–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, N., D.M. Brantley, and J. Chen. 2002. The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev. 13:75–85. [DOI] [PubMed] [Google Scholar]
- Cho, E.A., L.T. Patterson, W.T. Brookhiser, S. Mah, C. Kintner, and G.R. Dressler. 1998. Differential expression and function of cadherin-6 during renal epithelium development. Development. 125:803–812. [DOI] [PubMed] [Google Scholar]
- Coulthard, M.G., J.D. Lickliter, N. Subanesan, K. Chen, G.C. Webb, A.J. Lowry, S. Koblar, C.D. Bottema, and A.W. Boyd. 2001. Characterization of the Epha1 receptor tyrosine kinase: expression in epithelial tissues. Growth Factors. 18:303–317. [DOI] [PubMed] [Google Scholar]
- da Silva, J.S., and C.G. Dotti. 2002. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat. Rev. Neurosci. 3:694–704. [DOI] [PubMed] [Google Scholar]
- Dalva, M.B., M.A. Takasu, M.Z. Lin, S.M. Shamah, L. Hu, N.W. Gale, and M.E. Greenberg. 2000. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 103:945–956. [DOI] [PubMed] [Google Scholar]
- Davies, J., M. Lyon, J. Gallagher, and D. Garrod. 1995. Sulphated proteoglycan is required for collecting duct growth and branching but not nephron formation during kidney development. Development. 121:1507–1517. [DOI] [PubMed] [Google Scholar]
- Davis, S., N.W. Gale, T.H. Aldrich, P.C. Maisonpierre, V. Lhotak, T. Pawson, M. Goldfarb, and G.D. Yancopoulos. 1994. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 266:816–819. [DOI] [PubMed] [Google Scholar]
- Ethell, I.M., F. Irie, M.S. Kalo, J.R. Couchman, E.B. Pasquale, and Y. Yamaguchi. 2001. Ephb/syndecan-2 signaling in dendritic spine morphogenesis. Neuron. 31:1001–1013. [DOI] [PubMed] [Google Scholar]
- Eph Nomenclature Committee. 1997. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell. 90:403–404. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and A. Hall. 2001. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 106:489–498. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature. 420:629–635. [DOI] [PubMed] [Google Scholar]
- Fisher, C.E., L. Michael, M.W. Barnett, and J.A. Davies. 2001. Erk MAP kinase regulates branching morphogenesis in the developing mouse kidney. Development. 128:4329–4338. [DOI] [PubMed] [Google Scholar]
- Flanagan, J.G., and P. Vanderhaeghen. 1998. The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 21:309–345. [DOI] [PubMed] [Google Scholar]
- Furge, K.A., Y.W. Zhang, and G.F. Vande Woude. 2000. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 19:5582–5589. [DOI] [PubMed] [Google Scholar]
- Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science. 279:509–514. [DOI] [PubMed] [Google Scholar]
- Holder, N., and R. Klein. 1999. Eph receptors and ephrins: effectors of morphogenesis. Development. 126:2033–2044. [DOI] [PubMed] [Google Scholar]
- Irie, F., and Y. Yamaguchi. 2002. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat. Neurosci. 5:1117–1118. [DOI] [PubMed] [Google Scholar]
- Jou, T.S., and W.J. Nelson. 1998. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J. Cell Biol. 142:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi, K., S. Kuroda, and M. Amano. 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68:459–486. [DOI] [PubMed] [Google Scholar]
- Karihaloo, A., S.A. Karumanchi, J. Barasch, V. Jha, C.H. Nickel, J. Yang, S. Grisaru, K.T. Bush, S. Nigam, N.D. Rosenblum, et al. 2001. Endostatin regulates branching morphogenesis of renal epithelial cells and ureteric bud. Proc. Natl. Acad. Sci. USA. 98:12509–12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma, R., S. Ahmed, A. Best, and L. Lim. 1995. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 15:1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger, D.A., and A.F. Horwitz. 1996. Cell migration: a physically integrated molecular process. Cell. 84:359–369. [DOI] [PubMed] [Google Scholar]
- Lindberg, R.A., and T. Hunter. 1990. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol. Cell. Biol. 10:6316–6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubarsky, B., and M.A. Krasnow. 2003. Tube morphogenesis. Making and shaping biological tubes. Cell. 112:19–28. [DOI] [PubMed] [Google Scholar]
- Luo, L. 2000. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1:173–180. [DOI] [PubMed] [Google Scholar]
- Miao, H., E. Burnett, M.S. Kinch, E. Simon, and B. Wang. 2000. EphA2 kinase activation suppresses integrin function and causes focal adhesion kinase dephosphorylation. Nat. Cell Biol. 2:62–69. [DOI] [PubMed] [Google Scholar]
- Miao, H., B.R. Wei, D.M. Peehl, Q. Li, T. Alexandrou, J.R. Schelling, J.S. Rhim, J.R. Sedor, E. Burnett, and B. Wang. 2001. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat. Cell Biol. 3:527–530. [DOI] [PubMed] [Google Scholar]
- Mitchell, K.J., K.I. Pinson, O.G. Kelly, J. Brennan, J. Zupicich, P. Scherz, P.A. Leighton, L.V. Goodrich, X. Lu, B.J. Avery, et al. 2001. Functional analysis of secreted and transmembrane proteins critical to mouse development. Nat. Genet. 28:241–249. [DOI] [PubMed] [Google Scholar]
- Montesano, R., K. Matsumoto, T. Nakamura, and L. Orci. 1991. a. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 67:901–908. [DOI] [PubMed] [Google Scholar]
- Montesano, R., G. Schaller, and L. Orci. 1991. b. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. 28. Cell. 66:697–711. [DOI] [PubMed] [Google Scholar]
- Nelson, W.J., and P.J. Veshnock. 1986. Dynamics of membrane-skeleton (fodrin) organization during development of polarity in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 103:1751–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes, C.D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 81:53–62. [DOI] [PubMed] [Google Scholar]
- Pandey, A., D.F. Lazar, A.R. Saltiel, and V.M. Dixit. 1994. Activation of the Eck receptor protein tyrosine kinase stimulates phosphatidylinositol 3-kinase activity. J. Biol. Chem. 269:30154–30157. [PubMed] [Google Scholar]
- Penzes, P., R.C. Johnson, R. Sattler, X. Zhang, R.L. Huganir, V. Kambampati, R.E. Mains, and B.A. Eipper. 2001. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 29:229–242. [DOI] [PubMed] [Google Scholar]
- Piscione, T.D., and N.D. Rosenblum. 2002. The molecular control of renal branching morphogenesis: current knowledge and emerging insights. Differentiation. 70:227–246. [DOI] [PubMed] [Google Scholar]
- Pohl, M., R.O. Stuart, H. Sakurai, and S.K. Nigam. 2000. Branching morphogenesis during kidney development. Annu. Rev. Physiol. 62:595–620. [DOI] [PubMed] [Google Scholar]
- Potempa, S., and A.J. Ridley. 1998. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol. Biol. Cell. 9:2185–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, X.D., W.B. Kiosses, and M.A. Schwartz. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, C., A. Ebner, and M. Affolter. 2002. In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. Dev. Cell. 2:677–683. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J. 1999. Stress fibres take shape. Nat. Cell Biol. 1:E64–E66. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., H.F. Paterson, C.L. Johnston, D. Diekmann, and A. Hall. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 70:401–410. [DOI] [PubMed] [Google Scholar]
- Royal, I., and M. Park. 1995. Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J. Biol. Chem. 270:27780–27787. [DOI] [PubMed] [Google Scholar]
- Santos, O.F., and S.K. Nigam. 1993. HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta. Dev. Biol. 160:293–302. [DOI] [PubMed] [Google Scholar]
- Saxen, L. 1987. Organogenesis of the kidney. Cambridge University Press, Cambridge, MA.. 173 pp.
- Shamah, S.M., M.Z. Lin, J.L. Goldberg, S. Estrach, M. Sahin, L. Hu, M. Bazalakova, R.L. Neve, G. Corfas, A. Debant, and M.E. Greenberg. 2001. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 105:233–244. [DOI] [PubMed] [Google Scholar]
- Soriano, J.V., M.S. Pepper, T. Nakamura, L. Orci, and R. Montesano. 1995. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J. Cell Sci. 108:413–430. [DOI] [PubMed] [Google Scholar]
- Sutherland, D., C. Samakovlis, and M.A. Krasnow. 1996. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 87:1091–1101. [DOI] [PubMed] [Google Scholar]
- Tepass, U., K. Truong, D. Godt, M. Ikura, and M. Peifer. 2000. Cadherins in embryonic and neural morphogenesis. Nat. Rev. Mol. Cell Biol. 1:91–100. [DOI] [PubMed] [Google Scholar]
- Tuzi, N.L., and W.J. Gullick. 1994. eph, the largest known family of putative growth factor receptors. Br. J. Cancer. 69:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio, S., and Y. Lin. 2002. Coordinating early kidney development: lessons from gene targeting. Nat. Rev. Genet. 3:533–543. [DOI] [PubMed] [Google Scholar]
- Wahl, S., H. Barth, T. Ciossek, K. Aktories, and B.K. Mueller. 2000. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 149:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, A.Z., J.C. Wang, G.K. Ojakian, and W.J. Nelson. 1994. Determinants of apical membrane formation and distribution in multicellular epithelial MDCK cysts. Am. J. Physiol. 267:C473–C481. [DOI] [PubMed] [Google Scholar]
- Weidner, K.M., M. Sachs, and W. Birchmeier. 1993. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J. Cell Biol. 121:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, D.G. 2000. Eph receptors and ephrins: regulators of guidance and assembly. Int. Rev. Cytol. 196:177–244. [DOI] [PubMed] [Google Scholar]
- Wilkinson, D.G. 2001. Multiple roles of EPH receptors and ephrins in neural development. Nat. Rev. Neurosci. 2:155–164. [DOI] [PubMed] [Google Scholar]